Abstract
The diverse physiological actions of somatostatin are mediated by a family of G-protein coupled receptors (SSTRs). Several peptide analogues of somatostatin such as octreotide have been developed for therapeutic use, including treatment of gastrointestinal disorders such as secretory diarrhoea. However, their development as anti-diarrhoeal agents has been limited by poor oral bioavailability, necessitating parenteral administration. This in vitro study investigated the anti-secretory potential of a group of novel, non-peptide, somatostatin-receptor agonists that selectively activate specific SSTR subtypes to assess their potential for oral administration.
The ability of the agonists to inhibit forskolin-stimulated chloride secretion was measured using a sensitive bioassay system in isolated rat colonic mucosa.
The SSTR-2 selective agonist, L-779,976 was 10-times more potent than octreotide as an inhibitor of secretion when added to the basolateral surface of rat colon. Non-peptide agonists selective for SSTR1 (L-797,591), SSTR3 (L-796,778), SSTR4 (L-803,087) or SSTR5 (L-817,818) showed little or no anti-secretory activity in this preparation.
L-779,976 was able to inhibit secretion when applied to the luminal surface at sub-micromolar concentrations suggesting that it can cross the colonic epithelium. The anti-secretory potency of luminal L-779,976 was increased 3 fold in the presence of GF120918, a known inhibitor of P-glycoprotein.
Non-peptide somatostatin receptor agonists may provide a basis for the development of new, orally available anti-diarrhoeal therapies.
Keywords: Somatostatin; octreotide; secretory diarrhoea; L-779,976
Introduction
Somatostatin (SST) is a regulatory peptide with diverse physiological functions that are mediated by a family of G-protein coupled receptors (SSTRs) (Patel, 1999; Csaba & Dournaud, 2001). In the large intestine, somatostatin is a potent inhibitor of fluid and electrolyte secretion, primarily by activation of SSTR2 receptors, although the contribution of SSTR1 remains unclear (McKeen et al., 1995; Warhurst et al., 1995; 1996).
Peptide-based analogues of somatostatin, such as octreotide (Pless et al., 1986), have been developed for use in clinical therapies to overcome the short therapeutic half-life of somatostatin. These analogues have been shown to be highly stable, to exhibit greater potency in the inhibition of gastrointestinal secretion than native somatostatin (Lembcke et al., 1987), and to be highly effective in the treatment of gastrointestinal disorders including bleeding angiodysplasia and varices, endocrine tumours, gastrointestinal fistulas, short bowel syndrome and severe or intractable diarrhoea (Farthing, 1996; Guarino et al., 1998; Murao et al., 1999; Nightingale et al., 1989; Oberg, 1996; Orsi et al., 2001; Torres et al., 1992; Wang et al., 2001; Zidan et al., 2001). However, their use as anti-diarrhoeal agents is limited by their poor oral bioavailability, which necessitates parenteral administration (Fuessl et al., 1987) and marked pain at the sites of injection (Creutzfeldt et al., 1987). A key objective in developing new somatostatin-based therapies has therefore been to identify molecules that can selectively activate particular SSTR subtypes and which have the potential for oral administration. Such a group of non-peptide compounds has been developed using combinatorial libraries based on molecular modelling of known peptide agonists (Rohrer et al., 1998).
The current study had two objectives. Firstly, using an in vitro bioassay of intestinal secretion in isolated rat distal colon, to determine the ability of the compounds described by Rohrer et al. (1998) to inhibit fluid and electrolyte secretion when applied to the serosal surface of the intestine. Secondly, as an indicator of their potential for oral delivery, to assess whether these analogues display anti-secretory activity when applied to the luminal surface of the intestine. The results show that the SSTR2-selective agonist, L-779,976, is a highly potent inhibitor of intestinal secretion when applied serosally. In addition, evidence is presented that this compound can permeate functionally intact across the intestinal epithelium when applied to the luminal surface, and that interaction with drug efflux processes may limit this ability.
Methods
Non-peptide somatostatin receptor subtype-specific agonists
A series of non-peptide somatostatin receptor agonists that bind with high affinity and selectivity to the five somatostatin receptor subtypes, SSTR 1 – 5 were used in these studies. The identification through combinatorial chemistry and screening for receptor selectivity has been described previously (Rohrer et al., 1998) and is summarized in Table 1 as inhibition constant (Ki) determined using a receptor binding assay against recombinant human SSTR subtypes. The molecular weights of the compounds are also shown.
Table 1.
