Abstract
The common mechanism of action of non-steroidal anti-inflammatory drugs (NSAIDs) is the inhibition of the enzyme cyclo-oxygenase (COX), however, this inhibition is not enough to completely account for the efficacy of these agents in several models of acute pain.
It has been demonstrated that cholinergic agents can induce antinociception, but the nature of the interaction between these agents and NSAIDs drugs has not been studied. The present work evaluates, by isobolographic analysis, the interactions between the cholinergic indirect agonist neostigmine (NEO) and NSAIDs drugs, using a chemical algesiometric test.
Intraperitoneal (i.p.) or intrathecal (i.t.) administration of NEO and of the different NSAIDs produced dose-dependent antinociception in the acetic acid writhing test of the mouse.
The i.p. or i.t. co-administration of fixed ratios of ED50 fractions of NSAIDs and NEO, resulted to be synergistic or supra-additive for the combinations ketoprofen (KETO) and NEO, paracetamol (PARA) and NEO) and diclofenac (DICLO) and NEO administered i.p. However, the same combinations administered i.t. were only additive. In addition, the combinations meloxicam (MELO) and NEO and piroxicam (PIRO) and NEO, administered either i.p. or i.t., were additive.
The results suggest that the co-administration of NEO with some NSAIDs (e.g. KETO, PARA or DICLO) resulted in a synergistic interaction, which may provide evidence of supraspinal antinociception modulation by the increased acetylcholine concentration in the synaptic cleft of cholinergic interneurons. The interaction obtained between neostigmine and the NSAIDs could carry important clinical implications.
Keywords: Writhing test, NSAIDs, neostigmine, isobolographic analysis, antinociception
Introduction
It is generally accepted that the common mechanism of action of non-steroidal anti-inflammatory drugs (NSAIDs) is the inhibition of the enzyme cyclo-oxygenase (COX). Two isoforms of COX, referred to as COX-1 and COX-2, have been identified (Vane et al., 1998; Marnett & Kalgutkar, 1999). Each enzyme is encoded by a separate gene with a different pattern of expression and function. COX-1 is constitutively expressed and high levels can be found in most tissues, whereas COX-2 is inducible and usually, under physiological conditions, low levels are detectable in some tissues, but levels are rapidly increased during inflammation (Vane et al., 1998; Marnett & Kalgutkar, 1999). Several works have demonstrated that the inhibition of COX mediates the anti-inflammatory, antipyretic and analgesic effects of NSAIDs (Cryer & Feldman, 1998; Warner et al., 1999; Mitchell & Warner, 1999; Smith et al., 2000; Paik et al., 2000). In spite of this emphasis on the COX mediated-antinociceptive activity of NSAIDs, the selective inhibition of COX seems not to be enough to completely account for the efficacy of these agents, since in several models of pain without inflammation, such as models of chemical and thermal acute pain, NSAIDs are powerful analgesics (Miranda et al., 2001; Pinardi et al., 2001).
The role of the cholinergic system in nociception has been recognized. Thus, it has been suggested that acetylcholine is an endogenous antinociceptive compound that may act through monoaminergic pathways (Gillberg et al., 1989; Iwamoto & Marion, 1993; Guimaraes & Prado, 1994). Cholinergic agents such as neostigmine (NEO), produce analgesia after systemic and intrathecal administration (Eisenach & Gebhart, 1995; Naguib & Yaksh, 1997; Chiari et al., 1999; Eisenach, 1999). On the other hand, cholinergic agonists exert a positive modulatory action enhancing analgesia induced by opioid or clonidine (Abram & Winne, 1995; Eisenach & Gebhart, 1995; Xu et al., 1996; Hood et al., 1997).
Although previous studies have demonstrated that cholinergic agents can induce antinociception, a study of the characteristics of the interaction between these agents and NSAIDs has not been performed. The purpose of the current study was to examine, by isobolographic analysis, the interactions between the cholinergic indirect agonist NEO and NSAIDs drugs, using a chemical algesiometric test.
