Abstract
To study the mechanisms involved in the action of Z-338, a newly synthesized gastroprokinetic agent, experiments were performed with the paratracheal ganglion cells acutely dissociated from 2-week-old Wistar rats. The effects of Z-338 on both nicotinic and muscarinic responses of the ganglion cells were studied by nystatin perforated patch recording configuration under the current- and voltage-clamp conditions.
Acetylcholine (ACh) or nicotine, and muscarine or oxotremorine-M (OX-M) induced membrane depolarization with rapid and slow time courses respectively, followed by repetitive generation of action potentials in the ganglion cell. Corresponding to the membrane depolarization induced by cholinergic agents, ACh induced biphasic inward currents with rapid and slow time courses under the voltage-clamp condition. Nicotine and muscarine or OX-M evoked inward currents with rapid and slow time courses, respectively. The rapid and slow inward currents were accompanied by increase and decrease in the membrane conductance, respectively. In addition, OX-M dose-dependently suppressed the M-type K+ current evoked in response to hyperpolarizing voltage-steps from VH of −25 mV to −50 mV, indicating that the activation of muscarinic acetylcholine receptors inhibits M-type K+ current, thus inducing inward current in the ganglion cell.
Z-338 competitively suppressed the inward currents induced by OX-M through M1 ACh receptor, and uncompetitively suppressed the currents induced by nicotine.
The inhibitory actions of Z-338 on the membrane depolarization and corresponding inward currents mediated by M1-muscarinic and neuronal nicotinic ACh receptors in the isolated ganglion cells were discussed in relation to the inhibitory actions on autoreceptors in the parasympathetic nerve terminals, which would explain the gastroprokinetic actions of Z-338.
Keywords: Z-338, muscarinic response, nicotinic response, perforated patch recording, airway parasympathetic neuron
Introduction
N-(N′,N′-diisopropylaminoethyl)-[2-(2-hydroxy-4,5-dimethoxy-benzoyl-amino)-1,3-thiazole-4-yl] carboxyamide monohydrochloride trihydrate (Z-338) is a newly synthesized gastroprokinetic agent that enhances gastrointestinal motility in rats and dogs (Ueki et al., 1998). It is known that Z-338 has various pharmacological actions on the gastrointestinal tract (GI tract). For example, Z-338 enhances electrically stimulated contraction and the release of acetylcholine (ACh) in the [3H]-choline-preincubated gastric antrum and body at doses greater than 10−6 M (Ueki et al., 1998). Although the half-maximum inhibitory concentration (IC50) is about 100 fold less than that of neostigmine and physostigmine, Z-338 reversibly inhibits human erythrocyte acetylcholinesterase activity (Ueki et al., 1998). Therefore, under the conditions where the cholinesterase activity was inhibited by physostigmine, the enhancing effect of Z-338 on ACh release was examined and it turned out that Z-338 enhances electrically stimulated release of ACh to a greater extent when physostigmine was present in the superfusion solution, indicating that Z-338 does not enhance the apparent ACh release by inhibiting acetylcholinesterase (Ueki et al., 1998). Recently, we found that Z-338 (⩾10−8 M) specifically enhances excitatory neuro-effector transmission mainly through prejunctional mechanisms, with no effect on the non-adrenergic non-cholinergic inhibitory neuro-effector transmission in the guinea-pig stomach (Nakajima et al., 2000). In supporting this view, it was also reported that Z-338 (3 – 30×10−6 M) enhances electrically stimulated contractions and the release of ACh, that was tetrodotoxin sensitive and extracellular Ca2+ dependent, in gastric strips of guinea-pig stomach (Ogishima et al., 2000). These observations indicate that the gastrointestinal prokinetic effect of Z-338 is mainly due to its facilitatory action on ACh release from enteric nerve endings by blocking the muscarinic autoreceptors (Nakajima et al., 2000; Ogishima et al., 2000).
However the precise mechanisms by which Z-338 enhances ACh release from the nerve terminal remains to be clarified. The direct way to study the presynaptic actions of Z-338 would be to observe the action of Z-338 on the cholinegic auto-inhibitory responses in the prejunctional nerve terminals, but, these experiments are not technically feasible. Therefore, we observed the effects of Z-338 on cholinergic responses of isolated rat paratracheal ganglion cells, since this was used as the model to study the prejunctional modulations of excitatory neuro-effector transmission (Murai et al., 1998; Ito et al., 2000). In the present experiments, firstly we observed the effects of ACh on isolated paratracheal ganglion cells and then studied the effects of Z-338 on these responses of the ganglion cells to ACh. We found that Z-338 inhibits the inward currents mediated by nicotinic and muscarinic receptors in the isolated paratracheal ganglion cells in an uncompetitive and competitive manner, respectively. These findings strongly indicate that Z-338 suppresses the autoinhibitory mechanisms involved in the ACh release from the nerve terminals, thereby enhancing excitatory neuro-effector transmission.
Methods
Dissociation of paratracheal ganglion cells
This study was conducted under ‘Guiding Principles for the Care and Use of Laboratory Animals' approved by the Japanese Pharmacological Society.
The procedure for obtaining dissociated paratracheal ganglion neurons is similar to that described elsewhere (Aibara et al., 1992; Ishibashi et al., 2001). Briefly, the paratracheal ganglia were rapidly removed from the trachea of 2-week-old Wistar rats under ether anaesthesia, and the rats were subsequently killed by exsanguination. The isolated ganglia were treated with the normal external solution containing 0.3% collagenase and 0.3% trypsin for 45 min at 35°C. Following this enzyme treatment, the ganglion neurons were dissociated mechanically by gentle triturating using a fire-polished Pasteur pipette in a culture dish. The dissociated neurons adhered to the bottom of the dish within 20 min. The neurons were then prepared for electrophysiological experiments.
Electrophysiological recordings
Electrical measurements were performed using the nystatin perforated patch recording configuration (Akaike & Harata, 1994). Patch pipettes were prepared using a vertical micropipette puller (PB-7, Narishige, Tokyo, Japan). The resistance between the patch pipette filled with the internal solution and the reference electrode in the normal external solution was 2 – 4 MΩ and was compensated in same manner explained by Ishibashi et al. (2001).
Ionic currents were measured with a patch clamp amplifier (EPC-7, List-Medical), low-pass filtered at 1 kHz (E-3210A, NF Electronic Instruments, Tokyo, Japan) and monitored on a digital storage oscilloscope (VC-6025, Hitachi, Tokyo, Japan). Data were stored on magnetic tape using digital audio tape recorder (RE-130TE, TEAC, Tokyo, Japan) for subsequent computer analysis using the pCLAMP system (Axon Instruments). All experiments were carried out at room temperature (21 – 24°C). Membrane conductance was measured by applying the hyperpolarizing step pulses from a holding potential (VH) of −40 mV to −55 mV every 15 s. Experimental values are presented as mean±standard error of the mean (s.e.mean). Statistical significance was determined using the paired or unpaired Student's t-test.
Solutions and chemicals
The ionic composition of the normal external solution was (in mM): NaCl 150, KCl 5, MgCl2 1, CaCl2 2, N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES) 10 and glucose 10. The pH was adjusted to 7.4 with tris (hydroxymethyl) aminomethane (Tris-OH). The composition of patch pipette (internal) solution was (in mM): K-methanesulphonate 80, KCl 70 and HEPES 10. Nystatin was dissolved in methanol, resulting in a 10 mg/ml stock solution, and added to the internal solution to give a final concentration of 100 μg/ml, just before use. Drugs were applied with the ‘Y-tube' technique, as described elsewhere (Nakagawa et al., 1990).
