Abstract
Sigma (σ) receptors have generated a great deal of interest on the basis of their possible roles in various pathologies, including cytoprotection. Although the exact function of sigma-1 (σ1) receptors is not yet known, their role in the regulation of intracellular Ca2+ levels and sterol biosynthesis, functions that could be assigned to mitochondria, are the only mechanisms described.
Using preparations of purified rat liver and brain mitochondria we demonstrate herein the presence of σ-like binding sites. [3H](+)-pentazocine, a σ1 radioligand was used to label these sites.
In the liver, [3H](+)-pentazocine labelled one class of binding sites with high affinity (Kd=3 nM), similar to that observed in liver microsomes and synaptic membranes. These sites were located on the outer mitochondrial membranes and displayed high affinity for other σ1 ligands namely, haloperidol, ifenprodil, carbetapentane or 1,3-di(2-tolyl)guanidine (DTG).
The presence of σ1 receptors on liver mitochondria was confirmed using double fluorescence immunostaining.
[3H](+)-pentazocine binding sites were also found on brain mitochondria but they appeared pharmacologically distinct to the liver ones as [3H](+)-pentazocine and typical σ1 ligands displayed lower affinities for these sites. Nevertheless, [3H](+)-pentazocine binding on both liver and brain mitochondria was modulated by progesterone, a putative endogenous ligand for σ receptors.
Our data demonstrates the presence of [3H](+)-pentazocine binding sites with pharmacological characteristics identical to σ1 receptors on rat liver mitochondrial membranes. The pharmacological significance of these sites and their role on mitochondrial function remain unknown.
Keywords: (+)-Pentazocine, specific binding sites, sigma receptors, rat liver mitochondria, brain mitochondria
Introduction
First described by Martin et al. (1976), sigma (σ) binding sites were postulated to be new opioid receptor subtypes. Now, it is established that these sites are distinct from any other known receptor. Based on biochemical studies, it is generally accepted that at least two different subtypes of σ receptors exist, termed sigma-1 (σ1) and sigma-2 (σ2). The first one shows high affinity for benzomorphans, a stereoselectivity for the (+)-isomers, and is usually labelled with the radioligand [3H](+)-pentazocine (Dehaven-Hudkins et al., 1992; Cagnotto et al., 1994) whereas the second exhibits a lower affinity for (+)-pentazocine and a reverse stereoselectivity. Usually σ2 binding sites are probed with 1,3-di(2-tolyl)guanidine (DTG) which binds to both subtypes 1 and 2 in roughly equal amounts.
Whatever their subtypes, σ receptors were shown to bind a variety of compounds belonging to different chemical and pharmacological classes including cytochrome P450 inhibitors, Ca2+ channel antagonists, antipsychotic and anti-convulsant agents, cocaine and steroids. Among these hormones, progesterone is considered as a possible, although controversial, endogenous ligand (Su et al., 1988; Schwarz et al., 1989; Bergeron et al., 1999). It appears to be the more potent inhibitor of [3H](+)-pentazocine binding in in vitro studies and a close relationship between steroids and the σ1 receptor subtype was established in different physiological and behavioural tests (Maurice et al., 1999).
σ Receptors are expressed in various areas of the brain but also in peripheral organs including liver, testis, kidney, spleen and intestine. Subcellular distribution studies indicated that σ1 receptors are highly concentrated and mainly located on microsomal and plasma membranes (McCann & Su, 1990), but some studies also suggest that they could be associated with other organelles of the cell (McCann et al., 1994; Dehaven-Hudkins et al., 1994).
Extensive studies have been performed to elucidate the role of these receptors. The σ1 subtype has been the most studied and characterized. Although this receptor was recently cloned (Hanner et al., 1996), no clear biological functions have yet been attributed to it. It has been suggested that it modulates several neurotransmitter systems (Maurice et al., 1999) and that it is involved both in CNS pathologies such as schizophrenia, dementia, depression and peripheral nervous system diseases (Pascaud et al., 1990; Matsuno & Mita, 1998; Bowen, 2000). σ, especially σ1, receptor ligands were also shown to possess anti-ischaemic properties (Matsuno & Mita, 1998; Harukuni et al., 2000) and be effective neuroprotective agents in different in vitro and in vivo models tested (Maurice & Lockhart, 1997).
The reported role of σ1 binding sites in ischaemia brought our interest to this protein. We recently evidenced antiischaemic effects of trimetazidine on various experimental models and observed that these effects were related to a preservation of main mitochondrial functions both in brain and liver of the rat (Elimadi et al., 1998; Zini et al., 1996). It was also shown that [3H]-trimetazidine specifically bound to mitochondrial membranes but these binding sites have not been yet identified (Morin et al., 1998; 2000). Interestingly, among the drugs able to inhibit [3H]-trimetazidine binding, σ ligands competed with high affinity (Morin et al., 2000). Thus the hypothesis was raised that trimetazidine binding sites might be related to σ receptors.
In this study, we demonstrate the presence of σ1 like binding sites on purified mitochondria from rat brain and liver, using [3H](+)-pentazocine as a ligand. This localization was confirmed by means of double fluorescence studies on rat liver sections.
Methods
Preparation of purified rat liver and brain mitochondria
Rat brain mitochondria were isolated by differential centrifugation and purified on a Percoll gradient according to Sims (1990). Male Wistar rats weighing approximately 250 to 300 g were decapitated. Brains were removed, sliced and washed in a buffer containing (mM): sucrose 320, 2-amino-2-hydroxymethyl-propan-1,3-diol (Tris) 10 and ethylenediaminetetracetic acid (EDTA) 1, pH 7.4 at 4°C. The brains were then homogenized in 10 ml per g of tissue of the same buffer and centrifuged 3 min at 1330×g (Sorvall® RC 28 S). The supernatants (S1) were kept on ice and the pellets were suspended in 5 ml per g of tissue of the same buffer and centrifuged at 1330×g for 3 min. The pellets were discarded, the supernatants S2 pooled to S1 and centrifuged at 21 200×g for 10 min. The resulting supernatants (S3) were kept on ice. The final pellets were suspended in 15% (v v−1) percoll (10 ml g−1 of tissue originally homogenized) and layered on a discontinuous density gradient of 3.5 ml each of 23 and 40% percoll. The tubes were centrifuged at 30 700×g for 5 min. The purified mitochondrial rings were recovered at the interface of the 23 – 40% fractions using a Pasteur pipette and rinsed in the isolation buffer. Microsomal fractions were obtained by centrifugating the supernatants S3 at 100 000×g for 60 min. Protein contents were determined by the method of Lowry et al. (1951).
