Abstract
Recent work has demonstrated the production of reactive oxygen and nitrogen species in the vasculature of aging animals. Oxidant induced cell injury triggers the activation of nuclear enzyme poly(ADP ribose) polymerase (PARP) leading to endothelial dysfunction in various pathophysiological conditions (reperfusion, shock, diabetes). Here we studied whether the loss of endothelial function in aging rats is dependent upon the PARP pathway within the vasculature. Young (3 months-old) and aging (22 months-old) Wistar rats were treated for 2 months with vehicle or the PARP inhibitor PJ34. In the vehicle-treated aging animals there was a significant loss of endothelial function, as measured by the relaxant responsiveness of vascular rings to acetylcholine. Treatment with PJ34, a potent PARP inhibitor, restored normal endothelial function. There was no impairment of the contractile function and endothelium-independent vasodilatation in aging rats. Furthermore, we found no deterioration in the myocardial contractile function in aging animals. Thus, intraendothelial PARP activation may contribute to endothelial dysfunction associated with aging.
Keywords: Aging, cardiac function, endothelial dysfunction, poly(ADP ribose) polymerase
Introduction
Endothelial dysfunction represents a predominant feature of aging. Endothelial dysfunction makes the aging population prone to cardiovascular complications, and micro-thrombus formation. There is emerging evidence that the endothelial dysfunction associated with aging is related to the local formation of reactive oxygen and nitrogen species within and in the vicinity of the vascular endothelium (Inoue & Inoue, 1996; Rodriguez-Martinez et al., 1998; van der Loo et al., 2000; Hamilton et al., 2001). While the link between oxygen free radicals and vascular aging is well established, the downstream cellular mechanisms have not yet been explored in detail. Oxidant induced cell injury triggers the activation of nuclear enzyme poly(ADP ribose) polymerase (PARP) leading to endothelial dysfunction in various pathophysiological conditions including reperfusion, shock, diabetes (Zingarelli et al., 1997; 1998; Thiemermann et al., 1997; Szabo et al., 1997; Eliasson et al., 1997; Burkart et al., 1999; Liaudet et al., 2000; Soriano et al., 2001a, 2001b; Pacher et al., 2002). Here we studied whether the loss of endothelial function in aging rats is dependent upon the PARP pathway within the vasculature.
Methods
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by US National Institutes of Health (NIH Publication No. 85-23 revised 1985) and was performed with the approval of the local Institutional Animal Care and Use Committee.
Animals, treatment protocols
Retired ex-breeder male Wistar rats (22 months-old) were treated with vehicle (n=16) or the PARP inhibitor PJ34 (n=16; 20 mg kg−1/day PO) for 2 months. This dose regimen was found to effectively inhibit vascular PARP activation in previous studies (Soriano et al., 2001a, 2001b; Pacher et al., 2002). Three-month old male Wistar rats treated with vehicle (n=16) or PJ34 (n=16; 20 mg kg−1/day PO) for 2 months were used as controls.
Measurement of vascular reactivity in isolated aortic rings of rats
The method was described previously (Soriano et al., 2001a, 2001b). Briefly, the thoracic aorta was cleared from peri-adventitial fat and cut into 3 – 4 mm width rings using operation microscope, mounted in organ baths filled with warmed (37°C) and oxygenated (95% O2, 5% CO2) Krebs' solution (mM: CaCl2 1.6; MgSO4 1.17; EDTA 0.026; NaCl 130; NaHCO3 14.9; KCl 4.7; KH2PO4 1.18; Glucose 11). Isometric tension was measured with isometric transducers (Kent Scientific Corporation, Litchfield, CT, U.S.A.), digitized using a MacLab A/D converter and stored and displayed on a MacIntosh computer. A tension of 1.5 g was applied and the rings were equilibrated for 60 min, followed by measurements of the concentration-dependent contraction to epinephrine (10−10 to 3×10−5 M), and in rings precontracted with epinephrine (10−6 M), relaxation to acetylcholine (10−9 to 3 – 10−4 M) and sodium nitroprusside (10−12 – 10−5 M). Experiments were conducted in 8 – 10 pairs of rings in each experimental group.
