Abstract
Motor effects produced by tachykinins were studied in human isolated corpus spongiosum and cavernosum. In quiescent preparations neurokinin A caused potent contractions (pD2=8.3 – 7.9 respectively) prevented by the NK2 receptor-selective antagonist nepadutant, whereas [Sar9]SP sulfone and senktide (NK1 and NK3 receptor-selective agonists) produced no effect or spare contractions. In KCl-precontracted corpus spongiosum septide (pD2=7.1) and [Sar9]SP sulfone (pD2=7.7) produced tetrodotoxin-resistant relaxations, abolished by the tachykinin NK1 receptor-selective antagonist SR 140333. [Sar9]SP sulfone (1 μM) produced similar relaxations in precontracted corpus cavernosum. Electrical field stimulation (EFS) elicited tetrodotoxin-sensitive relaxations, which were additive to those produced by [Sar9]SP sulfone. Nω-nitro-L-arginine (L-NOARG) totally prevented both [Sar9]SP sulfone- and EFS-induced relaxations. These results show that tachykinin NK1 and NK2 receptors mediate opposite motor effects in human penile tissues, suggesting a possible modulatory role of tachykinins on smooth muscle tone in these organs.
Keywords: Tachykinins, tachykinin receptors, human corpus spongiosum, human corpus cavernosum
Introduction
Tachykinins are a family of neuropeptides distributed in the mammalian central and peripheral nervous system which produce a wide range of biological effects through the stimulation of at least three distinct receptor types, termed NK1, NK2 and NK3 (Regoli et al., 1989; Maggi et al., 1993).
Tachykinins are well-established nonadrenergic noncholinergic (NANC) excitatory neurotransmitters in the mammalian genitourinary tract; at this level they produce smooth muscle contraction in the renal pelvis, ureter, urinary bladder, vas deferens, uterus and urethra (Maggi, 1995; Santicioli & Maggi, 1998 for review). Tachykinin NK1 and/or NK2 receptors mediate the effects of tachykinins in these organs, depending on the species considered. In the human genitourinary tract the NK2 receptor is the main, if not the sole mediating contractile responses to tachykinins. Substance P (SP)-immunoreactive nerve fibres have been described in human penile tissues (Andersson et al., 1983; Gu et al., 1983; Hauser-Kronberger et al., 1994), most of them being localized beneath and within the epithelium of glans penis and urethra. However, few studies are up to now available reporting on the effects produced by tachykinins on penile tissues. Motor effects produced by SP were studied in the human corpus cavernosum and corpus spongiosum urethrae by Andersson et al. (1983) and Hedlund & Andersson (1985). These authors reported that SP contracted both tissues at resting tension, whereas transient relaxations were observed on precontracted strips. Azadzoi et al. (1992) reported that relaxations produced by SP in human and rabbit corpora cavernosa were prevented by L-NMMA. However, neither the receptor(s) mediating the effects produced by SP, nor the mechanism(s) underlying smooth muscle contraction and relaxation in response to tachykinins in the penile tissues have been fully investigated yet. In this study we have characterized the motor effects produced by tachykinins in human corpora spongiosa and cavernosa by using tachykinin receptor-selective agonists and antagonists. We have also compared motor responses produced by electrical field stimulation (EFS) to responses produced by tachykinin receptor stimulation in the two penile tissues.
Methods
General
All experiments were performed on tissue strips (10 – 15 mm long) of human corpus cavernosum or corpus spongiosum dissected free from the tunica albuginea. Specimens of corpora cavernosa were obtained from two patients undergoing surgery for penile cancer (age 45 and 58 years). Specimens of corpora spongiosa were obtained from three patients undergoing surgery for urethral stricture (age 42 – 48 years). No patient received radio- or chemotherapy before intervention. In all patients pre-anaesthetic medication was intramuscular atropine (1 mg) and diazepam (10 mg). Anaesthesia was induced by propofol 200 mg i.v. and maintained with N2O/O2 (1/2) and halothane (0.6 – 1%). The patients received pancuronium bromide (6 mg i.v.) during induction of anaesthesia. Four to eight strips could be obtained from each specimen. All specimens appeared macroscopically normal without signs of tumour or inflammation. All muscle strips were prepared immediately after surgical removal of the tissue and kept at 4°C overnight in ice-cold gassed (96% O2 and 4% CO2) Krebs – Henseleit solution of the following composition (mM): NaCl 119, NaHCO3 25, KH2PO4 1.2, MgSO4 1.5, CaCl2 2.5, KCl 4.7 and glucose 11. The next day, 20 – 24 h after excision, the strips were placed in 5-ml organ baths filled with oxygenated Krebs – Henseleit solution at 37°C and connected to isotonic force transducers under a resting tension of 5 mN. Electrical field stimulation (EFS; trains of stimuli of 10 Hz, 0.5 ms pulse width, 60 V, for 1 s) was delivered to preparations by means of two platinum wire electrodes placed at the top and the bottom of the organ bath, and connected to a Grass S88 stimulator. Motor responses evoked by drugs or EFS were digitized and stored on a Power MacIntosh PC by means of a Mac Lab/8e hardware device (AD Instruments, Castle Hill, Australia), and analysed by using Mac Lab Chart v. 3.6.1 software.
