Abstract
The effects of treatment with rolipram, a specific phosphodiesterase IV inhibitor, on learning and memory function and on the cyclic AMP/PKA/CREB signal transduction system were examined in rats with microsphere embolism (ME)-induced cerebral ischaemia.
Sustained cerebral ischaemia was induced by the injection of 900 microspheres (48 μm in diameter) into the right hemisphere of the rat brain. The animals were treated once daily with 3 mg kg−1 rolipram i.p. from 6 h after the onset of the operation for consecutive 10 days.
Microsphere-embolized rats showed prolongation of the escape latency in the water maze task starting from day 7 after the operation and lasting for 3 consecutive days. Treatment with rolipram reduced the escape latency.
ME decreased the cyclic AMP content, cytosolic PKA Cβ level, and nuclear PKA Cα and Cβ levels, as well as reduced the pCREB level and the DNA-binding activity of CREB in the cerebral cortex and hippocampus on day 10 after the operation. These alterations were attenuated by treatment with rolipram.
These results suggest that ME-induced failure in learning and memory function may be mediated by dysfunction of the cyclic AMP/PKA/CREB signal transduction system, that rolipram may ameliorate ME-induced impairment of learning and memory function, and that the drug effect may be partly attributed to activation of the cyclic AMP/PKA/CREB signal transduction system.
Keywords: Rolipram, microsphere embolism, learning and memory, cyclic AMP/PKA/CREB signal transduction system
Introduction
The cyclic AMP response element binding protein (CREB) plays an important role in the intracellular signal transduction that is considered to be responsible for the regulation of learning and memory function. A transcription factor, CREB is phosphorylated and activated by the cyclic AMP-dependent protein kinase (PKA), and consequently binds to the cyclic AMP response element (CRE) of target genes (Habener, 1990), which genes may play an important role in memory, especially long-term memory formation following new protein synthesis (Randt et al., 1982; Guzowski & McGaugh, 1997). In fact, injection of cyclic AMP into the lateral ventricle is protective against the experimental amnesia in mice (Chute et al., 1981). Also, intrahippocampal treatment with 8-bromo cyclic AMP, an analogue of cyclic AMP, accelerated inhibitory avoidance task and was coupled to an increased level of phosphorylated CREB (pCREB) in rats (Bernabeu et al., 1997). Moreover, in Aplysia sensory neurons, repeated pulses with serotonin gave rise to distinct phases of persistent activation of PKA (Müller & Carew, 1998) and prolonged the phosphorylation of CREB (Bartsch et al., 1998). These results suggest that defects of the cyclic AMP/PKA/CREB signal transduction system may lead to learning and memory dysfunction.
Cerebral ischaemia in the 4-vessel ligation (Imanishi et al., 1997) and middle cerebral artery occlusion (Yonemori et al., 1999) models was shown to induce deficits in learning and memory function, but the possible relationship between learning and memory dysfunction and cyclic AMP/PKA/CREB signal transduction system in the ischaemic brain remains to be proven.
Rolipram, 4-[3-(cyclopentyloxy)-4-methoxyphenyl]-2-pyrrolidinone, is a phosphodiesterase (PDE) inhibitor that is selective for the cyclic AMP-specific isozyme of PDE (PDE-IV, Wachtel, 1983). This agent has been shown to improve the working memory deficits caused by administration of scopolamine (Zhang & O'donnell, 2000) or MK-801 (Zhang et al., 2000). Rolipram has also been reported to attenuate neuronal cell death in the CA1 sector after transient ischaemia (Kato et al., 1995; Block et al., 1997). Moreover, rolipram improved the working memory deficits of rats caused by 4-vessel ligation (Imanishi et al., 1997). These reports suggest that treatment with rolipram may be beneficial for transient ischaemic brain. However, there are no reports whether rolipram may improve the learning and memory dysfunction induced by sustained cerebral ischaemia. To evaluate the feasibility of rolipram as a therapeutic for cognitive dysfunction, we examined the effects of rolipram on impairment of learning and memory function and on the cyclic AMP/PKA/CREB signal transduction system in the ischaemic rats.
In the present study, we used microsphere-embolized rats as an ischaemic model. The microsphere-induced cerebral embolism induces sustained cerebral ischaemia following the production of small, widespread embolic infarcts, especially in the cerebral cortex, hippocampus, and striatum (Miyake et al., 1993). It should be noted that this model is considered to mimic focal ischaemia-induced human stroke (Lyden et al., 1992) or multi-infarct dementia (Naritomi, 1991).
Methods
Microsphere embolism (ME)
Male Wistar rats (Charles River Japan Inc., Atsugi, Japan), weighing 180 to 220 g, were maintained in an animal room having a 12-h light/12-h darkness cycle at a temperature of 23±1°C and with a humidity of 55±5% throughout the experiment. The animals had free access to food and water according to the National Institute of Health Guide for the Care and Use of Laboratory Animals and the Guideline of Experimental Animal Care issued by the Prime Minister's Office of Japan. All efforts were made to minimize suffering by the animals, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available. The study protocol was approved by the Committee of Animal Care and Welfare of Tokyo University of Pharmacy & Life Science.
Microsphere-induced cerebral embolism was performed by the method described previously (Miyake et al., 1993) with some modification. In brief, rats were anaesthetized i.p. with 35 mg kg−1 of sodium pentobarbitone. The right external carotid and pterygopalatine arteries were temporarily occluded with strings. A needle connected to a polyethylene catheter (3 Fr., Atom Co., Tokyo) was then inserted into the right common carotid artery. Next, 900 microspheres (47.5±0.5 μm in diameter, NEN-005, New England Nuclear Inc., Boston, U.S.A.), suspended in 20% dextran solution (150 μl), were injected into the right internal carotid artery through the cannula. After the needle had been removed, and the puncture was repaired with surgical glue (Aron Alpha A, Sankyo, Co., Tokyo, Japan), the strings occluding the right external carotid and pterygopalatine arteries were released. It took 2 to 3 min to restart the blood flow to the areas supplied by the right external carotid and pterygopalatine arteries. The rats that underwent sham operation were injected with the same volume of vehicle without microspheres. Age-matched, non-operated animals were used as controls. Fifteen hours after the operation, the behaviour of the operated rats was scored on the basis of paucity of movement, truncal curvature, and forced circling during locomotion, which were considered to be typical symptoms of stroke in rats (Furlow & Bass, 1976; McGraw, 1977). The score of each item was rated from 3 to 0 (3 very severe, 2 severe, 1 moderate, 0 little or none). The rats with the total score of 7 – 9 points were used in the present study.