Purified rat liver mitochondria were obtained as previously described (Morin et al., 1998). Briefly, the livers were excised rapidly and placed in a medium containing (mM): sucrose 250, Tris 50 and ethylene glycol-bis(b-amino ethyl ether) tetraacetic acid (EGTA) 5, pH 7.8 at 4°C. Tissue samples (28 g) were minced and homogenized (10 g tissue in 60 ml medium) on ice using a Teflon Potter homogenizer. The homogenates were centrifuged at 600×g for 10 min (Sorvall® RC 28 S) and the supernatants were centrifuged for 10 min at 3300×g to obtain mitochondrial pellets. The resulting pellets were washed with medium from which EGTA was omitted and centrifuged for 10 min at 3300×g. The final pellets were suspended in 36 ml of Tris-sucrose buffer (without EGTA) and mitochondria were purified on a discontinuous sucrose gradient according to Morin et al. (1998).
The supernatant (from 10 min at 3300×g) was centrifuged at 15 000×g for 10 min and then at 170 000×g for 1 h (Sorvall® A841 rotor) to obtain a pellet corresponding to the ‘microsomal fraction'.
All animal procedures used in this study are in strict accordance with the European Community Council Directive of 24 November 1986 (86-609/EEC) and Decree of 20 October 1987 (87-848/EEC).
Preparation of synaptosomal membranes
Male Wistar rats were killed by decapitation, their brains were removed and rapidly dissected. The cerebral cortex was homogenized in 20 volumes of ice-cold sucrose buffer (Tris-HCl 50 mM, sucrose 0.32 M, pH=7.4 at 4°C) using a Teflon Potter homogenizer. The homogenate was centrifuged at 1000×g for 10 min and the pellet was discarded. The supernatant was centrifuged at 50,000×g for 20 min and the supernatant was discarded. This procedure was repeated two additional times. The final pellet was resuspended in 50 mM Tris-HCl buffer (pH=8 at 37°C).
Binding assays
Binding of [3H](+)-pentazocine to rat brain and liver mitochondria was measured as follows: Mitochondria (1 mg ml−1) were incubated with [3H](+)-pentazocine in 400 μl of 50 mM Tris-HCl buffer (pH=8) for 90 min at 37°C. Binding was stopped by the addition of ice-cold binding buffer, and bound and free ligands were separated by rapid filtration through Whatman GF/B glass fibre filters (presoaked in 0.1% polyethylenimine). Each filter was washed twice with an additional 5 ml of ice-cold Tris-buffer (50 mM) and counted in a liquid scintillation counter Packard 1600 TR with an efficiency of 45%. Non-specific binding was defined using 1 μM haloperidol in the liver and 10 μM (±)-pentazocine in the brain. For saturation experiments the range of concentrations of [3H](+)-pentazocine was 0.2 – 20 nM. For inhibition experiments 2 – 3 nM [3H](+)-pentazocine was incubated in the absence or in the presence of increasing concentrations (15) of the competing drug.
Enzyme assays
All enzymatic activities were determined by spectrophotometric methods using an Hitachi® UV-3000 spectrophotometer and were shown to be linear in the tissue and protein concentration range used. The determination of monoamine oxidase (EC 1.4.3.4) activity was performed according to the method of Bembenek et al. (1990) using kynuramine as a substrate and monitoring the formation of 4-hydroxyquinoline at 316 nm. Cytochrome c oxidase (EC 1.9.3.1) activity was assayed at 37°C according to the method of Rustin et al. (1994) by monitoring the oxidation of ferrocytochrome c (prepared from type III horse heart cytochrome c (Sigma) at 550 nm and NADPH cytochrome c reductase (EC 1.6.2.5) was assayed at room temperature as described by Graham (1993).
Immunocytochemistry
Cryosections from adult rat liver were fixed in 100% methanol for 10 min. Sections were incubated for 48 h at 4°C with two primary antibodies: σ1 receptor rabbit polyclonal antibody (diluted 1 : 200) (Alonso et al., 2000) and a mouse monoclonal antibody against the mitochondrial market protein Ab-1 (clone 113-1) (from Lab Vision Corporation, www.labvision.com, Cat No.: MS-627-p1). The Ab-1 antibody was used at a 1 : 100 dilution. Sections were rinsed in PBS three times and incubated for 2 h with two secondary antibodies, FITC-goat anti-rabbit IgG (Zymed, 1 : 150) and rhodamine Red-X-conjugated Affinipure goat anti-mouse IgG (Jackson Labs, 1 : 150). Antibodies were diluted in PBS containing 0.1% gelatin, 0.1% Triton X-100 and 1% normal goat serum. The slides were then mounted with the Prolong Antifade Kit (Molecular Probes Inc.) and observed under Olympus Fluoview laser scanning microscope.
Drugs
[3H](+)-pentazocine was obtained from New England Nuclear (Paris, France). Other drugs and chemicals were obtained from Sigma (St Quentin Fallavier, France) or Merck (Nogent-sur-Marne, France) and were of the highest purity available.
Data analysis
Equilibrium, namely Kd (dissociation constant) and Bmax (maximal density of binding sites), and inhibition (IC50) binding parameters were calculated by means of a non-linear regression method using a commercially available software (Micropharm, INSERM 1990; Urien, 1995) by modelling the data to one or two component models according to Hill equations as described previously (Morin et al., 1998).
A Hill coefficient (nH) value equal one corresponds to a competitive interaction and thus indicates the presence of one class of binding sites. In this case, inhibition constants (Ki) were calculated from the IC50 values according to the method of Cheng & Prusoff (1973). All data are presented as the mean±s.e.mean of three or more individual experiments.
