Abstract
Stem cell factor (SCF) is a major mast cell growth factor that promotes differentiation and chemotaxis of mast cells and inhibits their apoptosis.
We evaluated the effect of interleukin (IL)-1β, a major pro-inflammatory cytokine, on the constitutive expression of SCF and studied the effects of two glucocorticoids, budesonide and dexamethasone, on the IL-1β-enhanced SCF expression.
Human lung fibroblasts in culture were serum-starved for 48 h and treated with IL-1β, budesonide and/or RU486. SCF cDNA was quantified after total RNA reverse transcription by on-line fluorescent polymerase chain reaction. SCF protein was quantified by ELISA.
IL-1β induced an increase in SCF mRNA (+91% at 2.5 h) and protein production (+32%) by human lung fibroblasts in culture (P<0.001).
Budesonide inhibited IL-1β-induced SCF mRNA expression (−68%) at 2.5 h and even more so at 10 h (−192%) (P<0.001). The expression of SCF protein also decreased by 3.5-fold at 10 h. Results were similar with dexamethasone. The glucocorticoid antagonist RU486 cancelled the effects induced by the glucocorticoids.
Increased SCF mRNA levels were associated with increased stability of this mRNA as measured after treatment with actinomycin D (1.9-fold at 2.5 h). Budesonide decreased this IL-1β-enhanced stability by about 1.5-fold (P<0.001).
We conclude that in ‘inflammatory' conditions, mimicked in vitro by IL-1β, glucocorticoid treatment inhibits expression of the mast cell growth factor SCF. The reduced number and activation of mast cells observed in the bronchi of asthmatic patients treated by glucocorticoids may be due in part to this effect.
Keywords: SCF, IL-1β, budesonide, dexamethasone, fibroblasts, mast cells, lung, asthma
Introduction
Stem cell factor (SCF), also termed Kit ligand, steel factor, or mast cell growth factor (Huang et al., 1990; Martin et al., 1990; Zsebo et al., 1990), acts as an important growth factor for human mast cells (Galli et al., 1995). In vitro it induces the proliferation and differentiation of immature mast cells from bone marrow CD34+ progenitor cells (Rottem et al., 1994). SCF also aids mast cell survival by inhibiting their apoptosis (Iemura et al., 1994). Moreover, it is a potent chemotactic factor for mast cells (Nilsson et al., 1994) and promotes their adhesion to the extracellular matrix (Dastych & Metcalfe, 1994). In addition, in vivo, SCF induces mast cell hyperplasia after subcutaneous injection in humans (Costa et al., 1996). Finally, in vitro it greatly enhances antigen-induced degranulation of human lung-derived mast cells (Bischoff & Dahinden, 1992) as well as inducing mast cell degranulation itself alone in vitro (Takaishi et al., 1994) and in vivo (Costa et al., 1996). Therefore, SCF may be involved in many diseases associated with a local increase in the number and activation of mast cells, such as occurs in asthma (Ammit et al., 1997).
In asthmatic patients, large quantities of the pro-inflammatory cytokine interleukin (IL)-1β have been reported in bronchoalveolar lavage (Borish et al., 1992). IL-1β may be released by structural cells, such as epithelial cells (Devalia et al., 1993) and fibroblasts (Pan et al., 1996), as well as by infiltrated inflammatory cells, including eosinophils (Barnes, 1990) and mast cells (Moller et al., 1998). IL-1β is known to upregulate the expression of many genes involved in the inflammatory process, including RANTES (Saji et al., 2000), eotaxin (Ghaffar et al., 1999), IL-6 and IL-8 (Agarwal et al., 1995) and the IL-1β gene itself (Hiscott et al., 1993). However, the effect of IL-1β on SCF expression by pulmonary cells is not known.
Glucocorticoids remain the major treatment for asthma. This treatment is associated with a reduced number of infiltrated mast cells in the bronchial mucosa of asthmatic patients (Jeffery et al., 1992). Glucocorticoids affect cells in many ways, but their major therapeutic benefit is likely to occur through the regulation of the expression of genes involved in inflammation (for review, see Barnes & Adcock, 1993). In particular, as recently reported by Kassel et al. (1998), the regulation of the constitutive expression of SCF by glucocorticoids shows a time-dependent biphasic effect in human lung fibroblasts in culture.
Here we report our investigation of the regulation of SCF expression in inflammatory conditions mimicked by the addition of IL-1β, and the effect of the glucocorticoids budesonide and dexamethasone on the IL-1β-induced SCF expression by human pulmonary fibroblasts in culture.
Methods
Culture of human lung fibroblasts
Human lung derived fibroblasts were obtained by the explant technique, as previously described (Kassel et al., 1998). Briefly, macroscopically normal human lung tissue was separated into fragments within 1 h of resection for bronchocarcinoma. Fibroblasts were cultured in Dulbecco's modified Eagle's medium (DMEM)/F-12 (1 : 1) (GIBCO BRL, Cergy Pontoise, France), supplemented with 10% foetal calf serum (FCS), penicillin and streptomycin, and incubated in a humidified mixture of 95% air and 5% CO2 at 37°C. They were then replated in 25 cm2 tissue culture flasks (Costar, Acton, MA, U.S.A.) and were characterized as fibroblasts morphologically and by immunocytochemistry with an antifibroblast monoclonal antibody (5B5) (DAKO S.A., Trappes, France). They subsequently were split 1 : 4 at confluence and passaged. Fibroblasts were used at passage 7.