Treatment with rolipram
After stroke-like symptoms of microsphere-injected rats had been detected, the animals were randomly divided into the two groups, rolipram-treated and untreated groups. Rolipram at a dose of 3 mg kg−1, dissolved in distilled water containing 2% dimethylsulphoxide (DMSO), was administered i.p. The first application of rolipram was given 6 h after the onset of the operation, and the administration was conducted once daily over the next 9 days. Vehicle was administered to the untreated group. The dose employed in the present study was based on the data described by others (Block et al., 1997; Kato et al., 1995) and our dose-response data on the escape latency in the water maze task, obtained in a preliminary study.
Water maze test
The water maze test was performed according to the method described by other investigators (Morris, 1981; Takagi et al., 1997). The test was started on day 7 after the operation. ME and sham-operated animals were tested in the water maze using a three trial/day-regimen. To eliminate rats that could not swim due to injury following ME, we performed an habituation study by placing the surgically treated rats in a pool with a diameter of 100 cm on day 6 after the operation. No ME rats were eliminated due to a failure in swimming in the present study. The water maze apparatus (model TARGET/2, Neuroscience Co., Tokyo, Japan) consisted of a circular pool with a diameter of 170 cm, which was filled with water of 30-cm depth and having a temperature of 23±1°C. A clear acrylic platform circle with a diameter of 12 cm was placed 1.5 cm below the surface of the water and kept in a constant position in the centre of one of the four quadrants of the pool. The pool was surrounded by several cues such as circles, triangles, and squares drawn on the shield fence. When the rat mounted the platform, it was kept there for 30 s. If the rat did not reach the platform, it was transferred on the platform by hand and kept there for 30 s. Data collection was automated by an on-line video-tracking device designed to track the object in the field. Tracking was achieved by a system consisting of a black-and-white video camera with a 4.8-mm wide-angle lens mounted approximately 170 cm directly over the centre of the pool. The tracker digitized coordinate values were sampled in turn by a PC-9801 computer. The animals were released from three randomly assigned start locations (excluding a platform-existed quadrant). Escape latency (the time to reach onto the platform), path length (the distance traveled to reach onto the platform), and swimming speed (the distance that the animals swam divided by escape time) were determined for each trial with a behavioural tracing analyser (BAT-2, Neuroscience Co., Tokyo, Japan). The cut-off time for each trial was set at 180 s. Each trial was performed with approximately 1 h of inter-trial interval. On the next day after 3 days of trials, the probe test with visible platform above the surface of the water was also performed to examine the ability of spatial navigation of the operated animals.
The water maze test was carried out using the sham-operated and microsphere-embolized animals with and without rolipram treatment (n=16). After the probe test, the animals were subjected to biochemical studies. Among 16 animals, eight animals were used for measurement of the cyclic AMP content and eight animals for determination of PKA and CREB levels for studying the relation of learning and memory function with variables in the signal transduction system.
Measurement of cyclic AMP content
The procedure for rapid fixation of the brain to determine cyclic AMP content was the same as that reported elsewhere (Jones et al., 1974). In brief, the head of a rat was focally irradiated (5 kw; 0.85 s) by a microwave applicator (model TMW-6402C, Muromachi Kikai Co., Tokyo, Japan). After decapitation, the head of the animal was immersed into liquid nitrogen for 15 s, and the cerebral cortex and hippocampus were isolated from the cerebral hemispheres (Glowinski & Iversen, 1966). Each frozen brain tissue was homogenized in a solution of 0.2 M HClO4 and 0.01% ethylenediamine-N,N,N′,N′-tetra-acetic acid (EDTA) with a Polytron homogenizer (PT-10, Kinematica, Littau/Luzern, Switzerland). The homogenates were centrifuged at 10,000×g for 15 min at 4°C. The supernatant fluid was neutralized with Na2CO3, and centrifuged at 10,000×g for 15 min at 4°C. The cyclic AMP content in the supernatant fluid was determined with a commercially available kit (Amersham cyclic AMP EIA system, RPN. 225; Amersham, London, U.K.) based on an enzyme immunoassay (EIA) using a non-acetylation procedure. The pellets from the homogenate obtained after centrifugation were digested with 2 N NaOH at 70°C. Protein concentrations in the extract were determined by the method of Lowry et al. (1951).
Preparation of nuclear extracts and cytosol fraction
Nuclear extracts were prepared by the method of Schreiber et al. (1989) with minor modifications. In brief, after decapitation, the head of the animal was immersed into liquid nitrogen for 15 s, and the cerebral cortex and hippocampus were isolated from the cerebral hemispheres. Each brain was homogenized in 10 volumes of buffer A (in mM): HEPES-NaOH (pH 7.9) 10, containing KCl 10, EDTA 1, ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetra-acetic acid (EGTA) 1, dithiothreitol (DTT) 5, sodium fluoride (NaF) 10, sodium pyrophosphate (NaPPi) 10, sodium orthovanadate (Na3VO4 1, sodium β-glycerophosphate 10, 1.25 μg ml−1 pepstatin A, 10 μg ml−1 leupeptin, 2.5 μg ml−1 aprotinin, and 1 phenylmethylsulphonyl fluoride (PMSF). Following the addition of 10% Nonidet P-40 for a final concentration of 1%, the homogenates were centrifuged at 15,000×g for 5 min at 4°C. Pellets were washed in three volumes of buffer A, and centrifuged at 15,000×g for 5 min at 4°C. The pellets were then suspended in one volume of buffer B (in mM): HEPES-NaOH (pH 7.9) 20, NaCl 400, EDTA 1, EGTA 1, DTT 5, NaF 10, NaPPi 10, Na3VO4 1, sodium β-glycerophosphate 10, 1.25 μg ml−1 pepstatin A, 10 μg ml−1 leupeptin, 2.5 μg ml−1 aprotinin, and 1 mM PMSF), and centrifuged at 15,000×g for 5 min. Supernatants thus obtained were stored at −80°C as nuclear extracts. The supernatant after ultracentrifugation at 100,000×g for 20 min after homogenation was used as a cytosolic preparation. The protein concentration of the samples was determined by the method prescribed by the protein assay kit (Protein assay, Bio-Rad, U.S.A.) used, i.e., the method of Bradford (1976).