Results
Characterization of purified mitochondria
Given that σ binding sites were described to be highly enriched in liver and brain microsomes (McCann et al., 1994; Dehaven-Hudkins et al., 1994), we evaluated the possible contamination of our mitochondrial preparations by microsomal membranes. Thus, liver and brain mitochondria were characterized for purity for their content in monoamine oxidase (an outer mitochondrial membrane marker), cytochrome c oxidase (an inner mitochondrial membrane marker) and the microsomal marker, NADPH cytochrome c reductase and compared to the respective microsomal fractions. A high activity of the two mitochondrial markers was found in the mitochondrial fractions whereas NADPH cytochrome c reductase activity was very low and not detectable in purified liver and brain mitochondria, respectively (Table 1).
Table 1.
Characterization of purified mitochondria by means of marker enzymes
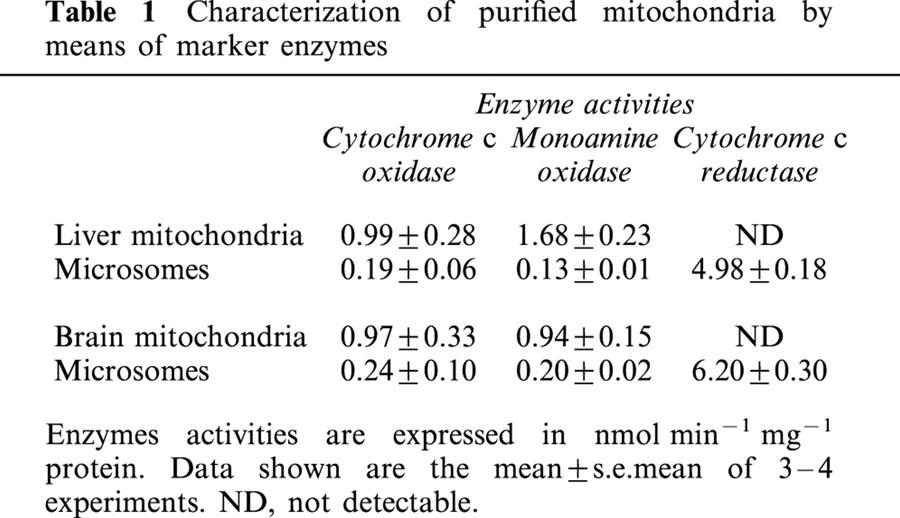
These results indicate that our purified mitochondrial fractions were devoid of microsomes and thus were appropriate to use in search of mitochondrial σ binding sites. These preparations corresponded to those previously described using the same mitochondrial purification procedures (Sims, 1990; Morin et al., 1998).
Characterization of [3H](+)-pentazocine binding sites on rat liver mitochondria
To identify σ like binding sites in mitochondria we used [3H](+)-pentazocine as a ligand. [3H](+)-pentazocine is a ligand of choice to label σ1 binding sites because it displays high affinity and good selectivity for this subtype (Quirion et al., 1992; Hellewell et al., 1994). Figure 1A shows that [3H](+)-pentazocine binds to liver mitochondria and reached equilibrium after 90 min incubation at 37°C. When intact mitochondria were used a non-saturable binding component was observed (Figure 1B). It was eliminated when mitochondria were subjected to hypo-osmotic shock and sonication (Figure 1A). This component probably corresponds to an uptake mechanism, which accumulates the ligand within the mitochondria without binding. Therefore all the following experiments were performed on broken mitochondria. Dilution of the suspension (1/100) induced a slow dissociation of the ligand from its binding sites demonstrating that the binding was reversible (Figure 1C).
Figure 1.
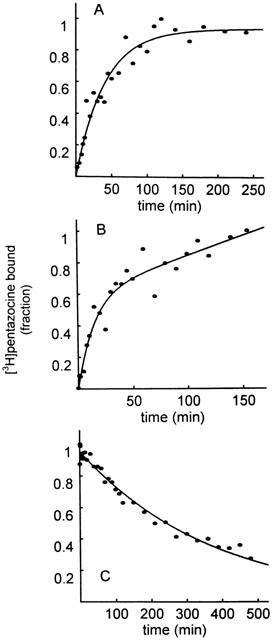
Association and dissociation kinetics of [3H](+)-pentazocine to rat liver mitochondria. Association experiment: Liver mitochondria (1 mg ml−1) were incubated with 2 nM [3H](+)-pentazocine at 37°C. Specific binding in intact (B) and broken mitochondria (A). Dissociation experiment (C): after equilibrium was reached, dissociation was induced by dilution and specific binding monitored for 500 min (C). Data shown are plotted as fractions of the maximal binding value ([3H](+)-pentazocine bound=1) and are from typical experiments representing the means of duplicate determinations.
Saturation binding experiments indicated that [3H](+)-pentazocine recognized a single population of binding sites (nH=1) over the range of concentration studies with mean Kd and Bmax values of 2.42±0.50 nM and 1.05±0.08 pmol mg−1 protein, respectively. The linear Scatchard plot confirmed this analysis of the data. A representative binding curve is shown on Figure 2.
Figure 2.
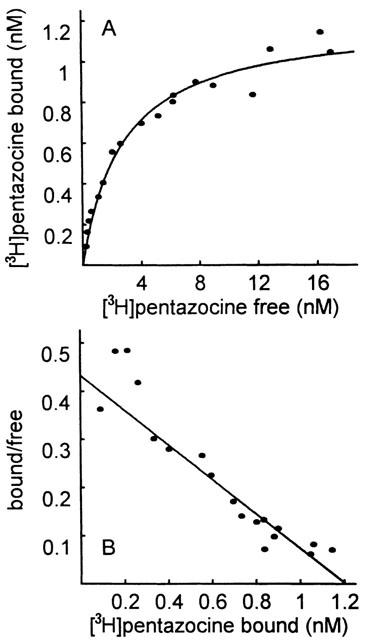
Representative equilibrium binding curve of [3H](+)-pentazocine binding to liver mitochondria. Concentration of [3H](+)-pentazocine (0.2 – 2 nM) was incubated with sonicated mitochondria (1 mg ml−1) for 90 min at 37°C and non-specific binding was defined using 1 μM haloperidol. Equilibrium parameters were estimated by a non-linear regression analysis. In this particular experiment, Kd and Bmax values were 2.78 nM and 1.20 pmol mg−1 protein, respectively. (A) Direct plot. (B) Scatchard plot.
In order to confirm the nature of the sites labelled by [3H](+)-pentazocine, several σ reference ligands were examined for their potencies to inhibit its binding (Table 2, Figure 3). Among the drugs tested haloperidol and (±)-pentazocine were the most potent with Ki values of 1.93±0.76 and 5.27±0.62 nM, respectively.