Methods
CF-1 mice, weighing 27±2 g, were used throughout the experimental work. The animals were acclimatized to the laboratory environment for at least 2 h before being used and ethical standard guidelines were followed as previously described (Pinardi et al., 1998) and were approved by the local ethical commission. In particular, the duration of the experiments was as short as possible, the number of animals involved was kept to a minimum and the animals were killed immediately after the recording period. Each animal was used only once and received only one dose of the drugs tested. All drugs were freshly prepared by dissolving them in normal saline or in a slightly hyperbaric solution of glucose (6%). All observations during the assay were performed by the authors in a randomized and blind manner. Evaluation of antinociceptive activity was accomplished as previously reported (Pinardi et al., 1998). Briefly, intraperitoneal (i.p.) administration was done by injecting the total dose in a constant volume of 10 ml kg−1, 30 min before the algesiometric test. For intrathecal (i.t.) administration, the Hylden & Wilcox (1980) technique was used, and the doses of the drugs were injected 15 min before the algesiometric test, in a constant volume of 5 μl, dissolved in a slightly hyperbaric solution of glucose (6%) to limit diffusion. The procedure was performed rapidly with a high degree of accuracy and reproducibility. The times of drug administration before the algesiometric test (30 min for i.p. and 15 min for i.t.) were found in previous experiments to be near the time of onset of the maximum analgesic effect. Control animals (saline or 6% glucose) were run interspersed concurrently with the drug-treated animals, which prevented all the controls being run on a single group of mice at one time during the course of the investigation.
Algesiometric test (writhing test)
Mice were injected i.p. with 10 ml kg−1 of 0.6% acetic acid and the number of writhes was counted during a 5 min period, starting 5 min after the administration of the acetic acid solution. A writhe was defined as a contraction of the abdominal muscles accompanied by an elongation of the body and extension of the hindlimbs.
Evaluation of antinociception
Dose-response curves, determined near the time of peak effect, were constructed in order to assess the antinociceptive action of the different NSAIDs and of NEO administered either intraperitoneally or intrathecally. Eight animals were used at each of at least four doses to determine a dose-response curve. The dose that produced 50% of antinociception (ED50: 50% reduction of control writhes) was calculated using standard linear regression analysis of the log dose-response curve. Antinociceptive activity was expressed as per cent inhibition of the usual number of writhes observed in i.p. saline (19.77±0.30, n=60) or i.t. glucose (20.1±0.43, n=53) control animals.
Isobolographic analysis
The interaction of the cholinergic agent neostigmine (NEO) with the antinociceptive effects of NSAIDs was evaluated by the simultaneous administration of fixed proportions of NEO with each NSAID, and performing an isobolographic analysis for the different combinations, as described by Tallarida et al. (1989). The isobologram was constructed by connecting the ED50 of the corresponding NSAID plotted on the abscissa with the ED50 of NEO plotted on the ordinate to obtain the additivity line. For each drug mixture, the ED50 and its associated 95% confidence intervals were determined by linear regression analysis of the log dose-response curve (eight animals at each of at least four doses) and compared by a ‘t'-test to a theoretical additive ED50 obtained from the calculation: ED50 add=ED50 NSAID/(P1+R·P2), where R is the potency ratio of the NSAID alone to NEO alone, P1 is the proportion of NSAID and P2 is the proportion of NEO in the total mixture. In the present study, fixed-ratio proportions were selected by first combining the ED50 of each compound and then constructing a dose-response curve in which ED50 fractions (½, ¼ and ⅛) of NEO-NSAID combinations were administered; in the equation above, ED50 add is the total dose and the variance of ED50 add was calculated from the fraction of the ED50's (i.e. 0.5) in the combination as: Var ED50 add=(0.5)2·Var ED50 NSAID +(0.5)2Var ED50 NEO (Pinardi et al., 2001). From these variances, confidence limits are calculated and resolved according to the ratio of the individual drugs in the combination. Supra-additivity or synergistic effect is defined as the effect of a drug combination which is higher and statistically different (ED50 significantly lower) than the theoretical calculated equieffect of a drug combination with the same proportions. When the drug combination gives an experimental ED50 not statistically different from the theoretically calculated ED50, the combination has an additive effect and additivity means that each constituent contributes to the effect in accord with its own potency and the less potent drug is acting as though it is merely a diluted form of the other (Tallarida, 2001).
Drugs
All drugs used were dissolved in saline solution for i.p. administration or in a slightly hyperbaric solution of glucose (6%) to limit diffusion, for i.t. administration. The drugs, provided by local laboratories, were: diclofenac (DICLO), ketoprofen (KETO), meloxicam (MELO), paracetamol (PARA) and piroxicam (PIRO). Neostigmine bromide (NEO) was purchased from Sigma Chemical Co., (St. Louis, MO, U.S.A.).
Statistics
Results are presented as ED50 values with 95% confidence limits. Student's ‘t'-test for independent means was used to assess statistical significance (P<0.05).
