Abstract
The tetanus toxin seizure model, which is associated with spontaneous and intermittent generalized and non-generalized seizures, is considered to reflect human complex partial epilepsy. The purpose of the present study was to investigate and compare the anticonvulsant effects of carbamazepine with that of levetiracetam, a new anti-epileptic drug in this model.
One μl of tetanus toxin solution (containing 12 mLD50 μl−1 of tetanus toxin) was placed stereotactically into the rat left hippocampus resulting in generalized and non-generalized seizures. Carbamazepine (4 mg kg−1 h−1) and levetiracetam (8 and 16 mg kg−1 h−1) were administered during a 7 day period via an osmotic minipump which was placed in the peritoneal cavity.
Carbamazepine (4 mg kg−1 h−1) exhibited no significant anticonvulsant effect, compared to control, when the entire 7 day study period was evaluated but the reduction in generalized seizures was greater (35.5%) than that for non-generalized seizures (12.6%). However, during the first 2 days of carbamazepine administration a significant reduction in both generalized seizure frequency (90%) and duration (25%) was observed. Non-generalized seizures were unaffected. This time-dependent anticonvulsant effect exactly paralleled the central (CSF) and peripheral (serum) kinetics of carbamazepine in that steady-state concentrations declined over time, with the highest concentrations achieved during the first 2 days. Also there was a significant 27.3% reduction in duration of generalized seizures during the 7 day study period (P=0.0001).
Levetiracetam administration (8 and 16 mg kg−1 h−1) was associated with a dose-dependent reduction in the frequency of both generalized (39 v 57%) and non-generalized (36 v 41%) seizures. However, seizure suppression was more substantial for generalized seizures. Also a significant dose-dependent reduction in overall generalized seizure duration was observed.
These data provide experimental evidence for the clinical efficacy of levetiracetam for the management of patients with complex partial seizures. Furthermore, levetiracetam probably does not act by preventing ictogenesis per se but acts to reduce seizure severity and seizure generalization.
Keywords: Epilepsy, carbamazepine, levetiracetam, new anti-epileptic drug, tetanus toxin model, complex partial seizures
Introduction
Levetiracetam (Keppra®, ucb LO59, (S)-α-ethyl-2-oxo-pyrrolidine acetamide) is a new anti-epileptic drug which has been recently approved for clinical use in 21 countries world wide, including the USA and in Europe, as adjunctive treatment for patients with partial epilepsy that is refractory to other antiepileptic drugs. In clinical trials, levetiracetam was associated with a favourable therapeutic index consequent to its substantial antiepileptic efficacy and good tolerability profile (Kasteleijn-Nolst Trenite et al., 1996; Sharief et al., 1996; Betts et al., 2000; Cereghino et al., 2000; Ben-Menachem & Falter, 2000; Shorvon et al., 2000; Grant & Shorvon, 2000). Pre-clinical studies show no evidence of carcinogenic, mutagenic or teratogenic potential (Genton & Van Vleymen, 2000). Furthermore, levetiracetam exhibits antiepileptogenic effects in the rat kindling model of temporal lobe epilepsy (Loscher et al., 1998). The pharmacokinetic profile of levetiracetam in humans is highly favourable: it is rapidly and almost completely absorbed, pharmacokinetics are linear and it is not susceptible to kinetic interaction with concomitant antiepileptic drugs (Patsalos, 2000). Thus, levetiracetam appears to be an important addition to the current range of anti-epileptic drugs.
The exact mechanism of action of levetiracetam is not known. Although it significantly decreases spontaneous substantia nigra pars reticulata neuronal activity, which is dependent on strong GABAergic (γ-aminobutyric acid) input from the brain (Loscher et al., 1996), levetiracetam is thought to exert its anticonvulsant effect via non-GABAergic mechanisms (Margineanu & Wulfert, 1997; Sills et al., 1997). Furthermore, only the S-enantiomer of levetiracetam exhibits anticonvulsant properties and the three metabolites of levetiracetam (representing 27% of an administered levetiracetam dose) are not pharmacologically active (Klitgaard et al., 1998). The finding that the anticonvulsant activity of levetiracetam was stereoselective led to the identification of a specific binding site for levetiracetam in the rat central nervous system (Noyer et al., 1995). However, the site remains to be characterized, and its exact role in the anticonvulsant action of levetiracetam is unknown.
Levetiracetam was originally discovered by random screening to have potent efficacy against seizures induced in audiogenic mice (Gower et al., 1992). In contrast to other established anti-epileptic drugs, levetiracetam has no activity in the acute maximal electroshock seizure test or the pentylenetetrazole seizure test, models assumed to predict clinical efficacy in man (Loscher & Schmidt, 1988). However, levetiracetam, at doses well below those inducing adverse effects, is very efficacious in animal models of chronic epilepsy, including genetic (Genetic Absence Epilepsy Rat from Strasbourg, GAERS) and kindled (amygdala) rats (Gower et al., 1995; Loscher & Honack, 1993). This selective suppression of seizures in animal models of chronic epilepsy further suggests a unique mechanism and profile of action for levetiracetam.
A single injection of tetanus toxin into the dorsal hippocampus of the rat leads to a chronic epileptic syndrome in which both generalized and non-generalized seizures occur spontaneously and intermittently. This model is considered to reflect human complex partial epilepsy, in terms of type of seizures that occur, the incidence and nature of the behavioural abnormalities and the fact that anti-epileptic drugs used in the treatment of complex partial seizures, are effective in this model (Mellanby et al., 1977; 1981; 1984; 1985; Hawkins & Mellanby, 1986; 1987). Carbamazepine, considered clinically to be the drug of choice in the treatment of complex partial seizures, is particularly effective in this model (Hawkins et al., 1985).
In the present study we have used the rat tetanus toxin model of complex partial epilepsy to study the temporal anticonvulsant actions of levetiracetam. Efficacy was ascertained by measurement of EEG activity using video telemetry with concurrent measurement of blood and CSF kinetics of levetiracetam. Because the anticonvulsant action of carbamazepine is well documented in this model, we compared the action of carbamazepine with that of levetiracetam. The present study demonstrates that levetiracetam has marked anticonvulsant action at pharmacokinetically determined levetiracetam concentrations. Pilot data from this study have been previously published in abstract form (Doheny et al., 1995; 1997a, 1997b).
Methods
Animals
Male Sprague-Dawley rats (Harlen Olac, Bicester, U.K.), weighing 250 – 350 g, were housed in groups of four for 7 – 14 days prior to surgery and were allowed free access to normal laboratory diet (22 F diet, Labsure, Poole, U.K.) and water. A 12 hour light/dark cycle (light on at 06 : 00 h) was maintained.
Tetanus toxin
Tetanus toxin, as a lyophilized powder (Wellcome Research Laboratories, Beckenham, U.K.) was reconstituted under aseptic conditions using saline to a stock solution of 1200 mLD50 μl−1 and stored at −20°C. Aliquots were subsequently diluted to a final concentration of 12 mLD50 μl−1 with phosphate buffered saline which contained 0.05 M NaH2PO4/NaPO4 (pH 7.4) and 0.2% bovine serum albumin (Sigma, Poole, Dorset, U.K.) and stored at 4 – 10°C for periods up to 4 weeks.