Table 2.
Inhibition of [3H](+)-pentazocine by various sigma reference ligands
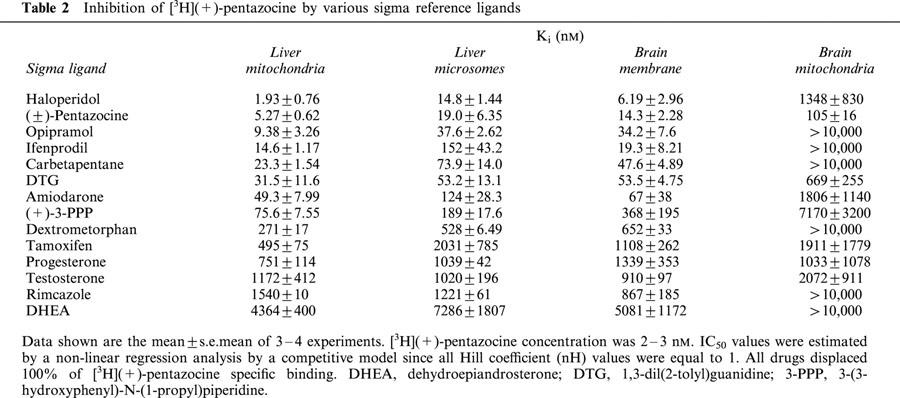
Figure 3.
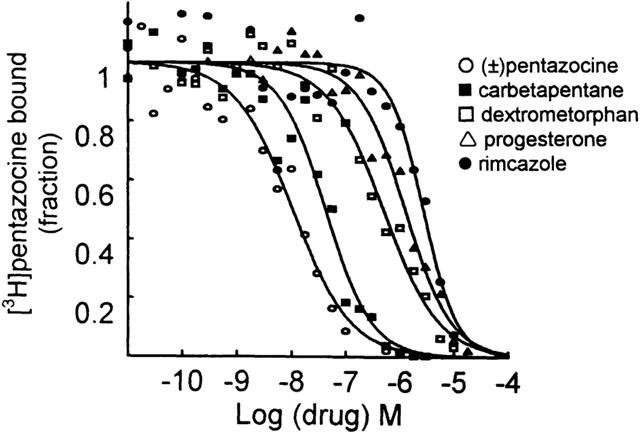
Effect of several σ receptor ligands on [3H](+)-pentazocine binding to rat liver mitochondria. Incubations were carried out with 2 – 3 nM [3H](+)-pentazocine at 37°C for 90 min. Data shown are from typical experiments and are plotted as fractions of the control binding value (in the absence of inhibitor: [3H](+)-pentazocine bound=1).
The Ki value for (±)-pentazocine was slightly higher than the Kd value determined from equilibrium experiments (2.42±0.50 nM) but it is probably due to the use of the (+)-pentazocine radioligand which has higher affinity than the racemic compound (Bowen et al., 1989; Quirion et al., 1992). Opipramol, ifenprodil, carbetapentane, DTG and amiodarone were found to be very potent inhibitors (Ki<100 nM) of [3H](+)-pentazocine binding whereas progesterone and testosterone showed moderate inhibition with Ki values around 1 μM. The other steroids hormones were less active with Ki values higher than 4 μM.
The rank order of potency at the [3H](+)-pentazocine binding sites is similar between the liver mitochondria and the well-characterized brain membranes (Cagnotto et al., 1994; Dehaven-Hudkins et al., 1994; McCann et al., 1994; Table 2) showing a strong correlation (r=0.975, P<0.0001; Figure 4). A similar relation was found between liver mitochondria and liver microsomes (r=0.966; P<0.0001; Figure 4). These observations provided strong evidence that [3H](+)-pentazocine binding sites located on mitochondria are similar to σ1 receptors.
Figure 4.
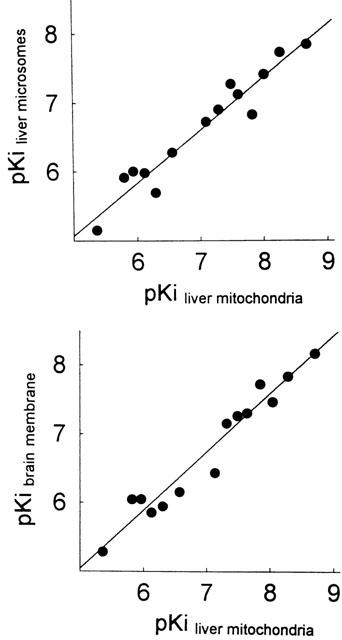
Relationship between the affinity of drugs for [3H](+)-pentazocine binding sites from liver mitochondria, liver microsomes and brain membranes. Plotted are pKi values listed in Table 2. Regression line was obtained by least squares fit analysis of the data points.
Modulation of progesterone on [3H](+)-pentazocine binding to liver mitochondria
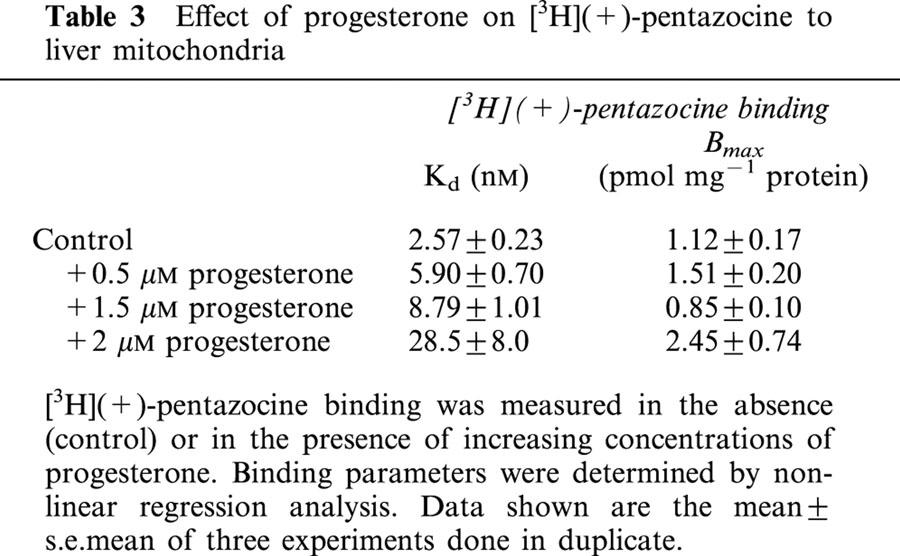
Progesterone has been proposed as a putative endogenous ligand for σ binding sites. We investigated the interaction of progesterone with the liver mitochondrial [3H](+)-pentazocine binding sites. Inhibition experiments (Table 2) showed that progesterone competed with [3H](+)-pentazocine binding sites (nH=1). The results displayed on Table 3 reinforced the significance of these data. Increasing concentrations of progesterone increased the Kd for [3H](+)-pentazocine binding without altering its Bmax value in a significant manner.