Western immunoblotting for quantification of various'proteins
Western immunoblot analysis was carried out after 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS – PAGE). One sample containing 5 – 20 μg of protein was applied to each lane in a slab gel for SDS – PAGE. Following electrophoresis, the proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Immobilon PVDF, Millipore Co., Bedford, MA, U.S.A.). The membrane was incubated with one of the following primary antibodies: anti-pCREB (Cell Signaling Technology; corresponding to pCREB and directed against residues 129 – 137; 1 : 1000), anti-CREB (Cell Signaling Technology; directed against the CREB residues 123 – 137; 1 : 1000), anti-PKA catalytic subunit α (Cα., Santa Cruz Biotechnology; 1 : 1000), anti-PKA catalytic subunit β (Cβ., Santa Cruz Biotechnology; 1 : 1000), or anti-PKA regulatory IIα (RIIα., Santa Cruz Biotechnology; 1 : 1000). After the membrane had been incubated with horseradish peroxidase-conjugated anti-rabbit antibodies, the blots were developed by the enhanced chemiluminescence (ECL) detection method (Amersham Pharmacia Biotech, Buckinghamshire, U.K.). Quantification of the immunoreactive bands was performed by densitometric scanning of autoradiograms by using an Image Analysis System (NIH 1.61). For minimization of between-blot variability, an aliquot of pooled ‘control' membranes was run on one lane of every gel, and the immunolabelling was calculated relative to this standard. Immunoblotting of Cα, Cβ, and RIIα was also performed after preabsorption of these antibodies with their respective synthetic complementary peptides according to the method of Yamamoto et al. (1996).
Electrophoretic mobility shift assay (EMSA)
Double-stranded oligonucleotide (Promega; 5′-AGAGATTGCCTGACGTCAGAGAGCTAG-3′) containing the cyclic AMP-response element (CRE) consensus sequence (indicated in bold letters) was labelled with [γ-32P]-ATP by use of T4 polynucleotide kinase. The assays were performed as reported previously (Kornhauser et al., 1992). In brief, nuclear extracts were incubated with 35 fmol of 32P-labelled probe for 20 min at room temperature before electrophoresis on a 4% nondenaturing polyacrylamide gel in 0.5×TBE buffer containing 45 mM Tris-borate (pH 8.0) and 1 mM EDTA. In competition assays, a 25× excess of the unlabelled probe for CREB or activator protein-1 (AP-1) was added to the reaction mixture 10 min prior to the addition of the labelled probe. Supershift experiments were performed by preincubating the extracts during 30 min at room temperature with anti-CREB antibody. After electrophoresis, the gels were dried and exposed to X-ray film. Radioreactive bands were quantified by a liquid scintillation counter (LSC-3500, Aloka, Tokyo, Japan).
Statistical analysis
The results were presented as means±s.e.mean. Differences in values for the water maze test between sham-operated and rolipram-treated, sham-operated groups were evaluated statistically by using one-way analysis of variance (ANOVA) for repeated measures, and those in escape latency, distance travelled, and swimming speed between ME, rolipram-treated ME, and sham-operated groups, by using two-way ANOVA for repeated measures followed by Fisher's post-hoc protected least-significant differences (PLSD). Differences in the time to reach onto the visible platform and the distance travelled in the probe test between the sham-operated and microsphere-embolized animals with and without rolipram treatment were statistically evaluated by two-way ANOVA for repeated measures. Differences in biochemical variables between ME, rolipram-treated ME, sham-operated, and rolipram-treated sham-operated groups were evaluated by using two-way ANOVA followed by post-hoc Fisher's PLSD. Differences with a probability of 5% or less were considered to be significant (P<0.05).
Results
Operation
Among 55 microsphere-injected rats, eight animals (15%) died by day 1 after surgery. Forty-two of the surviving rats (76%) showed stroke-like symptoms with a total score of 7 – 9 points and five animals (9%) showed the symptoms of stroke to be less than seven points as described in Methods. Among the animals with the total score of 7 – 9, four rats (10%) died within 10 days after the operation. There were no stroke-like symptoms or mortality in the sham-operated animals (n=32).
Effects of rolipram on results of water maze task
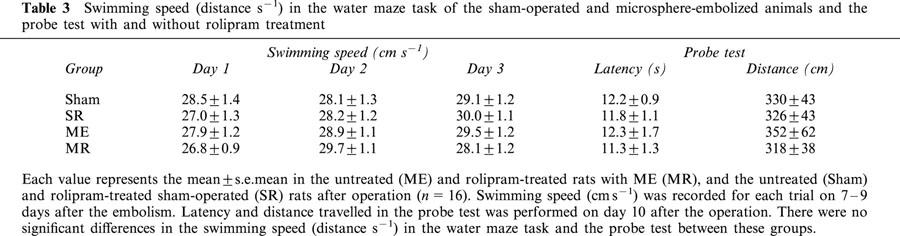
In the first set of experiments, the effect of rolipram on learning and memory function after ME was examined. The escape latency in the water maze task was measured on the day 7 to 9 after the operation. The escape latency of sham-operated rats was similar to that of the rolipram-treated, sham-operated ones (F[1,8]=0.272, P=0.9745, Table 1). The escape latency in the water maze task was significantly longer in the ME rats than in sham-operated rats (F[1,8]=11.963, P<0.0001). Rolipram-treated ME rats showed a significant attenuation in the prolongation of the escape latency compared with ME rats (F[1,8]=4.238, P<0.0001, Figure 1). The swimming distance of sham-operated rats was similar to that of the rolipram-treated, sham-operated ones (F[1,8]=0.076, P=0.9997). The distance in the water maze task of the ME rats was significantly longer than that of sham-operated rats (F[1,8]=5.400, P<0.0001). Rolipram-treated ME rats showed a significant shortening in the swimming distance compared with ME rats (F[1,8]=2.221, P=0.0346, Table 2). The swimming speed (the distance that the animals swam divided by escape time) in the water maze task was similar in all groups examined. Furthermore, the escape latency and distance of the probe test did not differ in all of the four groups (Table 3).