Surgical procedures
Experimental procedures were licensed under the Animals (Scientific Procedures) Act 1986, and performed in accordance with Home Office guidelines (U.K.). Rats were anaesthetized with hypnorm/hypnovel in water (2 : 2 : 4, 3.33 ml kg−1 i.p.). One μl of tetanus toxin solution (containing 12 mLD50 μl−1 of tetanus toxin) was placed stereotactically into the left hippocampus (co-ordinates 3.0 mm posterior, 3.5 mm lateral to bregma and 3.3 mm below the neocortical surface) according to Paxinos & Watson (1986). Placement of toxin was undertaken manually using a Hamilton 7102N syringe over a 1-min period. Subsequently, bipolar recording electrodes (constructed from twisted Teflon coated stainless steel wire (diameter 0.125 mm, Medwire Corp., New York, U.S.A.) with one tip cut back to 500 – 750 μm) were placed stereotactically in the right hippocampus. The placement co-ordinates were: 3.1 mm posterior, 2.9 mm lateral and 2.5 mm below the neocortical surface (Paxinos & Watson, 1986). A third wire was connected to one of the skull anchor screws and was used to earth the animal during electroencephalographic recordings. Finally, the three wires were crimped to contact pins (AWP Electronics Ltd, Redhill, Surrey, U.K.), wires fixed to the skull using dental cement and animal sutured. Post surgery animals were housed individually in perspex cages with free access to food and water. A 12 h light/dark cycle was maintained.
Electoencephalographic (EEG) recordings
One to two days post surgery, when complete recovery from the surgical procedure occurred, EEG recordings were commenced. The headstage contacts were connected, via a slip ring to allow free movement, to a Neurolog System comprising of an AC preamplifier, an AC/DC amplifier and finally a filter (Digitimer, U.K.). The amplified hippocampal EEG signal was then digitized (1401 plus, Cambridge Electronic Design, Cambridge, U.K.) and stored on computer for off-line analysis using the SPIKE2 software (Cambridge Electronic Design, Cambridge, U.K.). The animals (six per study group) were filmed continuously during an EEG recording session using a Panasonic infra-red camera and a time-lapse video recorder so that seizures could be reviewed and categorized.
Minipump placement
Five to seven days after the onset of the tetanus toxin induced seizure syndrome, rats were re-anaesthetized, as described above, and an Alzet osmotic minipump (Charles River, U.K.) containing carbamazepine (n=6; Sigma, Poole, Dorset, U.K.), constituted in DMSO, propylene glycol and ethyl alcohol (42.5 : 42.5 : 15, v : v : v), or levetiracetam (n=6; UCB SA, Chemin du Foriest, Belgium), constituted in saline, was placed in the peritoneal cavity. Control animals (n=6) had minipumps implanted that only contained the corresponding drug vehicle.
Jugular vein and cisterna magna canulation
In order to determine the peripheral pharmacokinetics (blood) and the central neuropharmacokinetics (CSF) of levetiracetam and carbamazepine separate experiments were undertaken in normal non-seizing rats. Rats were anaesthetized with hypnorm/hypnovel in water (2 : 2 : 4, 3.33 ml kg−1 i.p.), an Alzet osmotic minipump was placed into the peritoneal cavity and catheters implanted in the cisterna magna, for CSF sampling, and the right jugular vein, for blood sampling, as previously described (Patsalos et al., 1992). In these experiments, the minipump was adapted in that a short coiled tubing (PE 40) was attached to the outlet of the minipump which represented a dead volume of osmotic diffusion of 2 days. The length of the coil was established from pilot experiments and was designed so as to delay drug delivery into the peritoneal cavity until 2 days after the placement of the minipump, at which time animals had recovered from the surgical procedure.
Carbamazepine and levetiracetam administration and blood and CSF sampling
The implanted minipumps were set to deliver into the peritoneal cavity 4 mg kg−1 h−1 carbamazepine and 8 or 16 mg kg−1 h−1 levetiracetam. Two days after surgical implantation, when complete recovery was achieved, CSF (30 μl) and blood (50 μl) samples were collected at 30 min intervals for 9 h (denoted as day 1). On four additional days (denoted as days 2, 4, 6 and 7), blood and CSF sampling was undertaken at 30 min intervals for 3.5 h. Blood and CSF samples were collected in 0.5 ml polypropylene centrifuge tubes (Treff Lab, Degersheim, Switzerland). Blood samples were centrifuged for 5 min at 11,000 g to separate the cells from the sera. Sera were transferred to new tubes and sera and CSF were stored at −70°C until required for analysis. Carbamazepine and carbamazepine epoxide, the primary pharmacologically active metabolite of carbamazepine, concentrations in sera and CSF were determined using high performance liquid chromatography (HPLC) with ultraviolet detection as previously described (Sokomba et al., 1988). Levetiracetam concentrations were similarly determined using HPLC with ultraviolet detection by the method of Ratnaraj et al. (1996).
Data analysis
All data are expressed as mean±s.e.mean, or median± interquartile range (IQR). Statistical analysis of changes in seizure frequency was performed using a two-way non-parametric analysis of variance. Seizure severity, as demonstrated by seizure duration, was analysed using a t-test. Data that were not normally distributed were analysed using the Mann – Whitney Rank Sum Test.
Results
Behaviour during the seizure syndrome and seizure course
The tetanus toxin-induced seizure syndrome typically began 1 – 3 days post administration of tetanus toxin, and onset of seizures was accompanied by a general increase in irritability and difficulty with handling. Seizures themselves produced a variety of stereotyped behavioural changes, which altered as the seizure syndrome progressed. The seizures were divided into two types: non-generalized and generalized. Non-generalized seizures included behaviours such as immobility, vibrissal or eyelid twitching and gentle head nodding and effectively corresponded to a partial seizure. During such seizures the animals were generally not aware of their surroundings, as determined by their unresponsiveness to any external stimuli or handling. Generalized seizures encompassed not only the behaviour described above, but in addition rearing with or without forelimb clonus or falling also occurred and this was considered to be equivalent to a secondarily generalized seizure.
Typically, seizures tended to cluster, with the first cluster starting 1 – 2 days post tetanus toxin administration and the second starting 3 – 5 days post tetanus toxin administration and lasting for 7 – 10 days. Peak seizure frequency occurred approximately 5 – 6 days post tetanus toxin administration (i.e. during the second cluster). Seizure type evolved during each seizure cluster so that non-generalized seizures evolved into generalized seizures. This pattern of seizure generalization also pertained when the seizure syndrome became established. Furthermore, as the seizure type evolved, the duration of the seizures became progressively longer. Thus, at the beginning of the seizure syndrome the duration of non-generalized seizures was substantially shorter than that of generalized seizures. However, as the seizure syndrome progressed and became established, the difference in seizure duration between non-generalized and generalized seizures became minimal. Seizures did not exhibit any diurnal pattern, since there was no significant difference in seizure frequency when the day and night time periods were compared (mean±s.e.mean seizures; day=655±48.7, night=691±51.3; P=0.908, Mann – Whitney rank sum test).