Cell treatment
At confluence, fibroblasts were placed into a quiescent state by reducing the FCS content to 0.3% (starving medium) for 48 h. IL-1β (ROCHE Diagnostics, Meylan, France), prepared from a 2 μg ml−1 stock solution, was added at a final concentration of 400 pg ml−1. The glucocorticoid budesonide (kindly provided by Dr R. Brattsand, Astra, Lund, Sweden) was prepared from a 10 mM stock solution in absolute ethanol and used at 0.1 μM. Dexamethasone (Sigma Chemicals, St Louis, MO, U.S.A.) was used at 1 μM in similar conditions. The glucocorticoid antagonist RU486 (Sigma Chemicals) was prepared from a 10 mM stock solution in absolute ethanol and incubated at 1 μM for 1 h before IL-1β and glucocorticoid treatments. Similar experiments were performed with staggered treatment starts, to verify the effect of the duration of the culture. Supernatants were sampled and stored at −80°C until the SCF protein assay. Fibroblasts were harvested for total RNA extraction.
For analysis of the transcript stability, confluent fibroblasts were treated with solvent alone (ethanol; 1 μM), IL-1β (400 pg ml−1), budesonide (0.1 μM) or the combination of both for 2.5 or 10 h. Supernatants were replaced by the starving medium, which contained 5 μg ml−1 actinomycin D (Sigma Chemicals). Cells were harvested at every hour from 0 to 4 h and total RNA was extracted.
SCF ELISA
Immunoreactive SCF released in the supernatant of fibroblasts treated with cytokine, glucocorticoid, and/or RU486, and the control fibroblasts treated only with solvent was quantified by a sensitive ELISA procedure. It used a capture anti-human SCF monoclonal antibody (clone 13302.6; R&D Systems Europe, Abingdon, U.K.) and a biotinylated detection anti-human SCF polyclonal antibody (R&D Systems Europe), revealed by extravidin-horseradish peroxidase (Sigma) and a 3,3′,5,5′-tetramethylbenzidine liquid substrate system (Sigma Chemicals). Standard curves were generated with recombinant human SCF (R&D Systems Europe) diluted in the starving medium and were linear from 3.9 to 500 pg ml−1.
Extraction of total RNA and reverse transcription
Total RNA was extracted from control or treated fibroblasts with TriReagent® (Molecular Research Center Inc., Cincinnati, OH, U.S.A.). Isolated RNA was diluted in RNase-free water and quantified by absorbance measurement at 260 nm. Four μg of total RNA were incubated with 0.5 μg of random primers for 5 min at 70°C and allowed to cool down to room temperature. RNA was subsequently reverse transcribed in 1× reverse transcription (RT) buffer (75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, and 50 mM Tris-HCl, pH 8.3) that contained 1 unit μl−1 RNasin ribonuclease inhibitor, 1 mM of each dNTP, and 10 units μl−1 RNase H(−)-Moloney leukaemia virus reverse transcriptase (all reagents from Promega, Madison, WI, U.S.A.). The reaction was conducted for 1 h at 37°C, and the reverse transcriptase was then heat-inactivated at 99°C for 5 min.
Quantification of SCF cDNA
Quantification of SCF cDNA was performed by polymerase chain reaction (PCR), and the results were normalized to total cDNA.
PCR of SCF cDNA
SCF cDNA was amplified from total cDNA obtained after reverse transcription by online fluorescent PCR (LightCycler™-SYBR Green I, ROCHE Diagnostics), with primers that lead to a single 149 bp PCR product. PCR reactions were performed in 1× PCR reaction buffer (2 μl of the reaction mix containing FastStart Taq DNA polymerase, dNTP mix, SYBR Green I, 3 mM MgCl2) (all from ROCHE Diagnostics), and 10 pmol of each primer: sense primer: 5′-TGGATAAGCGAGATGGTAGT-3′, antisense primer: 5′-TTTTCTTTCACGCACTCCAC-3′, in a 20 μl final volume for 35 cycles. Each cycle consisted of 15 s denaturation at 95°C, 10 s annealing at 53°C, and 10 s extension at 72°C. Amplified SCF cDNA was analysed on-line by fluorescence, and quantified during the exponential state.
The standard SCF cDNA was obtained from pulmonary fibroblast total cDNA. After amplification by PCR with the primers described above, the 149 bp-PCR products were electrophoresed on a 2% agarose gel stained with ethidium bromide, purified on QIAEX II (QIAEX II gel extraction kit, QIAGEN, Courtaboeuf, France), and quantified by fluorescence (PicoGreen®, Molecular Probes Inc.) according to a standard curve obtained with double-stranded phage λ DNA (0 – 1 μg ml−1). This purified SCF cDNA was used as a standard curve from 1 to 300 fg ml−1.
Quantification of total cDNA
One μl of total cDNA was diluted (1 : 15) and added to 250 μl Tris-EDTA (TE) 1× buffer (10 mM Tris-HCl, pH 7.8, 1 mM EDTA, pH 8). This reaction mix was incubated with 250 μl OliGreen® (1 : 200) (Molecular Probes Inc., Eugene, Oregon, U.S.A.) for 10 min in the dark. Simultaneously, a standard single-stranded M13 phage DNA (Sigma) curve (0 – 500 ng ml−1) was obtained in the same conditions. The total cDNA was quantified in a fluorometer (Hitachi F-2000, Tokyo, Japan) at an excitation wavelength of 480 nm and an emission wavelength of 520 nm. Total cDNA remained unchanged over time and for all treatments.
Expression of results and statistical analysis
SCF cDNA was calculated as fg SCF cDNA/ng total cDNA, and SCF protein was calculated in pg ml−1. The values after treatment were expressed as a percentage of the control values. Results are the means±s.e.mean of n=4 experiments performed in duplicate. Statistical analysis of the results used Student's t-test, or Student-Newman-Keuls when more than two variables were compared. Values of P<0.05 were considered significant.