Results
Antinociceptive activity of NSAIDs and NEO in the writhing test
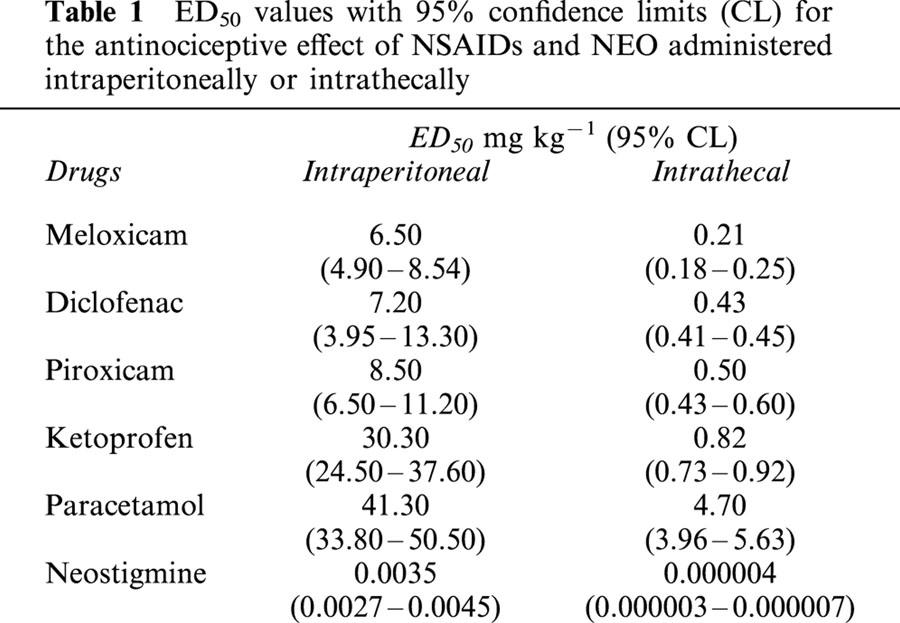
The i.p. or i.t. administration of NEO and the different NSAIDs produced dose-dependent antinociceptive effects in the algesiometric assay of acetic acid-induced writhes, with different potencies. The dose-response curves obtained were characterized by equal efficacy, and the corresponding ED50 values with their 95% confidence limits are shown in Table 1. Animals tested with the different doses of NSAIDs or NEO used did not exhibit significant behavioural or motor dysfunctions.
Table 1.
ED50 values with 95% confidence limits (CL) for the antinociceptive effect of NSAIDs and NEO administered intraperitoneally or intrathecally
Interactions of NSAIDs and neostigmine
The antinociceptive activity induced by the co-administration of fixed ratios of ED50 fractions of NSAIDs and NEO was examined by the analysis of the corresponding isobolograms obtained after i.p. or i.t. administration. The ED50 values for the combinations and their 95% confidence intervals are shown in Tables 2 and 3. Figure 1 shows an example of the log dose-response curves obtained, determined for DICLO and the combination DICLO/NEO administered i.p. (Figure 1A) and i.t. (Figure 1B), from where the experimental ED50's for the combinations were calculated. The isobolographic analysis for the combination DICLO/NEO administered i.p., resulted in a supra-additive interaction (Figure 1C), however, the same combination administered i.t. was only additive (Figure 1D), since the experimental point was not statistically different from the calculated theoretical additive point. In addition, KETO/NEO and PARA/NEO, administered either i.p. or i.t., resulted to be synergistic or supra-additive (Figure 2A,B,C,D). The combinations MELO/NEO and PIRO/NEO, administered either i.p. or i.t., were only additive (Figure 3A,B,C,D).
Table 2.
Theoretical and experimental antinociceptive ED50 values with 95% confidence limits (CL) for combinations of NSAIDs with NEO administered intraperitoneally
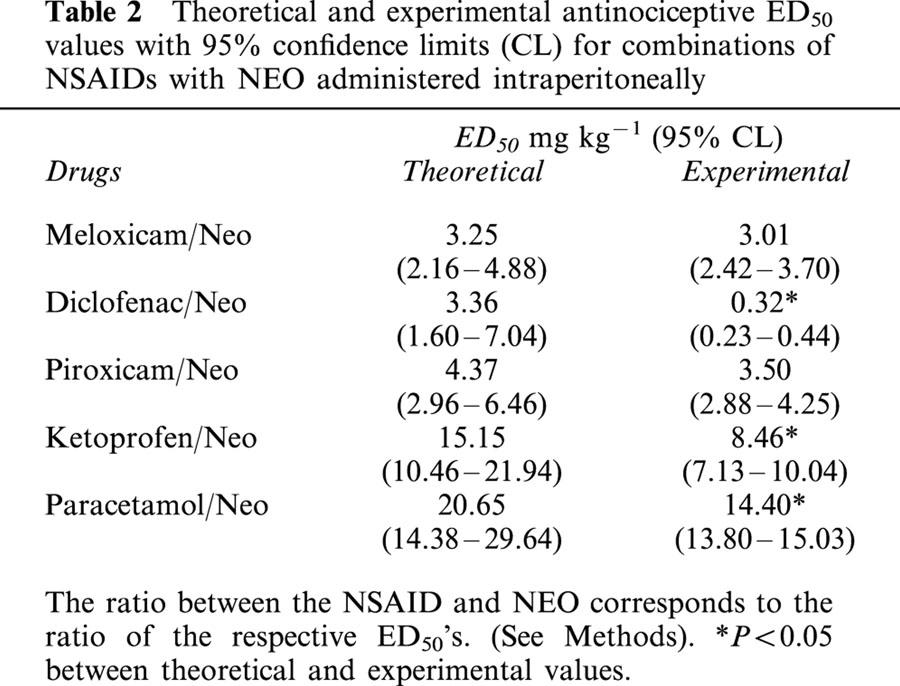
Table 3.