Table 3.
Effect of progesterone on [3H](+)-pentazocine to liver mitochondria
Sub-mitochondrial location of [3H](+)-pentazocine binding sites
The sub-mitochondrial localization of [3H](+)-pentazocine binding sites was determined using a digitonin solubilization protocol to separate the outer from the inner mitochondrial membrane as described by Anholt et al. (1986). Intact liver mitochondria were incubated with a low digitonin concentration (0.6 mg digitonin mg−1 protein), which resulted in the extraction of monoamine oxidase (an outer membrane marker) without affecting cytochrome c oxidase activity (an inner membrane marker, Figure 5), confirming previous results (Anholt et al., 1986; Morin et al., 1998). Under these conditions, [3H](+)-pentazocine binding to mitochondria was present in the digitonin extract enriched in outer membranes only (Figure 5), suggesting that [3H](+)-pentazocine binding sites are located on the outer mitochondrial membrane.
Figure 5.
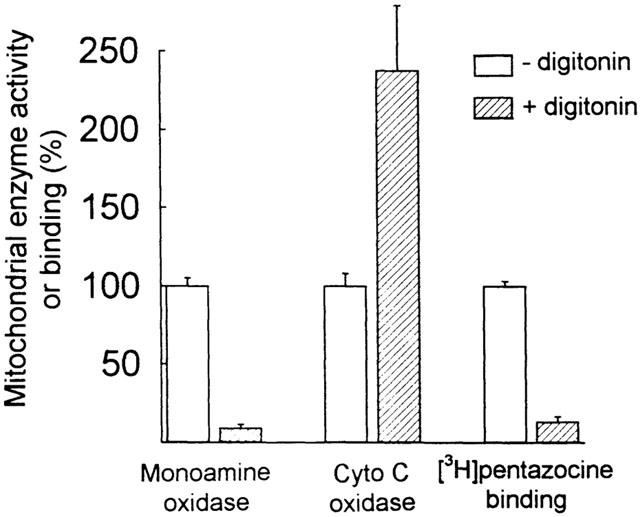
[3H](+)-pentazocine binding sites are located on the outer mitochondrial membranes. Freshly isolated mitochondria (8 mg ml−1) were treated or not with 0.6 mg digitonin mg−1 protein in 1 ml Tris-sucrose buffer for 15 min at 4°C. After incubation the mitochondrial suspension was diluted and centrifuged at 15,000×g for 7 min. Cytochrome c oxidase activity, monoamine oxidase activity and [3H](+)-pentazocine (2 nM) binding were then assayed in the pellet. The maximal activities of the markers (100% values) were 1.57 nmol min−1 mg−1 protein for monoamine oxidase, 0.87 nmol min−1 mg−1 protein for cytochrome c oxidase, and 0.41 pmol mg−1 protein for [3H](+)-pentazocine binding. Each data point is the mean±s.e.mean of duplicate measurements obtained from 3 – 4 experiments.
Immunostaining for σ1 receptors of liver mitochondria
In order to confirm the presence of σ receptors on mitochondria, liver sections were double immunostained for σ1 receptors and a mitochondria marker (mitochondria Ab-1 primary antibody). Using conventional immunofluorescence the presence of σ1 receptor (green) and mitochondria Ab-1 (red) in rat liver was clear (Figure 6). When confocal images of σ1 receptor staining and mitochondria Ab-1 staining were merged, a colocalization (yellow) of their respective antigens was observed.
Figure 6.
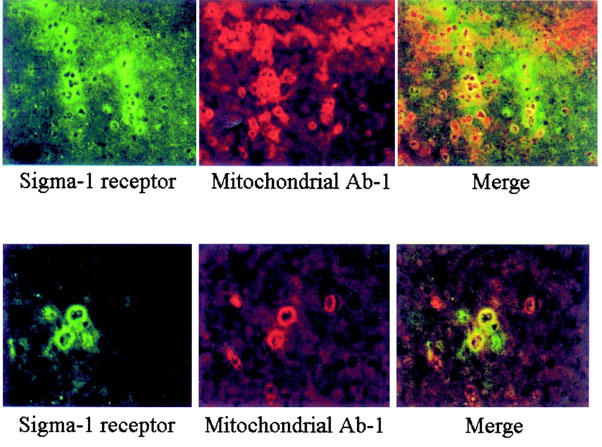
Colocalization of σ1 receptor and mitochondrial Ab-1 marker in rat liver. As described under Methods rat liver sections were incubated with σ1 receptor and mitochondria Ab-1 primary antibodies. Using conventional immunofluorescence the presence of σ1 receptor (green) and mitochondria Ab-1 (red) in rat liver is clear. Confocal images of σ1 receptor staining and mitochondria Ab-1 staining were merged, showing a colocalization (yellow) of their respective antigens. Magnification: ×20 (top) and ×40 (bottom).
Study of [3H](+)-pentazocine binding on rat brain mitochondria
To investigate whether the mitochondrial location of [3H](+)-pentazocine binding sites is exclusive to liver, we examined whether [3H](+)-pentazocine binding was present in rat brain mitochondria. Indeed, [3H](+)-pentazocine binding was found in rat brain mitochondria. However, these sites differ from those observed on brain membrane fraction and on liver mitochondria. Although the number of sites was similar in brain (Bmax=1.26±0.09 pmol mg−1 protein) and liver (Bmax=1.05±0.08 pmol mg−1 protein), [3H](+)-pentazocine had 15-fold lower affinity in brain (Kd=31.6±3.9 nM) compared to the liver. Inhibition experiments confirmed this difference as all the σ compounds tested were less potent when tested on brain than on liver mitochondria (Table 2). In addition, the selective antagonist rimcazole was without effect (Table 2). The only notable exceptions were steroid hormones, which exhibit the same affinities in both liver and brain mitochondria preparations.