Inhibition constant (Ki) against recombinant human SSTR (taken from Rohrer et al., 1998) and molecular weights of the non-peptide SSTR-selective agonists

In vitro measurement of ion secretion in rat colon
The anti-secretory properties of non-peptide somatostatin receptor agonists were assessed by their ability to inhibit forskolin (FSK)-stimulated short circuit current (Isc) in rat colonic mucosa in vitro (Warhurst et al., 1996). Increases in Isc stimulated by cyclic AMP agonists such as FSK have previously been shown to be indicative of Cl− secretion in this model. Non-fasting male Sprague-Dawley rats were killed by cervical dislocation in accordance with Home Office regulations, the distal colon (or proximal colon, where indicated) was isolated and its muscle layers removed by blunt dissection. Segments of mucosa were mounted in Ussing chambers with an exposed tissue area of 0.64 cm2 and bathed on luminal and serosal surfaces with a bicarbonate-buffered Ringer solution containing (in mM) NaCl, 121; NaHCO3, 25; K2HPO4, 1.2; KH2PO4, 0.2; KHCO3, 1.6; CaCl2, 1.2, MgCl2, 1.2 and glucose 10 (pH 7.4). Bathing solutions were continuously oxygenated with 95% O2/5% CO2 and maintained at 37°C. Tissues were equilibrated for ≈45 min to allow stabilization of electrical parameters. The spontaneous transmucosal electrical potential difference (PD) was measured via 3 M KCl in 3% agar bridges and matched calomel electrodes. Isc was measured using silver/silver chloride electrodes connected to a voltage clamp for automatic short circuiting. Tissue resistance was calculated from PD and Isc according to Ohm's law. Under these conditions, distal colon exhibited a mean Isc of 22.8±4.8 μA.cm−2 (n=35) and transepithelial electrical resistance, Rt of 72.5±3.1 ohms.cm2 (n=35). Following equilibration, FSK was added to the serosal bathing medium to give a final concentration of 0.25 μM. At the peak of the resulting Isc response, somatostatin receptor agonists were added to either serosal or mucosal chambers and the change in Isc monitored. Where indicated, tissues were pre-treated for 15 min with GF 120918 (30 μM) added to both luminal and serosal surfaces.
Statistical methods
Values are expressed as mean±s.e.mean for the number of tissues indicated in each group. These were taken from a minimum of three animals. Statistical significance was assessed using Student's t-test with a P value of <0.05 taken as significant.
Materials
The somatostatin receptor subtype-specific non-peptide agonists, L-797,591 (SSTR1), L-779,976 (SSTR2), L-796,778 (SSTR3), L-803,087 (SSTR4) and L-817,818 (SSTR5) were kindly provided by Dr Susan Rohrer of Merck Research Laboratories (Rahway, U.S.A.). GF120918 was provided by GlaxoSmithKline (Ware, U.K.) and octreotide by Novartis (Basel, Switzerland). Native somatostatin (SST-14) and forskolin were purchased from Sigma-Aldrich Chemical Co. Ltd. (Poole, Dorset, U.K.).
Results
Anti-secretory properties of non-peptide somatostatin agonists
The anti-secretory effects of the non-peptide SSTR-selective agonists were studied in rat colon stimulated by the cyclic AMP-mediated secretagogue, forskolin (FSK). In colonic mucosa, 0.25 μM FSK induced a sustained increase in Isc (Control: 16.7±4.2 μA.cm−2, FSK: 47.2±5.5 μA.cm−2 n=36, P<0.01). Serosal addition of SSTR2 agonist L-779,976 at a concentration of 1 nM caused a rapid and almost complete inhibition of FSK-stimulated Isc within 10 min. Octreotide at the same concentration inhibited secretion by 44±9.5% while 1 nM SST-14 induced a very small inhibitory effect (Figure 1). The non-peptide agonists for receptor types 1 (L-797,591) and 5 (L-817,818), subtypes that are also expressed in intestinal mucosa at the mRNA level, exhibited very weak anti-secretory activity at concentrations in the micromolar range (Figure 2). It is likely that at high concentrations the response to these agonists reflects cross-reactivity with the type 2 receptor (see Table 1). The type 4 (L-803,087) and type 3 (L-796,778) selective agonists showed little or no activity respectively at concentrations up to 10 μM (Figure 2).
Figure 1.
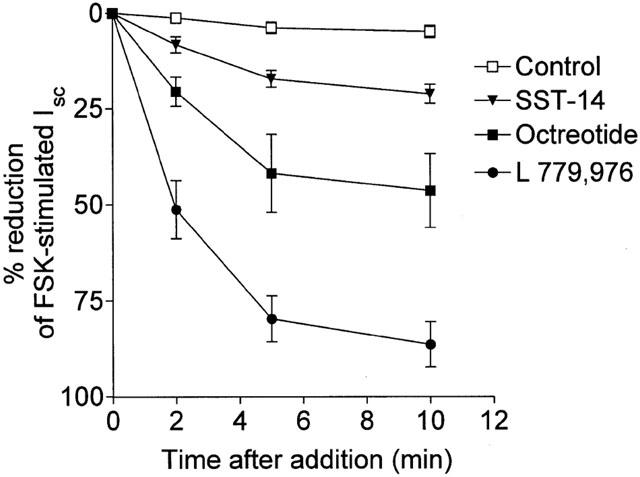
Inhibition of forskolin (FSK)-stimulated ion secretion in rat colon in vitro by serosal addition of somatostatin and analogues. Tissues were stimulated by addition of FSK to the serosal surface to a final concentration of 0.25 μM. The mean basal Isc and the mean FSK-stimulated increase in Isc were 16.7±4.2 μA.cm−2 and 30.4±2.3 μA.cm−2 respectively. At the peak of the short circuit current (Isc) response (shown as t=0), somatostatin, octreotide, L-779,976 (all at 1 nM) or control buffer were added serosally. Results show per cent decrease in the FSK-stimulated Isc and represent mean±s.e.mean for n=6 – 12 tissues in each group.