Table 1.
Escape latency (s) in sham-operated and rolipram-treated, sham-operated rats
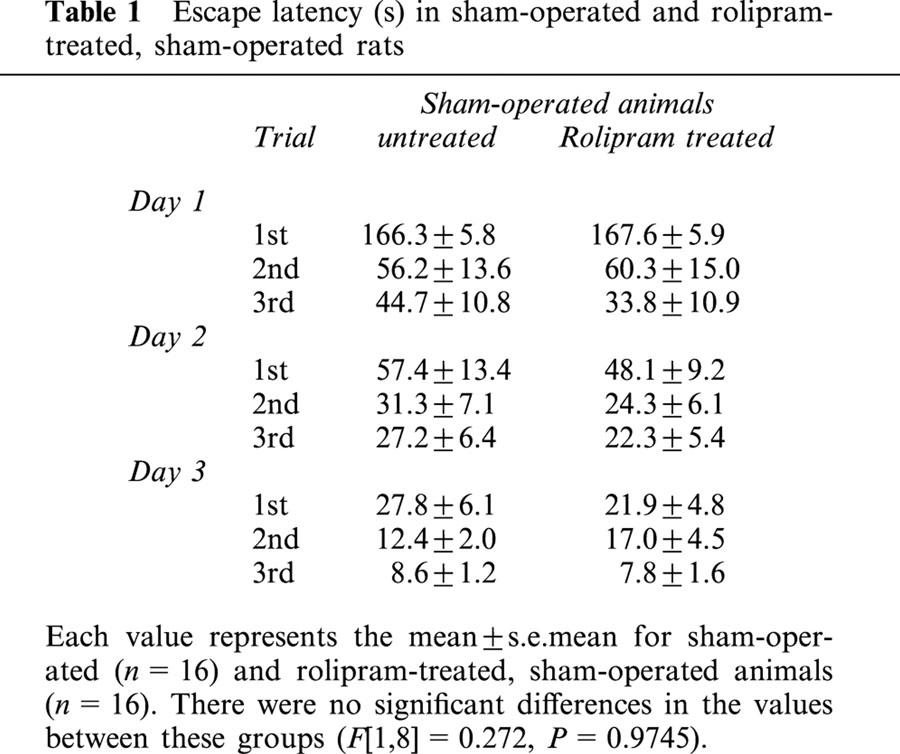
Figure 1.
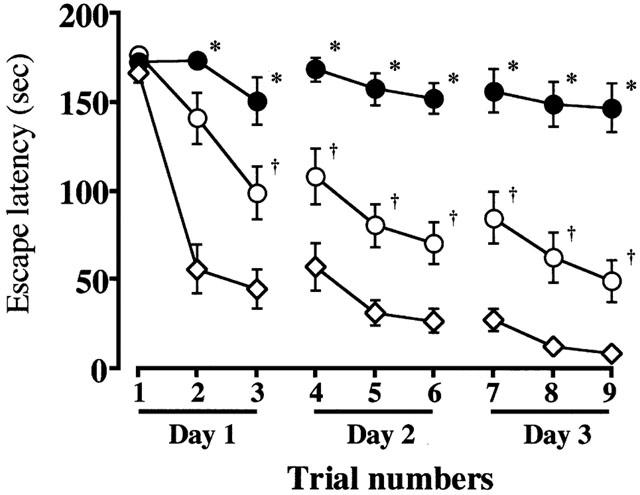
Changes in escape latency of the water maze task of the untreated (n=16, ME: closed circles) and rolipram-treated rats with ME (n=16, MR: open circles), and the untreated sham-operated rats (n=16; S: open diamonds). The escape latency was determined from day 7 to 9 after the ME. Each value represents the mean±s.e.mean. Two-way ANOVA of the data revealed significant differences in the escape latency between the sham-operated and ME rats (P<0.0001), and between untreated and rolipram-treated rats with ME (P<0.0001). *Significantly different (P<0.05) from the sham-operated group when estimated by Fisher's post-hoc protected least-significant differences (PLSD). †Significantly different (P<0.05) from the corresponding untreated ME group when estimated by Fisher's PLSD.
Table 2.
Distance (cm) in the water maze test of the sham-operated and microsphere-embolized animals with and without rolipram treatment
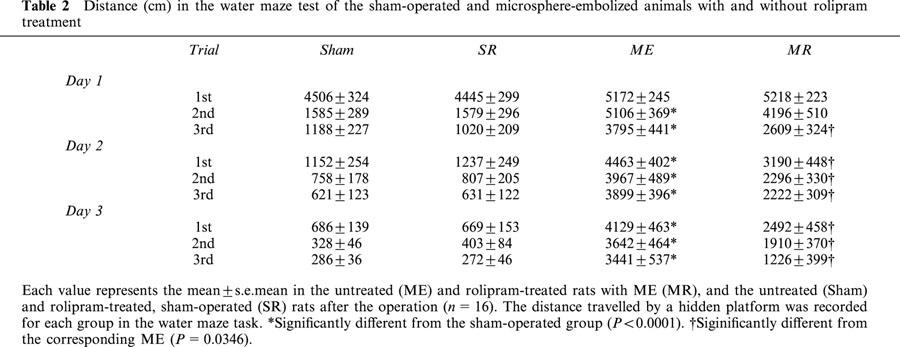
Table 3.