Haemodynamic measurements in rats
Analysis of left ventricular performance was measured in rats anaesthetized with i.p. injection of thiopentone sodium (60 mg kg−1). Animals were placed on controlled heating pads, and core temperature measured via a rectal probe was maintained at 36 – 38°C. A microtip catheter transducer (SPR-524; Millar Instruments, Houston, TX, U.S.A) was inserted into the right carotid artery and advanced into the left ventricle under pressure control. After stabilization for 15 – 20 min, the pressure signal was continuously recorded using a MacLab A/D converter (AD Instruments, Mountain View, CA, U.S.A.), and stored and displayed on an Apple Macintosh personal computer. The heart rate, the left ventricular systolic and end-diastolic pressures (LVSP and LVEDP) were measured and the maximal slope of systolic pressure increment (+dP dt−1) and diastolic pressure decrement (−dP dt−1), and indexes of contractility and relaxation, were calculated. After these measurements, the catheter was pulled back into the aorta for the measurement of arterial blood pressure. After the haemodynamic measurements were made, animals were sacrificed by lethal injection of thiopentone sodium. Haemodynamic measurements were conducted in 7 – 9 animals in each group.
Statistical analysis
Results are reported as mean±s.e.mean. Statistical significance between two measurements was determined by the two-tailed unpaired Student's t-test, and among groups it was determined by analysis of variance with Bonferroni's correction. Probability values of P<0.05 were considered significant.
Reagents
All reagents were obtained from Sigma/Aldrich (St. Louis, MO, U.S.A.), unless indicated otherwise. The potent, novel, water soluble phenantridinone derivative PARP inhibitor, PJ34-the hydrochloride salt of N-(-oxo-5,6-dihydro-phenanthridin-2-yl)-N,N-dimethylacetamide, was synthesized as described (Soriano et al., 2001a).
Results
Body weight
Body weight significantly increased with age (g) (475±21.4; (n=9) and 924±35.2; (n=9), P<0.01, 5 and 24 months old respectively). PJ34 treatment for 2 months did not influence the body weight of control and aging animals (g) (458±17.5 vs 475±21.4; (n=9) and 893±38.4 vs 924±35.2; (n=9) respectively).
Aging induces a PARP-dependent endothelial dysfunction in rat
Ex vivo experiments demonstrated the loss of endothelial function, as measured by the relaxant responsiveness of pre-contracted vascular rings to the endothelium-dependent vasodilator, NO liberating hormone acetylcholine (ACh) in aging rats (24 months old) (Figure 1A). In contrast to the 24-month-old rats, aortic rings taken from 12-month-old rats (n=5) did not show impaired endothelium-dependent dilatory response to ACh (data not shown). Inhibition of PARP activation was achieved by chronic treatment with the potent, water-soluble phenantridinone derivative PARP inhibitor PJ34 for 2 months. This treatment restored normal vascular function (Figure 1A) in aging animals. The endothelium-independent relaxant response to sodium nitroprusside was unchanged (Figure 1C), indicating the ability of the endothelium to release NO, rather than the ability of the smooth muscle to relax to NO is impaired in aging. The contractile responsiveness of the thoracic aorta in aging rats was unchanged (Figure 1B). PJ34 treatment had no significant effects on contractile or endothelium-dependent and independent relaxant responses in control animals (Figure 1).
Figure 1.
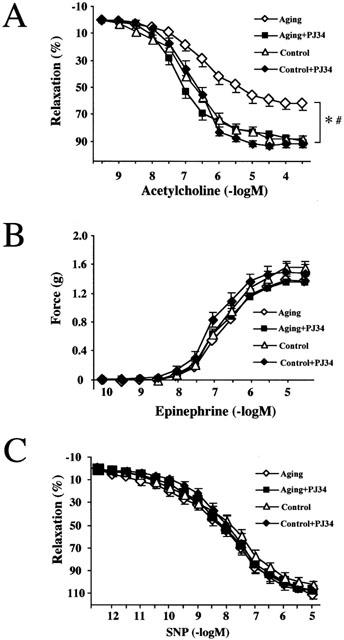
Reversal of aging-induced endothelial dysfunction by pharmacological inhibition of PARP in rats. Epinephrine-induced contractions (B), Ach-induced endothelium-dependent relaxation (A) and SNP-induced endothelium-independent relaxations (C). Each point of the curve represents mean±s.e.mean of 7 – 10 experiments in vascular rings. *P<0.05 vs control; #P<0.05 vs aging.