EEG Activity
The EEG of control animals (minipumps containing drug vehicle only) showed baseline low frequency field potential oscillations. The amplitude of the oscillations was dependent on the activity of the animal so that when the animal was sleeping the baseline was relatively flat while movement around the recording cage resulted in large amplitude field potential oscillations in the 4 – 12 Hz range. Three types of epileptiform activity were recorded: inter-ictal spikes, polyspikes and seizures, and these are similar to those previously reported (Finnerty & Jefferys, 2000). Inter-ictal spikes and polyspikes, were not accompanied by any obvious behavioural changes. Inter-ictal spikes lasted 25 to 100 ms and consisted of a high frequency oscillation. Polyspikes lasted 0.4 – 2 s and consisted of a number of inter-ictal spikes conjoined. The electrographic trace recorded during a seizure was composed of units which resembled inter-ictal or polyspikes with the difference that seizure activity continued for longer periods. Furthermore, the normal baseline hippocampal field potential oscillations, seen when recording freely moving activity, disappeared leaving a flat EEG trace between inter-ictal type events during a seizure.
Carbamazepine and carbamazepine-epoxide concentrations in serum and CSF
Carbamazepine was detectable in both the serum and CSF compartments at 0.5 h after initiation of peritoneal administration. In contrast, carbamazepine-epoxide was simultaneously detected in serum and CSF, somewhat later at 2.5 h after carbamazepine administration. Cmax values for serum and CSF carbamazepine and carbamazepine-epoxide were achieved at 2.5 – 3.3 h and at 8.0 – 9.0 h after initiation of carbamazepine administration. During the subsequent 7 day period, steady-state serum and CSF concentrations of carbamazepine and carbamazepine-epoxide declined and the data for days 1, 2, 4, 6 and 7 after 4 mg kg−1 h−1 carbamazepine administration are shown in Figure 1.
Figure 1.
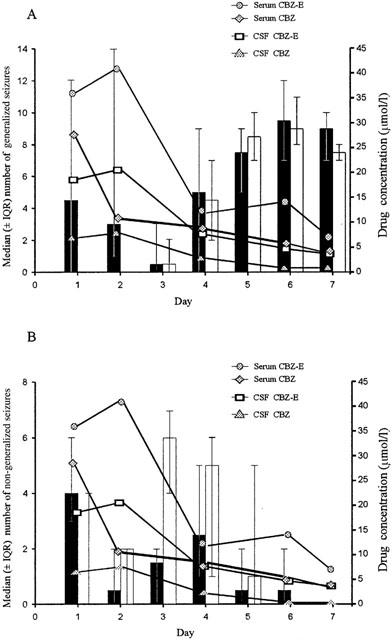
The temporal relationship between the daily median number (± IQR) of generalized (A) and non-generalized (B) seizures in control (shaded) and carbamazepine (CBZ; non-shaded) treated rats and CBZ and carbamazepine epoxide (CBZ-E) concentration in serum and cerebrospinal fluid (CSF) in CBZ administrated rats (4 mg kg−1 h−1; n=6 per group).
Levetiracetam concentrations in serum and CSF
Levetiracetam was detectable in the serum compartment at 1.0 h after initiation of intraperitoneal administration. However, levetiracetam was not detectable in the CSF compartment until 1.5 h after levetiracetam administration. Both serum and CSF levetiracetam concentrations rose linearly and dose-dependently with Cmax values being achieved at 5.5 – 9.0 h, at which time steady-state was achieved. The mean steady-state serum and CSF levetiracetam concentrations for days 1, 2, 4, 6 and 7 after 8 mg kg−1 h−1 and 16 mg kg−1 h−1 levetiracetam administration are shown in Figures 3 and 4 respectively.
Figure 3.
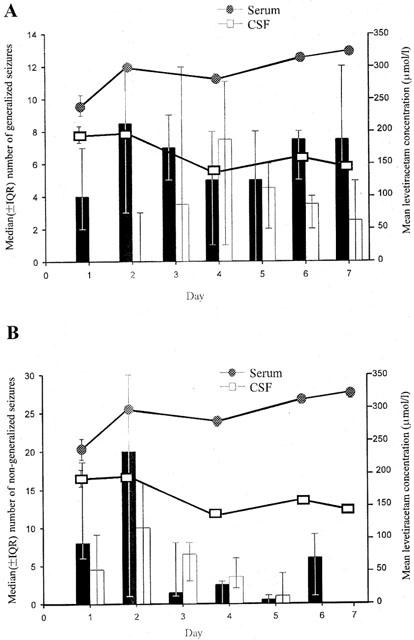
The temporal relationship between the daily median number (±IQR) of generalized (A) and non-generalized (B) seizures in control (shaded) and levetiracetam (non-shaded) treated rats and levetiracetam concentration in serum and cerebrospinal fluid (CSF) in levetiracetam administrated rats (8 mg kg−1 h−1; n=6 per group).
Figure 4.
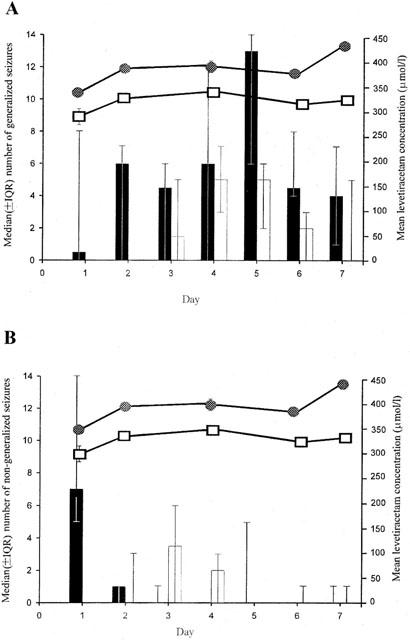
The temporal relationship between the daily median number (±IQR) of generalized (A) and non-generalized (B) seizures in control (shaded) and levetiracetam (non-shaded) treated rats and levetiracetam concentration in serum and cerebrospinal fluid (CSF) in levetiracetam administrated rats (16 mg kg−1 h−1; n=6 per group).
Anticonvulsant effect of carbamazepine
Figure 1 illustrates the temporal relationship between generalized and non-generalized seizures and mean steady-state carbamazepine and carbamazepine-epoxide serum and CSF concentrations in rats treated with 4 mg kg−1 h−1 carbamazepine compared with controls. During days 1 and 2 when both serum and CSF carbamazepine and carbamazepine-epoxide concentrations were relatively high, carbamazepine administration was clearly associated with a significant reduction in the frequency of generalized (P=0.0001) but not non-generalized seizures. During days 3 – 7 the effect of carbamazepine on generalized seizures dissipated and this was associated with concurrent reduction in carbamazepine and carbamazepine epoxide concentrations in both the serum and CSF compartments.