Figure 2.
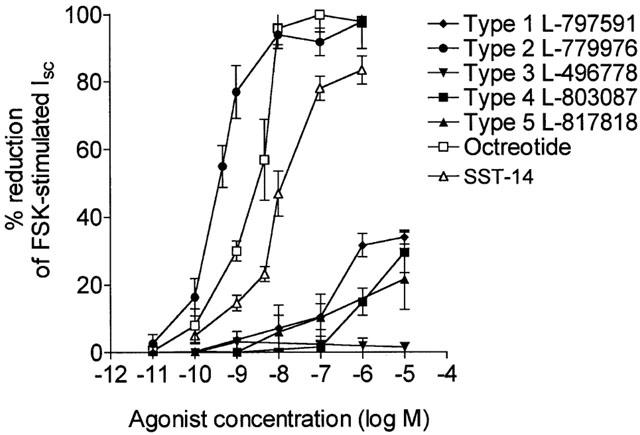
Dose response inhibition of FSK-stimulated secretion by non-peptide somatostatin analogues. Rat colonic mucosa stimulated by 0.25 μM FSK (final conc.) were exposed serosally to several concentrations of L-797,591 (SSTR1), L-779,976 (SSTR2), L-796,778 (SSTR3), L-803,087 (SSTR4), L-817,818 (SSTR5), octreotide and SST-14. Results show the decrease in Isc plotted as a percentage of the FSK-stimulated response measured 10 min after addition of the analogues. Data are shown as mean±s.e.mean for n=5 – 7 tissues in each group.
Dose response studies confirmed the SSTR2-selective agonist L-779,976 as an extremely potent inhibitor of intestinal ion secretion (Figure 2) producing half-maximal inhibition of the FSK-stimulated Isc response at 0.37 nM. This is 10 fold lower than that observed for octreotide (3.8 nM) and almost 50 fold lower than the native peptide SST-14 (18 nM). These data show that L-779,976 is the most potent anti-secretory agent so far identified and that activation of the other four SSTR subtypes produces no significant anti-secretory effects.
Mucosal action of L-779,976
The high potency and relatively small size (645 Da) of the SSTR2 agonist L-779,976 suggested that it may have the potential to cross the intestinal epithelium and inhibit secretion when applied to the mucosal (or luminal) surface. To investigate this possibility, L-779,976 was added to the mucosal bathing medium following activation of secretion by 0.25 μM FSK, and the effect on Isc monitored over a 60 min period. Mucosal L-779,976 induced a marked dose- and time-dependent inhibition of FSK-stimulated Isc (Figure 3). At the highest concentration (1 μM) L-779,976 essentially abolished secretion but, compared with serosal addition, the time-course was much slower, with maximal inhibition occurring after 20 min (c.f. 5 – 10 min following serosal addition). At lower concentrations, the time needed to attain maximal Isc inhibition was considerably longer (50 min with 10 nM mucosal L-779,976). In contrast, SST-14 had no significant effect on Isc when applied mucosally at concentrations of up to 1 μM (Figure 3). Since SST receptors are known to be expressed only on the basolateral membrane of colonocytes (Warhurst et al., 1995, 1996), these results suggest that L-779,976 is able to permeate the intestinal epithelium. Comparison of the dose response curves for serosal (Ki – 0.38 nM) and mucosal (Ki – 48 nM) addition of L-779-976 indicates that approximately 0.4% of the agonist crosses the colonic epithelium within 30 min (Figure 4).
Figure 3.