Drugs used in the present experiment were acetylcholine chloride, α-bungarotoxin, collagenase, hexamethonium, mecamylamine, muscarine, nicotine, nystatin, oxotremorine-M (OX-M), pirenzepine and trypsin [Sigma, Ct. Louis, MO, U.S.A.], and 11-[[2-[(dimethylamino)methyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one) (AF-DX116) [Tocris Cookson Inc, Bristol, U.K.], muscarinic toxin 7 (MT-7) [Peptide Institute Inc, Osaka, Japan], and Z-338 (a gift from Zeria Pharmaceutical Co. Ltd., Tokyo, Japan).
Results
Effects of cholinergic agents on the membrane potential of isolated paratracheal ganglion cell
To study the actions of ACh on the single paratracheal neuron, nystatin-perforated patch recording was performed on neurons isolated from the rat paratracheal ganglia.
Firstly, the effects of ACh, nicotine and muscarine were tested under current-clamp conditions. The mean input resistance of the isolated paratracheal ganglia was 48±6 MΩ and the resting membrane potential was −65.7±3.1 mV (n=7), and these values were similar to those previously reported in the airway parasympathetic neurons of guinea-pigs (Myers et al., 1990). As shown in Figure 1Aa, ACh (10−5M) induced a rapid membrane depolarization followed by repetitive generation of action potential in all neurons examined (eight cells). In increased concentration, ACh (10−4 M) further depolarized the membrane and repetitive generation of action potentials was inhibited (n=4, Figure 1Ab). After rinsing ACh, the frequency of the repetitive firings gradually reduced and ceased completely within 30 – 60 s. Application of nicotine (10−5 M) or muscarine (10−6 M) also depolarized the membrane which was followed by repetitive action potentials (Figure 1Ba,b). However the time course of the membrane depolarization was slower in the case of muscarine, compared to those of ACh and nicotine. Furthermore, the generation of action potential ceased immediately after washing out nicotine, but it lasted for more than 30 – 60 s in case of ACh (Figure 1A) and muscarine (Figure 1Bb).
Figure 1.
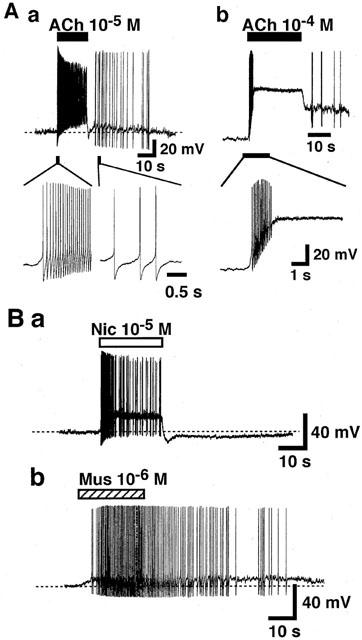
Effects of cholinergic agonists on the membrane potential of the isolated rat paratracheal ganglion cells. Recordings were made under the current-clamp conditions. (A) Depolarizing response of ACh. Horizontal closed bars above the upper traces indicate the application of ACh. The lower traces in Aa & b shows the effects of ACh on the membrane potential of the isolated ganglion cells in faster time course. (B) Effect of nicotine (Nic, 10−5 M) and muscarine (Mus, 10−6 M). Horizontal open and hatched bars above the traces indicate the application of nicotine and muscarine, respectively. Each trace shows the representative of seven reproducible experiments.
Inward currents evoked by cholinergic agents
To study the mechanisms involved in the membrane depolarization induced by cholinergic agents further, the membrane currents during application of ACh, nicotine, muscarine and oxotremorine-M (OX-M) were measured in voltage-clamped paratracheal ganglion cells at a holding potential (VH) of −40 mV. As shown in Figure 2A, ACh in relatively low (3×10−8 – 3×10−7 M) and high (>10−6 M) concentrations evoked inward currents with slow and rapid time courses, respectively. The inward currents reached their peak value after 20 – 40 s and 5 – 15 s after application of 3×10−7 and 3×10−6 M ACh, respectively. Interestingly, the slow and rapid inward currents were accompanied by decrease and increase in the membrane conductance, respectively. Nicotine at low and high concentrations (3×10−6 – 3×10−4 M) evoked inward current with rapid time course as in the case of higher concentrations of ACh (Figure 2B). On the other hand, muscarine and OX-M at all concentrations examined (3×10−8 – 10−6 M) evoked slow inward current, which was similar to that evoked by relatively low concentrations of ACh (Figure 2C). During the slow inward currents evoked by muscarine or OX-M, the membrane conductance was decreased (not shown). Figure 2D shows the effect of atropine (3×10−7 M) on the currents induced by ACh (10−7 or 10−5 M) in the same cell. Atropine fully inhibited the slow inward current induced by 10−7 M ACh but it had little effect on the ACh (10−5 M)-induced rapid current in all neurons tested (n=4).
Figure 2.
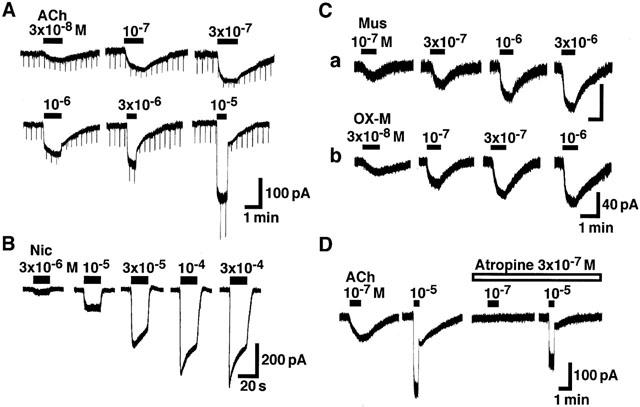
Inward currents induced by cholinergic agents. Current-recordings were made at a holding potential (VH) of −40 mV. (A) The inward currents induced by various concentrations (3×10−8 – 10−5 M) of ACh. The hyperpolarizing step pulses from a VH of −40 mV to −55 mV with 500 ms duration were applied every 15 s. (B) The inward currents induced by nicotine (3×10−6 – 3×10−4 M). (C) The inward currents elicited by muscarine (10−7 – 3×10−6 M) and oxotremorine-M (OX-M, 3×10−8 – 10−6 M). (D) Effect of atropine on the ACh response. Four consecutive responses to ACh (10−7 or 10−5 M) in the absence or presence of atropine (3×10−7 M). ACh was applied for 1 min (10−7 M) or 30 s (10−5 M) every 6 min. It is noted that the rapid component of ACh (10−5 M)-induced current was clearly observed when the agonist was washed out. The figure is representative of four reproducible experiments.
Figure 3 summarizes the relationships between dose and relative amplitude of the inward currents evoked by ACh, nicotine, muscarine and OX-M. The dose-response relationships for muscarine and OX-M and nicotine were sigmoidal curves. The minimum concentrations to evoke inward currents was relatively low for muscarine and OX-M (10−8 – 3×10−8 M), and higher for nicotine (10−6 – 3× 10−6 M). The maximum responses were obtained at 3× 10−6 M for muscarine and OX-M, and at 3×10−4 M for nicotine. On the other hand, the concentrations of ACh to evoke the minimum and maximam inward current were 10−8 and 3×10−4 M respectively, thereby showing wide range dose-response relationship compared to those of nicotine or muscarine and OX-M. Furthermore, the dose-response relationship for ACh was not a single sigmoid, showing a hump at around 3×10−7 M. Namely in the relatively low concentrations of ACh, the amplitude of the inward current dose-dependently increased, and reached a plateau value at a concentration of 3×10−7 M. At a dose higher than 3×10−7 M, ACh-induced inward current increased again in a dose-dependent manner, indicating another sigmoidal dose-response relationship between 3×10−7 and 3×10−4 M.