Data analysis
All values in the text, tables or figures are expressed as mean±s.e.mean. Statistical analysis was performed by means of Student's t-test for paired or unpaired data. Numerosity was expressed as n=number of observations/number of patients. Agonist activity is expressed as pD2(−log EC50). Antagonist affinity is expressed as pKB (−log of the antagonist dissociation constant). pKB values were estimated as the mean of the individual values obtained with the equation: pKB=log [dose ratio −1)]−log [antagonist concentration].
Drugs
Nepadutant (MEN 11420) (or: c{[(β-D-GlcNAc)Asn-Asp-Trp-Phe-Dpr-Leu]c(2β-5β)}) was synthesized at Menarini Laboratories, Florence, Italy, by conventional solid-phase methods. Neurokinin A, [Sar9]SP sulfone, senktide and septide were purchased from Peninsula Laboratories (St. Helens, U.K.), atropine from Serva (Heidelberg, Germany), tetrodotoxin from Sankyo (Japan), guanethidine from ICFI (Milan, Italy), Nω-nitro-L-arginine (L-NOARG) and capsaicin were from Sigma (St. Louis, U.S.A.). SR 140333, or [(S)1-{2-[3-(3,4-dichlorophenyl)-1-(3-isopropoxyphenylacetyl) piperidin-n-3yl]ethyl}-4-phenyl-1-azoniabi-cyclo [2,2,2] octane chloride] was kindly provided by Drs X. Emonds-Alt and G. Le Fur, Sanofi-Synthelabo (Montpellier, France).
Results
Quiescent preparations
The tachykinin NK1 receptor-selective agonists [Sar9]SP sulfone and septide (1 μM each) produced weak contractile responses (not exceeding 30% of maximal contraction produced by KCl 80 mM) in two strips obtained from one specimen of corpus cavernosum, whereas both peptides were ineffective on a second specimen of corpus cavernosum. In the corpus spongiosum, [Sar9]SP sulfone and septide (1 μM each) produced contraction in one out of three specimens examined, their effects not exceeding 25% of the maximal contractile response to KCl 80 mM, whereas they were ineffective on the others. The tachykinin NK3 receptor-selective agonist senktide (1 μM) failed to produce any motor response in all specimens of corpora spongiosa and cavernosa. In contrast, the tachykinin NK2 receptor agonist neurokinin A (NKA) produced potent contractile responses in all tissues examined (four strips from two corpora cavernosa and two strips from two corpora spongiosa).
Concentration-response curves to NKA (1 nM – 1 μM) could be constructed in the corpus cavernosum (pD2=7.9±0.1; n=4/2; Emax=77±8% of KCl 80 mM) (Figure 1) and spongiosum (pD2=8.3, n=2/2; Emax=86% of KCl 80 mM). The tachykinin NK2 receptor-selective antagonist nepadutant (0.1, 0.3 and 1 μM) rightward shifted concentration-response curves to NKA in the corpus cavernosum, without depressing agonist Emax (Figure 1). The estimated apparent affinity of nepadutant in the corpus cavernosum was: pKB=8.3±0.06 (n=3/2). Capsaicin (1 μM) failed to affect muscular tone in both corpus spongiosum and cavernosum (n=3/2 each tissue).
Figure 1.