Swimming speed (distance s−1) in the water maze task of the sham-operated and microsphere-embolized animals and the probe test with and without rolipram treatment
Effects of rolipram on cyclic AMP content
In the next experiments, the effects of rolipram on the cyclic AMP content of the cerebral cortex and hippocampus of the ME rats on the next day after the last water maze task (day 10 after the operation) were examined. A significant decrease in cyclic AMP content was seen in the cerebral cortex and hippocampus of the rats with ME. Treatment with rolipram partially attenuated the decrease in the cyclic AMP content in both regions (Figure 2).
Figure 2.

Cyclic AMP content of the cerebral cortex (a) and hippocampus (b) in the right hemisphere of the untreated (n=8, ME) and rolipram-treated rats with ME (n=8, MR), and in the untreated (n=8, S) and rolipram-treated sham-operated (n=8, SR) rats, on day 10 after the operation. Each value represents the mean±s.e.mean. *Significantly different from the sham-operated group (P<0.05). †Significantly different from the corresponding untreated ME group (P<0.05).
Effects of rolipram on PKA subunits in cytosolic and nuclear fractions
Changes in PKA subunits (RIIα, Cα, and Cβ) in the cytosol were examined in the cerebral cortex and hippocampus of the ME rat on the next day after the last water maze task (Figure 3). The immunoreactivity of cytosolic Cβ in the cerebral cortex and hippocampus after ME decreased (Table 3). Treatment with rolipram partially attenuated this decrease in both regions. No significant alterations in the immunoreactivity of cytosolic Cα and RIIα were seen in the ME and rolipram-treated ME animals (Table 3).
Figure 3.
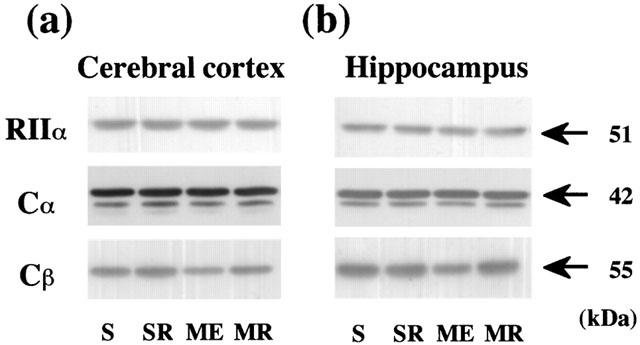
Western immunoblotting analysis of cytosolic PKA subunits (RIIα, Cα, and Cβ) in the cerebral cortex and hippocampus. Representative Western immunoblotting of cytosolic PKA subunits indicating the specific 51-kDa band for RIIα, 42-kDa one for Cα, and 55-kDa one for Cβ of the cerebral cortex (a) and hippocampus (b) from the right hemisphere of the untreated (ME) and rolipram-treated rats with ME (MR), and from that of the untreated (S) and rolipram-treated sham-operated (SR) rats on day 10 after the operation.
Representative immunoreactivities of PKA Cα and Cβ in nuclear extracts from ME and rolipram-treated rats are shown in Figure 4. The immunoreactivity of nuclear Cα and Cβ in the cerebral cortex and hippocampus after ME decreased. Treatment with rolipram partially attenuated these decreases in both regions (Figure 4).
Figure 4.
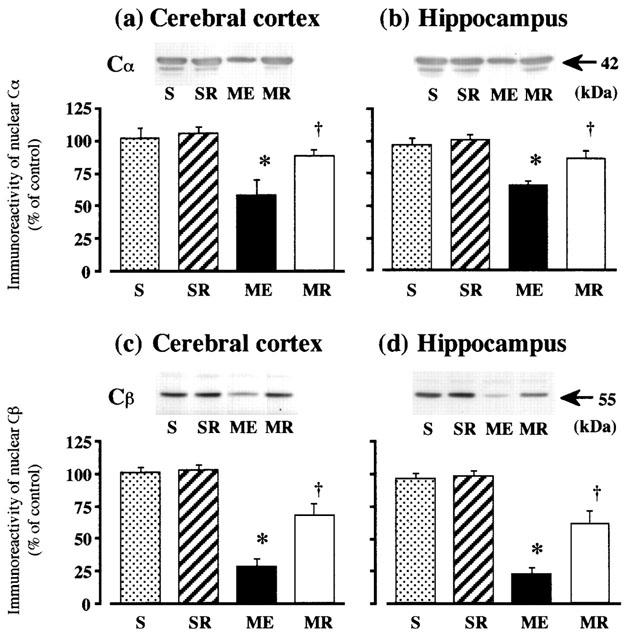
Western immunoblot analysis of nuclear PKA subunits (Cα and Cβ) in the cerebral cortex and hippocampus. Representative Western immunoblots in the upper panels indicate the specific 42-kDa band for Cα in the cerebral cortex (a) and hippocampus (b), and the 55-kDa band for Cβ in the cerebral cortex (c) and hippocampus (d) from the right hemisphere of the untreated (n=8, ME) and rolipram treated rats with ME (n=8, MR), and from that of the untreated (n=8, S) and rolipram-treated sham-operated (n=8, SR) rats, on day 10 after the operation. Quantified data for the levels of Cα and Cβ proteins in the cerebral cortex and hippocampus are shown in the lower panels. Each value represents the mean percentage of the control±s.e.mean. *Significantly different from the sham-operated group (P<0.05). †Significantly different from the corresponding untreated ME group (P<0.05).
Effects of rolipram on CREB
The effects of rolipram on immunoreactivities of pCREB and total CREB of the cerebral cortex and hippocampus from ME rats on the next day after the last water maze task were examined by Western immunoblotting. A significant decrease in immunoreactivities of pCREB was seen in the two regions after ME. Treatment with rolipram partially attenuated the decrease in the immunoreactivity of pCREB in both regions (Figure 5). The total CREB in the two regions of ME rats was not changed, and treatment with rolipram did not alter the total CREB, either (Figure 5).
Figure 5.