Figure 3.
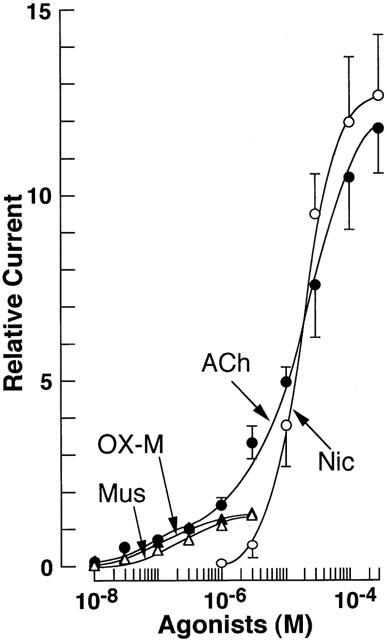
Concentration-response relationships for ACh-, nicotine-, muscarine- and oxotremorine-induced peak current component. All responses were normalized to the peak current induced by 3×10−7 M ACh. Each point shows the average of 4 – 8 experiments, and vertical bars indicate mean±s.e.mean. The continuous theoretical curves for the response to nicotine, muscarine and oxotremorine-M were drawn according to the following equation: I=ImaxCn/(Cn+Kn) where I is the observed agonist-induced current, Imax is the maximum current, C is the agonist concentration, n is the Hill coefficient, and K is the agonist concentration that evoked the half-maximal response. The curve for ACh response was drawn as sum of the two concentration-response curves according to the following equation: I=Imax1 Cn1/(Cn1+K1n1)+Imax2Cn2/(Cn2+K2n2) where Imax1 and Imax2 are the plateau amplitudes at lower and higher concentrations, and K1 and K2 are the agonist concentration (4.94×10−7 and 2.03×10−5 M, respectively) that evoked the half-maximal response of each current component, respectively.
These results indicate that both nicotinic and muscarinic ACh receptors co-exist on the membrane of individual neurons, and that the nicotinic and muscarinic responses are involved in the action of ACh on the neurons.
The current-voltage (I-V) relationships of nicotinic and muscarinic responses were examined. The I-V relationship of nicotine-induced current was made from the current induced by 10−5 M nicotine at various VHs. The I-V relationship of OX-M-induced currents was studied by using ramp-clamp method (Akaike et al., 1987). The patch-pipette solution contained 150 mM K+, and neurons were perfused with an external solution containing 5 mM K+. Current responses to voltage-ramps from a VH of −25 mV to −100 mV before and during application of OX-M were observed.
Figure 4A shows the I-V relationships for nicotine- and OX-M-induced currents (INIC and IOX-M, respectively). INIC showed typical I-V relationship with a marked inward rectifying property. Due to the inward rectification, accurate measurements of the reversal potential was not technically feasible, however the apparent reversal potential for INIC estimated from the I-V relationship was 3±2 mV (n=5). In contrast, as far as the IOX-M concerned, the current amplitude gradually decreased at membrane potentials negative to −30 mV. The I-V relationship for IOX-M did not show clear reversal potential, thereby indicating that the action of muscarinic agonist inhibits the M-type K+-current. It is known that M-current is inactivated at potentials negative to −70 mV (Constanti & Brown, 1981). Figure 4B shows the actual current and I-V relationships before and during application of OX-M. Similar I-V relationships were observed in the case of muscarine-induced current (n=3, not shown).
Figure 4.
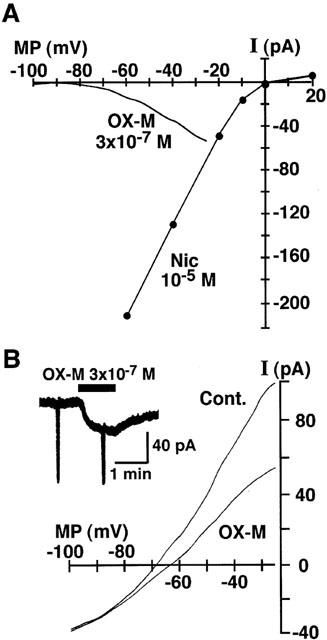
Current-voltage (I-V) relationships for nicotine- and OX-M-induced currents. Peak amplitude of INic activated by 10−5 M nicotine was measured at various VHs. In the case of OX-M response, the hyperpolarizing ramp commands from a VH of −25 mV to −100 mV of 3 s duration were applied before application of OX-M (3×10−7 M) and at a steady state of the current response (B). I-V relationship for IOX-M shown in (A) was obtained by subtracting the current response to the control ramp pulse from that during application of OX-M. All recordings were obtained from the same neuron. The figure is representative of five reproducible experiments.
The time-dependent inward currents evoked in response to hyperpolarizing voltage-steps of 800 ms from a VH of −25 mV to −50 mV were also studied in the absence or presence of OX-M. The OX-M dose-dependently suppressed the M-current with an IC50 value of 1.6×10−7 M (Figure 5).
Figure 5.
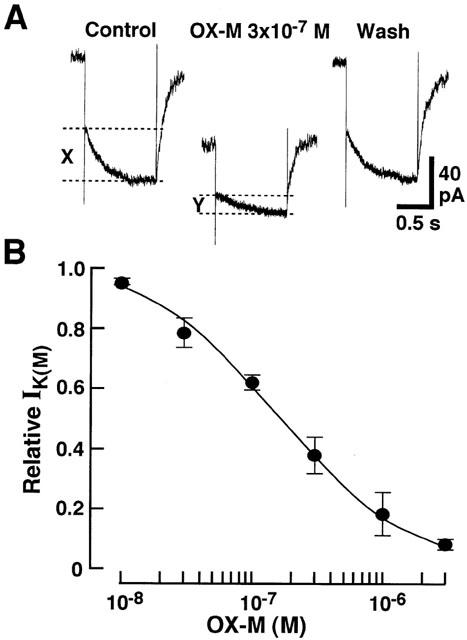
Effects of OX-M on M-type K+ current of the isolated paratracheal ganglion cells. (A) Representative M-current recordings for control, during 3×10−7 M OX-M application and after wash out. Hyperpolarizing voltage-steps from VH of −25 mV to ×50 mV were applied to evoke the time-dependent M-current deactivation. The amplitudes of M-current deactivation, before and during the application of OX-M, were shown as X and Y, respectively. (B) Concentration-dependent inhibitory effect of OX-M on the M-current. The continuous curves were drawn according to the following equation: I=1 – Cn/(Cn+Kn) where I is the normalized current amplitude, C is the antagonist concentration, K is the antagonist concentration that produced the half-maximal response, and n is the Hill coefficient. Each point is the average of 4 – 5 experiments, and vertical bars indicate ±s.e.mean.
To study the subtypes of muscarinic ACh receptors involved in the IOX-M, the effects of M1 (pirenzepine or MT-7) and M2-(AF-DX116) muscarinic antagonists were examined. As shown in Figure 6A, pirenzepine and AF-DX116 dose-dependently suppressed the IOX-M, and the IC50 values were 3.6×10−7 and 1.8×10−6 M respectively. MT-7 which is highly selective for M1-receptor subtype almost completely suppressed the 3×10−7 M OX-M-induced inward current at a dose of 5×10−8 M (Figure 6B). These results indicate that M1-receptors are involved in the IOX-M.