Theoretical and experimental antinociceptive ED50 values with 95% confidence limits (CL) for combinations of NSAIDs with NEO administered intrathecally.
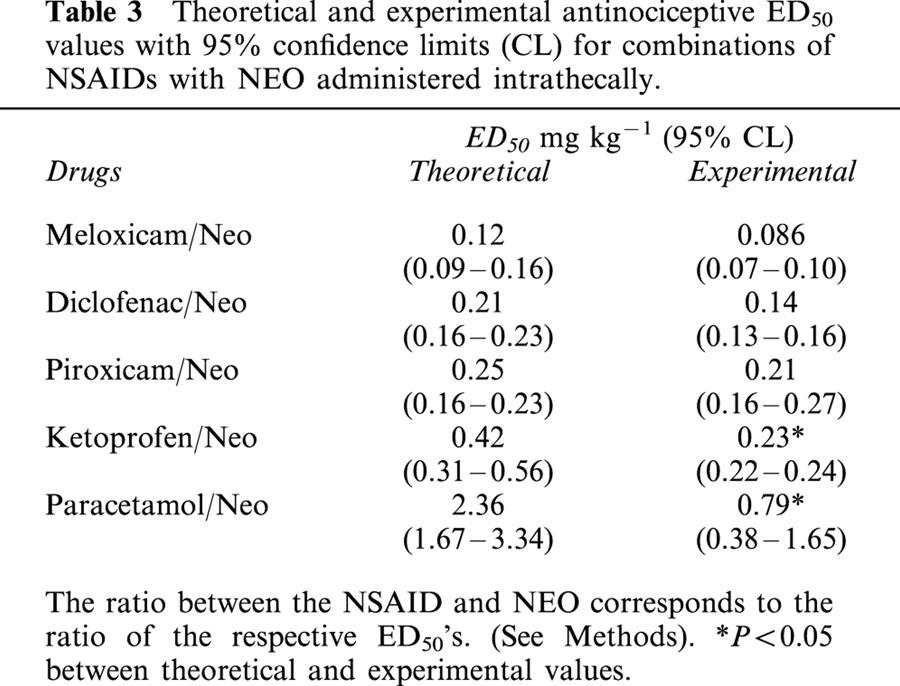
Figure 1.
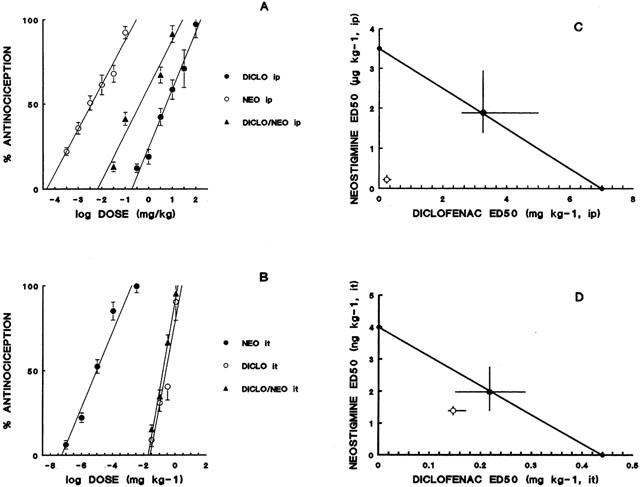
Dose-response curves for the administration of diclofenac (DICLO), neostigmine (NEO) and the simultaneous administration of DICLO+NEO (A: intraperitoneal, B: intrathecal). Isobologram for the simultaneous administration of diclofenac and neostigmine (C: intraperitoneal; D: intrathecal). Filled circles correspond to the theoretical ED50 with 95% confidence limits and open circles correspond to the experimental ED50 with 95% confidence limits. Ordinates of intraperitoneal (i.p.) and intrathecal (i.t.) isobolograms are on different scales.