Figure 2 summarizes the anticonvulsant effect of carbamazepine over the entire 7 day study period. It can be seen that whilst carbamazepine administration was associated with a 35.5% (P=0.617) reduction in the total number of generalized seizures, reduction in non-generalized seizures was only 12.6% (P=0.533). Neither reductions achieved statistical significance. However, statistical significance was achieved for the reduction in generalized seizures (90% reduction; P=0.008) when seizure frequency was compared to controls during the first 2 days of carbamazepine administration.
Figure 2.
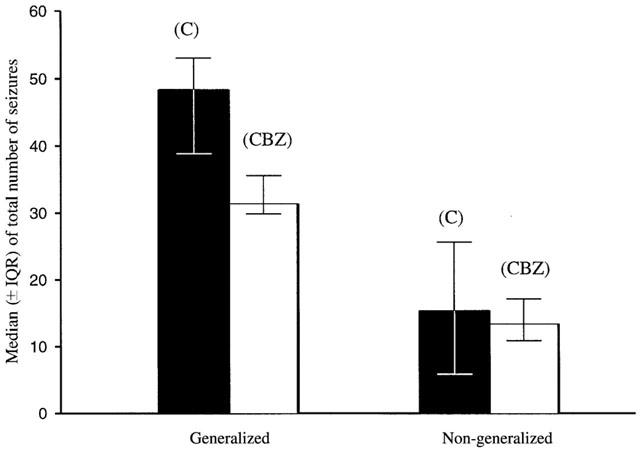
The overall effect of 4 mg kg−1 h−1 carbamazepine on generalized and non-generalized seizures compared to control during a 1 week period. C=control, CBZ=carbamazepine. (n=6 per group).
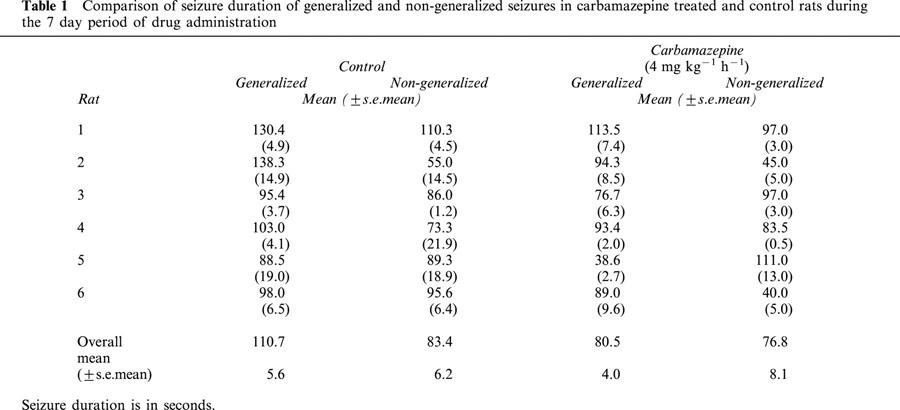
Table 1 shows the duration of generalized and non-generalized seizure in carbamazepine treated rats compared to controls. It can be seen that carbamazepine administration was associated with a significant 27.3% reduction in overall duration for generalized seizures compared to controls (P=0.0001). In contrast carbamazepine administration was associated with a statistically insignificant 7.9% reduction in non-generalized seizure duration (P=0.414).
Table 1.
Comparison of seizure duration of generalized and non-generalized seizures in carbamazepine treated and control rats during the 7 day period of drug administration
Anticonvulsant effect of levetiracetam
Figures 3 and 4 illustrate the temporal relationship between generalized and non-generalized seizures and mean steady-state levetiracetam serum and CSF concentrations in rats treated with levetiracetam (8 and 16 mg kg−1 h−1 respectively) compared with controls. Levetiracetam administration was clearly associated with an overall significant reduction in the frequency of both generalized and non-generalized seizures during the 7 day study period. At the lower levetiracetam dose, levetiracetam reduced the median number of generalized seizures on 6 of the 7 days and non-generalized seizures on 3 of the 7 days (Figure 3). However, seizure suppression was more substantial for generalized seizures compared with non-generalized seizures. Furthermore, at the higher levetiracetam dose, levetiracetam reduced generalized seizure frequency on each of the 7 days (P=0.0004) but had little additional effect on non-generalized seizures compared to the lower levetiracetam dose (Figure 4).
Figure 5 shows the anticonvulsant effect of levetiracetam over the entire 7 day study period. It can be seen that while there was a substantial reduction in the total number of generalized and non-generalized seizures after 8 mg kg−1 h−1 levetiracetam administration (Figure 3, 39.1% and 35.8%, respectively), the reductions did not attain statistical significance (P=0.071 and P=0.635, respectively). A dose-dependent reduction in the frequency of generalized (56.7%) and non-generalized (41.2%) seizures was observed following 16 mg kg−1 h−1 levetiracetam administration (Figure 4). However, whilst the decrease in the incidence of generalized seizures attained statistical significance (P=0.00004), the decrease in non-generalized seizures did not (P=0.525).
Figure 5.
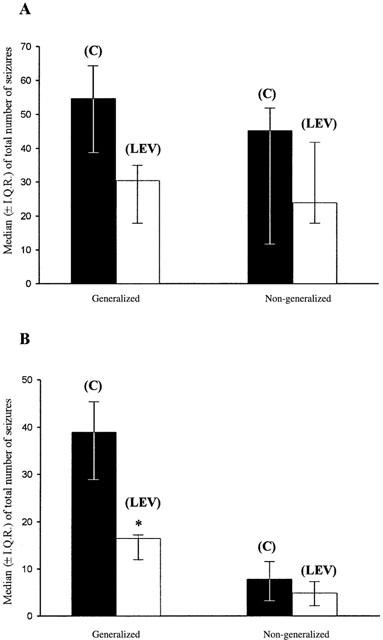
The overall effect of 8 mg kg−1 h−1 (A) and 16 mg kg−1 h−1 (B) levetiracetam on generalized and non-generalized seizures compared to control during a 1 week period. C=control, LEV=levetiracetam. (*=P<0.05, NPANOVA; n=6 per group).