Figure 6.
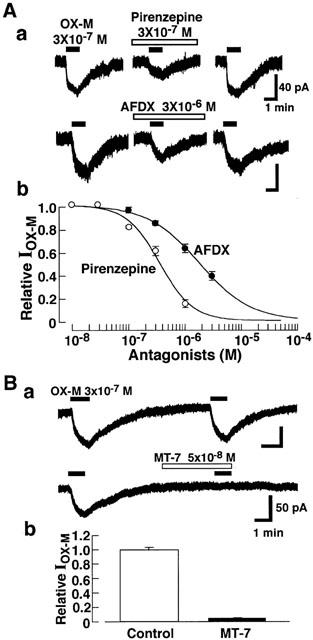
Involvement of the M1 receptor in muscarinic response. Recordings were made at a VH of −40 mV. Aa, Inward currents induced by OX-M (IOX-M) in the presence of pirenzepine (3×10−7 M), AF-DX116 (3×10−6 M) or Z-338 (3×10−5 M). Ab, Effect of various concentration of pirenzepine, AF-DX116 and Z-338 on IOX-M. Antagonists were applied 1 min before application of OX-M. Ba, Inhibitory effects of MT-7 (5×10−8 M) on OX-M (3×10−7 M)-induced inward currents. MT-7 was applied 3 min before application of OX-M. Each column shows the average of five cells, and vertical bars indicate s.e.mean.
As shown in Figure 7A, hexamethonium and mecamylamine dose-dependently suppressed the INIC, and the half maximal inhibitory concentrations (IC50) were 2.0×10−4 and 1.7×10−6 M, respectively. α-Bungarotoxin (3×10−7 M) had no effect on the INIC (Figure 7B). Taken together, these results indicate that the activation of neuronal nicotinic ACh receptor is involved in the generation of INIC.
Figure 7.
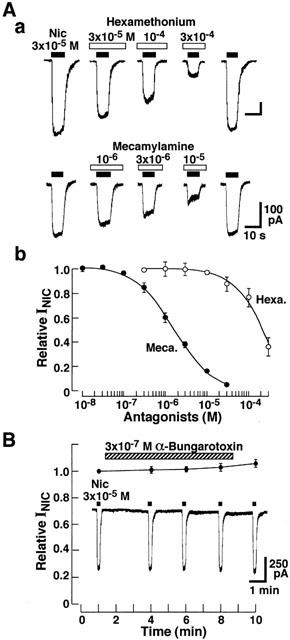
Effects of hexamethonium, mecamylamine and α-bungarotoxin on the nicotine-induced current (INIC). (Aa) Concentration-dependent suppression of INIC by mecamylamine and hexamethonium. Nicotine (3×10−5 M) was applied every 3 min. The antagonists were applied 1 min before nicotine application. VH was −40 mV. (Ab) Concentration-inhibition relationships of INIC by mecamylamine (Meca, closed circle) and hexamethonium (Hexa, open circle). Current amplitude in the presence of nicotinic antagonists was expressed as the relative value to the control response induced by 3×10−5 M nicotine alone. Each point is the average of 4 – 5 experiments. Vertical bars indicate ±s.e.mean. (B) Effects of α-bungarotoxin on INIC, and insert shows actual trace of INIC in the presence of 3×10−7 M α-bungarotoxin. Relative current amplitudes of INIC were calculated as ratios to that just before the toxin application in each cell. Each point is the average of four experiments.
Effects of Z-338 on INIC
The effects of Z-338 on INIC was firstly investigated at a VH of −40 mV. Figure 8A shows the time course of the inhibitory action of Z-338 (3×10−5 M) on the INIC induced by 3×10−5 M nicotine. Z-338 suppressed both of the peak and steady currents (20 s after the peak current) induced by nicotine. However the steady current was suppressed more effectively. The IC50 values for the peak and steady currents of Z-338 were 2.7×10−5 and 7.7×10−6 M, respectively (Figure 8B). Figure 8C shows the effect of Z-338 on INIC at various VHs. The membrane hyperpolarization enhanced the Z-338 block of the nicotinic response (Figure 8D).
Figure 8.
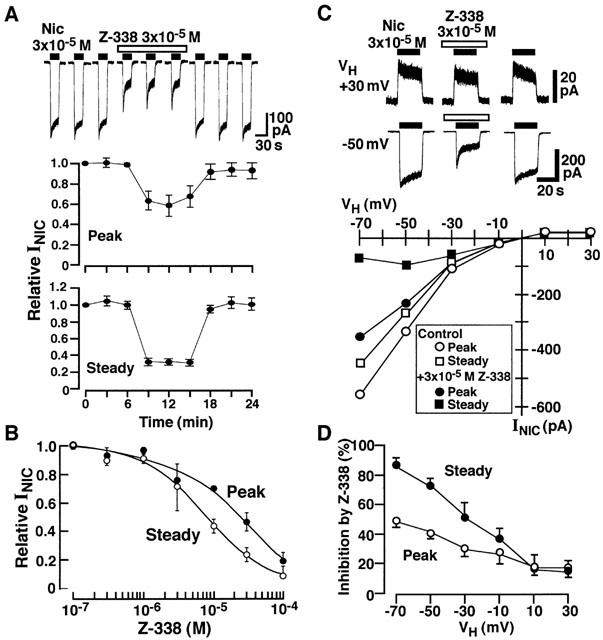
Effects of Z-338 on INic. (A) Current traces showing INic induced by 3×10−5 M nicotine in the absence and presence of 3×10−5 M Z-338. Nicotine was applied for 20 s every 3 min. Z-338 was applied for 8 min from 1 min before the application of nicotine as indicated by open bar. Lower two panels show time course of the action of Z-338 on peak and steady INIC. (B) Concentration-inhibition curve for Z-338 on 3×10−5 M nicotine-induced peak and steady currents. Steady current was arbitrarily measured 20 s after application of nicotine. Each point is the average of 4 – 5 experiments. Vertical bars indicate ±s.e.mean. (C) effect of Z-338 on INIC at various VHs. All recordings were from the same neuron. (D) Voltage-dependent effect of Z-338 on INIC. Percentage inhibition produced by Z-338 was plotted against VHs.
To study the inhibitory action of Z-338 on INIC further, the effect of Z-338 on the dose-response relationship of INIC was studied. As shown in Figure 9, Z-338 (10−5 M) suppressed the peak INIC without affecting the threshold concentration of nicotine to induce the current and suppressed the maximum response. EC50 value for nicotine to induce the peak current was 1.6×10−5 in the control condition. EC50 values for nicotine-induced peak current in the presence of 10−5 or 3×10−5 M Z-338 were 2.9×10−5 and 2.9×10−5, respectively. Thus, it seemed that Z-338 inhibits the peak currents induced by nicotine in a non-competitive manner (Figure 9B). On the other hand, Z-338 accelerated the current decay, and this change seemed to be increased with a rise in the nicotine concentration (Figure 9A). Thus, it is possible to presume that Z-338 acts as an uncompetitive blocker on nicotinic response, since the action of an uncompetitive inhibitor depends on prior activation of the receptor by the agonist, and therefore the degree of blockade increases as the level of agonist increases (Matsumoto et al., 1980; Chen & Lipton, 1997). On the other hand, in the case of non-competitive antagonism, the change produced by inhibitor does not depend on the agonist concentration. Therefore, the effect of 10−5 M Z-338 on the continuous presence of various concentrations of nicotine was also investigated. As shown in Figure 10, Z-338 produced greater suppression of the steady INIC as the concentration of nicotine increased, indicating that the interaction between Z-338 and nicotinic ACh receptors is not non-competitive but is uncompetitive.