Discussion
During the past few years σ1 recognition sites have been extensively studied. These studies were almost exclusively carried out on microsomal and plasma membranes because σ1 recognition sites are more abundant in these cellular fractions whatever the organ studied. However, several observations have suggested that these sites could be also present on other organelles (Cagnotto et al., 1994; Dehaven-Hudkins et al., 1994; Alonso et al., 2000) but no clear demonstration and pharmacological characterization using purified preparations were performed. In the present study, we demonstrate the presence of [3H](+)-pentazocine binding sites on purified preparation of mitochondria and provide the pharmacological profile of these mitochondrial binding sites, which proves their similarity with σ1 receptors.
In liver mitochondria, [3H](+)-pentazocine labels one class of high affinity (Kd=3 nM) binding sites, similar to that observed in synaptic membranes (Dehaven-Hudkins et al., 1994; Nguyen et al., 1996). The mitochondrial sites also display high affinity for other σ1 ligands such as haloperidol, opipramol, ifenprodil or carbetapentane. The binding inhibition profile obtained with these reference compounds is similar to those reported previously for synaptic membranes (McCann et al., 1994; Dehaven-Hudkins et al., 1994) and a close relationship exists between the Ki values obtained from liver mitochondria and either liver microsomes or synaptic membranes. On the other hand, [3H](+)-pentazocine binding is modulated by progesterone, a putative endogenous ligand for σ1 receptors, in agreement with previous findings on other biological materials (Su et al., 1988; Schwarz et al., 1989; Bergeron et al., 1999). Taken together, these results indicate that rat liver mitochondrial [3H](+)-pentazocine binding sites present pharmacological characteristics identical to the σ1 receptors. Interestingly, this is not the case for [3H](+)-pentazocine binding sites found on brain mitochondria. Indeed, [3H](+)-pentazocine and typical σ1 ligands display lower affinities for these sites. For example, haloperidol is 700-fold less potent on brain than on liver mitochondria and opipramol, ifenprodil and carbetapentane were ineffective on the former. This indicates that mitochondrial brain sites differ from the liver ones and from the typical peripheral σ1 receptors. In addition, they do not appear to be related to σ2 receptors because the σ2 binding ligand DTG showed a moderate affinity for these sites (Ki=0.6 μM). Although these sites are distinct from σ1, they exhibit common binding recognition properties for steroid hormone ligands and the antisteroid agent tamoxifen, which have similar affinities for liver and brain mitochondrial [3H](+)-pentazocine binding sites. This observation indicates that these sites could be related entities.
In support of our binding data, double fluorescence studies, using both an anti-σ1 antibody (Alonso et al., 2000) and a mitochondrial marker, clearly show the presence of immunoreactive σ1 protein(s) on liver mitochondrial membranes.
The pharmacological significance of these binding sites is unknown. The same comment can be made for the σ receptors. Indeed, although numerous pharmacological effects have been associated with σ1 receptors (reviewed by Matsuno & Mita, 1998; Maurice et al., 1999; Bowen, 2000), the cellular transduction mechanism and the functional role of these receptors remain unclear. Among the effects described, σ ligands exert cytoprotective and more particularly neuroprotective properties (Maurice & Lockhart, 1997). This function of the ligands relates to their ability to directly inhibit the excitatory neurotransmitter system (Maurice & Lockhart, 1997). More recently, several lines of evidence indicated that σ receptors could mediate some of their effects by altering intracellular effects. For example, they were shown to modulate intracellular calcium and more particularly facilitate the mobilization of intracellular calcium pools (Hayashi et al., 2000). This increase, due to the release of calcium from endoplasmic reticulum stores, has been suggested to contribute to the cytoprotective effect, reducing indirectly presynaptic glutamate release (Maurice & Lockhart, 1997). These data indicate that σ ligands might use calcium signal as cellular transduction mechanism and may participate to the control of cellular calcium homeostasis. Since mitochondria are known to store calcium (Gunter et al., 1994) and are supposed to play a role in the transduction of the calcium signal (Ichas et al., 1997) we can speculate that mitochondrial [3H](+)-pentazocine binding sites might be a pharmacological site regulating calcium stores.
A possible relationship between σ receptors and sterol metabolism has also been evoked. Indeed, the σ1 receptor has been cloned in several species (Hanner et al., 1996; Kekuda et al., 1996; Seth et al., 1997) and shows a 30% identity and a 60% homology to a yeast sterol C8-C7 isomerase suggesting a role for this drug-binding protein in sterol synthesis (Moebius et al., 1997). In addition, certain neurosteroids have been shown to exhibit modulatory effects via σ receptors (Monnet et al., 1995; Bergeron et al., 1996). Interestingly, another drug-receptor, the peripheral-type benzodiazepine receptor, is precisely localized on the outer mitochondrial membrane where it is involved in the transport of cholesterol into mitochondria, the first step in steroid biosynthesis (Papadopoulos et al., 1997; Li et al., 2001). Although the role of peripheral-type benzodiazepine receptors in cholesterol transport into mitochondria of steroidogenic cells has been shown, it is important to note that a more general role for this ubiquitous receptor protein in intracellular cholesterol compartmentalization has been proposed (Li & Papadopoulos, 1998). The recent finding that this receptor is a high-affinity cholesterol binding protein (Li et al., 2001; Lacapere et al., 2001) further supports its function in cholesterol compartmentalization.
Given that our data demonstrate that [3H](+)-pentazocine binding sites are also located on the outer membrane of mitochondria and are modulated by several steroid hormones, especially progesterone which is considered to be a putative endogenous ligand (Su et al., 1988; Schwarz et al., 1989; Bergeron et al., 1999), a possible modulation of steroid biosynthesis and/or cholesterol movement across mitochondrial membrane, initial step in membrane biogenesis and steroid biosynthesis, by σ ligands cannot be excluded. This might also contribute to the cytoprotective effect of these drugs. Although the role of σ1 receptors has not yet been defined, their involvement in this process seems plausible. The effect of steroids and Ca2+ on σ1 receptors together with their presence on the mitochondrial membranes, presented herein, support this hypothesis. Studies to test these hypotheses are in progress in our laboratory. In conclusion, we report herein the presence and the pharmacological characteristics of mitochondrial σ1 binding sites. The assignment of these drug-binding sites to mitochondria, considering the vital role of these organelles in respiration, apoptosis, sterol metabolism etc, opens a new area for the application of σ1 drug ligands in search of a role of this drug-binding protein.