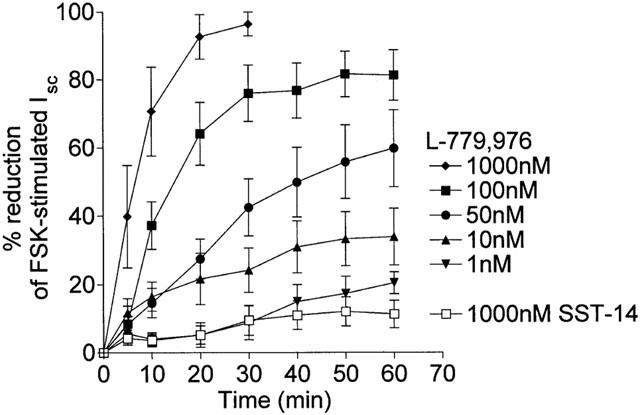
Anti-secretory action of L-779,976 added to the mucosal surface of FSK-stimulated rat colon. L-779,976 (1 – 1000 nM) or SST-14 (1000 nM) was added to the mucosal surface of FSK-stimulated tissue at the peak of the FSK response and the change in Isc monitored over 60 min. Data are shown as the decrease in Isc plotted as a percentage of the FSK-stimulated response. Values are mean±s.e.mean for 6 – 8 tissues in each group.
Figure 4.
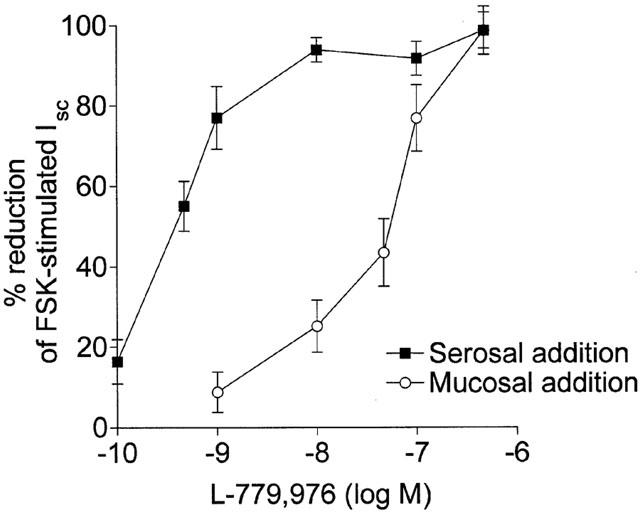
Comparison of the dose response curves for inhibition of FSK-stimulated Isc by serosal and mucosal L-779,976 in rat colon. Values were measured 10 min after addition of the agonist to the serosal surface or 30 min after addition to the mucosal (luminal) surface of the tissue. Data are shown as the decrease in Isc plotted as a percentage of the FSK-stimulated response. Values are mean±s.e.mean for 6 – 8 tissues in each group.
To investigate further whether Isc inhibition was due to L-779,976 permeation from the mucosal to the serosal reservoir, fluid was removed from the serosal reservoir of tissues that had been exposed on the mucosal surface to 1 μM L-779,976 for 60 min. The retrieved serosal fluid, at a dilution of 1 : 5, was then added to the serosal reservoir of fresh FSK-activated tissues where it elicited a rapid inhibition of Isc (61±8% 10 min after addition). By reference to the dose response curve for serosal application of L-779,976 in Figure 2 it was possible to calculate the concentration of agonist present in the retrieved serosal fluid as 3.7 nM or 0.37% of the amount originally placed in the mucosal reservoir. These data are further evidence that the mucosal actions of the non-peptide agonist are mediated by an effect on serosal SST receptors following permeation across the colonic epithelium.
Potentiation of mucosal effects of L-779,976 by GF120918
The polyspecific xenobiotic transporter, P-glycoprotein (PGP) may be a significant barrier to the absorption of orally administered drugs by pumping them back into the lumen of the gut (Wacher et al., 1998). To investigate the possible role of PGP in limiting absorption of non-peptide somatostatin agonists, the mucosal effects of L-779,976 on FSK-stimulated secretion were measured in the presence or absence of GF120918, a potent inhibitor of PGP (Figure 5). In proximal colon, pre-treatment with 30 μM GF120918 induced a small but not significant increase in the anti-secretory action of 30 nM L-779,976. However, GF120918 potentiated the inhibitory effect of mucosally applied L-779,976 in distal colon by almost 3 fold (Figure 5). In contrast, GF120918 did not change the antisecretory effect of serosally applied L-779,976 in distal colon (0.6 nM L-779,976 alone: 72.4±2.3%, 0.6 nM L-779,976+30 μM GF120918: 71.5±6.3% inhibition of FSK-stimulated Isc). Addition of GF120918 alone had no effect on FSK-stimulated Isc (data not shown). These data suggest that L-779,976 is a substrate for a GF120918-sensitive colonic efflux transporter, possibly PGP, and that inhibition of the transporter can markedly increase permeation of L-779,976 across distal colon.
Figure 5.