Figure 9.
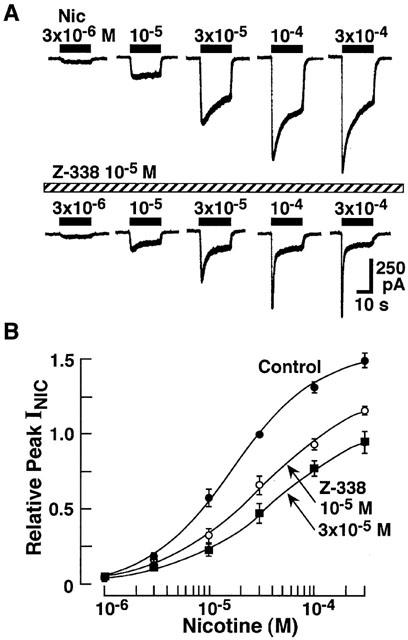
Effects of Z-338 (10−5 and 3×10−5 M) on the dose-response relationship of INIC. (A) INIC induced by various concentrations of nicotine in the presence or absence of Z-338 (10−5 M). (B) Concentration-response relationship of nicotine-induced peak currents in the presence or absence of Z-338. Each point is the average of 5 – 8 experiments, and vertical bars indicate ±s.e.mean.
Figure 10.
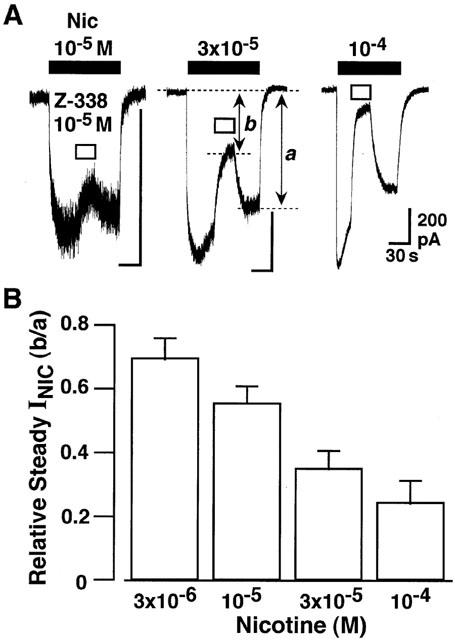
Effect of Z-338 on steady INIC. To evaluate the inhibitory action of Z-338 on the steady INIC, Z-338 was applied about 30 s after the peak INIC. Current recordings were made at a VH of −40 mV. Each nicotinic current in the presence of Z-338 was normalized to that just after wash out of Z-338, as indicated by b/a. Data are average of five experiments.
Effects of Z-338 on IOX-M
Figure 11A shows the effects of Z-338 on the IOX-M at a VH of −40 mV. Z-338 suppressed the IOX-M evoked by 3×10−7 M OX-M in a concentration-dependent manner with an EC50 of 1.4×10−5 M (Figure 11B). Z-338 shifted the concentration-response relationship of OX-M to the right without affecting the maximum current (Figure 11C), suggesting that Z-338 competitively inhibits the action of muscarinic response. The EC50 values in the absence or presence of 3×10−6, 10−5 and 3×10−5 M Z-338 were 1.4×10−7, 2.2×10−7, 5.8×10−7 and 1.1×10−6 M, respectively. The Shild plot constructed from these values yield a pA2 of 5.38 (Figure 11E). As shown in Figure 11D, the Lineweaver-Burk plot also confirmed that Z-338 inhibits the action of OX-M in a competitive manner, since the plots differ in slope and shared a common intercept on 1/R axis.
Figure 11.
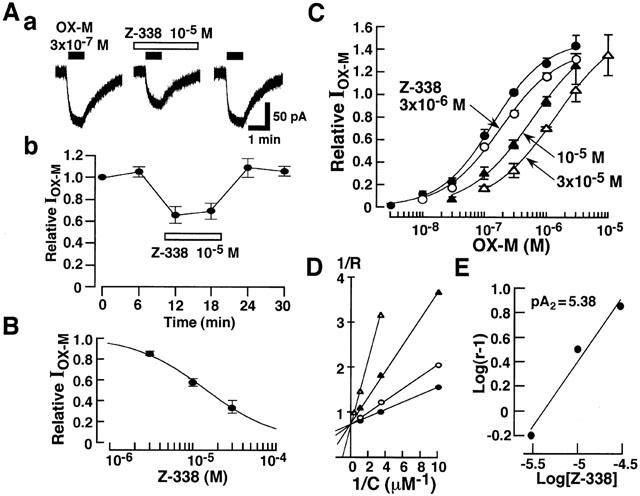
Effect of Z-338 on the IOX-M. (A) Effects of Z-338 (10−5 M) on the IOX-M. OX-M (3×10−7 M) was applied for 1 min every 6 min in the absence and presence of Z-338. Current recordings were made at a VH of −40 mV. (B) Concentration-dependent inhibitory effects of Z-338 on IOX-M. (C) Effects of Z-338 on the concentration response relationship of IOX-M. (D) Lineweaver-Burk plots for the inhibitory effects of Z-338 on IOX-M. Note the plots shared a common intercept on 1/R axis. (E) Shild plot analysis for Z-338. Shild plot was constructed from the data shown in (C) where r is the OX-M concentration ratio. The line was fitted by linear regression and yielded a pA2 for Z-338 of 5.38 with an unconstrained slope of 1.05 (r2=0.98).
The effect of Z-338 on IOX-M was also examined at various VHs. The inhibition of IOX-M by 10−5 M Z-338 at VHs of −60, −40, −20 and 0 mV was 46.5±2.6, 42.4±5.8, 41.0±2.2 and 43.7±4.9% (n=4), respectively. There is no statistical difference among these values.
Effects of Z-338 on M-current
As mentioned above, OX-M suppressed the M-type K+ current dose-dependently (Figure 5). Therefore it was of interest to observe the effects of Z-338 on the inhibitory action of OX-M on M-type K+ current. After the pretreatment with Z-338 (3×10−5 M), the inhibitory action of OX-M (3×10−7 M) on the M-type K+ current was greatly suppressed (Figure 12).
Figure 12.
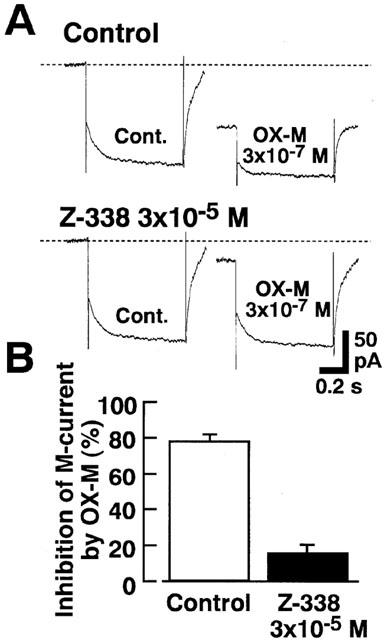
Effects of Z-338 on the inhibitory actions of OX-M on M type K+ current. Hyperpolarizing voltage-steps from a VH of −25 mV to −50 mV were applied to induce the M-current deactivation. In the presence of Z-338 (3×10−5 M) inhibitory action of OX-M was greatly relieved. The column show the mean value of four experiments, and vertical bars shows ±s.e.mean.