Results
Effect of IL-1β on SCF production
SCF mRNA
IL-1β (400 pg ml−1) significantly increased SCF mRNA expression by human lung fibroblasts in culture (Figure 1a,b). This increase reached +91% at 2.5 h (128.2±51.4 fg ng−1 total cDNA for control and 245.9±99.4 for IL-1β-treated fibroblasts; P<0.001, n=4) (Figure 1a). This effect decreased over time, but was still significant at 10 h (+39%; 75.3±23.7 fg ng−1 total cDNA for control and 102.6±36.6 for IL-1β-treated fibroblasts; P<0.01, n=4) (Figure 1b).
Figure 1.
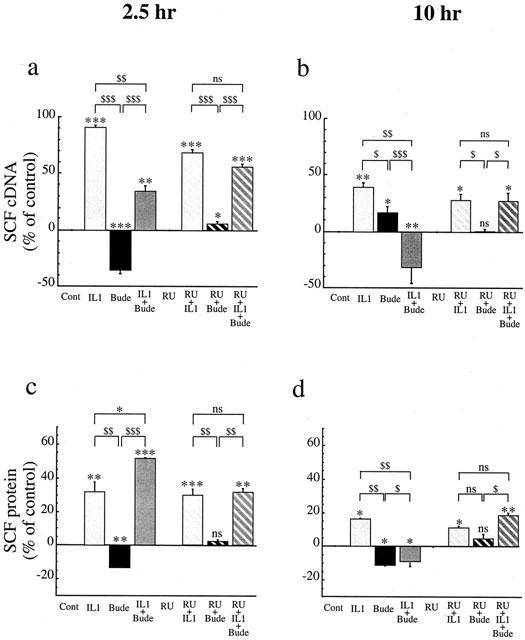
Effect of IL-1β (IL1, 400 pg ml−1: pale grey) or budesonide (bude, 0.1 μM: black) or IL-1β+budesonide (IL1+bude: dark grey) and/or RU486 (1 μM: hatched columns) on SCF mRNA (a,b) and protein (c,d) expression at 2.5 h (a,c) and 10 h (b,d) by human lung fibroblasts in culture. SCF cDNA was quantified after total RNA reverse transcription by online fluorescent PCR. Results were normalized to total cDNA and expressed as fg SCFcDNA/ng total cDNA; the values after treatment were expressed as a percentage of the control values. SCF protein levels were assessed in the supernatant by ELISA and the values after treatment expressed as a percentage of the control values, in pg ml−1. Results are mean values (blocks)≈plus;s.e.mean (bars) of four experiments performed in duplicate on fibroblasts from three different donors (*P<0.05; **P<0.01; ***P<0.001).
SCF protein
SCF protein production also increased after treatment with IL-1β (Figure 1c,d). At 2.5 h, this increase reached +32% (36.8±1.6 pg ml−1 for control and 48.7±3.3 for IL-1β-treated fibroblasts; P<0.01, n=4) (Figure 1c). At 10 h, the increase was less but still significant (+16%; 74.3±12.1 pg ml−1 for control and 85.7±14.6 for IL-1β-treated fibroblasts; P<0.05, n=4) (Figure 1d). Similar results were obtained when IL-1β treatment was staggered to stop the cell culture at the same time. This finding confirmed that the IL-1β effect we observed was not due to a modification in the cell phenotype over time.
Effect of budesonide on IL-1β-induced SCF production
SCF mRNA
Budesonide (0.1 μM) inhibited the IL-1β-induced increase in SCF mRNA expression (Figure 1a,b). This inhibition reached 68% at 2.5 h (165.7±64.1 fg ng−1 total cDNA; P<0.01, n=4) (Figure 1a) and continued so that this expression was below the baseline at 10 h (192% inhibition; 42.4±4.4 fg ng−1 total cDNA; P<0.01, n=4) (Figure 1b). Conversely, as previously shown (Kassel et al., 1998), budesonide alone caused SCF mRNA expression to decrease below baseline at 2.5 h (−36%; 85.7±35.7 fg ng−1 total cDNA; P<0.001, n=4) (Figure 1a), whereas it provoked a slight increase at 10 h (+17% compared with baseline expression; 91.5±30.1 fg ng−1 total cDNA; P<0.05, n=4) (Figure 1b).
SCF protein
The modulation by budesonide over time was different for the IL-1β-induced increase of SCF protein than for that of SCF mRNA (Figure 1c,d). A surprising increase over IL-1β-induced expression alone was observed at 2.5 h (61%; 56.0±1.0 pg ml−1 SCF; P<0.001, n=4) (Figure 1c). As was expected from the mRNA modulation, however, total inhibition of the effect of IL-1β was observed at 10 h (−9% as compared with baseline expression; 64.6±9.3 pg ml−1 SCF; P<0.05, n=4) (Figure 1d). Conversely, budesonide alone caused SCF protein expression to decrease at 2.5 h (−13%; 32.1±1.7 pg ml−1 SCF; P<0.01, n=4) (Figure 1c) and at 10 h (−11%; 66.0±13.4 pg ml−1 SCF; P<0.05, n=4) (Figure 1d).