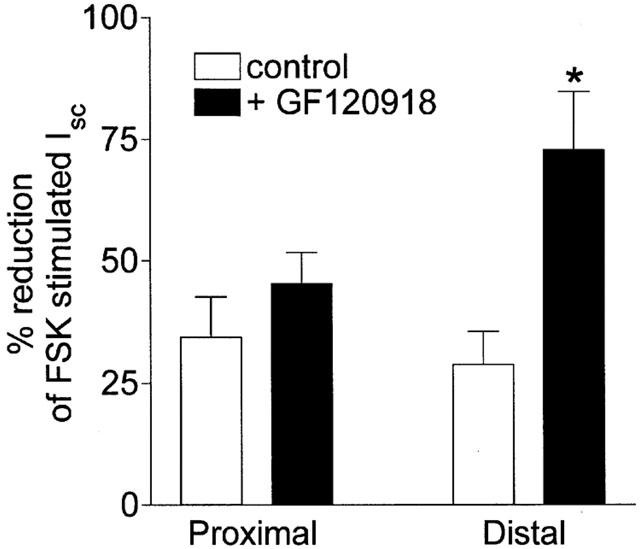
Anti-secretory effect of luminal L-779,976 is potentiated by GF120918 in distal colon. Inhibition by L-779,976 of FSK-stimulated Isc was measured 30 min after addition of the agonist at a concentration of 30 nM to the luminal surface of proximal or distal colonic mucosa in the presence or absence of 30 μM GF120918. GF120918 was added to both luminal and serosal compartments 15 min prior to addition of L-779,976. Data are shown as the decrease in Isc plotted as a percentage of the FSK-stimulated response. Values are mean±s.e.mean for n=6 tissues in each group. *P<0.025.
Discussion
The ability of somatostatin to regulate a wide range of cellular processes via multiple receptor subtypes offers considerable therapeutic potential. Although current peptide-based analogues such as octreotide have been of considerable therapeutic value, their use is limited by their pharmacokinetic properties. Octreotide is characterized by poor oral bioavailability, generally necessitating intravenous administration. It is also subject to excretion by multiple carrier-mediated systems resulting, for example, in poor penetration into the central nervous system (Kitazawa et al., 1998). As a result, development of non-peptide ligands with improved oral bioavailability and the ability to target different somatostatin receptor subtypes selectively has been an important objective of recent research (Freidinger, 1999).
Despite a detailed knowledge of the primary cellular mechanism responsible for secretory diarrhoea, octreotide and related peptide analogues of somatostatin are the only clinically available agents able to inhibit this pathway directly. The aim of the present work was to investigate the functionality of one group of non-peptide ligands as inhibitors of intestinal fluid secretion and as potential alternative anti-diarrhoeal agents. We employed a sensitive bioassay system in which cyclic AMP-mediated ion secretion in isolated rat colon was monitored continuously by measuring Isc. This approach also allowed the luminal and serosal effects of agents to be investigated. Using this system, the SSTR2-selective agonist L-779,976 was shown to be an extremely potent inhibitor of secretion when applied to the basolateral surface with an IC50 10 fold lower than that of octreotide. The finding that none of the other receptor-selective agents were able to inhibit secretion (except at very high concentrations) is in agreement with previous work that showed SSTR2 to be the primary mediator of somatostatin's anti-secretory effects in the intestine (McKeen et al., 1995; Warhurst et al., 1996). Of particular interest is the lack of effect of the SSTR1-selective agonist L-797,591. A previous study had suggested that SSTR1 receptors in cultured colonocytes may have anti-secretory properties (Warhurst et al., 1995), and thus SSTR1 may be an alternative target for anti-diarrhoeal therapy. However, this conclusion was based on indirect evidence and without access to an SSTR1-selective agonist. The results of the present study argue strongly that this receptor subtype, although present in colonic tissues (Warhurst et al., 1996), has no significant anti-secretory potential.
The ability of the SSTR2 agonist L-779,976 to inhibit forskolin-stimulated secretion when applied mucosally suggests that non-peptide agonists provide a potential route for the development of novel, orally administered anti-diarrhoeal drugs. The present study has not directly measured the concentration of L-779,976 appearing on the basolateral surface after mucosal addition. Nevertheless, the data presented here provide strong evidence that the anti-secretory activity of L-779,976 when applied mucosally is due to translocation across the epithelium, since SST receptors are functionally expressed only on the basolateral surface (Warhurst et al., 1996). Although the potency of the agonist was much lower when applied mucosally, concentrations in the 100 nM range were sufficient to produce virtually complete inhibition of secretion within 30 min. The bioavailability of L-779,976 from the mucosal surface is still relatively low (1%) but structural modifications such as those suggested by Pasternak et al. (1999) may produce similarly potent and selective compounds with substantially improved intestinal absorption, although the routes of translocation remain to be determined. Preliminary data suggest that polyethylene glycol molecules of a similar size to L-779,976 have a permeability consistent with that calculated for L-779,976 indicating a possible paracellular route (data not shown). However, interaction with P-glycoprotein points to the involvement of a transcellular process.