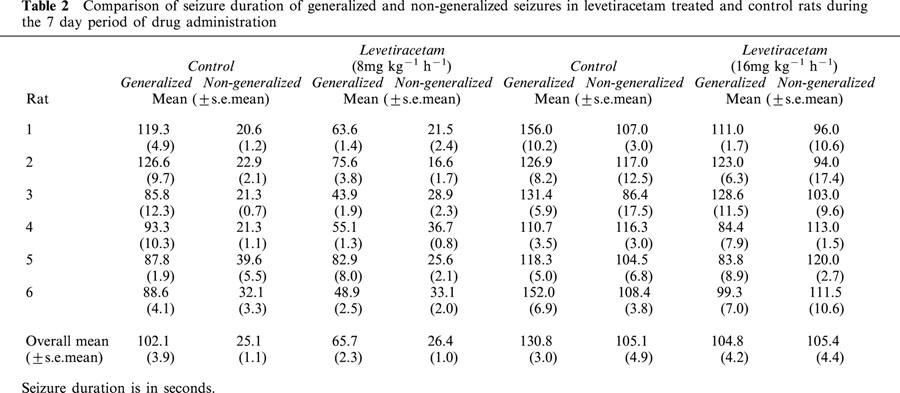
Table 2 shows the duration of generalized and non-generalized seizures in levetiracetam treated rats compared to controls. For generalized seizures, it can be seen that levetiracetam exhibited a dose-dependent and statistically significant reduction in overall seizure duration compared to controls (19.9 and 35.7% reduction for 8 and 16 mg kg−1 h−1 respectively, P=0.0001 for both doses). In contrast levetiracetam had no significant effect on non-generalized seizure duration.
Table 2.
Comparison of seizure duration of generalized and non-generalized seizures in levetiracetam treated and control rats during the 7 day period of drug administration
Discussion
The tetanus toxin model is a chronic model of complex partial seizures with features very similar to those seen in human epilepsy namely both non-generalized and generalized seizures occur over several weeks and seizures are intermittent in nature. Because the epilepsy is somewhat reversible in that the animals eventually stop seizing and the accompanying EEG abnormalities disappear, it is possible to distinguish between the effects of ongoing seizures and the long term consequences of seizures. Furthermore, since the focal seizures occur at a consistent rate for 4 – 6 weeks (Hawkins & Mellanby, 1987; Jefferys & Williams, 1987), it is possible to monitor seizure frequency for several days before, during and after the administration of an anticonvulsant drug. The model therefore constitutes an excellent experimental model for the study of not only seizures but also of epilepsy.
The tetanus toxin model used in this study is based on an adaptation of the model previously described by Mellanby et al. (1977; 1981). Because of differences in the experimental protocol it was necessary to further characterize the model and also to further develop, characterize and validate the EEG monitoring of the resultant seizures. The model adaptations made in the present study include: (1) The site of tetanus toxin administration; unilateral versus bilateral and dorsal versus ventral hippocampus; (2) Amount of tetanus toxin administered (12 mLD50 μl−1 versus 6 mLD50 μl−1); (3) Route of drug administration, continuous infusion via an intraperitoneal osmotic minipump over 7 days versus three times a day oral administration until a 7 day seizure remission period had occurred; (4) Timing of drug administration, 7 – 9 days after the induction of seizures versus first day of seizure occurrence. Even though there are differences in the procedures for inducing the seizure model, the time course of electroencephalographic features and indeed the behavioural manifestations were almost identical in the two studies (Mellanby et al., 1977; 1981). A notable observation is that in comparison to generalized seizures, non-generalized seizures exhibited considerable variation in baseline values between studies (Figures 2 and 5). However, the exact reason for this, which is typical of this model, is unknown and may be the consequence of differences in anatomical and physiological differences between different batches of animals and also variation in different aliquots of tetanus toxin.
In this tetanus toxin model, carbamazepine (4 mg kg−1 h−1) exhibited no significant anticonvulsant effect, compared to control, when the entire 7 day study period was evaluated but the reduction in generalized seizures was greater (35.5%) than that for non-generalized seizures (12.6%). However, when the first 2 days of carbamazepine administration are compared to those of vehicle controls, carbamazepine was associated with a significant reduction in both generalized seizure frequency (90%) and duration (25%). Non-generalized seizures were unaffected. Thus, carbamazepine exhibited a time-dependent anticonvulsant effect and this exactly paralleled the central (CSF) and peripheral (serum) kinetics of carbamazepine and carbamazepine-epoxide in that steady-state concentrations declined over time, with the highest concentrations achieved during the first 2 days (Figure 1). The decline in carbamazepine concentrations is attributable to autoinduction, a well described characteristic of carbamazepine (McNamara et al., 1979; Sumi et al., 1987; Wedlund & Levy, 1983).
The anticonvulsant effect of carbamazepine observed in the present study further validates this model of complex partial seizures, particularly since carbamazepine is effective clinically for the management of patients with complex partial seizures (Porter, 1987). However, the maximum anticonvulsant effect of carbamazepine was much greater than that previously reported (Hawkins et al., 1985). This can be attributed in part not only to the difference in the mode of model induction with the tetanus toxin (discussed above) but perhaps more significantly to the carbamazepine blood concentrations achieved. Thus, whilst carbamazepine mean steady-state serum concentrations of 29 and 11 μmol l−1 were achieved for days 1 and 2 respectively (Figure 1), Hawkins et al. (1985) achieved serum concentrations of approximately 6 – 9 μmol l−1. The clinical therapeutic (target) range for carbamazepine is 20 – 50 μmol l−1 (Patsalos, 2001). Hawkins et al. (1985) administered carbamazepine by mouth on a three times a day basis, with rough allowances made for any volume of drug not completely ingested by the rats. Not only is this approach inefficient and time consuming but also stressful to the animals, which in turn can influence seizure threshold. Perhaps, using an intraperitoneal osmotic minipump, particularly for drugs with a relatively short half-life, is a more reliable method for continuous drug administration, and indeed our animals did not exhibit any signs indicative of overt stress. A notable disadvantage of the osmotic minipump, however, is that it has a fixed volume and therefore limited duration of drug delivery.
Levetiracetam administration (8 and 16 mg kg−1 h−1) was associated with an overall significant reduction in the frequency of both generalized and non-generalized seizures during the 7 day study period. However, seizure suppression was more substantial for generalized seizures compared with non-generalized seizures. Furthermore, the effect of levetiracetam was dose-dependent so that after 8 mg kg−1 h−1 levetiracetam administration, generalized and non-generalized seizure frequency was reduced by 39.1 and 35.8% and after 16 mg kg−1 h−1 levetiracetam the equivalent reductions were 56.7 and 41.2%. However, the decrease in the frequency of non-generalized seizures was not statistically significant. Levetiracetam also exhibited a significant dose-dependent reduction in overall generalized seizure duration compared to controls (Table 2) but was without significant effect in relation to non-generalized seizure duration. These observations suggest that whereas levetiracetam does not effect ictogenesis per se it does reduce seizure severity and, particularly, seizure generalization.