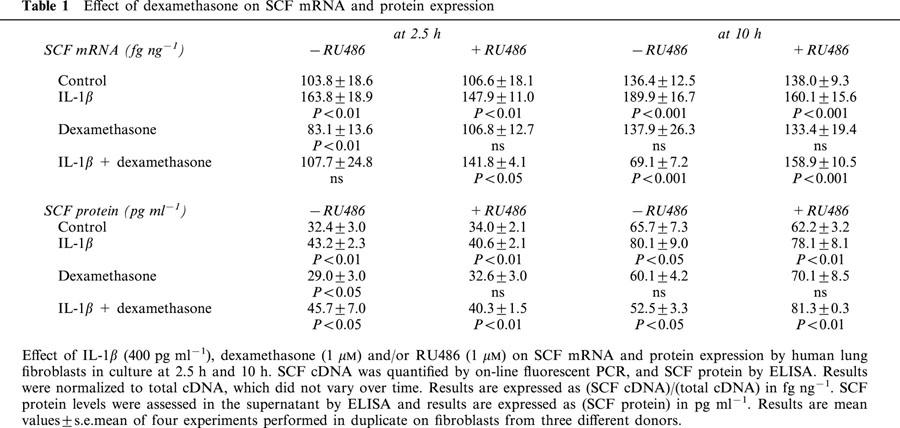
Results were similar with dexamethasone (Table 1): the effect of the glucocorticoids was totally opposite in constitutive and inflammatory conditions.
Table 1.
Effect of dexamethasone on SCF mRNA and protein expression
Effect of RU486 on SCF expression
To verify that these effects depended on the glucocorticoid receptor, we evaluated the effect of the glucocorticoid-receptor antagonist RU486 on the budesonide-induced effects.
SCF mRNA (Figure 1a,b)
RU486 (1 μM) by itself had no effect on SCF mRNA constitutive expression (130.3±53.0 fg ng−1 total cDNA; n=4 at 2.5 h; 79.0±23.1 fg ng−1 total cDNA, n=4 at 10 h). In addition, RU486 antagonized both effects of budesonide: the decrease in SCF mRNA expression at 2.5 h (138.2±56.7 fg ng−1 total cDNA for RU486+budesonide-treated fibroblasts; P<0.05, n=4) (Figure 1a), and the increase induced by budesonide alone at 10 h (75.1±21.1 fg ng−1 total cDNA; n=4) (Figure 1b), as well as the inhibiting effect of budesonide on the IL-1β-stimulated SCF mRNA expression at 2.5 h (197.7±3.8 fg ng−1 total cDNA; NS, n=4) (Figure 1a) and at 10 h (91.3±22.1 fg ng−1 total cDNA; NS, n=4) (Figure 1b). However, RU486 alone slightly decreased the IL-1β-stimulated SCF mRNA expression at 2.5 h by 28% (212.6±83.6 fg ng−1 total cDNA for RU486+IL-1β-treated fibroblasts; P<0.001, n=4) (Figure 1a), but not at 10 h (92.6±23.0 fg ng−1 total cDNA; NS, n=4) (Figure 1b).
SCF protein expression (Figure 1c,d)
RU486 had no effect on SCF constitutive protein expression (35.0±2.2 pg ml−1 SCF; n=4 at 2.5 h; 74.6±12.1 pg ml−1 SCF; n=4 at 10 h). Similarly, it totally cancelled out the effect of budesonide alone at 2.5 h (37.4±1.5 pg ml−1 SCF; n=4) (Figure 1c) and at 10 h (75.7±11.0 pg ml−1 SCF; n=4) (Figure 1d). It also cancelled out the effect of budesonide on the IL-1β-enhanced SCF protein expression at 2.5 h (48.0±0.3 pg ml−1 SCF; NS, n=4) (Figure 1c) and the inhibition induced at 10 h (87.8±0.7 pg ml−1 SCF; NS, n=4) (Figure 1d). Finally, RU486 had no effect on the IL-1β-enhanced SCF protein expression at 2.5 h (48.0±3.0 pg ml−1 SCF; P<0.001, n=4) (Figure 1c) or at 10 h (80.3±14.1 pg ml−1 SCF; P<0.05, n=4) (Figure 1d). Similar results were observed with dexamethasone (Table 1).
SCF mRNA stability
The mechanism of the glucocorticoid-induced effects in IL-1β-treated fibroblasts was analysed by evaluating the stability of the SCF mRNA by measuring its half-life (t1/2) after adding actinomycin D.
At 2.5 h (Figure 2a), the estimated half-life of SCF mRNA was 2.0 h in the control fibroblasts treated with solvent alone. IL-1β treatment significantly increased SCF mRNA stability to t1/2=3.8 h (compared with control, P<0.001). The addition of budesonide slightly decreased this enhanced stability, to t1/2=3.1 h, still significantly higher than the control (P<0.001). Budesonide alone, on the other hand, significantly decreased the stability of the SCF mRNA to t1/2=1.2 h (compared with control, P<0.001).
Figure 2.
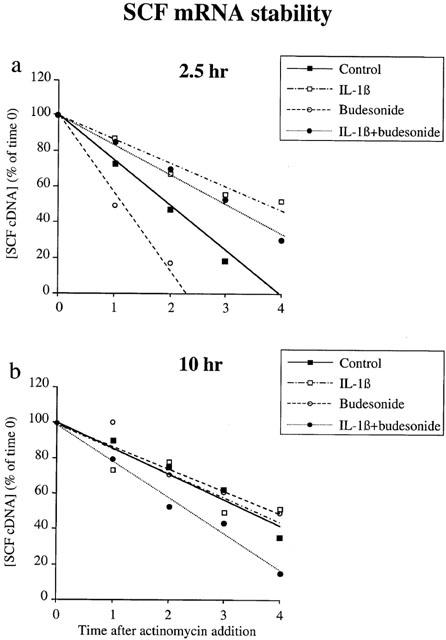
SCF mRNA stability. Fibroblasts were treated with IL-1β (400 pg ml−1), budesonide (0.1 μM), IL-1β plus budesonide or ethanol (1 μM; control) for 2.5 h (a) or 10 h (b) Fibroblasts were then washed and treated with 5 μg ml−1 actinomycin D for the indicated time. SCF cDNA was quantified by on-line fluorescent PCR. Results are expressed as a percentage of the quantity of SCF cDNA measured for each treatment at time 0 of actinomycin D addition, which is represented as the 100% of each treatment. Data are means of three experiments performed in duplicate on fibroblasts from three different donors.