The marked increase in the anti-secretory effects of mucosal L-779,976 when co-administered with GF120918 suggests that P-glycoprotein may be a factor limiting the intestinal permeability of this compound. GF120918 is a potent inhibitor of PGP (de bruin et al., 1999) that has been shown in co-administration studies to increase the oral bioavailability of several compounds (Letrent et al., 1998; Malingre et al., 2001). Octreotide, in common with other cyclic peptides, has been shown to be a substrate for PGP (Sharom et al., 1998; Yamada et al., 1998) and interaction between another transporter molecule, MRP2, and a fluorescent derivative of octreotide has also been reported (Gutman et al., 2000). These interactions may explain octreotide's low bioavailability (<1%) and high hepatic and renal elimination. In addition, previous work has shown that octreotide is preferentially absorbed in the proximal GI tract with little absorption seen in the distal GI tract (Fricker et al., 1991). L-779,976, although a considerably smaller molecule than octreotide, has distinct structural similarities (Figure 6) which may explain an interaction with colonic PGP. GF120918 has also been shown to inhibit breast cancer resistance protein (BCRP) which is expressed in intestinal tissues (Jonker et al., 2000). Although we cannot rule out the possibility that colonic BCRP may contribute to limiting the mucosal effects of L-779,976, a study of PGP- and BCRP-expressing cell lines suggests that GF120918 is at least 10 fold more potent an inhibitor of PGP than of BCRP (de bruin et al., 1999). Our data suggest that a GF120918-sensitive transport process, most likely PGP, limits absorption of L-779,976 significantly. This limitation is particularly clear in the distal colon, in a pattern similar to that of octreotide, since GF120918 increases the anti-secretory potency of L-779,976 in this region by a factor of three. However, there is clear evidence that L-779,976 can still be absorbed sufficiently in distal colon to have a marked anti-secretory action even at mucosal concentrations of 100 nM or less.
Figure 6.
Molecular structures of the SSTR2-selective non-peptide agonist L-779,976 and the peptide agonist octreotide.
In conclusion, this study shows that the novel non-peptide SSTR2-selective agonist, L-779,976 is, in terms of its ability to inhibit ion secretion in rat colonic mucosa, the most potent anti-secretory agent yet described. In contrast, an agonist selective for the other major subtype present in intestine, SSTR1, had little effect on ion secretion. The ability of L-779,976 to inhibit secretion when applied mucosally at sub-micromolar concentrations suggests that this molecule is able to cross the colonic epithelium, despite evidence of interaction with a GF120918-sensitive efflux transporter. The ability to deliver a non-peptide agent with such potent anti-secretory properties via the gut lumen could provide a basis for developing new anti-diarrhoeal therapies based on non-peptide agonists of the SSTR2 receptor. However, it will be important to test the activity of these compounds in human tissues in the light of recent data which suggests that activation of somatostatin receptors may have different effects on ion transport in rat and human colon (Hope et al., 2001).
Acknowledgments
The authors thank Dr Susan Rohrer, Merck Research Laboratories, Rahway, U.S.A. for providing the non-peptide SSTR agonists. This study was supported by a grant from the Sir Jules Thorn Charitable Trust.
Abbreviations
- Cyclic AMP
cyclic adenosine monophosphate
- FSK
Forskolin
- Isc
short-circuit current
- SST
somatostatin
- SSTR
somatostatin receptor
References
- CREUTZFELDT W., LEMBKE B., FOLSCH U.R., SCHLESER S., KOOP I. Effect of somatostatin analogue (SMS 201-995, Sandostatin) on pancreatic secretion in humans. Am. J. Med. 1987;82:49–54. doi: 10.1016/0002-9343(87)90426-8. [DOI] [PubMed] [Google Scholar]
- CSABA Z., DOURNAUD P. Cellular biology of somatostatin receptors. Neuropeptides. 2001;35:1–23. doi: 10.1054/npep.2001.0848. [DOI] [PubMed] [Google Scholar]