The kinetic profiles of levetiracetam correlated well with the observed anticonvulsant profile. Steady-state levetiracetam concentrations in serum and CSF were achieved by the second day of sampling and these concentrations were maintained for the entire study period. Unlike carbamazepine, levetiracetam exhibited no evidence of autoinduction. Both serum and CSF levetiracetam concentrations increased dose-dependently and essentially linearly and confirm previous data (Doheny et al., 1999; Tong & Patsalos, 2001). As this is the first study whereby the administration of levetiracetam was by osmotic minipump and other studies of the anticonvulsant efficacy and pharmacokinetics of levetiracetam comprised of single dose or multiple dose administration by intraperitoneal injection, there are no comparable data to compare directly. However, from the levetiracetam serum and CSF concentrations achieved in the present study the levetiracetam doses used (4 mg kg−1 h−1 and 8 mg kg−1 h−1; Figures 3 and 4) can be considered equivalent to 40 mg kg−1 and 60 mg kg−1 levetiracetam i.p., respectively (Doheny et al., 1999; Tong & Patsalos, 2001). Consequently, the levetiracetam doses used in the present study can be considered relevant, since in animal models of epilepsy, the effective doses at which 50% of animals respond (ED50) ranges from 5 to 30 mg kg−1 (Gower et al., 1992) and the ED50 for suppression of secondarily generalized seizures in the amygdala-kindled model of epilepsy is 40 mg kg−1 (Loscher & Honack, 1993). Furthermore, the levetiracetam doses used in the present study are comparable to those used clinically where levetiracetam at doses up to 60 mg kg−1 are used to treat patients with epilepsy (Patsalos, 2000).
Tetanus toxin is a potent clostridal neurotoxin, which blocks preferentially the presynaptic release of GABA and glycine, the two major inhibitory neurotransmitters of the CNS. Indeed, several studies have reported on the decrease in GABA release and of the associated reduction in the size of IPSPs (Whittington & Jefferys, 1994; Jordan & Jefferys, 1992; Empson & Jefferys, 1993). These effects are considered to be the consequence of the cleavage of synaptobrevin by tetanus toxin (Schiavo et al., 1992). However, the epileptogenic effect of tetanus toxin far outlasts the impairment of GABA release (Whittington & Jefferys, 1994). Furthermore, synaptic inhibition in the ipsilateral focus (at the site of injection of tetanus toxin) drops to 10% of controls during the first 2 weeks, but there is a subsequent recovery. In contrast, the loss of synaptic inhibition in the contralateral mirror focus never drops below 50% (Empson & Jefferys, 1993; Whittington & Jefferys, 1994). Thus, these studies suggest a more complex mechanism of action of the tetanus toxin and/or the induced focus than just the blockade of inhibitory (GABA and glycine) neurotransmission. A further mechanism may relate to inhibitory neurones not being recruited by the hippocampal network, either because they are less excitable or because the synapses that excite them are impaired (Bekenstein & Lothman, 1993; Sloviter, 1991; Whittington & Jefferys, 1994).
Levetiracetam exhibits a wide spectrum of anticonvulsant effects in various animal models of generalized seizures in rats and mice (Gower et al., 1992; 1995; Margineanu & Wulfert, 1995). Levetiracetam also retards the development of kindling induced in mice by subthreshold doses of pentylenetetrazole (Gower et al., 1992) and significantly reduces the severity and duration of both focal and secondary generalized seizures and also of afterdischarge duration in amygdala-kindled rats (Loscher et al., 1996). However, the exact mechanism of action of levetiracetam is unknown, although levetiracetam has recently been shown to inhibit high-voltage activated Ca2+ currents in hippocampal pyrimidal neurons (Niespodziany et al., 2000). A direct action on GABA is considered unlikely (Margineanu & Wulfert, 1995; Sills et al., 1997; Loscher et al., 1996) particularly since levetiracetam has no significant affinity for the GABAA receptor complex (Noyer et al., 1995) and extracellular concentrations of GABA in the frontal cortex or the hippocampus are unaffected by levetiracetam (Tong & Patsalos, 2001). Furthermore, since levetiracetam in the present study has been observed to have significant efficacy in the tetanus toxin model, these data further suggest perhaps an indirect effect on GABA by levetiracetam. Interestingly such an indirect action on GABA by levetiracetam was suggested from a recent in vitro study, which reported that levetiracetam reversed the inhibitory action of negative allosteric modulators on both GABA and glycine-gated currents in hippocampal neurons (Rigo et al., 2000). Indeed the study by Rigo et al. (2000) is particularly noteworthy in relation to the efficacy of levetiracetam in the tetanus toxin model since tetanus toxin induced seizures are the consequence of a block, in part, on the presynaptic release of both GABA and glycine.
The finding that levetiracetam has a specific binding site in the rat CNS (Noyer et al., 1995) needs comment. The site appears to be specifically confined to the synaptic plasma membranes of the hippocampus, cortex, cerebellum and striatum, as no specific binding was observed in a range of peripheral tissues. Levetiracetam binding affinity to hippocampal membranes is modest (Kd=780±115 nm) but is of high binding capacity (Bmax=9.1±1.2 pmol mg−1 protein). Similar Kd and Bmax values were obtained with the cortex, cerebellum and striatum. Since the Kd of levetiracetam for this binding site is approximately 1000 fold less than the concentrations achieved in the CSF compartment in the present study, it can be concluded that this binding site is unlikely to play a significant role in the anticonvulsant action of levetiracetam in this model.
In conclusion this study demonstrates that whilst levetiracetam significantly reduces the frequency of both generalized and non-generalized seizures, its effect is substantially greater on generalized seizures. Furthermore, levetiracetam acted to reduce the severity and duration of seizure generalization.
Acknowledgments
This work was supported in part by a Brain Research Trust Student Fellowship awarded to Helen C. Doheny. We are grateful to UCB SA, Chemin du Foriest, Belgium for the supply of levetiracetam and ∞,2,2-dimethyl-5-oxo-1-pyrrolidine acetamide.