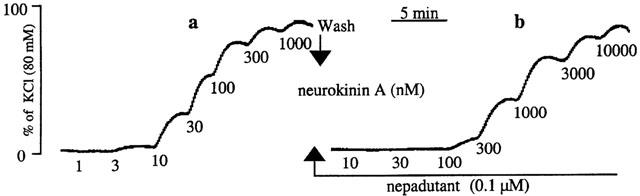
Typical tracing showing concentration-dependent contractile responses of the human isolated corpus cavernosum smooth muscle to neurokinin A in the absence (a) and presence (b) of the tachykinin NK2 receptor-selective antagonist nepadutant (15 min before).
Precontracted preparations
To investigate potential smooth muscle relaxant activity of tachykinins in the two tissues, further experiments were performed on strips whose tone had been raised by a submaximally effective concentration of KCl (16 mM), that produced stable contractions averaging 55±7% (n=8/3) and 60±9% (n=6/2) of that produced by KCl (80 mM) in the corpus spongiosum and cavernosum, respectively. In the corpus spongiosum, both [Sar9]SP sulfone and septide (1 nM – 1 μM each) produced concentration-dependent relaxations of precontracted strips (pD2=7.7±0.7, Emax=34±11% of maximal relaxation, n=4/3 and pD2=7.1±0.4, Emax=58±11% n=3/3, respectively) (Figure 2). In the corpus cavernosum, single concentrations (1 μM) of [Sar9]SP sulfone were administered, producing 27±5% of maximal relaxation (n=4/2). In both corpus cavernosum and spongiosum application of EFS to precontracted strips elicited prompt relaxations (50±5% of maximal relaxation in the spongiosum, n=8/3; 88±9% in the cavernosum, n=8/2), followed by rapid return to initial tone at the end of the stimulus (Figure 3). Addition of atropine (1 μM) and guanethidine (3 μM) or capsaicin (1 μM) to precontracted strips of both tissues did not affect the response to EFS or to NK1 receptor agonists (not shown). The inhibitory motor responses produced by EFS and by [Sar9]SP sulfone (1 μM) were partially additive in the corpus spongiosum and completely additive in the corpus cavernosum (Table 1; Figure 3). Tetrodotoxin (1 μM) abolished all responses to EFS, thus proving their neurogenic origin, whereas relaxations produced by [Sar9]SP sulfone were not affected (47±8% in the absence vs 49±9% in the presence, in the corpus spongiosum, n=3/2) (Figure 3). In contrast, the tachykinin NK1 receptor-selective antagonist SR 140333 (1 μM) completely prevented [Sar9]SP sulfone (1 μM)-induced relaxant effects in the corpus spongiosum (n=3/2), while leaving the response to EFS unaffected (Figure 4a). L-NOARG (100 μM; 30 min before) completely abolished relaxations induced either by EFS or [Sar9]SP sulfone (1 μM), in both tissues (n=3/2 each; Figure 4b).
Figure 2.
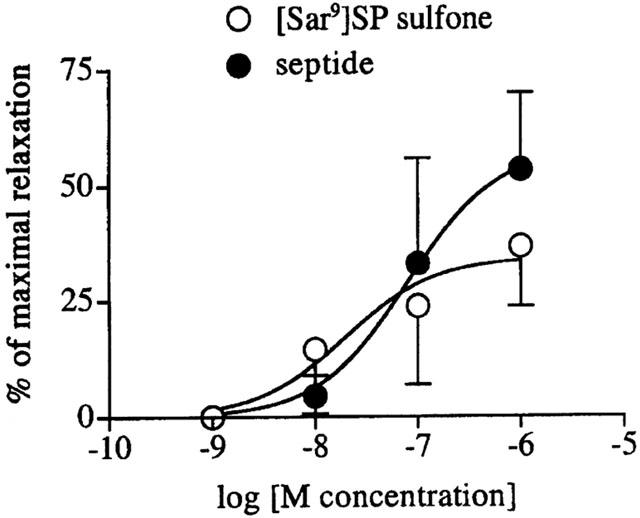
Log concentration-dependent relaxations produced by [Sar9]SP sulfone and septide on human isolated corpus spongiosum precontracted with KCl (16 mM). Each value is mean±s.e.mean of 3 – 4 experiments.
Figure 3.