Figure 2.
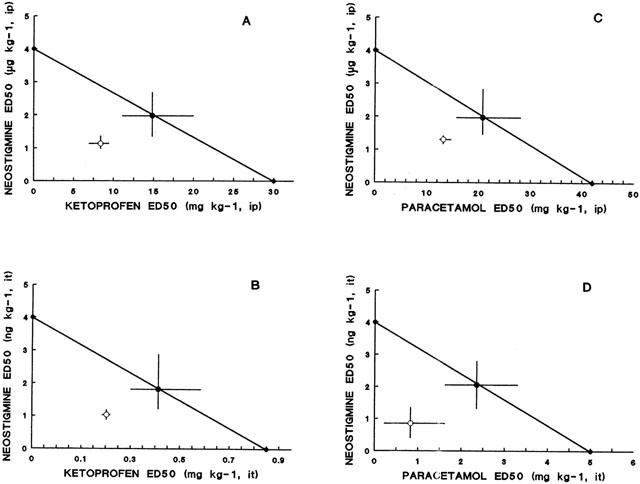
Isobologram for the simultaneous administration of ketoprofen and neostigmine (A: intraperitoneal; B: intrathecal) and paracetamol and neostigmine (C: intraperitoneal; D: intrathecal). Ordinates of intraperitoneal (i.p.) and intrathecal (i.t.) administration are on different scales. Symbols as in Figure 1.
Figure 3.
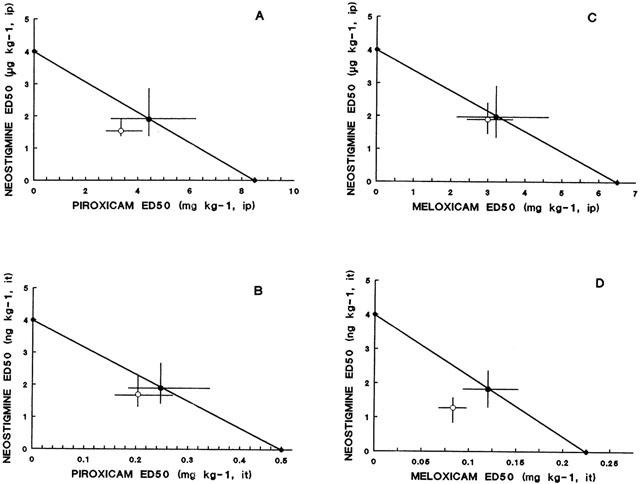
Isobologram for the simultaneous administration of piroxicam and neostigmine (A: intraperitoneal; B: intrathecal) and meloxicam and neostigmine (C: intraperitoneal; D: intrathecal). Ordinates of intraperitoneal (i.p.) and intrathecal (i.t) administration are on different scales. Symbols as in Figure 1.
Discussion
There is evidence that muscarinic receptor agonists, such as carbachol and acetylcholinesterase inhibitors, such as NEO, increase the pain threshold after systemic and spinal administration. The mechanism of spinal cholinergic antinociception is not known, but muscarinic interneurons in the pain transmission pathway and muscarinic desensitizing effects on peripheral nociceptors have been postulated to explain the interactions with other neurotransmitters (Hartvig et al., 1989; Bernardini et al., 2001). In the central nervous system (CNS), cholinergic antinociception seems to be mediated by activation of M1 post-synaptic muscarinic receptors, even if M2 muscarinic autoreceptors can also regulate pain by modulating acetylcholine release via a negative feedback mechanism (Ghelardini et al., 1990; Bartolini et al., 1992).
It has been postulated that the administration of the acetylcholinesterase inhibitor NEO, modulates central and peripheral sites of intrinsic cholinergic inhibitory pathways of pain perception (Buerkle et al., 1998). In the spinal cord, muscarinic receptors are concentrated in the superficial layers of the dorsal horn (Eisenach, 1999). Several mechanisms, such as hyperpolarization of neurons, reduction in the release of pro-nociceptive neurotransmitters and activation of the nitric oxide-cyclic guanosine monophosphate pathway, have been suggested as mediators of cholinergic antinociception, through the elevation of endogenous Ach (Yang et al., 1998). In addition, the central antinociception induced by cholinergic agonists seems to be mediated in part, by the subsequent activation of spinally-projecting noradrenergic neurons, located in the pontine A7 catecholamine cell group (Nuseir et al., 1999).