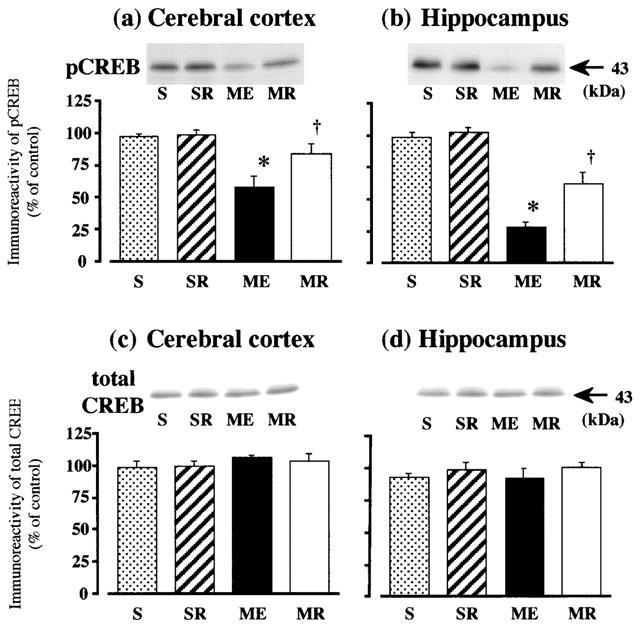
Western immunoblot analysis of pCREB and total CREB in the cerebral cortex and hippocampus. Representative Western immunoblots in the upper panels indicate the specific 43-kDa band for pCREB in the cerebral cortex (a) and hippocampus (b), and total CREB of the cerebral cortex (c) and hippocampus (d) in the right hemisphere of the untreated (n=8, ME) and rolipram-treated rats with ME (n=8, MR), and in that of the untreated (n=8, S) and rolipram-treated sham-operated (n=8, SR) rats, on day 10 after the operation. Quantified data for the levels of pCREB protein and total CREB protein of the cerebral cortex and hippocampus are shown in the lower panels. Each value represents the mean percentage of the control±s.e.mean. *Significantly different from the sham-operated group (P<0.05). †Significantly different from the corresponding untreated ME group (P<0.05).
Effects of rolipram on CRE-binding activity of CREB
The electrophoretic mobility shift assay (EMSA) performed with a CRE-containing oligonucleotide probe revealed a binding complex. Figure 6 shows EMSA using nuclear extracts prepared from the cerebral cortex and hippocampus of a control animal. The first lanes in Figure 6a,d show a retardation of probe mobility caused by CREB binding to the CRE oligonucleotide in the absence of an antibody or any competitor oligonucleotide. The addition of CREB antibody was able to supershift the entire retarded band (marked by the arrowhead). In addition, inclusion of excess cold CRE oligonucleotide blocked the binding of the CREB protein to the radioactive probe, whereas excess AP-1 oligonucleotide had no effect on the CREB binding (marked by arrow). These results indicate that the shift was caused by binding of CREB-related proteins. The effects of rolipram on immunoreactivities of CRE-binding activity of CREB of the cerebral cortex and hippocampus of ME on the next day after the last water maze task were examined (Figure 6b,c,e,f). A significant decrease in radioactivity representing CREB-binding was seen in the cerebral cortex and hippocampus after ME. Treatment with rolipram partially attenuated this decrease in both regions.
Figure 6.
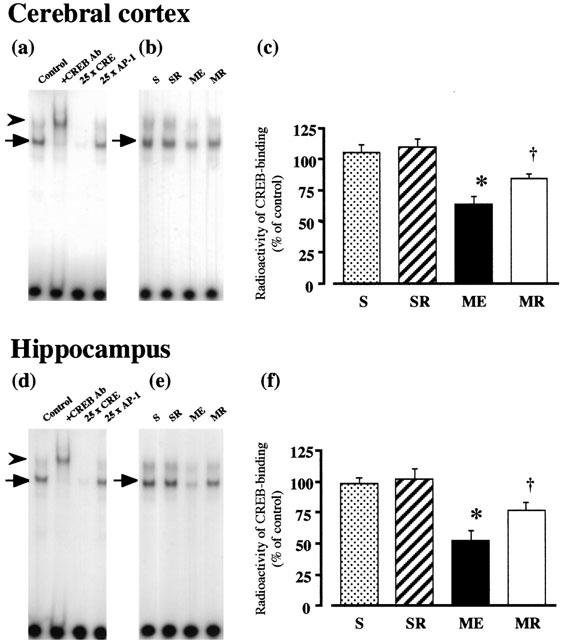
EMSA of CREB-binding activity in the cerebral cortex and hippocampus. Representative autoradiograms on the left showing CREB-binding in the cerebral cortex (a) and hippocampus (d). The first lane shows a retardation caused by CREB binding to the CRE oligonucleotide in the absence of an antibody or any competitor oligonucleotides. Addition of CREB antibody was able to supershift the entire shifted band (marked by the arrowhead). In addition, inclusion of excess cold CRE oligonucleotide blocked the binding of the CREB protein to the radioactive CREB band, whereas excess AP-1 oligonucleotide had no effect on the CREB binding (marked by the arrow), indicating that the shift was caused by binding of CREB-related proteins. Representative autoradiograms on the right indicate the specific band for CREB-CRE binding in samples from the cerebral cortex (b) and hippocampus (e), from the right hemisphere of the untreated (n=8, ME) and rolipram-treated rats with ME (n=8, MR), and from that of the untreated (n=8, S) and rolipram-treated sham-operated (n=8, SR) rats, on day 10 after the operation. Bands corresponding to CREB-CRE binding were cut out, and counted by liquid scintillation spectrometry. Quantified data for the level of CREB binding activity of the cerebral cortex (c) and hippocampus (f) are shown. Each value represents the mean percentage of the control±s.e.mean. *Significantly different from the sham-operated group (P<0.05). †Significantly different from the corresponding untreated ME group (P<0.05).