At 10 h (Figure 2b), the estimated half-life was 3.4 h in the control cells, thereby showing that SCF mRNA stability increases with time. Treatment with either IL-1β or budesonide did not significantly modify this stability (t1/2=3.5 h and 3.8 h, respectively, NS). The combination of IL-1β and budesonide markedly decreased it, however (t1/2=2.4 h, P<0.001).
Discussion
We present here evidence that (1) the pro-inflammatory cytokine IL-1β substantially increases the expression of SCF mRNA and protein by human lung fibroblasts in culture, and (2) that this increased expression is markedly regulated by the glucocorticoids budesonide and dexamethasone.
This first finding, that IL-1β, present in large amounts in the human airways of asthmatic patients (Borish et al., 1992), heightens SCF mRNA expression, is in agreement with the reported increase in the IL-1β-mediated expression of genes implicated in the inflammatory process, such as cyclooxygenase (COX)-2 mRNA expression by human pulmonary epithelial cells (Lin et al., 2000) or eotaxin mRNA expression in A549 airway epithelial cells (Jedrzkiewicz et al., 2000). Both reports propose a mechanism for this increased expression associated with the activation of the transcription factor nuclear factor (NF)-κB by IL-1β (Jedrzkiewicz et al., 2000; Lin et al., 2000); this mechanism would involve gene promoters with at least one NF-κB responsive element. One such element has been identified in the SCF gene promoter (Taylor et al., 1996), although its specific role in SCF gene expression is not yet known.
We also observed that when SCF expression increased, its mRNA became more stable (had a longer half-life). This is consistent with the increased stability of COX-2 mRNA observed in HeLa cells stimulated by IL-1β (Ridley et al., 1998) and of inducible nitric oxide synthase mRNA in cardiac myocytes (Oddis et al., 1995). Among the mechanisms involved in increased mRNA stability is the length of the poly(A)+ tail (Ross, 1995), and it has been proposed that Il-1β increases the process of polyadenylation (Stoeckle, 1992), which might play a part in this effect. Accordingly, SCF mRNA and protein behave like many other proinflammatory factor genes: their expression increases in the presence of IL-1β. These genes include COX-2 (Lin et al., 2000), RANTES (Saji et al., 2000), IL-6 and IL-8 (Agarwal et al., 1995), eotaxin (Ghaffar et al., 1999) and NGF (Olgart & Frossard, 2001).
Budesonide is the major anti-inflammatory compound used in the treatment of diseases such as asthma that are associated with an increased number of tissue mast cells. Here we studied its effect on SCF expression in inflammatory conditions created by IL-1β, rather than the previously-reported effects on its constitutive expression (Kassel et al., 1998). We showed here that budesonide inhibited the IL-1β-induced increase in SCF mRNA and protein expression in the short term (2.5 h) and even more so longer after treatment (10 h). This effect is consistent with many others reported in the literature, where glucocorticoids inhibit the expression of genes involved in the inflammatory process, including IL-4 and 5 (Bentley et al., 1996) or IL-1β (Sousa et al., 1997) (for review, see Barnes et al., 1998). We have demonstrated here that this inhibitory effect involves the destabilization of SCF mRNA in the short term and its aggravation after a longer period.
The precise mechanisms of glucocorticoids action in SCF mRNA stability is unclear. It has been suggested that AU-rich elements in the 3′-untranslated region play some role in the glucocorticoid-induced decrease in mRNA stability (Peppel et al., 1991; Ristimaki et al., 1996), but none have been found in the human SCF mRNA sequence (Martin et al., 1990). Hence, the observed SCF mRNA destabilization probably involves another mechanism, as yet unidentified. Possibilities include the down-regulation of Poly(A)+ binding proteins (PABP) or dysregulation of the 5′-Catabolite gene Activator Protein (CAP) that protects mRNA from degradation (Ross, 1995). Other currently unknown mechanisms may also play a role in the inhibition by budesonide of IL-1β-stimulated SCF expression. Since the SCF gene promoter has NF-κB and AP-1 responsive elements (Taylor et al., 1996), a direct interaction between activated GR and these transcription factors may be involved. This interaction has been proposed as a general mechanism (Adcock, 2001) and been shown to exist in specific cases between GR and NF-κB, involving repression of histone acetyl transferase activity activated by p65 (Ito et al., 2001) and between GR and AP-1 (Diamond et al., 1990; Kerppola et al., 1993). Nevertheless, we also noticed that the short-term increase in SCF protein expression induced by the combination of IL-1β and budesonide was substantially greater than that induced by IL-1β alone, conversely to the decreased SCF mRNA expression. This surprising effect may be related to an earlier increase in SCF mRNA expression, to a rapid increase of the translational process after short term treatment by glucocorticoids, or to both. However, SCF gene expression is not described in the literature as an early gene and thus not regulated in the same way as the early gene family. The mechanism of this interesting event requires further investigation.