The synergism obtained after the i.p. administration of the muscarinic indirect agonist NEO combined with KETO, PARA or DICLO can be interpreted as a signal of supraspinal antinociception modulation by the increased acetylcholine concentration in the synaptic cleft, with activation of descending inhibitory noradrenergic and serotonergic pathways, probably by modulating cholinergic interneurons (Gillberg et al., 1989; Iwamoto & Marion, 1993; Guimaraes & Prado, 1994). Synergistic mechanisms between drugs may be influenced by pharmacodynamics interactions (at receptor or second messenger levels) and by functional pharmacokinetic interactions due to different activity at diverse anatomic sites (Solomon & Gebhart, 1994), and this consideration might explain the differences between the effects observed with different NSAIDs.
The present findings, in which differences were found between i.p. and i.t. administrations, are in agreement with previous works, in which synergistic antinociceptive interactions were obtained after systemic administration, but not after intrathecal administration, since systemically administered drugs reach both supraspinal and spinal sites and intrathecal drugs have limited diffusion and a more local effect (Yeung & Rudy, 1980; Roerig et al., 1984). In addition, it has been suggested that drug interactions may involve the activation of different pathways of pain inhibition; thus, systemic administration may stimulate descending pain inhibitory pathways, usually mediated by norepinephrine and serotonin, while these inhibitory pathways should not be activated to the same degree by intrathecal administration (Suh & Tseng, 1988; Roerig et al., 1988). The fact that intraperitoneal co-administration of NEO with NSAIDs results mainly in supra-additive interactions can then be related to the different distribution of the drugs in the CNS. As a result, supra-additive interactions could be ascribed to the activation of complementary central and peripheral mechanisms of antinociception, since the activation of a common mechanism should presumably produce only an additive interaction. Thus, cholinergic stimulation would imply the activation of several circuits, perhaps cholinergic, noradrenergic, serotonergic, opioid, and/or nitridergic, that modulate the afferent nociceptive information.
The results obtained in the present study are in agreement with previous results that suggest that drug interactions between other type of drugs, i.e. opioids, may be additive or synergistic, depending on the route of administration and the nociceptive test used (Ossipov et al., 1990). In addition, these results indicate that NSAIDs have a powerful effect upon spinal nociceptive processing, in agreement with previous works (Malmberg & Yaksh, 1993). The interactions obtained between NEO and the NSAIDs could carry important clinical implications for the treatment of pain states, in the same way that those observed between cholinergic agents and α2-adrenergic agonists or opioid drugs (Bouaziz et al., 1995; Abram & Winne, 1995; Eisenach & Gebhart, 1995; Xu et al., 1996; Hood et al., 1997).
Acknowledgments
Work supported by Fondecyt project No 1990842. The authors thank J. López and A. Correa for outstanding technical assistance.
Abbreviations
- CNS
central nervous system
- COX
cyclo-oxygenase
- DICLO
diclofenac
- KETO
ketoprofen
- MELO
meloxicam
- NEO
neostigmine
- NSAID
non-steroidal anti-inflammatory drug
- PARA
paracetamol
- PIRO
piroxicam
References
- ABRAM S.E., WINNE R.P. Intrathecal acetyl cholinesterase inhibitors produce analgesia that is synergistic with morphine and clonidine in rats. Anesth. Analg. 1995;81:501–507. doi: 10.1097/00000539-199509000-00013. [DOI] [PubMed] [Google Scholar]