Discussion
In the present experiments, using nystatin perforated patch recording mode, we found that ACh induces slow and fast inward currents through the activation of muscarinic M1 and neuronal nicotinic ACh receptors, both of which produce membrane depolarization in freshly dissociated rat paratracheal ganglion neurons. The sensitivity of nicotinic and muscarinic M1 receptors of paratracheal ganglion neurons to cholinergic agonists was similar to those observed by conventional whole-cell recording in other autonomic ganglion neurons. For example, EC50 values for nicotine- and OX-M-induced currents were 1.6×10−5 and 1.4×10−7 M in paratracheal neurons, and 7.5×10−5 and 3.0×10−7 M in rat superior cervical ganglion neurons, respectively (Nakazawa, 1994; Bernheim et al., 1992).
The release of ACh from cholinergic nerve terminals is modulated by prejunctional muscarinic receptors that serve to limit (inhibitory autoreceptor) or enhance (facilitatory autoreceptor) the release of ACh. The previous studies in the enteric nervous system have indicated that the release of ACh from guinea-pig mesenteric and submucous plexus neurons is inhibited by the M2 receptor in rat antral mucosal/submucosal neurons (Ren & Harty, 1994). Since prejunctional muscarinic inhibitory autoreceptors attenuate the release of ACh from the vagus nerve terminals which innervate airway smooth muscle in the guinea-pig (Freyer & Maclagan, 1984; Kilbinger et al., 1995), rat (Zhu et al., 1997), dog (Ito & Yoshitomi, 1988) and human (Minette & Barnes, 1988), it was proposed that prejunctional inhibitory muscarinic autoreceptors play an important role as a ‘braking system' in excitatory cholinergic neuro-effector transmission in the airway (Freyer & Maclagan, 1984; Ito & Yoshitomi, 1988; Murai et al., 1998). On the other hand, the functional role of prejunctional muscarinic M1 receptor in ACh release from prejunctional terminal is somewhat controversial. In airway, the release of ACh from guinea-pig mesenteric and submucous plexus neurons is inhibited by prejunctional M1 receptor (Kawashima et al., 1988; Kilbinger et al., 1993; Dietrich & Kilbinger, 1995). It is also reported that prejunctional M1 receptors act as excitatory autoreceptors which facilitate the ACh release in rat urinary bladder (Somogyi & de Groat, 1999), guinea-pig gallbladder (Parkman et al., 1999) and guinea-pig ileum (Izzo et al., 1999). Therefore, muscarinic M1 receptors on different locations may serve distinct physiological functions.
Assuming M1 muscarinic and neuronal nicotinic ACh receptors are distributed on the cholinergic nerve terminals, activation of these receptors by released ACh would depolarize the nerve terminal as shown in Figure 1A. If concentration of released ACh is low, ACh produce a mild depolarization via muscarinic M1 receptors, leading to the facilitation of the ACh release. On the other hand, providing the concentration of released ACh is high, ACh will lead a greater depolarization through an activation of nicotinic receptors, and the generation of action potentials should be attenuated because of inactivation of voltage-dependent Na+ channels as shown in Figure 1Ab. This may lead the decrease in ACh release from presynaptic nerve terminals. Thus, it is possible to speculate that nicotinic ACh receptor acts as an inhibitory autoreceptor on cholinergic nerve terminals. The presynaptic nicotinic ACh receptor of α-bungarotoxin-insensitive type is also thought to be an inhibitory autoreceptor on rat phrenic nerve terminal (Tian et al., 1997; Prior & Singh, 2000).
In addition, we have also found that ACh selectively reduces both N- and R-type high-voltage-activated (HVA) Ca2+-currents in the isolated paratracheal ganglia, by activating pertussis toxin sensitive G-protein through the M2 muscarinic receptor (Murai et al., 1998). Schild plot analysis of the agonist concentration-ratios yielded pA2 values of 6.85 and 8.57 for pirenzepine and methoctramine, respectively. The low affinity for pirenzepine suggests any contribution of muscarinic M1 receptors is small, whilst the high affinity for methoctramine favours the involvement of muscarinic M2 and/or M4 receptors. The pA2 values for these antagonists on paratracheal ganglion cells are similar to those estimated by ligand binding studies on heterologously expressed M2 receptors. The pKi values are 6.04 – 6.70 and 7.88 – 8.44 respectively (Buckley et al., 1989; Dörje et al., 1991). However these results do not exclude the possible involvement of muscarinic M4 receptors, if present, in the inhibitory effects of ACh on the Ca2+-current in the paratracheal ganglia. Thus, provided N- or R-type Ca2+ channels are present in the nerve terminals and that these receptors are coupled with muscarinic ACh-receptors through pertussis toxin-sensitive G-proteins, as observed in the cell body, the inhibition of these Ca2+ channels by ACh would also reduce the amount of ACh released from the nerve terminals in response to single or repetitive stimulation, thereby operating as a ‘braking system'.
One of the new findings in the present experiments is that Z-338 inhibits muscarinic and nicotinic ACh receptor-mediated inward currents competitively and uncompetitively, respectively. The form of antagonism by which the antagonist combines primarily with the active agonist-receptor complex is equivalent to uncompetitive antagonism (Pennefather & Quastel, 1982). The inhibitory actions of Z-338 on the inhibitory autoreceptors would facilitate the excitatory cholinergic neuro-effector transmission, due to the removal of the autoinhibitory mechanisms of ACh. In gastrointestinal tissues, M1, M2 and M3 receptors are reported to be present on the neurons and/or smooth muscle cells. The neuronal muscarinic receptors appear to be located on the cholinergic nerve terminals and to operate as autoreceptors (Starke et al., 1989). A recent study showed that pirenzepine and AF-DX116 enhanced the electrically stimulated ACh release, and the authors concluded that facilitation of ACh release in the presence of pirenzepine and AF-DX116 can be attributed to blockade of the prejunctional M1 and M2 autoreceptors which are activated by ACh released from the nerve terminals (Ogishima et al., 2000). Z-338 acts as an antagonist at M1 and M2 receptors in the oocytes expressing either M1 or M2 muscarinic receptors, since Z-338 at concentrations 10−6 M inhibited the ACh-induced inwards currents mediated by stimulation of M1 and M2 muscarinic receptors although Z-338 alone did not produce any currents (Ogishima et al., 2000). On the other hand, Z-338 at low concentrations (10−8 – 10−6 M) dose-dependently enhances the amplitude of twitch-like contractions and excitatory junction potentials (EJPs) evoked by single or repetitive electrical field stimulation without affecting the non-adrenergic non-cholinergic (NANC) relaxation and inhibitory junction potentials (IJPs) in the circular muscle strips of the guinea-pig stomach (Nakajima et al., 2000). The EC50 of the Z-338 concentration response curve on the EJPs was 4.7×10−8 M (Nakajima et al., 2000). This EC50 value is markedly lower than that observed in the Z-338 action on muscarinic responses. These observation suggest that Z-338 may enhance the EJP amplitude through blockade of nicotinic autoreceptors. This may be possible provided that concentration of released ACh from nerve terminal is high at the junctional cleft, since the concentration-inhibition curve shifts to the left as the concentration of agonist increases when the relative change in agonist-induced response is plotted against concentration of the uncompetitive antagonist in logarithmic scale (Matsumoto et al., 1980). However the existence and possible role of nicotinic ACh receptors in the nerve terminal is yet to be clarified in the autonomic nervous system, in terms of modulation of the transmitter release. In the motor nerve terminals, on the other hand, it is known that ACh release is negatively regulated by nicotinic ACh autoreceptors, and the neuronal nicotinic antagonists, hexamethonium and methyllycaconitine, increase the evoked ACh release from phrenic nerve terminals (Tian et al., 1997; Prior & Singh, 2000).