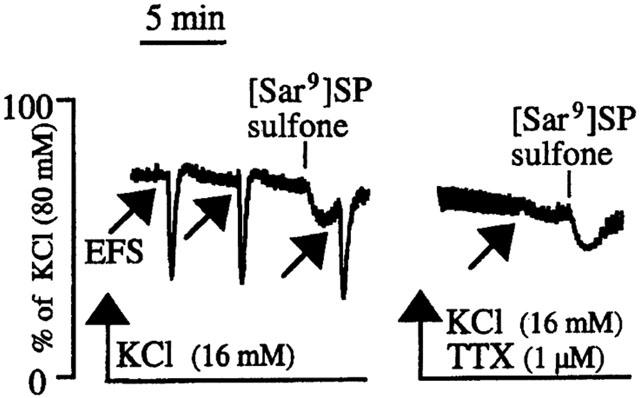
Typical tracings showing smooth muscle relaxations produced by electrical field stimulation (EFS, at arrows) and [Sar9]SP sulfone (1 μM) in the human isolated corpus spongiosum precontracted with KCl (16 mM) before and after addition of tetrodotoxin (TTX, 1 μM).
Table 1.
Additive relaxant responses produced by electrical field stimulation (EFS) and [Sar9]SP sulfone on precontracted corpus cavernosum (CC) and spongiosum (CS)
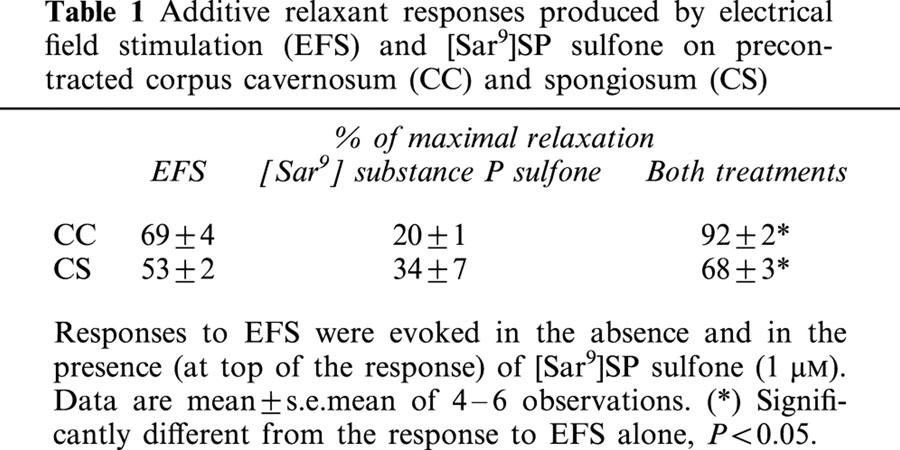
Figure 4.

Typical tracings showing smooth muscle relaxations produced by electrical field stimulation (EFS, at arrows) and [Sar9]SP sulfone (1 μM) in: (a) human isolated corpus spongiosum precontracted with KCl (16 mM) before and after addition of SR 140333 (1 μM), and (b) human isolated corpus cavernosum precontracted with KCl (16 mM) before and after addition of Nω-nitro-L-arginine (L-NOARG, 100 μM).