Abbreviations
- CSF
cerebrospinal fluid
- CNS
central nervous system
- EEG
electroencephalograph
- HPLC
high performance liquid chromatography
References
- BEKENSTEIN J.W., LOTHMAN E.W. Dormancy of inhibitory interneurons in a model of temporal lobe epilepsy. Science. 1993;259:97–100. doi: 10.1126/science.8093417. [DOI] [PubMed] [Google Scholar]
- BEN-MENACHEM E., FALTER U. Efficacy and tolerability of levetiracetam 3000 mg/day in patients with refractory partial seizures: a multicentre, double-blind, responder-selected study evaluating monotherapy. Epilepsia. 2000;41:1276–1283. doi: 10.1111/j.1528-1157.2000.tb04605.x. [DOI] [PubMed] [Google Scholar]
- BETTS T., WAEGEMANS T., CRAWFORD P. A multicentre, double-blind, randomized, parallel group study to evaluate the tolerability and efficacy of two oral doses of levetiracetam, 2000 mg daily and 4000 mg daily, without titration in patients with refractory epilepsy. Seizure. 2000;9:80–87. doi: 10.1053/seiz.2000.0380. [DOI] [PubMed] [Google Scholar]
- CEREGHINO J.J., BITON V., ABOU-KHALIL B., DREIFUSS F., GAUER L.J., LEPPIK I., and the United States Levetiracetam Study Group Levetiracetam for partial seizures. Results of a double-blind, randomized clinical trial. Neurology. 2000;55:236–242. doi: 10.1212/wnl.55.2.236. [DOI] [PubMed] [Google Scholar]
- DOHENY H.C., RATNARAJ N., JENKINS L., JEFFERYS J.G.R., PATSALOS P.N. The kinetics of carbamazepine in a steady state model. Epilepsia. 1995;36 Suppl. 3:S36. [Google Scholar]
- DOHENY H.C., JEFFERYS J.G.R., PATSALOS P.N. Chronic administration of carbamazepine in a limbic model of epilepsy. Epilepsia. 1997a;38 Suppl. 3:223. [Google Scholar]
- DOHENY H.C., WHITTINGTON M.A., JEFFERYS J.G.R., PATSALOS P.N. Levetiracetam in a chronic limbic model of epilepsy. Epilepsia. 1997b;38 Suppl. 8:30. [Google Scholar]
- DOHENY H.C., RATNARAJ N., WHITTINGTON M.A., JEFFERYS J.G.R., PATSALOS P.N. Blood and cerebrospinal fluid pharmacokinetics of the novel anticonvulsant levetiracetam (ucb LO59) in the rat. Epilepsy Res. 1999;34:161–168. doi: 10.1016/s0920-1211(98)00104-1. [DOI] [PubMed] [Google Scholar]
- EMPSON R.M., JEFFERYS J.G.R. Synaptic inhibition in primary and secondary chronic epileptic foci induced by intrahippocampal tetanus toxin in the rat. J. Physiol. Lond. 1993;465:595–614. doi: 10.1113/jphysiol.1993.sp019695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINNERTY G.T., JEFFERYS J.G.R. 9–16 Hz oscillation precedes secondary generalization of seizures in the rat tetanus toxin model of epilepsy. J. Neurophysiol. 2000;83:2217–2226. doi: 10.1152/jn.2000.83.4.2217. [DOI] [PubMed] [Google Scholar]
- GENTON P., VAN VLEYMEN B. Piracetam and levetiracetam: close structural similarities but different pharmacological and clinical profiles. Epileptic Disord. 2000;2:99–105. [PubMed] [Google Scholar]
- GOWER A.J., NOYER M., VERLOES R, GOBERT J., WULFERT E. ucb L059, a novel anti-convulsant drug: pharmacological profile in animals. Eur. J. Pharmacol. 1992;222:193–203. doi: 10.1016/0014-2999(92)90855-x. [DOI] [PubMed] [Google Scholar]
- GOWER A.J., HIRSCH E., BOEHRER A., NOYER M., MARESCAUX C. Effects of levetiracetam, a novel antiepileptic drug, on convulsant activity in two genetic rat models of epilepsy. Epilepsy Res. 1995;22:207–213. doi: 10.1016/0920-1211(95)00077-1. [DOI] [PubMed] [Google Scholar]
- GRANT R., SHORVON S.D. Efficacy and tolerability of 1000–4000 mg per day of levetiracetam as add-on therapy in patients with refractory epilepsy. Epilepsy Res. 2000;42:89–95. doi: 10.1016/s0920-1211(00)00158-3. [DOI] [PubMed] [Google Scholar]
- HAWKINS C.A., MELLANBY J.H., BROWN J. Antiepileptic and antiamnesic effect of carbamazepine in experimental limbic epilepsy. J. Neurol. Neurosurg. Psychiat. 1985;48:459–468. doi: 10.1136/jnnp.48.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWKINS C.A., MELLANBY J.H. Piracetam potentiates the antiepileptic action of carbamazepine in chronic experimental limbic epilepsy. Acta Neurol. Scand. 1986;109 Suppl:117–121. doi: 10.1111/j.1600-0404.1986.tb04871.x. [DOI] [PubMed] [Google Scholar]
- HAWKINS C.A., MELLANBY J.H. Limbic epilepsy induced by tetanus toxin: a longitudinal electroencephalographic study. Epilepsia. 1986;28:431–444. doi: 10.1111/j.1528-1157.1987.tb03669.x. [DOI] [PubMed] [Google Scholar]
- JEFFERYS J.G.R., WILLIAMS S.F. Physiological and behavioural consequences of seizures induced in the rat by intrahippocampal tetanus toxin. Brain. 1987;110:517–532. doi: 10.1093/brain/110.2.517. [DOI] [PubMed] [Google Scholar]
- JORDAN S.J., JEFFERYS J.G.R. Sustained and selective block of IPSPs in brain slices from rats made epileptic by intrahippocampal tetanus toxin. Epilepsy Res. 1992;11:119–129. doi: 10.1016/0920-1211(92)90046-v. [DOI] [PubMed] [Google Scholar]
- KASTELEIJN-NOLST TRENITE D.G.A., MARESCAUX C., STODIECK J., EDELBROEK P.M., OOSTING P.M. Photosensitive epilepsy: a model to study the effect of antiepileptic drugs. Evaluation of the piracetam analogue levetiracetam. Epilepsy Res. 1996;25:225–230. doi: 10.1016/s0920-1211(96)00031-9. [DOI] [PubMed] [Google Scholar]
- KLITGAARD H., MATAGNE A., GOBERT J., WULFERT E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur. J. Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- LOSCHER W., HONACK D. Profile of ucb LO59, a novel anticonvulsant drug, in models of partial and generalised epilepsy in mice and rats. Eur. J. Pharmacol. 1993;232:147–158. doi: 10.1016/0014-2999(93)90768-d. [DOI] [PubMed] [Google Scholar]
- LOSCHER W., HONACK D., BLOMS-FUNKE P. The novel antiepileptic drug levetiracetam (ucb L059) induces alterations in GABA metabolism and turnover in discrete areas of rat brain and reduces neuronal activity in substantia nigra pars reticulata. Brain Res. 1996;735:208–216. doi: 10.1016/0006-8993(96)00587-2. [DOI] [PubMed] [Google Scholar]
- LOSCHER W., HONACK D., RUNDFELDT C. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb LO59) in the kindling model of temporal lobe epilepsy. J. Pharmacol. Exp. Ther. 1998;284:474–479. [PubMed] [Google Scholar]
- LOSCHER W., SCHMIDT D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res. 1988;2:145–181. doi: 10.1016/0920-1211(88)90054-x. [DOI] [PubMed] [Google Scholar]