Ventricular function in rats
Aging in rats was characterized by a significant (P<0.05) increase in LVEDP and decrease in diastolic −dP dt−1 (Figure 2). There was no change in heart rate, mean BP, LVSP and systolic +dP dt−1 (Figure 2). PJ34 treatment did not significantly influence the depression in diastolic −dP dt−1 in aging animals, however significantly decreased the LVEDP. PJ34 treatment in control rats had no significant effects on haemodynamic parameters (Figure 2).
Figure 2.
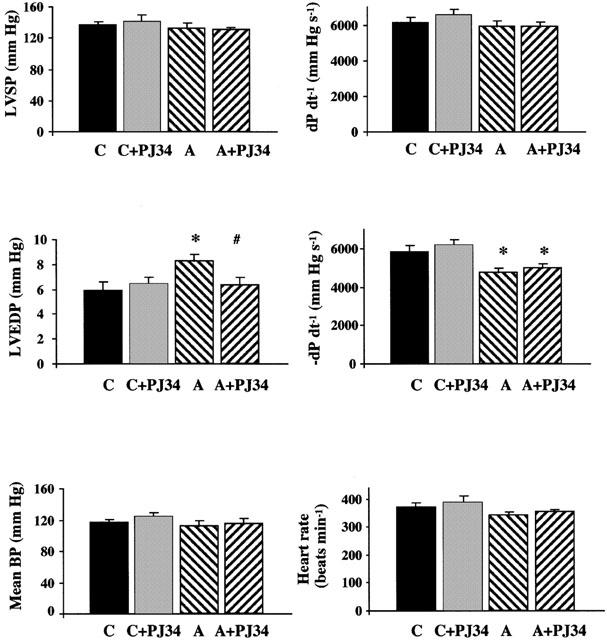
The effects of aging (A) and pharmacological inhibition of PARP on cardiac function. Effect of aging and PJ34 on left ventricular systolic pressure (LVSP), left ventricular end diastolic pressure (LVEDP), left ventricular +dP dt−1, left ventricular −dP dt−1, mean BP and heart rate in rats. C, control; A, aging; C+PJ34 control treated with PJ34 (for 2 months); A+PJ34, aging treated with PJ34 (for 2 months). Results are mean±s.e.mean. *P<0.05 vs C# P<0.05 vs A.
Discussion
There is overwhelming evidence demonstrating an aging-associated development of endothelial dysfunction (the reduced ability of blood vessels to relax in response to endothelium-dependent relaxants) in animals or humans (Hatake et al., 1990; Taddei et al., 1995; Küng & Lüscher, 1995; Higashi et al., 1997; Imaoka et al., 1999; Heymes et al., 2000; van der Loo et al., 2000; Matz et al., 2000; Hamilton et al., 2001). The mechanisms responsible for this age-related endothelial dysfunction have not yet been clearly established.
This impairment is, at least in part, related to the increased local formation of reactive oxygen and nitrogen species within and in the vicinity of the vascular endothelium (Inoue & Inoue, 1996; Rodriguez-Martinez et al., 1998; van der Loo et al., 2000; Hamilton et al., 2001). Superoxide anion interacts with nitric oxide, forming the oxidant peroxynitrite (ONOO), which attacks various biomolecules, leading, among others, the production of a modified amino acid (nitrotyrosine) (Beckman & Koppenol, 1996). Although nitrotyrosine was initially considered a specific marker of peroxynitrite generation, other pathways can also induce tyrosine nitration (Eiserich et al., 1998). Thus, nitrotyrosine is now generally considered a collective index of reactive nitrogen species, rather than a specific indicator of peroxynitrite formation (Eiserich et al., 1998; Halliwell, 1997). Indeed, increased nitrotyrosine formation was reported in the endothelium of aorta in aging animals, which was localized in the nucleus, the cytosol and the mitochondria (van der Loo et al., 2000).
Oxidative stress accompanied by increased formation of hydrogen peroxide, superoxide anion and peroxynitrite are endogenous inducers of DNA single-strand breakage and DNA single strand breakage is the obligatory trigger of PARP activation (Pieper et al., 2000; Szabo et al., 1997; Szabo, 2000), which in turn may results in rapid depletion of the intracellular NAD+ and ATP pools, slowing the rate of glycolysis and mitochondrial respiration, eventually leading to cellular dysfunction and necrosis. The protective effect of pharmacological inhibition of PARP or lack of PARP gene in preventing vascular dysfunction has been demonstrated in experimental models of shock, reperfusion injury and diabetes (another condition where oxidative stress plays a key pathogenetic role) (Zingarelli et al., 1997; 1998; Thiemermann et al., 1997; Szabo et al., 1997; Liaudet et al., 2000; Soriano et al., 2001a, 2001b; Pacher et al., 2002).