Discussion
Vasodilatation is one of the most prominent effects elicited by SP and related tachykinins which produce transient hypotension in vivo, and relaxation of precontracted blood vessels in vitro. The vasodilator effect of tachykinins requires the activation of tachykinin receptors of the NK1 type and is strictly dependent on the presence of intact endothelium, from which nitric oxide (NO) and other mediators are released to produce vascular smooth muscle relaxation (Maggi, 1995, for review). Although most of the studies have been performed in vivo or on isolated arteries, there is evidence that tachykinin NK1 receptor stimulation also leads to endothelium-dependent, NO-mediated smooth muscle relaxation in veins (Patacchini & Maggi, 1995). Besides their vasodilator effect, tachykinins can produce vasoconstriction in certain blood vessels by stimulating receptors of the NK1, NK2 or NK3 type (Maggi, 1995, for review). The corpus cavernosum is a unique vascular bed composed of cavernosal spaces lined by vascular endothelium containing bundles of smooth muscle in a framework of collagen, elastin and fibroblasts (Andersson & Wagner, 1995). Relaxation of cavernosal smooth muscle is an essential step in the process of penile erection, while the corpus spongiosum urethrae (histologically similar to the cavernosum except for a higher content of elastic fibres) seems to play a major role in ejaculation (Andersson & Wagner, 1995; Moreland et al., 2001). Among the local factors that have been proposed as responsible for cavernosal smooth muscle relaxation and penile erection, NO, either released from nitrergic nerves or produced by the endothelium, is regarded as a/the major mediator of these effects (Burnett et al., 1992; Andersson & Wagner, 1995). A number of neuropeptides have been found to possess either contractile (e.g. endothelins) or relaxant (e.g. VIP, CGRP) effects on the cavernosal smooth muscle, while both effects were reported in response to SP (Andersson et al., 1983; Hedlund & Andersson, 1985). In the present study we have shown that tachykinins produce smooth muscle contraction or relaxation of human corpus spongiosum and cavernosum depending on the type of tachykinin receptor activated and muscular tone of the tissue. In particular, the use of tachykinin receptor-selective agonists and antagonists and other selective inhibitors has allowed to establish that: (1) tachykinin NK2 receptors mediate smooth muscle contraction, as observed in (almost) all smooth muscles of human origin (Maggi, 1995). In favour of this conclusion is that NKA-induced contractions in the corpus cavernosum were anatagonized by the NK2 receptor-selective antagonist nepadutant (Catalioto et al., 1998), with apparent affinity (pKB=8.3) similar to that measured against NK2 receptor-mediated contractions in the human isolated urinary bladder, ileum and colon (pKB=8.3−8.5; Patacchini et al., 2000). (2) Tachykinin NK1 receptors mediate smooth muscle relaxation via NO generation, as observed in the majority of blood vessels. The effectiveness of SR 140333 (Emonds-Alt et al., 1993) in preventing relaxations produced by [Sar9]SP sulfone supports the above conclusion. Although neither the source of the released NO, nor the location of NK1 receptors mediating relaxant effects was investigated directly, it is conceivable it could be endothelial in both cases. The latter hypothesis stems from the observations that (i) tachykinin NK1 receptor-mediated relaxations are non-neurogenic (as they are unaffected by tetrodotoxin); and (ii) they were additive with the neurogenic relaxations elicited by EFS. On the other hand, spare contractions were recorded in about 1/3 preparations upon administration of NK1 receptor-selective agonists at high concentrations. Further investigation on these latter effects could not be performed, because of the limited number of available specimens. However, it may be speculated that the contractile effects produced by [Sar9]SP sulfone and SP (Andersson et al., 1983; Hedlund & Andersson, 1985) are mediated by NK1 receptors present on smooth muscle cells, although a contribution given by NK2 receptors, activated by the high concentrations employed (1 – 10 μM), cannot be ruled out. (3) Tachykinin NK3 receptors, even though present, do not participate in motor effects produced by tachykinins. Also capsaicin was without effect, providing no evidence for the presence of capsaicin-sensitive (tachykinin-containing) primary afferent nerve terminals in these tissues.
In conclusion, our study is the first demonstration that tachykinin NK1 and NK2 receptors are functionally present in the human corpus cavernosum and spongiosum, and mediate opposite motor effects, i.e. relaxation vs contraction, respectively. Owing to the high morphological similarity of the two tissues and to the observation that opposite motor responses to tachykinins were recorded in specimens obtained from all donors (n=5 patients) it can be proposed that these effects of tachykinins occur in the whole erectile smooth muscle tissue of the human penis. These findings extend our knowledge on the mediators affecting penile erection/detumescence, and possibly could disclose a new area for development of drugs for the treatment of erectile dysfunction. To this regard, further studies are needed to assess a possible (patho)physiological role of tachykinins and tachykinin receptors in normal or impaired penile erection.