Discussion
In the present study, we examined the effects of rolipram, a PDE-IV inhibitor, on ME-induced learning and memory dysfunction and damage to cyclic AMP/PKA/CREB signal transduction. Several investigations have suggested the possible involvement of the parietal cortex and hippocampus in spatial learning (Dimattia & Kesner, 1988; Save et al., 1992), and a selective role of the hippocampus in a higher-order form of memory (O'keefe & Nadel, 1978; Jarrard, 1993). Furthermore, we observed in a previous study that the cerebral cortex and hippocampus were severely impaired by ME (Miyake et al., 1993). Therefore, we examined learning and memory function of ME rat in association with biochemical alterations in the cyclic AMP/PKA/CREB signal transduction system in the cerebral cortex and hippocampus of the brain.
Several phosphodiesterase inhibitors have been shown to enhance learning and memory function and to ameliorate experimentally induced amnesia or cognitive dysfunction in both animals and humans. For example, vinpocetin, which selectively inhibits Ca2+/calmodulin-dependent PDE, suppressed scopolamine- and hypoxia-induced retrieval deficits of the passive avoidance response in rats (Denoble et al., 1986), and enhanced memory in normal healthy volunteers (Subhan & Hindmarch, 1985). Propentofylline, which is a non-selective PDE inhibitor, improved the decreased ability to learn shuttle avoidance behaviour seen in spontaneously hypertensive rats (Goto et al., 1987). Also, nonspecific PDE inhibitors, such as papaverine and isobutylmethylxanthine, were found to improve memory in passive avoidance tasks in rodents (Villiger & Dunn, 1981). Moreover, in rats, rolipram improved the working memory deficits induced by scopolamine or MK-801 (Zhang & O'donnell, 2000; Zhang et al., 2000) as well as by transient ischaemia (Imanishi et al., 1997). These results suggest that PDE inhibitors may play a significant role in learning and memory function. In the present study, the results of the water maze task showed that rolipram improved the learning and memory dysfunction induced by ME in rats. These results suggest that the anti-amnesic effect of rolipram could be related to cyclic AMP levels. However, there is no information about the effect of rolipram on downstream components of cyclic AMP signal transduction in cerebral ischaemia.
There is increasing evidence for a relationship between the cyclic AMP/PKA/CREB signal transduction system and learning and memory function in the mammalian brain (Bernabeu et al., 1997; Silva et al., 1998). We studied the effect of rolipram on pathophysiological changes in the cyclic AMP/PKA/CREB signal transduction system of ME rats after the animals had been examined in the water maze task. At first, we observed that rolipram attenuated the decrease in cyclic AMP content after ME (Figure 2). It was earlier demonstrated that rolipram elevated the brain cyclic AMP level in vivo (Randt et al., 1982; Schneider, 1984), but there is no report that rolipram attenuated the decrease in cyclic AMP content after cerebral ischaemia in association with recovery of learning and memory function. Our results suggest that rolipram may improve ME-induced learning and memory dysfunction due to an increase in cyclic AMP.
Next, we studied the effect of rolipram treatment on the immunoreactivity of PKA subunits (Cα, Cβ, and RIIα) in ME animals. The protein level of Cβ in the cytosol of the ipsilateral hemisphere was reduced after ME, and rolipram attenuated this decrease (Figure 3, Table 1). There were, however, no alterations in the protein levels of Cα, another catalytic subunit, or of RIIα, the regulatory subunit irrespective of ME or rolipram treatment (Figure 3, Table 1). We observed that treatment with rolipram attenuated the ME-induced reduction in the nuclear Cα and Cβ levels (Figure 4). We detected a very small amount of RIIα in the nuclear fraction (data not shown), which is consistent with the earlier finding that RII subunits did not translocate to the nucleus (Ventra et al., 1996). As cytosolic PKA activated by cyclic AMP dissociates the regulatory subunit from the catalytic one, which action is followed by the subsequent translocation of the catalytic subunit into the nucleus (Bacskai et al., 1993; Hagiwara et al., 1993), our results suggest that PKA catalytic subunits cannot translocate from the cytosol to the nucleus after ME because of a decrease in the cyclic AMP level. This may lead to impairments of this signal transduction. Treatment with rolipram may thus improve the cyclic AMP signal transduction system of ME animals.
Previously, the PKA catalytic subunit was shown to translocate into the nucleus, where it phosphorylated CREB (Hagiwara et al., 1993). We analysed the immunoblotting profile of pCREB and CREB with specific antibodies after ME in rats with and without rolipram treatment. We observed a decrease in pCREB in the cerebral cortex and hippocampus of the ME rat on day 10. Rolipram attenuated the decrease in pCREB after ME (Figure 5). In contrast, there were no alterations in the protein level of total CREB (Figure 5). Such results suggest that the attenuation of the ME-induced decrease in pCREB by treatment with rolipram may be due to restoration of PKA catalytic subunits in the nucleus.
To further characterize ischaemic alterations in the activity of CREB in the brain, we studied, by EMSA, changes in CRE-DNA binding activity of CREB of ME rats. We observed a decrease in the CRE-binding activity in the cerebral cortex and hippocampus of the ME rat on day 10, and showed that rolipram attenuated this decrease (Figure 6), which attenuation was coincided with that of the reduced level of pCREB. These results suggest that ME may impair new protein transcription for learning and memory via CREB and that treatment with rolipram improved this function.
Several studies suggest a possible connection between the cyclic AMP/PKA/CREB signal transduction system and learning and memory. For example, overexpression of the R(AB) inhibitory subunit of PKA in transgenic mice led to deficits in spatial learning and in long-term context-dependent fear conditioning (Abel et al., 1997). PKA inhibitors hindered long-term memory for inhibitory avoidance in the chick brain (Zhao et al., 1995). In CREB knockout mice, long-term memory of fear conditioning and spatial learning was disrupted, whereas acquisition and short-term memory were normal (Bourtchuladze et al., 1994; Kogan et al., 1996). Also, intrahippocampal infusion of CREB antisense oligonucleotides several hours prior to learning reduced the level of CREB protein in the hippocampus and disrupted long-term spatial memory (Guzowski & McGaugh, 1997). It was also noted that the pCREB level was decreased in the hippocampus of patients with dementia of the Alzheimer's disease but that the total CREB level was unchanged (Yamamoto et al., 1999). Furthermore, age-dependent decrease in CRE-binding activity was shown by EMSA (Asanuma et al., 1996). We found learning and memory dysfunction in ME rats in the present study. Taken together, the data strongly suggest that reductions in the pCREB level and CRE-binding activity may play an important role in the mechanism generating of learning and memory dysfunction.