So far, little information is available about the uncompetitive inhibition of nicotinic response since no specific inhibitor was identified which would interact with nicotinic ACh receptor in the uncompetitive mode. On the other hand, uncompetitive antagonists of the NMDA receptor are known to bind the channel site in a ‘use-dependent' manner (Parsons et al., 1999). This means that they block the channel when the channel is open state. In the present study, when nicotine was applied for 20 s every 3 min in the presence of Z-338, Z-338 seemed to have no use-dependent block of peak INIC (Figure 8A). However, Z-338 accerelated the current decay and suppressed steady INIC greater than peak INIC (Figures 8 and 9). This is one of the characteristics of open channel blockade. The degree of use-dependent blockade of peak current depends on interval of agonist application. Thus, the inter-response interval of 3 min used in the present study may be too long to evaluate the use-dependent suppression of peak INIC by Z-338. The effect of Z-338 on INIC evoked by high frequency application of nicotine should be examined in a future study.
Previously it was reported that ACh induces inward current consisting of an initial transient peak and a successive steady-state plateau in the dissociated rat paratracheal ganglion cells (Aibara & Akaike, 1991). The authors concluded that the ACh-induced inward current (IACh) is mediated mainly by nicotinic receptor activation for the following reasons: (i) it was mimicked by nicotine, (ii) in a K+-free solution, EACh was close to ENa, (iii) the current-voltage relationship for the IACh showed inward rectification as previously reported in other preparations (Mathie et al., 1990; Tateishi et al., 1990), and iv) the peak and steady components of IACh was blocked by either D-tubocurarine or atropine. Atropine inhibited the IACh by a non-competitive manner, and the authors suggested that atropine could block the nicotinic response as reported in bullfrog sympathetic ganglion cells (Conor et al., 1983), frog sympathetic neurons (Sadoshima et al., 1990) and parasympathetic neurons of frog heart (Tateishi et al., 1990). However, the present experiments revealed that muscarinic agonists evoke a slow inward current through activation of muscarinic ACh receptors by inhibition of the M type K+ current.
In conclusion, the present experiments revealed that ACh induces slow and fast inward currents through activation of M1 muscarinic and neuronal nicotinic ACh receptors in isolated paratracheal ganglion cells, and Z-338 inhibits both slow and fast inward currents induced by ACh in a competitive and uncompetitive manner, respectively.
Acknowledgments
We thank Dr K.E. Creed for critical reading of the study. This work supported by grants from the Ministry of Education and Sciences (No.12470020 to Y. Ito & No. 13307003 to N Akaike).
Abbreviations
- ACh
acetylcholine
- EC50
half-maximum effective concentration
- IC50
half-maximum inhibitory concentration
- INIC
nicotine-induced current
- IOX-M
oxotremorine-M-induced current
- OX-M
oxotremorine-M
- VH
holding potential
- Z-338
N-(N′,N′-diisopropylaminoethyl)-[2-(2-hydroxy-4,5-dimethoxy-benzoyl-amino)-1,3-thiazole-4-yl] carboxyamide monohydrochloride trihydrate
References
- AIBARA K., AKAIKE N. Acetylcholine-activated ionic currents in isolated paratracheal ganglion cells of the rat. Brain Res. 1991;558:20–26. doi: 10.1016/0006-8993(91)90709-5. [DOI] [PubMed] [Google Scholar]
- AIBARA K., EBIHARA S., AKAIKE N. Voltage-dependent ionic currents in dissociated paratracheal ganglion cells of the rat. J. Physiol. 1992;457:591–610. doi: 10.1113/jphysiol.1992.sp019396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKAIKE N., HARATA H. Nystatin perforated patch recording and its applications to analysis of intracellular mechanisms. Jpn. J. Physiol. 1994;44:433–473. doi: 10.2170/jjphysiol.44.433. [DOI] [PubMed] [Google Scholar]
- AKAIKE N., INOMATA N., TOKUTOMI N. Contribution of chloride shifts to the fade of γ-aminobutyric acid-gated currents in frog dorsal root ganglion cells. J. Physiol. 1987;391:219–234. doi: 10.1113/jphysiol.1987.sp016735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNHEIM L., MATHIE A., HILLE B. Characterization of muscarinic receptor subtypes inhibiting Ca2+ current and M current in rat sympathetic neurons. Proc. Natl. Acad. Sci. U.S.A. 1989;89:9544–9548. doi: 10.1073/pnas.89.20.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCKLEY N.J., BONNER T.T., BUCKLEY C.M., BRANN M.R. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-KI cells. Mol. Pharmacol. 35:469–476. [PubMed] [Google Scholar]
- CHEN H.-S.V., LIPTON S.A. Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism. J. Physiol. (Lond.) 1997;499:27–46. doi: 10.1113/jphysiol.1997.sp021909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONOR E.A., LEVY S.M., PARSONS R.L. Kinetic analysis of atropine-induced alterations in bullfrog ganglionic fast synaptic currents. J. Physiol. 1983;337:137–158. doi: 10.1113/jphysiol.1983.sp014616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONSTANTI A., BROWN D.A. M-currents in voltage-clamped mammalian sympathetic neurons. Neurosci. Lett. 1981;24:289–294. doi: 10.1016/0304-3940(81)90173-7. [DOI] [PubMed] [Google Scholar]
- DIETRICH C., KILBINGER H. Prejunctional M1 and postjunctional M3 muscarinic receptors in the circular muscle of guinea-pig ileum. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;351:237–243. doi: 10.1007/BF00233242. [DOI] [PubMed] [Google Scholar]
- DÖRJE F., WESS J., LAMBRECHT G., TACKE R., MUTSCHLER E., BRANN M.R. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J. Pharmacol. Exp. Ther. 1991;256:727–733. [PubMed] [Google Scholar]
- FREYER A.D., MACLAGAN J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerve in the guinea pig. Br. J. Pharmacol. 1984;83:973–978. doi: 10.1111/j.1476-5381.1984.tb16539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIBASHI H., MOCHIDOME T., OKAI J., ICHIKI H., SIMADA H., TAKAHAMA K. Activation of potassium conductance by ophiopogonin-D in acutely dissociated rat paratracheal neurones. Br. J. Pharmacol. 2001;132:461–466. doi: 10.1038/sj.bjp.0703818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO Y., MURAI Y., ISHIBASHI H., ONOUE H., AKAIKE N. The prostaglandin E series modulates high-voltage-activated calcium channels probably through the EP3 receptor in rat paratracheal ganglia. Neuropharmacol. 2000;39:181–190. doi: 10.1016/s0028-3908(99)00142-2. [DOI] [PubMed] [Google Scholar]