Acknowledgments
This work was supported by the Ministère de l'Education Nationale (DRED EA 427), and the Réseau de Pharmacologie Clinique. We gratefully acknowledge Felipe Pires for his skilful technical assistance.
Abbreviations
- DHEA
dehydroepiandrosterone
- DTG
1,3-di(2-tolyl)guanidine
- EDTA
ethylenediaminetetracetic acid
- EGTA
ethylene glycol-bis(b-amino ethyl ether) tetraacetic acid
- 3-PPP
3-(3-hydroxyphenyl)-N-(1-propyl)piperidine
- Tris
2-amino-2-hydroxymethyl-propan-1,3-diol
References
- ALONSO G., PHAN V.L., GUILLEMAIN I., SAUNIER M., LEGRAND A., ANOAL M., MAURICE T. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- ANHOLT R.R., PEDERSEN P.L., DE SOUZA E.B., SNYDER S.H. The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J. Biol Chem. 1986;261:576–583. [PubMed] [Google Scholar]
- BEMBENEK M.E., ABELL C.W., CHRISEY L.A., ROZWADOWSKA M.D., GESSNER W., BROSSI A. Inhibition of monoamine oxidases A and B by simple isoquinoline alcaloids: racemic and optically active 1,2,3,4-tetrahydro, 3,4-dihydro and fully aromatic isoquinolines. J. Med. Chem. 1990;33:147–152. doi: 10.1021/jm00163a025. [DOI] [PubMed] [Google Scholar]
- BERGERON R., DE MONTIGNY C., DEBONNEL G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via sigma receptors. J. Neurosci. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGERON R., DE MONTIGNY C., DEBONNEL G. Pregnancy reduces brain sigma receptor function. Br. J. Pharmacol. 1999;127:1769–1776. doi: 10.1038/sj.bjp.0702724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWEN W.D. Sigma receptors: recent advances and new clinical potentials. Pharm. Acta Helv. 2000;74:211–218. doi: 10.1016/s0031-6865(99)00034-5. [DOI] [PubMed] [Google Scholar]
- BOWEN W.D., HELLEWELL S.B., MCGARRY K.A. Evidence for a multi-site model of the rat brain sigma receptor. Eur. J. Pharmacol. 1989;163:309–318. doi: 10.1016/0014-2999(89)90200-8. [DOI] [PubMed] [Google Scholar]
- CAGNOTTO A., BASTONE A., MENNINI T. [3H](+)-pentazocine binding to rat brain sigma 1 receptors. Eur. J. Pharmacol. 1994;266:131–138. doi: 10.1016/0922-4106(94)90102-3. [DOI] [PubMed] [Google Scholar]
- CHENG Y., PRUSOFF W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- DEHAVEN-HUDKINS D.L., FLEISSNER L.C., FORD-RICE F.Y. Characterization of the binding of [3H](+)-pentazocine to sigma recognition sites in guinea pig brain. Eur. J. Pharmacol. 1992;227:371–378. doi: 10.1016/0922-4106(92)90153-m. [DOI] [PubMed] [Google Scholar]
- DEHAVEN-HUDKINS D.L., LANYON L.F., FORD-RICE F.Y., ATOR M.A. Sigma recognition sites in brain and peripheral tissues. Characterization and effects of cytochrome P450 inhibitors. Biochem. Pharmacol. 1994;47:1231–1239. doi: 10.1016/0006-2952(94)90395-6. [DOI] [PubMed] [Google Scholar]
- ELIMADI A., SETTAF A., MORIN D., SAPENA R., LAMCHOURI F., CHERRAH Y., TILLEMENT J.P. Trimetazidine counteracts the hepatic injury associated with ischemia-reperfusion by preserving mitochondrial function. J. Pharmacol. Exp. Ther. 1998;286:23–28. [PubMed] [Google Scholar]
- GRAHAM J.M. Methods in molecular biology Biomembrane Protocols 1993Totowa, New Jersey: Humana Press; 1–18.eds. Graham J.M., Higgins J.A. pp [Google Scholar]
- GUNTER T.E., GUNTER K.K., SHEU S.S., GAVIN C.E. Mitochondrial calcium transport: physiological and pathological relevance. Am. J. Physiol. 1994;267:C313–C339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- HANNER M., MOEBIUS F.F., FLANDORFER A., KNAUS H.G., STRIESSNIG J., KEMPNER E., GLOSSMANN H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARUKUNI I., BHARDWAJ A., SHAIVITZ A.B., DEVRIES A.C., LONDON E.D., HURN P.D., TRAYSTMAN R.J., KIRSCH J.R., FARACI F.M. Sigma(1)-receptor ligand 4-phenyl-1-(4-phenylbutyl)-piperidine affords neuroprotection from focal ischemia with prolonged reperfusion. Stroke. 2000;31:976–982. doi: 10.1161/01.str.31.4.976. [DOI] [PubMed] [Google Scholar]
- HAYASHI T., MAURICE T., SU T.P. Ca(2+) signaling via sigma(1)-receptors: novel regulatory mechanism affecting intracellular Ca(2+) concentration. J. Pharmacol. Exp. Ther. 2000;293:788–798. [PubMed] [Google Scholar]
- HELLEWELL S.B., BRUCE A., FEINSTEIN G., ORRINGER J., WILLIAMS W., BOWEN W.D. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur. J. Pharmacol. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- ICHAS F., JOUAVILLE L.S., MAZAT J.P. Mitochondria are excitable organelles capable of generating the conveying electrical and calcium signals. Cell. 1997;89:1145–1153. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- KEKUDA R., PRASAD P.D., FEI Y.J., LEIBACH F.H., GANAPATHY V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1) Biochem. Biophys. Res. Commun. 1996;229:553–558. doi: 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- LACAPERE J.J., DELAVOIE F., LI H., PERANZI G., MACCARIO J., PAPADOPOULOS V., VIDIC B. Structural and functional study of reconstituted peripheral benzodiazepine receptor. Biochem. Biophys. Res. Commun. 2001;284:536–541. doi: 10.1006/bbrc.2001.4975. [DOI] [PubMed] [Google Scholar]