- DE BRUIN M., MIYAKE K., LITMAN T., ROBEY R., BATES S.E. Reversal of resistance by GF120918 in cells expressing the ABC half-transporter, MXR. Cancer Lett. 1999;146:117–126. doi: 10.1016/s0304-3835(99)00182-2. [DOI] [PubMed] [Google Scholar]
- FARTHING M.J. Chronic diarrhoea: Current concepts on mechanisms and management. Eur. J. Gastro. Hepatol. 1996;8:157–167. [PubMed] [Google Scholar]
- FREIDINGER R.M. Non-peptidic ligands for peptide and protein receptors. Curr. Opin. Chem. Biol. 1999;3:395–406. doi: 10.1016/S1367-5931(99)80060-X. [DOI] [PubMed] [Google Scholar]
- FRICKER G., BRUNS C., MUNZER J., BRINER U., ALBERT R., KISSEL T., VONDERSCHER J. Intestinal absorption of the octapeptide SMS-201-995 visualised by fluorescence derivatisation. Gastroenterology. 1991;100:1544–1552. doi: 10.1016/0016-5085(91)90651-z. [DOI] [PubMed] [Google Scholar]
- FUESSL H.S., DOMIN J., BLOOM S.R. Oral absorption of the somatostatin analogue SMS 201-995: theoretical and practical implications. Clin. Sci. 1987;72:255–257. doi: 10.1042/cs0720255. [DOI] [PubMed] [Google Scholar]
- GUARINO A., CANANI R.B., SPAGNUOLO M.I., BISCEGLIA M., BOCCIA M.C., RUBINO A. In vivo and in vitro efficacy of octreotide for treatment of enteric cryptosporidiosis. Dig. Dis. Sci. 1998;43:436–441. doi: 10.1023/a:1018839329759. [DOI] [PubMed] [Google Scholar]
- GUTMAN H., MILLER D.S., DROULLE A., DREWE J., FAHR A., FRICKER G. P-glycoprotein and MRP-2 mediated octreotide transport in renal proximal tubule. Br. J. Pharmacol. 2000;129:251–256. doi: 10.1038/sj.bjp.0703003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPE N., BUTT G., ROSS I., WARHURST G., ARN M., GRIGOR M., LUBCKE R., BARBEZAT G.O. Somatostatin enhances cAMP-dependent short-circuit current in human colon via somatostatin receptor subtype-2. Dig. Dis. Sci. 2001;46:2499–2503. doi: 10.1023/a:1012392307462. [DOI] [PubMed] [Google Scholar]
- JONKER J.W., SMIT J.W., BRINKHUIS R.F., MALIEPAARD M., BEIJNEN J.H., SCHELLENS J.H.M., SCHINKEL A.H. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J. Natl. Canc. Inst. 2000;92:1651–1656. doi: 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- KITAZAWA T., TERASAKI T., SUZUKI H., KAKEE A., SUGIYAMA Y. Efflux of taurocholic acid across the blood brain barrier: interaction with cyclic peptides. J. Pharm Exp. Ther. 1998;286:890–895. [PubMed] [Google Scholar]
- LEMBCKE B., CREUTZFELDT W., SCHLESER S., EBERT R., SHAW C., KOOP I. Effect of the somatostatin analogue (SMS 201-995) on gastrointestinal, pancreatic and biliary function and hormone release in normal men. Digestion. 1987;36:108–124. doi: 10.1159/000199408. [DOI] [PubMed] [Google Scholar]
- LETRENT S.P., POLLACK G.M., BROUWER K.R., BROUWER K.L.R. Effect of GF120918, a potent P-glycoprotein inhibitor, on morphine pharmacokinetics and pharmacodynamics in the rat. Pharm. Res. 1998;15:599–605. doi: 10.1023/a:1011938112599. [DOI] [PubMed] [Google Scholar]
- MCKEEN E.S., FENIUK W., HUMPHREY P.P. Somatostatin receptors mediating inhibition of basal and stimulated electrogenic ion transport in rat isolated distal colonic mucosa. Naunyn Schmiedebergs Arch. Pharmacol. 2001;352 4:402–411. doi: 10.1007/BF00172777. [DOI] [PubMed] [Google Scholar]
- MALINGRE M.M., BEIJNEN J.H., ROSING H., KOOPMAN F.J., JEWELL R.C., PAUL E.M., HUININCK W.W.T., SCHELLENS J.H.M. Co-administration of GF120918 significantly increases the systemic exposure to oral paclitaxel in cancer patients. Br. J. Cancer. 1995;84:42–47. doi: 10.1054/bjoc.2000.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAO S., HIRATA K., ISHIDA T., TAKAHARA J. Severe diabetic diarrhea successfully treated with octreotide, a somatostatin analogue. Endocrin. J. 1999;46:477–478. [PubMed] [Google Scholar]
- NIGHTINGALE J.M.D., WALKER E.R., BURNHAM W.R., FARTHING M.J.G., LENNARD-JONES J.E. Octreotide (a somatostatin analogue) improves the quality of life in some patients with a short intestine. Aliment. Pharmacol.Therap. 1989;3:367–373. doi: 10.1111/j.1365-2036.1989.tb00223.x. [DOI] [PubMed] [Google Scholar]