- BARTOLINI A., GHELARDINI C., FANTETTI L., MALCANGIO M., MALMBERG-AIELLO P., GIOTTI A. Role of muscarinic receptor subtypes in central antinociception. Br. J. Pharmacol. 1992;105:77–82. doi: 10.1111/j.1476-5381.1992.tb14213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNARDINI N., SAUER S.K., HABERBERGER R., FISCHER M.J., REEH P.W. Excitatory nicotinic and desensitizing muscarinic (M2) effects on C-nociceptors in isolated rat skin. J. Neurosci. 2001;21:3295–3302. doi: 10.1523/JNEUROSCI.21-09-03295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUAZIZ H., HEWITT C., EISENACH J.C. Subarachnoid neostigmine potentiation of alpha 2-adrenergic agonist analgesia. Dexmedetomidine versus clonidine. Reg. Anesth. 1995;20:121–127. [PubMed] [Google Scholar]
- BUERKLE H., BOSCHIN M., MARCUS M.A., BRODNER G., WUSTEN R., VAN ANKEN H. Central and peripheral analgesia mediated by acetylcholinesterase-inhibitor neostigmine in the rat inflamed knee joint model. Anesth. Analg. 1998;86:1027–1032. doi: 10.1097/00000539-199805000-00023. [DOI] [PubMed] [Google Scholar]
- CHIARI A., TOBIN J.R., PAN H.L., HOOD D.D., EISENACH J.C. Sex differences in cholinergic analgesia. I: a supplemental nicotinic mechanism in normal females. Anesthesiology. 1999;91:1447–1454. doi: 10.1097/00000542-199911000-00038. [DOI] [PubMed] [Google Scholar]
- CRYER B., FELDMAN N. Cyclo-oxygenase-1 and cyclo-oxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998;104:413–421. doi: 10.1016/s0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- EISENACH J.C. Muscarinic-mediated analgesia. Life Sci. 1999;64:549–554. doi: 10.1016/s0024-3205(98)00600-6. [DOI] [PubMed] [Google Scholar]
- EISENACH J.C., GEBHART G.F. Intrathecal amitryptiline. Antinociceptive interactions with intravenous morphine and intrathecal clonidine, neostigmine and carbamylcholine in rats. Anesthesiology. 1995;83:1036–1045. doi: 10.1097/00000542-199511000-00017. [DOI] [PubMed] [Google Scholar]
- GHELARDINI C., FANTETTI L., MALCANGIO M., MALMBERG-AIELLO P., GIOTTI A., BARTOLINI A. Methoctramine increases cholinergic neurotransmission by acting presynaptically. Pharmacol. Res. 1990;22:227. doi: 10.1016/1043-6618(92)90523-e. [DOI] [PubMed] [Google Scholar]
- GILLBERG P.G., GORDH T., JR, HARTVIG O., JANSSON I., PETTERSSON J., POST C. Characterization of the antinociception induced by intrathecally administered carbachol. Pharmacol. Toxicol. 1989;64:340–343. doi: 10.1111/j.1600-0773.1989.tb00660.x. [DOI] [PubMed] [Google Scholar]
- GUIMARAES A.P., PRADO W.A. Antinociceptive effects of carbachol microinjected into different portions of the mesencephalic periaqueductal gray matter of the rat. Brain Res. 1994;674:220–230. doi: 10.1016/0006-8993(94)91321-8. [DOI] [PubMed] [Google Scholar]
- HARTVIG P., GILLBERG P.G., GORDH T., JR, POST C. Cholinergic mechanisms in pain and analgesia. Trends Pharmacol. Sci. 1989. pp. 75–79. [PubMed]
- HOOD D.K., MALLAK K.A., JAMES R.L., TUTTLE R., EISENACH J.C. Enhancement of analgesia from systemic opioid in humans by spinal cholinesterase inhibition. J. Pharmacol. Exp. Ther. 1997;282:86–92. [PubMed] [Google Scholar]
- HYLDEN J.L.K., WILCOX G.L. Intrathecal morphine in mice: a new technique. Eur. J. Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- IWAMOTO E.T., MARION L. Characterization of the antinociception produced by intrathecally administered muscarinic agonists in rats. J. Pharmacol. Exp. Ther. 1993;266:329–338. [PubMed] [Google Scholar]
- MALMBERG A.B., YAKSH T.L. Pharmacology of the spinal action of ketorolac, morphine, ST-91, U50488H, and L-PIA on the formalin test and an isobolographic analysis of the NSAID interaction. Anesthesiology. 1993;79:270–281. doi: 10.1097/00000542-199308000-00012. [DOI] [PubMed] [Google Scholar]
- MARNETT L.J., KALGUTKAR A.S. Cyclooxygenase 2 inhibitors: discovery, selectivity and the future. Trends. Pharmacol. Sci. 1999;20:465–469. doi: 10.1016/s0165-6147(99)01385-1. [DOI] [PubMed] [Google Scholar]
- MIRANDA H.F., SIERRALTA F., PINARDI G. An isobolographic analysis of the adrenergic modulation of diclofenac antinociception. Anesth. Analg. 2001;93:430–435. doi: 10.1097/00000539-200108000-00039. [DOI] [PubMed] [Google Scholar]
- MITCHELL J.A., WARNER T.D. Cyclo-oxygenase2: pharmacology, physiology, biochemistry and relevance to NSAID therapy. Brit. J. Pharmacol. 1999;128:1121–1132. doi: 10.1038/sj.bjp.0702897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGUIB M., YAKSH T.L. Characterization of muscarinic receptor subtypes that mediates antinociception in the rat spinal cord. Anesth. Analg. 1997;85:847–853. doi: 10.1097/00000539-199710000-00025. [DOI] [PubMed] [Google Scholar]