The present study demonstrates that the chronic treatment with PARP inhibitor PJ34 normalizes the impaired endothelium-dependent dilatory response to Ach in aortic rings from 2-year-old aging Wistar rats (Figure 1). Aging was also associated with significant impairment of diastolic but not systolic function of the heart (Figure 2). PARP inhibitor treatment did not affect the aging-associated decrease in diastolic −dP dt−1 (an index of ventricular relaxation) but normalized increased LVEDP.
Based on the results of the current study, we conclude that the reactive oxygen/nitrogen species – PARP pathway plays a pathogenetic role in the development of endothelial dysfunction in aging.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01GM60915). P. Pacher is on leave from the Department of Pharmacology and Pharmacotherapy, Semmelweis University, Budapest. L. Liaudet is on leave from the Critical Care Division, Department of Internal Medicine, University Hospital, Lausanne, Switzerland.
Abbreviations
- BP
blood pressure
- +dP dt−1
maximal slope of systolic pressure increment
- −dP dt−1
maximal slope of diastolic pressure decrement
- LVEDP
left ventricular end-diastolic pressure
- LVSP
left ventricular systolic pressure
- PARP
poly (ADP-ribose) polymerase
References
- BECKMAN J.S., KOPPENOL W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. C1424. 1996;271 doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- BURKART V., WANG Z.Q., RADONS J., HELLER B., HERCEG Z., STINGL L., WAGNER E.F., KOLB H. Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic beta-cell destruction and diabetes development induced by streptozotocin. Nat. Med. 1999;5:314–319. doi: 10.1038/6535. [DOI] [PubMed] [Google Scholar]
- EISERICH J.P., HRISTOVA M., CROSS C.E., JONES A.D., FREEMAN B.A., HALLIWELL B., VAN DER VLIET A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- ELIASSON M.J., SAMPEI K., MANDIR A.S., HURN P.D., TRAYSTMAN R.J., BAO J., PIEPER A., WANG Z.Q., DAWSON T.M., SNYDER S.H., DAWSON V.L. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat. Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo. FEBS. Lett. 1997;411:157–160. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- HAMILTON C.A., BROSNAN M.J., MCINTYRE M., GRAHAM D., DOMINICZAK A.F. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- HATAKE K., KAKISHITA E., WAKABAYASHI I., SAKIYAMA N., HISHIDA S. Effect of aging on endothelium-dependent vascular relaxation of isolated human basilar artery to thrombin and bradykinin. Stroke. 1990;21:1039–1043. doi: 10.1161/01.str.21.7.1039. [DOI] [PubMed] [Google Scholar]
- HEYMES C., HABIB A., YANG D., MATHIEU E., MAROTTE F., SAMUEL J., BOULANGER C.M. Cyclo-oxygenase-1 and -2 contribution to endothelial dysfunction in ageing. Br. J. Pharmacol. 2000;131:804–810. doi: 10.1038/sj.bjp.0703632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGASHI Y., OSHIMA T., OZONO R., MATSUURA H., KAJIYAMA G. Aging and severity of hypertension attenuate endothelium-dependent renal vascular relaxation in humans. Hypertension. 1997;30:252–258. doi: 10.1161/01.hyp.30.2.252. [DOI] [PubMed] [Google Scholar]
- IMAOKA Y., OSANAI T., KAMADA T., MIO Y., SATOH K., OKUMURA K. Nitric oxide-dependent vasodilator mechanism is not impaired by hypertension but is diminished with aging in the rat aorta. J. Cardiovasc. Pharmacol. 1999;33:756–761. doi: 10.1097/00005344-199905000-00012. [DOI] [PubMed] [Google Scholar]
- INOUE M., INOUE K. Role of free radicals in and around vascular endothelial cells in the mechanism of aging. Ann. N.Y. Acad. Sci. 1996;786:224–232. doi: 10.1111/j.1749-6632.1996.tb39065.x. [DOI] [PubMed] [Google Scholar]