- OBERG K. Neuroendocrine gastrointestinal tumours. Ann. Oncol. 1996;7:453–463. doi: 10.1093/oxfordjournals.annonc.a010633. [DOI] [PubMed] [Google Scholar]
- ORSI P., GUATTI-ZULIANI C., OKOLICSANYI L. Long-acting octreotide is effective in controlling re-bleeding angiodysplasia of the gastrointestinal tract. Digest. Liver. Dis. 2001;33:330–334. doi: 10.1016/s1590-8658(01)80087-6. [DOI] [PubMed] [Google Scholar]
- PASTERNAK A., PAN Y., MARINO D., SANDERSON P.E., MOSLEY R., ROHRER S.P., BIRZIN E.T., HUSKEY S-E.W., JACKS T., SCLEIM K.D., CHEN K., SCHAEFFER J.M., PATCHETT A.A., YANG L. Potent, orally bioavailable somatostatin agonists: good absorption achieved by urea backbone cyclisation. Bioorg. Med. Chem. Lett. 1999;9:491–496. doi: 10.1016/s0960-894x(99)00016-5. [DOI] [PubMed] [Google Scholar]
- PATEL Y.C. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- PLESS J., BAUER W., BRINER U., DOEPFNER W., MARBACH P., MAURER R., PETCHER T.J., REUBI J.C., VONDERSCHER J. Chemistry and pharmacology of SMS 201-995, a long-acting octapeptide analogue of somatostatin. Scand. J. Gastroenterol. 1986;21 Suppl 119:54–64. doi: 10.3109/00365528609087432. [DOI] [PubMed] [Google Scholar]
- ROHRER S.P., BIRZIN E.T., MOSLEY R.T., BERK S.C., HUTCHINS S.M., SHEN D.M., XIONG Y.S., HAYES E.C., PARMAR R.M., FOOR F., MITRA S.W., DEGRADO S.J., SHU M., KLOPP J.M., CAI S.J., BLAKE A., CHAN W.W.S., PASTERNAK A., YANG L.H., PATCHETT A.A., SMITH R.G., CHAPMAN K.T., SCHAEFFER J.M. Rapid identification of subtype-selective agonists of the somatostatin receptor through combinatorial chemistry. Science. 1998;282:737–740. doi: 10.1126/science.282.5389.737. [DOI] [PubMed] [Google Scholar]
- SHAROM F.J., LU P., LIU R., YU X.H. Linear and cyclic peptides as substrates and modulators of P-glycoprotein: peptide binding and effects on drug transport and accumulation. Biochem. J. 1998;333:621–630. doi: 10.1042/bj3330621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRES A.J., LANDA J.I., MORENO-AZCOITA M., ARGUELLO J.M., SILECCHIA G., CASTRO J., HERNANDEZ-MERLO F., JOVER J.M., MORENO-GONZALES E., BALIBREA J.L. Somatostatin in the management of gastrointestinal fistulas. A multicenter trial. Arch. Surg. 1992;127:97–100. doi: 10.1001/archsurg.1992.01420010115018. [DOI] [PubMed] [Google Scholar]
- WACHER V.J., SILVERMAN J.A., ZHANG Y.C., BENET L.Z. Role of P-glycoprotein and cytochrome P450 3A in limiting oral absorption of peptides and peptidomimetics. J. Pharm. Sci. 1998;87:1322–1330. doi: 10.1021/js980082d. [DOI] [PubMed] [Google Scholar]
- WANG J.R., ZHENG H., HAUER-JENSEN M. Influence of short term octreotide administration on chronic tissue injury, transforming growth factor beta (TGF-beta) overexpression, and collagen accumulation in irradiated rat intestine. J. Pharm. Exp. Ther. 2001;297:35–42. [PubMed] [Google Scholar]
- WARHURST G., BARBEZAT G.O., HIGGS N.B., REYL-DESMARS F., LEWIN M.J.M., COY D.H., ROSS I., GRIGOR M.R. Expression of somatostatin receptor genes and their role in inhibiting Cl− secretion in HT-29cl.19A colonocytes. Am. J. Physiol. 1995;269:G729–G736. doi: 10.1152/ajpgi.1995.269.5.G729. [DOI] [PubMed] [Google Scholar]
- WARHURST G., HIGGS N.B., FAKHOURY H., WARHURST A.C., GARDE J., COY D.H. Somatostatin receptor subtype 2 mediates somatostatin inhibition of ion secretion in rat distal colon. Gastroenterology. 1996;111:325–333. doi: 10.1053/gast.1996.v111.pm8690197. [DOI] [PubMed] [Google Scholar]
- YAMADA T., KATO Y., KUSUHARA H., LEMAIRE M., SUGIYAMA Y. Characterisation of the transport of a cationic octapeptide, octreotide, in rat bile canalicular membrane: possible involvement of P-glycoprotein. Biol. Pharm. Bull. 1998;21:874–878. doi: 10.1248/bpb.21.874. [DOI] [PubMed] [Google Scholar]
- ZIDAN J., HAIM N., BENY A., STEIN M., GEZ E., KUTEN A. Octreotide in the treatment of severe chemotherapy-induced diarrhoea. Ann. Oncol. 2001;12:227–229. doi: 10.1023/a:1008372228462. [DOI] [PubMed] [Google Scholar]