Additionally, we have demonstrated here that all the results obtained with budesonide were glucocorticoid-receptor dependent, since RU486, a glucocorticoid-receptor antagonist (Cairns et al., 1993), cancelled out all the effects induced by budesonide on SCF mRNA and protein expression, whether or not IL-1β was present. RU486 alone exerted a slight inhibitory effect on IL-1β-induced SCF mRNA expression. Since RU486 had no effect on control cells or on SCF mRNA stability (not shown), we may hypothesize that upon IL-1β stimulation, RU486 interacts with the transductional pathways activated by IL-1β. In accordance with this hypothesis, it has been reported that RU486, after binding to the glucocorticoid receptor (GR), interacts with the transcription factor NF-κB and inhibits NF-κB activity without affecting the latter's binding to DNA (Hofmann et al., 1998; Liden et al., 1997), through interaction with a histone acetyltransferase/NF-κB complex (Ito et al., 2001). A similar mechanism may thus be proposed to explain the decrease in IL-1β-enhanced SCF mRNA expression shown in this study.
In conclusion, our study clearly shows that IL-1β, a pro-inflammatory cytokine present in large quantities in the airways of asthmatic patients, and here mimicking the inflammatory conditions in human lung fibroblasts in vitro, increases SCF expression, at least partly by increasing the stability of its mRNA. Moreover, anti-inflammatory glucocorticoids decreased this IL-1β-enhanced SCF expression and the stability of its mRNA. This may be of major importance in the mechanism of glucocorticoid treatment of diseases associated with an increased number of mast cells in tissues, such as asthma. Our results thus suggest a new mechanism of glucocorticoid action, through the regulation of the expression of the mast cell growth factor SCF.
Abbreviations
- COX-2
cyclooxygenase type-2
- DMEM
Dulbecco's modified eagle's medium
- ELISA
enzyme-linked immuno sorbent assay
- FCS
foetal calf serum
- GR
glucocorticoid-receptor
- IL-1β
interleukin (IL)-1β
- NF-βB
nuclear factor-kappa B
- PCR
polymerase chain reaction
- RT
reverse transcription
- SCF
stem cell factor
References
- ADCOCK I.M. Glucocorticoid-regulated transcription factors. Pulm. Pharmacol. Ther. 2001;14:211–219. doi: 10.1006/pupt.2001.0283. [DOI] [PubMed] [Google Scholar]
- AGARWAL S., BARAN C., PIESCO N.P., QUINTERO J.C., LANGKAMP H.H., JOHNS L.P., CHANDRA C.S. Synthesis of proinflammatory cytokines by human gingival fibroblasts in response to lipopolysaccharides and interleukin-1 beta. J. Periodontal. Res. 1995;30:382–389. doi: 10.1111/j.1600-0765.1995.tb01291.x. [DOI] [PubMed] [Google Scholar]
- AMMIT A.J., BEKIR S.S., JOHNSON P.R., HUGHES J.M., ARMOUR C.L., BLACK J.L. Mast cell numbers are increased in the smooth muscle of human sensitized isolated bronchi. Am. J. Respir. Crit. Care Med. 1997;155:1123–1129. doi: 10.1164/ajrccm.155.3.9116997. [DOI] [PubMed] [Google Scholar]
- BARNES P.J. Effect of corticosteroids on airway hyperresponsiveness. Am. Rev. Respir. Dis. 1990;141:S70–S76. [PubMed] [Google Scholar]
- BARNES P.J., ADCOCK I. Anti-inflammatory actions of steroids: molecular mechanisms [see comments] Trends Pharmacol. Sci. 1993;14:436–441. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., PEDERSEN S., BUSSE W.W. Efficacy and safety of inhaled corticosteroids. New developments. Am. J. Respir. Crit. Care Med. 1998;157:S1–S53. doi: 10.1164/ajrccm.157.3.157315. [DOI] [PubMed] [Google Scholar]
- BENTLEY A.M., HAMID Q., ROBINSON D.S., SCHOTMAN E., MENG Q., ASSOUFI B., KAY A.B., DURHAM S.R. Prednisolone treatment in asthma. Reduction in the numbers of eosinophils, T cells, tryptase-only positive mast cells, and modulation of IL-4, IL-5, and interferon-gamma cytokine gene expression within the bronchial mucosa. Am. J. Respir. Crit. Care Med. 1996;153:551–556. doi: 10.1164/ajrccm.153.2.8564096. [DOI] [PubMed] [Google Scholar]
- BISCHOFF S.C., DAHINDEN C.A. c-kit ligand: a unique potentiator of mediator release by human lung mast cells. J. Exp. Med. 1992;175:237–244. doi: 10.1084/jem.175.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORISH L., MASCALI J.J., DISHUCK J., BEAM W.R., MARTIN R.J., ROSENWASSER L.J. Detection of alveolar macrophage-derived IL-1 beta in asthma. Inhibition with corticosteroids. J. Immunol. 1992;149:3078–3082. [PubMed] [Google Scholar]
- CAIRNS C., CAIRNS W., OKRET S. Inhibition of gene expression by steroid hormone receptors via a negative glucocorticoid response element: evidence for the involvement of DNA-binding and agonistic effects of the antiglucocorticoid/antiprogestin RU486. DNA Cell. Biol. 1993;12:695–702. doi: 10.1089/dna.1993.12.695. [DOI] [PubMed] [Google Scholar]