Abbreviations
- EFS
electrical field stimulation
- L-NOARG
Nω-nitro-L-arginine
- NANC
nonadrenergic noncholinergic
- NKA
neurokinin A
- NO
nitric oxide
- SP
substance P
References
- AZADZOI K.M., KIM N., BROWN M.L., GOLDSTEIN I., COHEN R.A., DE TEJADA I.S. Endothelium-derived nitric oxide and cyclooxygenase products modulate corpus cavernosum smooth muscle tone. J. Urol. 1992;147:220–225. doi: 10.1016/s0022-5347(17)37201-4. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.-E., HEDLUND H., MATTIASSON A., SJORGEN C., SUNDLER F. Relaxation of isolated human corpus spongiosum induced by vasoactive intestinal polypeptide, substance P, carbachol and electrical field stimulation. World J. Urol. 1983;1:203–208. [Google Scholar]
- ANDERSSON K.-E., WAGNER G. Physiology of penile erection. Physiol. Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- BURNETT A.L., LOWENSTEIN C.J., BREDT D.S., CHANG T.S.K., SNYDER S.H. Nitric oxide: a physiological mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- CATALIOTO R.-M., CRISCUOLI M., CUCCHI P., GIACHETTI A., GIANNOTTI D., GIULIANI S., LECCI A., LIPPI A., PATACCHINI R., QUARTARA L., RENZETTI A.R., TRAMONTANA M., ARCAMONE F., MAGGI C.A. MEN 11420 (Nepadutant), a novel glycosylated bicyclic peptide tachykinin NK2 receptor antagonist. Br. J. Pharmacol. 1998;123:81–91. doi: 10.1038/sj.bjp.0701587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMONDS-ALT X., DOUTREMEPUICH J.D., HEAULME M., NELIAT G., SANTUCCI V., STEINBERG R., VILAIN P., BICHON D., DUCOUX J.P., PROIETTO E., VAN BROECK D., SOUBRIÉ P., LE FUR G., BRELIÉRE J.C. In vitro and in vivo biological activities of SR 140,333, a novel potent nonpeptide tachykinin NK1 receptor antagonist. Eur. J. Pharmacol. 1993;250:403–413. doi: 10.1016/0014-2999(93)90027-f. [DOI] [PubMed] [Google Scholar]
- GU J., POLAK M., PROBERT L., ISLAM N., MARANGOS P.J., MINA S., ADRIAN T.E., MCGREGOR G.P., O'SHAUGHNESS D.J., BLOOM S.R. Peptidergic innervation of the human male genital tract. J. Urol. 1983;130:386–391. doi: 10.1016/s0022-5347(17)51174-x. [DOI] [PubMed] [Google Scholar]
- HAUSER-KRONBERGER C., HACKER G.W., GRAF A.-H., MACK D., SUNDLER F., DIETZE O., FRICK J. Neuropeptides in the human penis: an immunohistochemical study. J. Androl. 1994;15:510–520. [PubMed] [Google Scholar]
- HEDLUND H., ANDERSSON K.-E. Effects of some peptides on isolated human penile erectile tissue and cavernous artery. Acta Physiol. Scand. 1985;124:413–419. doi: 10.1111/j.1748-1716.1985.tb07677.x. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Progress Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., PATACCHINI R., ROVERO P., GIACHETTI A. Tachykinin receptors and tachykinin receptor subtypes. J. Autonom. Pharmacol. 1993;13:23–93. doi: 10.1111/j.1474-8673.1993.tb00396.x. [DOI] [PubMed] [Google Scholar]
- MORELAND R.B., HSIEH G., NAKANE M., BRIONI J.D. The biochemical and neurologic basis for the treatment of male erectile dysfunction. J. Pharmacol. Exp. Ther. 2001;296:225–234. [PubMed] [Google Scholar]
- PATACCHINI R., GIULIANI S., TURINI A., NAVARRA G., MAGGI C.A. Effect of nepadutant at tachykinin NK2 receptors in human intestine and urinary bladder. Eur. J. Pharmacol. 2000;398:389–397. doi: 10.1016/s0014-2999(00)00346-0. [DOI] [PubMed] [Google Scholar]
- PATACCHINI R., MAGGI C.A. Tachykinin NK1 receptors mediate both vasoconstrictor and vasodilator responses in the rabbit isolated jugular vein. Eur. J. Pharmacol. 1995;283:233–240. doi: 10.1016/0014-2999(95)00361-n. [DOI] [PubMed] [Google Scholar]
- REGOLI D., DRAPEAU G., DION S., D'ORLEANS-JUSTE P. Receptors for substance P and related neurokinins. Pharmacology. 1989;38:1–15. doi: 10.1159/000138512. [DOI] [PubMed] [Google Scholar]
- SANTICIOLI P., MAGGI C.A. Myogenic and neurogenic factors in the control of pyeloureteral motility and ureteral peristalsis. Pharmacol. Rev. 1998;50:683–721. [PubMed] [Google Scholar]