- ITO Y., YOSHITOMI T. Autoregulation of acetylcholine release from vagus nerve terminals through activation of muscarinic receptors in the dog trachea. Br. J. Pharmacol. 1988;93:636–646. doi: 10.1111/j.1476-5381.1988.tb10321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., DI CARLO G., CAPASSO F. Ascending neural pathways in the isolated guinea-pig ileum: effect of muscarinic M1, M2 and M3 cholinergic antagonists. Neuroscience. 1999;91:1575–1580. doi: 10.1016/s0306-4522(98)00641-1. [DOI] [PubMed] [Google Scholar]
- KAWASHIMA K., FUJIMOTO K., SUZUKI T., OOHATA H. Direct determination of acetylcholine release by radioimmunoassay and presence of presynaptic M1 muscarinic receptors in guinea pig ileum. J. Pharmacol. Exp. Ther. 1988;244:1036–1039. [PubMed] [Google Scholar]
- KILBINGER H., DIETRICH C., VON BARDELEBEN R.S. Functional relevance of presynaptic muscarine autoreceptors. J. Physiol. (Paris) 1993;87:77–81. doi: 10.1016/0928-4257(93)90001-a. [DOI] [PubMed] [Google Scholar]
- KILBINGER H., VON BARDELEBEN R., SIEFKEN H., WOLF D. Prejunctional muscarinic receptors regulating neuro-transmitter release in airways. Life Sci. 1995;56:981–987. doi: 10.1016/0024-3205(95)00037-7. [DOI] [PubMed] [Google Scholar]
- MATHIE A., COLQUHOUN D., CULL-CANDY S.G. Rectification of currents activated by nicotinic acetylcholine receptors in rat sympathetic ganglion neurones. J. Physiol. 1990;427:625–655. doi: 10.1113/jphysiol.1990.sp018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUMOTO M., SASAKI K., SOMEI K., SATO M. Dose-inhibition curve and its application to the analysis of ACh-receptor activity. Jpn. J. Physiol. 1980;30:743–750. doi: 10.2170/jjphysiol.30.743. [DOI] [PubMed] [Google Scholar]
- MINETTE P.A., BARNES P.J. Prejunctional inhibitory muscarinic receptors on cholinergic nerves in human and guinea-pig airways. J. Appl. Physiol. 1988;64:2532–2537. doi: 10.1152/jappl.1988.64.6.2532. [DOI] [PubMed] [Google Scholar]
- MURAI Y., ISHIBASHI H., AKAIKE N., ITO Y. Acetylcholine modulation of high-voltage-activated calcium channels in the neurones dissociated from rat paratracheal ganglia. Br. J. Pharmacol. 1998;123:1441–1449. doi: 10.1038/sj.bjp.0701753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS A.C., UNDEM J.B., WEINREICH D. Electrophysiological properties of neurons in guinea pig broncheal parasympathetic ganglia. Am. J. Physiol. 1990;259:L403–L409. doi: 10.1152/ajplung.1990.259.6.L403. [DOI] [PubMed] [Google Scholar]
- NAKAGAWA T., SHIRASAKI T., WAKAMORI M., FUKUDA A., AKAIKE N. Excitatory amino acid response in isolated nucleus tractus solitaric neurons of the rat. Neurosci. Res. 1990;8:114–123. doi: 10.1016/0168-0102(90)90063-k. [DOI] [PubMed] [Google Scholar]
- NAKAJIMA T., NAWATA H., ITO Y. Z-338, a newly synthetized carboxyamide derivative, stimulates gastric motility through enhancing the excitatory neuortransmission. J. Smooth Muscle Res. 2000;36:69–81. doi: 10.1540/jsmr.36.69. [DOI] [PubMed] [Google Scholar]
- NAKAZAWA K. ATP-activated current and its interaction with acetylcholine-activated current in rat sympathetic neurons. J. Neurosci. 1994;14:740–750. doi: 10.1523/JNEUROSCI.14-02-00740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGISHIMA M., KAIBARA M., UEKI S., KURIMOTO T., TANIYAMA K. Z-338 facilitates acetylcholine release from enteric neurons due to blockade of muscarinic autoreceptors in guinea pig stomach. J. Pharmacol. Exp. Ther. 2000;294:33–37. [PubMed] [Google Scholar]
- PARKMAN H.P., PAGANO A.P., PYAN J.P. Subtypes of muscarinic receptors regulating gallbladder cholinergic contractions. Am. J. Physiol. 1999;276:G1243–G1250. doi: 10.1152/ajpgi.1999.276.5.G1243. [DOI] [PubMed] [Google Scholar]
- PARSONS C.G., DANYSZ W., BARTMANN A., SPIELMANNS P., FRANKIEWICZ T., HESSELINK M., EILBACHER B., QUACK G. Amino-alkyl-cyclohexanes are novel uncompetitive NMDA receptor antagonists with strong voltage-dependency and fast blocking kinetics: in vitor and in vivo characterization. Neuropharmacology. 1999;38:85–108. doi: 10.1016/s0028-3908(98)00161-0. [DOI] [PubMed] [Google Scholar]
- PENNEFATHER P., QUASTEL D.M.J. Modification of dose-response curves by effector blockade and uncompetitive antagonism. Mol. Pharmacol. 1982;22:369–380. [PubMed] [Google Scholar]
- PRIOR C., SINGH S. Factors influencing the low-frequency associated nicotinic ACh autoreceptor-mediated depression of ACh release from rat motor nerve terminals. Br. J. Pharmacol. 2000;129:1067–1074. doi: 10.1038/sj.bjp.0703161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REN J., HARTY R.F. Presynaptic muscarinic receptors modulate acetylcholine release from rat antral mucosal/submucosal nerves. Dig. Dis. Sci. 1994;39:1099–1106. doi: 10.1007/BF02087564. [DOI] [PubMed] [Google Scholar]
- SADOSHIMA J., OYAMA Y., AKAIKE N. Inhibition of nicotinic acetylcholine response by atropine in frog isolated sympathetic neurons. Brain Res. 1990;508:147–151. doi: 10.1016/0006-8993(90)91128-4. [DOI] [PubMed] [Google Scholar]
- SOMOGYI G.T., DE GROAT W.C. Function, signal transduction mechanisms and plasticity of presynaptic muscarinic receptors in the urinary bladder. Life Sci. 1999;64:411–418. doi: 10.1016/s0024-3205(98)00580-3. [DOI] [PubMed] [Google Scholar]
- STARKE K., GÖTHERT M., KILBINGER H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol. Rev. 1989;69:864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- TATEISHI N., KIM D.K., AKAIKE N. Acetylcholine-activated ionic currents in parasympathetic neurons of bullfrog heart. J. Neurophysiol. 1990;63:1052–1059. doi: 10.1152/jn.1990.63.5.1052. [DOI] [PubMed] [Google Scholar]
- TIAN L., PRIOR C., DEMPSTER J., MARSHALL I.G. Hexamethonium- and methyllycaconitine-induced changes in acetylcholine release from rat motor nerve terminals. Br. J. Pharmacol. 1997;122:1025–1034. doi: 10.1038/sj.bjp.0701481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UEKI S., MATSUNAGA Y., MATSUNAURA T., HORI Y., YONETA T., KURIMOTO T., TAMAKI H., ITOH Z. Z-338, a novel prokinetic agent, stimulates gastrointestinal motor activity and improves delayed gastric emptying in the dog and rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358 Suppl. 1:R351. [Google Scholar]
- ZHU F.-X., ZHANG X.-Y., ROBINSON N.E. Origin and modulation of ACh release from rat airway cholinergic nerves. Am. J. Physiol. 1997;272:L8–L4. doi: 10.1152/ajplung.1997.272.1.L8. [DOI] [PubMed] [Google Scholar]