- COSTA J.J., DEMETRI G.D., HARRIST T.J., DVORAK A.M., HAYES D.F., MERICA E.A., MENCHACA D.M., GRINGERI A.J., SCHWARTZ L.B., GALLI S.J. Recombinant human stem cell factor (kit ligand) promotes human mast cell and melanocyte hyperplasia and functional activation in vivo. J. Exp. Med. 1996;183:2681–2686. doi: 10.1084/jem.183.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DASTYCH J., METCALFE D.D. Stem cell factor induces mast cell adhesion to fibronectin. J. Immunol. 1994;152:213–219. [PubMed] [Google Scholar]
- DEVALIA J.L., CAMPBELL A.M., SAPSFORD R.J., RUSZNAK C., QUINT D., GODARD P., BOUSQUET J., DAVIES R.J. Effect of nitrogen dioxide on synthesis of inflammatory cytokines expressed by human bronchial epithelial cells in vitro. Am. J. Respir. Cell. Mol. Biol. 1993;9:271–278. doi: 10.1165/ajrcmb/9.3.271. [DOI] [PubMed] [Google Scholar]
- DIAMOND M.I., MINER J.N., YOSHINAGA S.K., YAMAMOTO K.R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990;249:1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- GALLI S.J., TSAI M., WERSHIL B.K., TAM S.Y., COSTA J.J. Regulation of mouse and human mast cell development, survival and function by stem cell factor, the ligand for the c-kit receptor. Int. Arch. Allergy Immunol. 1995;107:51–53. doi: 10.1159/000236928. [DOI] [PubMed] [Google Scholar]
- GHAFFAR O., HAMID Q., RENZI P.M., ALLAKHVERDI Z., MOLET S., HOGG J.C., SHORE S.A., LUSTER A.D., LAMKHIOUED B. Constitutive and cytokine-stimulated expression of eotaxin by human airway smooth muscle cells. Am. J. Respir. Crit. Care Med. 1999;159:1933–1942. doi: 10.1164/ajrccm.159.6.9805039. [DOI] [PubMed] [Google Scholar]
- HISCOTT J., MAROIS J., GAROUFALIS J., D'ADDARIO M., ROULSTON A., KWAN I., PEPIN N., LACOSTE J., NGUYEN H., BENSI G., FENTON M. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol. Cell. Biol. 1993;13:6231–6240. doi: 10.1128/mcb.13.10.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFMANN T.G., HEHNER S.P., BACHER S., DROGE W., SCHMITZ M.L. Various glucocorticoids differ in their ability to induce gene expression, apoptosis and to repress NF-kappaB-dependent transcription. FEBS Lett. 1998;441:441–446. doi: 10.1016/s0014-5793(98)01609-3. [DOI] [PubMed] [Google Scholar]
- HUANG E., NOCKA K., BEIER D.R., CHU T.Y., BUCK J., LAHM H.W., WELLNER D., LEDER P., BESMER P. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- IEMURA A., TSAI M., ANDO A., WERSHIL B.K., GALLI S.J. The c-kit ligand, stem cell factor, promotes mast cell survival by suppressing apoptosis. Am. J. Pathol. 1994;144:321–328. [PMC free article] [PubMed] [Google Scholar]
- ITO K., JAZRAWI E., COSIO B., BARNES P.J., ADCOCK I.M. p65-activated histone acetyltransferase activity is repressed by glucocorticoids: mifepristone fails to recruit HDAC2 to the p65-HAT complex. J. Biol. Chem. 2001;276:30208–30215. doi: 10.1074/jbc.M103604200. [DOI] [PubMed] [Google Scholar]
- JEDRZKIEWICZ S., NAKAMURA H., SILVERMAN E.S., LUSTER A.D., MANSHARAMANI N., IN K.H., TAMURA G., LILLY C.M. IL-1beta induces eotaxin gene transcription in A549 airway epithelial cells through NF-kappaB. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L1058–L1065. doi: 10.1152/ajplung.2000.279.6.L1058. [DOI] [PubMed] [Google Scholar]
- JEFFERY P.K., GODFREY R.W., ADELROTH E., NELSON F., ROGERS A., JOHANSSON S.A. Effects of treatment on airway inflammation and thickening of basement membrane reticular collagen in asthma. A quantitative light and electron microscopic study. Am. Rev. Respir. Dis. 1992;145:890–899. doi: 10.1164/ajrccm/145.4_Pt_1.890. [DOI] [PubMed] [Google Scholar]
- KASSEL O., SCHMIDLIN F., DUVERNELLE C., DE BLAY F., FROSSARD N. Up- and down-regulation by glucocorticoids of the constitutive expression of the mast cell growth factor stem cell factor by human lung fibroblasts in culture. Mol. Pharmacol. 1998;54:1073–1079. doi: 10.1124/mol.54.6.1073. [DOI] [PubMed] [Google Scholar]
- KERPPOLA T.K., LUK D., CURRAN T. Fos is a preferential target of glucocorticoid receptor inhibition of AP-1 activity in vitro. Mol. Cell. Biol. 1993;13:3782–3791. doi: 10.1128/mcb.13.6.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIDEN J., DELAUNAY F., RAFTER I., GUSTAFSSON J., OKRET S. A new function for the C-terminal zinc finger of the glucocorticoid receptor. Repression of RelA transactivation. J. Biol. Chem. 1997;272:21467–21472. doi: 10.1074/jbc.272.34.21467. [DOI] [PubMed] [Google Scholar]
- LIN C.H., SHEU S.Y., LEE H.M., HO Y.S., LEE W.S., KO W.C., SHEU J.R. Involvement of protein kinase C-gamma in IL-1beta-induced cyclooxygenase-2 expression in human pulmonary epithelial cells. Mol. Pharmacol. 2000;57:36–43. [PubMed] [Google Scholar]