- LI H., PAPADOPOULOS V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology. 1998;139:4991–4997. doi: 10.1210/endo.139.12.6390. [DOI] [PubMed] [Google Scholar]
- LI H., YAO Z., DEGENHARDT B., TEPER G., PAPADOPOULOS V. Cholesterol binding at the cholesterol recognition/interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc. Natl. Acad. Sci. 2001;98:1267–1272. doi: 10.1073/pnas.031461598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;93:265–275. [PubMed] [Google Scholar]
- MCCANN D.J., SU T.P. Haloperidol competitively inhibits the binding of (+)-[3H]SKF-10,047 to sigma sites. Eur. J. Pharmacol. 1990;80:361–364. doi: 10.1016/0014-2999(90)90322-w. [DOI] [PubMed] [Google Scholar]
- MCCANN D.J., WEISSMAN A.D., SU T.P. Sigma-1 and Sigma-2 sites in rat brain: comparison of regional, ontogenic, and subcellular patterns. Synapse. 1994;17:182–189. doi: 10.1002/syn.890170307. [DOI] [PubMed] [Google Scholar]
- MARTIN W.R., EADES C.G., THOMPSON J.A., HUPPLER R.E., GILBERT P.E. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- MATSUNO K., MITA S. SA4503: a novel sigma1 receptor agonist. CNS Drugs Rev. 1998;4:1–24. [Google Scholar]
- MAURICE T., LOCKHART B.P. Neuroprotective and anti-amnesic potentials of sigma (σ) receptors ligands. Prog. Neuropsychopharmacol. Biol. Psychiat. 1997;21:69–102. doi: 10.1016/s0278-5846(96)00160-1. [DOI] [PubMed] [Google Scholar]
- MAURICE T., PHAN V.L., URANI A., KAMEI H., NODA Y., NABESHIMA T. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn. J. Pharmacol. 1999;81:125–155. doi: 10.1254/jjp.81.125. [DOI] [PubMed] [Google Scholar]
- MOEBIUS F.F., STRIESSNIG J., GLOSSMANN H. The mysteries of sigma receptors: new family members reveal a role in cholesterol synthesis. Trends Pharmacol. Sci. 1997;18:67–70. doi: 10.1016/s0165-6147(96)01037-1. [DOI] [PubMed] [Google Scholar]
- MONNET F.P., MAHE V., ROBEL P., BAULIEU E.E. Neurosteroids, via sigma receptors, modulate the [3H]norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc. Natl. Acad. Sci. 1995;92:3774–3778. doi: 10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORIN D., ELIMADI A., SAPENA R., CREVAT A., CARRUPT P.A., TESTA B., TILLEMENT J.P. Evidence for the existence of [3H]trimetazidine binding sites involved in the regulation of the mitochondrial permeability transition pore. Br. J. Pharmacol. 1998;123:1385–1394. doi: 10.1038/sj.bjp.0701755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORIN D., SAPENA R., ELIMADI A., TESTA B., LABIDALLE S., LE RIDANT A., TILLEMENT J.P. [(3)H]-trimetazidine mitochondrial binding sites: regulation by cations, effect of trimetazidine derivatives and other agents and interaction with an endogenous substance. Br. J. Pharmacol. 2000;130:655–663. doi: 10.1038/sj.bjp.0703348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGUYEN V.H., KASSIOU M., JOHNSTON G.A., CHRISTIE M.J. Comparison of binding parameters of sigma 1 and sigma 2 binding sites in rat and guinea pig brain membranes: novel subtype-selective trishomocubanes. Eur. J. Pharmacol. 1996;311:233–240. doi: 10.1016/0014-2999(96)00395-0. [DOI] [PubMed] [Google Scholar]
- PAPADOPOULOS V., AMRI H., BOUJRAD N., CASCIO C., CULTY M., GARNIER M., HARDWICK M., LI H., VIDIC B., BROWN A.S., REVERSA J.L., BERNASSAU J.M., DRIEU K. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997;62:21–28. doi: 10.1016/s0039-128x(96)00154-7. [DOI] [PubMed] [Google Scholar]
- PASCAUD X., DEFAUX J.P., ROZE C., JUNIEN J.L. Effect of selective sigma ligands on duodenal alkaline secretion in the rat. J. Pharmacol. Exp. Ther. 1990;255:1354–1359. [PubMed] [Google Scholar]
- QUIRION R., BOWEN W.D, , ITZHAK Y., JUNIEN J.L., MUSACCHIO J.M., ROTHMAN R.B., SU T.P., TAM S.W., TAYLOR D.C. A proposal for the classification of sigma binding sites. Trends Pharmacol. Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- RUSTIN P., CHRETIEN D., BOURGERON T., GERARD B., ROTIG A., SAUDUBRAY J.M., MUNNICH A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Act. 1994;228:35–51. doi: 10.1016/0009-8981(94)90055-8. [DOI] [PubMed] [Google Scholar]
- SCHWARZ S., POHL P., ZHOU G.Z. Steroid binding at sigma-‘opioid' receptors. Science. 1989;246:1635–1638. doi: 10.1126/science.2556797. [DOI] [PubMed] [Google Scholar]
- SETH P., LEIBACH F.H., GANAPATHY V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem. Biophys. Res. Commun. 1997;241:535–540. doi: 10.1006/bbrc.1997.7840. [DOI] [PubMed] [Google Scholar]
- SIMS N.R. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J. Neurochem. 1990;55:698–707. doi: 10.1111/j.1471-4159.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- SU T.P., LONDON E.D., JAFFE J.H. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- URIEN S. Micropharm-K, a microcomputer interactive program for the analysis and simulation of pharmacokinetic processes. Pharm. Res. 1995;12:1225–1230. doi: 10.1023/a:1016280430580. [DOI] [PubMed] [Google Scholar]
- ZINI R., SIMON N., MORIN C., D'ATHIS P., TILLEMENT J.P. Inhibition of rat cerebral mitochondrial respiration by cyclosporins A, D, and G and restoration with trimetazidine. C R Acad. Sci. III. 1996;319:1087–1092. [PubMed] [Google Scholar]