- MARGINEANU D.G., WULFERT E. Ucb LO59, a novel anticonvulsant, reduces bicuculline-induced hyperexcitability in rat hippocampal CA3 in vivo. Eur. J. Pharmacol. 1995;286:321–326. doi: 10.1016/0014-2999(95)00597-8. [DOI] [PubMed] [Google Scholar]
- MARGINEANU D.G., WULFERT E. Inhibition by levetiracetam of a non-GABAA receptor-associated epileptiform effect of bicuculline in rat hippocampus. Br. J. Pharmacol. 1997;122:1146–1150. doi: 10.1038/sj.bjp.0701476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNAMARA P.J., COLBURN W.A., GIBALDI M. Time course of carbamazepine self-induction. J. Pharmacokinet. Biopharm. 1979;7:63–68. doi: 10.1007/BF01059441. [DOI] [PubMed] [Google Scholar]
- MELLANBY J.H., GEORGE G., ROBINSON A., THOMPSON P. Epileptiform syndrome in rats produced by injecting tetanus toxin into the hippocampus. J. Neurol. Neurosurg. Psychiat. 1977;40:404–414. doi: 10.1136/jnnp.40.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELLANBY J.H., HAWKINS C., BAILLIE-HAMILTON S., BOURNE M., SHEPHERD L., STROUD C.Kindling, behaviour and anticonvulsant drugs The psychopharmacology of epilepsy 1985Chichester: John Wiley & Sons Ltd; 17–31.ed. Trimble, M.T. pp [Google Scholar]
- MELLANBY J.H., HAWKINS C., MELLANBY H., RAWLINS J.N., IMPEY M.E. Tetanus toxin as a tool for studying epilepsy. J. Physiol. Paris. 1984;79:207–215. [PubMed] [Google Scholar]
- MELLANBY J.H., STRAWBRIDGE P., COLIINGRIDGE G.I., GEORGE G., RANDS G., STROUD C., THOMPSON P. Behavioural correlates of an experimental hippocampal epileptiform syndrome in rats. J. Neurol. Neurosurg. Psychiat. 1981;44:1084–1093. doi: 10.1136/jnnp.44.12.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIESPODZIANY I., KLITGAARD H., MARGINEANU D.G. Levetiracetam: modulation of high voltage-activated Ca2+ current in CA1 pyramidal neurons of rat hippocampal slices. Epilepsia. 2000;41 Suppl 7:37. doi: 10.1016/s0304-3940(01)01884-5. [DOI] [PubMed] [Google Scholar]
- NOYER M., GILLARD M., MATAGNE A., HENICHART J.P., WULFERT E. The novel antiepileptic drug levetiracetam (ucb L059) appears to act via a specific binding site in CNS membranes. Eur. J. Pharmacol. 1995;286:137–146. doi: 10.1016/0014-2999(95)00436-o. [DOI] [PubMed] [Google Scholar]
- PATSALOS P.N. Pharmacokinetic profile of levetiracetam: Toward ideal characteristics. Pharmacol. Therapeut. 2000;85:77–85. doi: 10.1016/s0163-7258(99)00052-2. [DOI] [PubMed] [Google Scholar]
- PATSALOS P.N. Therapeutic drug monitoring in epilepsy – principle and concepts. Epilepsy Monit. 2001;5:1–6. [Google Scholar]
- PATSALOS P.N., ALAVIJEH M.S., SEMBA J., LOLIN Y.I. A freely moving and behaving rat model for the chronic and simultaneous study of drug pharmacokinetics (blood) and neuropharmacokinetics (cerebrospinal fluid): hematological and biochemical characterization and kinetic evaluation using carbamazepine. J. Pharmacol. Toxicol. Methods. 1992;28:21–28. doi: 10.1016/1056-8719(92)90061-5. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C.The Rat Brain in Stereotactic Coordinates 1986San Diego: Academic Press; 2nd edn [Google Scholar]
- PORTER R.J. How to initiate and maintain carbamazepine therapy in children and adults. Epilepsia. 1987;28 suppl. 3:S59–S63. doi: 10.1111/j.1528-1157.1987.tb05779.x. [DOI] [PubMed] [Google Scholar]
- RATNARAJ N., DOHENY H.C., PATSALOS P.N. A micromethod for the determination of the new antiepileptic drug levetiracetam (ucb LO59) in serum or plasma by high performance liquid chromatography. Ther. Drug Monit. 1996;18:154–157. doi: 10.1097/00007691-199604000-00008. [DOI] [PubMed] [Google Scholar]
- RIGO J.M., NGUYEN L., BELACHEW S., MULGRANGE B., LEPRINCE P., MOONEN G., SELAK I., MATAGNE A., KLITGAARD H. Levetiracetam: novel modulation of ionotrophic inhibitory receptors. Epilepsia. 2000;41 Suppl 7:35. [Google Scholar]
- SCHIAVO G., BENFENATI F., POULAIN B., ROSSETTO O., POLVERINO DE LAURETO P., DASGUPTA B.R., MONTECUCCO C. Tetanus and botulinum-b neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- SHARIEF M.K., SINGH P., SANDER J.W.A.S., PATSALOS P.N., SHORVON S.D. Efficacy and tolerability study of ucb LO59 in patients with refractory epilepsy. J. Epilepsy. 1996;9:106–112. [Google Scholar]
- SHORVON S.D., LOWENTHAL A., JANZ D., BIELEN E., LOISEAU P. Multicentre double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory seizures. Epilepsia. 2000;41:1179–1186. doi: 10.1111/j.1528-1157.2000.tb00323.x. [DOI] [PubMed] [Google Scholar]
- SILLS G.J., LEACH J.P., FRASER C.M., FORREST G., PATSALOS P.N., BRODIE M.J. Neurochemical studies with the novel anticonvulsant levetiracetam in mouse brain. Eur. J. Pharmacol. 1997;325:35–40. doi: 10.1016/s0014-2999(97)00105-2. [DOI] [PubMed] [Google Scholar]
- SLOVITER R.S. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- SOKOMBA E.N., PATSALOS P.N., LOLIN Y.I., CURZON G. Concurrent monitoring of central carbamazepine and transmitter amine metabolism and motor activity in individual unrestrained rats using repetitive withdrawal of cerebrospinal fluid. Neuropharmacol. 1988;27:209–415. doi: 10.1016/0028-3908(88)90150-5. [DOI] [PubMed] [Google Scholar]
- SUMI M., WATARI N., UMEZAWA O., KANENIWA N. Pharmacokinetic study of carbamazepine and its epoxide metabolite in humans. J. Pharmacobio. Dyn. 1987;10:652–661. doi: 10.1248/bpb1978.10.652. [DOI] [PubMed] [Google Scholar]
- TONG X., PATSALOS P.N. A microdialysis study of the novel antiepileptic drug levetiracetam: extracellular pharmacokinetics and effect on taurine in rat brain. Br. J. Pharmacol. 2001;133:867–874. doi: 10.1038/sj.bjp.0704141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEDLUND P.J., LEVY R.H. Time-dependent kinetics VII: effect of diurnal oscillations on time course of carbamazepine autoinduction in the rhesus monkey. J. Pharm. Sci. 1983;72:905–908. doi: 10.1002/jps.2600720816. [DOI] [PubMed] [Google Scholar]
- WHITTINGTON M.A., JEFFERYS J.G.R. Epileptic activity outlasts disinhibition after intrahippocampal tetanus toxin in the rat. J. Physiol. Lond. 1994;481:593–604. doi: 10.1113/jphysiol.1994.sp020466. [DOI] [PMC free article] [PubMed] [Google Scholar]