- NUSEIR K., HEIDENRICH B.A., PROUDFIT H.K. The antinociception produced by microinjection of a cholinergic agonist in the ventromedial medulla is mediated by noradrenergic nurons in the A7 catecholamines cell group. Brain Res. 1999;822:1–7. doi: 10.1016/s0006-8993(98)01195-0. [DOI] [PubMed] [Google Scholar]
- OSSIPOV M.H., HARRIS S., LLOYD P., MESSINEO E. An isobolographic analysis of the antinociceptive effect of systemically and intrathecally administered combinations of clonidine and opioids. J. Pharmacol. Exp. Ther. 1990;255:1107–1116. [PubMed] [Google Scholar]
- PAIK J.H., JU J.H., LEE J.Y., BOUDRAEU M.D., HWANG D.H. Two opposing effects of non steroidal anti-inflammatory drugs on the expression of the inducible cyclooxygenase. Mediation through different signaling pathways. J. Biol. Chem. 2000;275:28173–28179. doi: 10.1074/jbc.M002329200. [DOI] [PubMed] [Google Scholar]
- PINARDI G., PELISSIER T., MIRANDA H.F. Interactions in the antinociceptive effect of tramadol in mice: an isobolographic analysis. Enz. J. Pain. 1998;2:343–350. doi: 10.1016/s1090-3801(98)90032-5. [DOI] [PubMed] [Google Scholar]
- PINARDI G., SIERRALTA F., MIRANDA H.F. Interaction between the antinociceptive effect of ketoprofen and adrenergic modulatory systems. Inflammation. 2001;25:233–239. doi: 10.1023/a:1010923820109. [DOI] [PubMed] [Google Scholar]
- ROERIG S.C., FUJIMOTO J.M., TSENG L.F. Comparisons of descending pain inhibitory pathways activated by β-endorphin and morphine as characterized by supraspinal and spinal antinociceptive interactions in mice. J. Pharmacol. Exp. Ther. 1988;247:1107–1113. [PubMed] [Google Scholar]
- ROERIG S.C., O'BRIEN S., FUJIMOTO J.M., WILCOX G.L. Tolerance to morphine analgesia: Decreased multiplicative interaction between spinal and supraspinal sites. Brain Res. 1984;308:360–363. doi: 10.1016/0006-8993(84)91078-3. [DOI] [PubMed] [Google Scholar]
- SMITH A.L., DEWITT D.L., GARAVITO R.M. Cyclooxygenases: structural, cellular, and molecular biology. Ann. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- SOLOMON R.E., GEBHART G.F. Synergistic antinociceptive interactions among drugs administered to the spinal cord. Anesth. Analg. 1994;78:1164–1172. doi: 10.1213/00000539-199406000-00025. [DOI] [PubMed] [Google Scholar]
- TALLARIDA R.J. Drug synergism: its detection and applications. J. Pharmacol. Exp. Ther. 2001;298:865–872. [PubMed] [Google Scholar]
- TALLARIDA R.J., PORRECA F., COWAN A. Statistical analysis of drug-drug and site-site interactions with isobolograms. Life Sci. 1989;45:947–961. doi: 10.1016/0024-3205(89)90148-3. [DOI] [PubMed] [Google Scholar]
- VANE J.R., BAKHLE Y.S., BOTTING R.M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- WARNER T.D., GIULIANO F., VOJNOVIC I., BUKASA A., MITCHELL J.A., VANE J.R. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: A full in vitro analysis. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG L.C., CHEN L.M., WANG C.J., BUERKLE H. Postoperative analgesia by intra-articular neostigmine in patients undergoing knee arthroscopy. Anesthesiology. 1998;88:334–339. doi: 10.1097/00000542-199802000-00010. [DOI] [PubMed] [Google Scholar]
- YEUNG J.C., RUDY T.A. Multiplicative interactions between narcotic agonism expressed at spinal and supraspinal sites of action as revealed by concurrent intrathecal and intracerebroventricular injections of morphine. J. Pharmacol. Exp. Ther. 1980;215:633–642. [PubMed] [Google Scholar]
- SUH H.H., TSENG L.F. Intrathecal beta-funaltrexine antagonizes intracerebroventricular beta-endorphin but not morphine-induced analgesia in mice. J. Pharmacol. Exp. Ther. 1988;245:587–593. [PubMed] [Google Scholar]
- XU Z., LI P., TONG C., FIGUEROA J., TOBIN J.R., EISENACH J.C. Location and characteristics of nitro oxide synthase in sheep spinal cord and its interaction with alpha(2)-adrenergic and cholinergic antinociception. Anesthesiology. 1996;84:890–899. doi: 10.1097/00000542-199604000-00017. [DOI] [PubMed] [Google Scholar]