- KÜNG C.F., LÜSCHER T.F. Different mechanisms of endothelial dysfunction with aging and hypertension in rat aorta. Hypertension. 1995;25:194–200. doi: 10.1161/01.hyp.25.2.194. [DOI] [PubMed] [Google Scholar]
- LIAUDET L., SORIANO F.G., SZABO E., VIRAG L., MABLEY J.G., SALZMAN A.L., SZABO C. Protection against hemorrhagic shock in mice genetically deficient in poly (ADP-ribose) polymerase. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10203–10208. doi: 10.1073/pnas.170226797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATZ R.L., DE SOTOMAYOR M.A., SCHOTT C., STOCLET J.C., ANDRIANTSITOHAINA R. Vascular bed heterogeneity in age-related endothelial dysfunction with respect to NO and eicosanoids. Br. J. Pharmacol. 2000;131:303–311. doi: 10.1038/sj.bjp.0703568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACHER P., LIAUDET L., SORIANO F.G., MABLEY J.G., SZABÓ E., SZABÓ C. The role of poly(ADP-ribose) polymerase in the development of cardiovascular dysfunction in diabetes mellitus. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- PIEPER A.A., WALLES T., WEI G., CLEMENTS E.E., VERMA A., SNYDER S.H., ZWEIER J.L. Myocardial postischemic injury is reduced by poly (ADPribose) polymerase-1 gene disruption. Mol. Med. 2000;6:271–282. [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ-MARTINEZ M.A., ALONSO M.J., REDONDO J., SALAICES M., MARIN J. Role of lipid peroxidation and the glutathione-dependent antioxidant system in the impairment of endothelium-dependent relaxations with age. Br. J. Pharmacol. 1998;123:113–121. doi: 10.1038/sj.bjp.0701595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SORIANO F.G., VIRAG L., JAGTAP P., SZABO E., MABLEY J.G., LIAUDET L., MARTON A., HOYT D.G., MURTHY K.G., SALZMAN A.L., SOUTHAN G.J., SZABO C. Diabetic endothelial dysfunction: the role of poly (ADP-ribose) polymerase activation. Nat. Med. 2001a;7:108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- SORIANO G.F., PACHER P., MABLEY J., LIAUDET L., SZABO C. Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-Ribose) polymerase. Circ. Res. 2001b;89:684–691. doi: 10.1161/hh2001.097797. [DOI] [PubMed] [Google Scholar]
- SZABO C. Cell Death: The role of PARP 2000Boca Raton: CRC Press; Ed [Google Scholar]
- SZABO C., CUZZOCREA S., ZINGARELLI B., O'CONNOR M., SALZMAN A.L. Endothelial dysfunction in a rat model of endotoxic shock. Importance of the activation of poly (ADP-ribose) synthetase by peroxynitrite. J. Clin. Invest. 1997;100:723–735. doi: 10.1172/JCI119585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TADDEI S., VIRDIS A., MATTEI P., GHIADONI L., GENNARI A., FASOLO C.B., SUDANO I., SALVETTI A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- THIEMERMANN C., BOWES J., MYINT F.P., VANE J.R. Inhibition of the activity of poly(ADP ribose) synthetase reduces ischemia-reperfusion injury in the heart and skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 1997;94:679–683. doi: 10.1073/pnas.94.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER LOO B., LABUGGER R., SKEPPER J.N., BACHSCHMID M., KILO J., POWELL J.M., PALACIOS-CALLENDER M., ERUSALIMSKY J.D., QUASCHNING T., MALINSKI T., GYGI D., ULLRICH V., LUSCHER T.F. Enhanced peroxynitrite formation is associated with vascular aging. J. Exp. Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINGARELLI B., CUZZOCREA S., ZSENGELLER Z., SALZMAN A.L., SZABO C. Protection against myocardial ischemia and reperfusion injury by 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase. Cardiovasc. Res. 1997;36:205–215. doi: 10.1016/s0008-6363(97)00137-5. [DOI] [PubMed] [Google Scholar]
- ZINGARELLI B., SALZMAN A.L., SZABO C. Genetic disruption of poly (ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ. Res. 1998;83:85–94. doi: 10.1161/01.res.83.1.85. [DOI] [PubMed] [Google Scholar]