- MARTIN F.H., SUGGS S.V., LANGLEY K.E., LU H.S., TING J., OKINO K.H., MORRIS C.F., MCNIECE I.K., JACOBSEN F.W., MENDIAZ E.A., BIRKETT N.C., SMITH K.A., JOHNSON M.J., PARKER V.P., FLORES J.C., PATEL A.C., FISHER E.F., ERJAVEC H.O., HERRERA C.J., WYPYCH J., SACHDEV R.J., POPE J.A., LESLIE A., WEN D., LIN C.H., CUPPLES R.L., ZSEBO K.M. Primary structure and functional expression of rat and human stem cell factor DNAs. Cell. 1990;63:203–211. doi: 10.1016/0092-8674(90)90301-t. [DOI] [PubMed] [Google Scholar]
- MOLLER A., HENZ B.M., GRUTZKAU A., LIPPERT U., ARAGANE Y., SCHWARZ T., KRUGER-KRASAGAKES S. Comparative cytokine gene expression: regulation and release by human mast cells. Immunology. 1998;93:289–295. doi: 10.1046/j.1365-2567.1998.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILSSON G., BUTTERFIELD J.H., NILSSON K., SIEGBAHN A. Stem cell factor is a chemotactic factor for human mast cells. J. Immunol. 1994;153:3717–3723. [PubMed] [Google Scholar]
- ODDIS C.V., SIMMONS R.L., HATTLER B.G., FINKEL M.S. cAMP enhances inducible nitric oxide synthase mRNA stability in cardiac myocytes. Am. J. Physiol. 1995;269:H2044–H2050. doi: 10.1152/ajpheart.1995.269.6.H2044. [DOI] [PubMed] [Google Scholar]
- OLGART C., FROSSARD N. Human lung fibroblasts secrete nerve growth factor: effect of inflammatory cytokines and glucocorticoids. Eur. Respir. J. 2001;18:115–121. doi: 10.1183/09031936.01.00069901. [DOI] [PubMed] [Google Scholar]
- PAN Z.K., ZURAW B.L., LUNG C.C., PROSSNITZ E.R., BROWNING D.D., YE R.D. Bradykinin stimulates NF-kappaB activation and interleukin 1beta gene expression in cultured human fibroblasts. J. Clin. Invest. 1996;98:2042–2049. doi: 10.1172/JCI119009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEPPEL K., VINCI J.M., BAGLIONI C. The AU-rich sequences in the 3′ untranslated region mediate the increased turnover of interferon mRNA induced by glucocorticoids. J. Exp. Med. 1991;173:349–355. doi: 10.1084/jem.173.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIDLEY S.H., DEAN J.L., SARSFIELD S.J., BROOK M., CLARK A.R., SAKLATVALA J. A p38 MAP kinase inhibitor regulates stability of interleukin-1-induced cyclooxygenase-2 mRNA. FEBS Lett. 1998;439:75–80. doi: 10.1016/s0014-5793(98)01342-8. [DOI] [PubMed] [Google Scholar]
- RISTIMAKI A., NARKO K., HLA T. Down-regulation of cytokine-induced cyclooxygenase-2 transcript isoforms by dexamethasone: evidence for post-transcriptional regulation. Biochem. J. 1996;318:325–331. doi: 10.1042/bj3180325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS J. mRNA stability in mammalian cells. Microbiol. Rev. 1995;59:423–450. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTTEM M., OKADA T., GOFF J.P., METCALFE D.D. Mast cells cultured from the peripheral blood of normal donors and patients with mastocytosis originate from a CD34+/Fc epsilon RI- cell population. Blood. 1994;84:2489–2496. [PubMed] [Google Scholar]
- SAJI F., NONAKA M., PAWANKAR R. Expression of RANTES by IL-1 beta and TNF-alpha stimulated nasal polyp fibroblasts. Auris Nasus Larynx. 2000;27:247–252. doi: 10.1016/s0385-8146(00)00052-3. [DOI] [PubMed] [Google Scholar]
- SOUSA A.R., TRIGG C.J., LANE S.J., HAWKSWORTH R., NAKHOSTEEN J.A., POSTON R.N., LEE T.H. Effect of inhaled glucocorticoids on IL-1 beta and IL-1 receptor antagonist (IL-1 ra) expression in asthmatic bronchial epithelium. Thorax. 1997;52:407–410. doi: 10.1136/thx.52.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOECKLE M.Y. Removal of a 3′ non-coding sequence is an initial step in degradation of gro alpha mRNA and is regulated by interleukin-1. Nucleic Acids Res. 1992;20:1123–1127. doi: 10.1093/nar/20.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAISHI T., MORITA Y., HIRAI K., YAMAGUCHI M., OHTA K., NODA E., MORITA T., ITO K., MIYAMOTO T. Effect of cytokines on mediator release from human dispersed lung mast cells. Allergy. 1994;49:837–842. doi: 10.1111/j.1398-9995.1994.tb00784.x. [DOI] [PubMed] [Google Scholar]
- TAYLOR W.E., NAJMABADI H., STRATHEARN M., JOU N.T., LIEBLING M., RAJAVASHISTH T., CHANANI N., PHUNG L., BHASIN S. Human stem cell factor promoter deoxyribonucleic acid sequence and regulation by cyclic 3′,5′-adenosine monophosphate in a Sertoli cell line. Endocrinology. 1996;137:5407–5414. doi: 10.1210/endo.137.12.8940364. [DOI] [PubMed] [Google Scholar]
- ZSEBO K.M., WILLIAMS D.A., GEISSLER E.N., BROUDY V.C., MARTIN F.H., ATKINS H.L., HSU R.Y., BIRKETT N.C., OKINO K.H., MURDOCK D.C., JACOBSEN F.W., LANGLEY K.E., SMITH K.A., TAKEISHI T., CATTANACH B.M., GALLI S.J., SUGGS S.V. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]