In this study, we observed that treatment with rolipram attenuated the dysfunction of cyclic AMP/PKA/CREB signal transduction system in the ME animal. It is considered that inhibition of PDE IV by rolipram treatment leads to an increase in the level of cyclic AMP, activation of PKA, and subsequent phosphorylation of CREB. This phosphorylated CREB may then bind to the CRE element of the promoter regions of various genes, and consequently may promote the transcription of proteins involved in learning and memory function.
In summary, we demonstrated the anti-amnesia effects of rolipram in the spatial memory of ME rats. Rolipram partially attenuated the ME-induced decreases in cyclic AMP content, cytosolic PKA Cβ, nuclear PKA Cα and Cβ, pCREB, and CRE-binding activity. These results suggest that rolipram improves impairment of learning and memory and attenuates the deleterious changes in the cyclic AMP/PKA/CREB signal transduction system in the ME rat. Rolipram was reported earlier to attenuate the neuronal damage to the CA1 sector seen after transient ischaemia (Kato et al., 1995; Block et al., 1997). Our study has shown an additional effect of rolipram on learning and memory function and on the cyclic AMP/PKA/CREB signal transduction system.
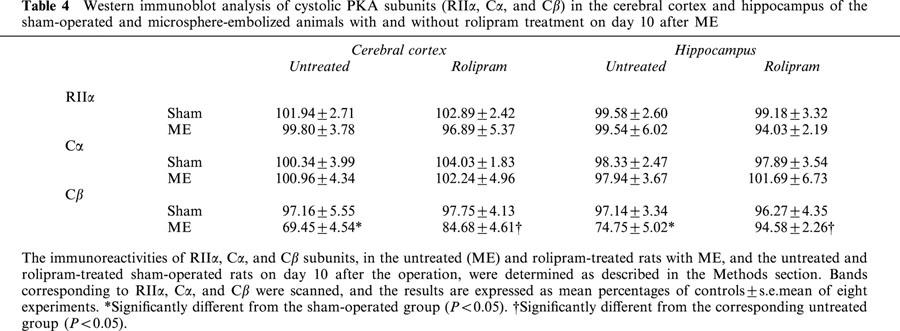
Table 4.
Western immunoblot analysis of cystolic PKA subunits (RIIα, Cα, and Cβ) in the cerebral cortex and hippocampus of the sham-operated and microsphere-embolized animals with and without rolipram treatment on day 10 after ME
Acknowledgments
We gratefully acknowledge Meiji Seika Co., Ltd., Tokyo, Japan, for their kind gift of rolipram (ME3167), for physicochemical and biochemical information about rolipram, and for helpful advice.
Abbreviations
- ANOVA
analysis of variance
- AP-1
activator protein-1
- CRE
cyclic AMP response element
- CREB
cyclic AMP response element binding protein
- DMSO
dimethylsulphoxide
- DTT
dithiothreitol
- ECL
enhanced chemiluminescence
- EDTA
ethylenediamine-N,N,N′,N′-tetra-acetic acid
- EGTA
ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetra-acetic acid
- EIA
enzyme immunoassay
- EMSA
electrophoretic mobility shift assay
- HEPES
2-[4-2(hydroxyethyl)-1-piperazinyl]ethanesulphonic acid
- HRP
horseradish peroxidase
- i.p.
intraperitoneal
- LTP
long-term potentiation
- ME
microsphere embolism
- ME rat
microsphere-embolized rat
- NIH
National Institutes of Health
- NaPPi
sodium pyrophosphate
- NaF
sodium fluoride
- Na3VO4
sodium orthovanadate
- pCREB
phosphorylated CREB
- PDE
phosphodiesterase
- PKA
protein kinase A
- PMSF
phenylmethylsulphonyl fluoride
- PLSD
post-hoc protected least-significant differences
- PVDF
polyvinylidene difluoride
- SDS – PAGE
sodium dodecylsulphate-polyacrylamide gel electrophoresis
References
- ABEL T., NGUYEN P.V., BARAD M., DEUEL T.A.S., KANDEL E.R., BOURTCHOULADZE R. Genetic demonstration of a role for PKA in the late phase of LTP and hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- ASANUMA M., NISHIBAYASHI S., IWATA E., KONDO Y., NAKANISHI T., VARGAS M.G., OGAWA N. Alterations of cAMP response element-binding activity in the aged rat brain in response to administration of rolipram, a cAMP-specific phosphodiesterase inhibitor. Mol. Brain Res. 1996;41:210–215. doi: 10.1016/0169-328x(96)00098-8. [DOI] [PubMed] [Google Scholar]
- BACSKAI B.J., HOCHNER B., MAHAUT-SMITH M., ADAMS S.R., KAANG B.K., KANDEL E.R., TSIEN R.Y. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993;260:222–226. doi: 10.1126/science.7682336. [DOI] [PubMed] [Google Scholar]
- BARTSCH D., CASADIO A., KARL K.A., SERODIO P., KANDEL E.R. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- BERNABEU R., BEVILAQUA L., ARDENGHI P., BROMBERG E., SCHMITZ P., BIANCHIN M., IZQUIERDO I., MEDINA J.H. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc. Natl. Acad. Sci. U.S.A. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOCK F., TONDAR A., SCHMIDT W., SCHWARZ M. Delayed treatment with rolipram protects against neuronal damage following global ischaemia in rats. NeuroReport. 1997;8:3829–3832. doi: 10.1097/00001756-199712010-00033. [DOI] [PubMed] [Google Scholar]
- BOURTCHULADZE R., FRENGUELLI B., BLENDY J., CIOFFI D., SCHUTZ G., SILVA A.J. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CHUTE D.L., VILLIGER J.W., KIRTON N.F. Testing cyclic AMP mediation of memory: reversal of α-methyl-ρ-tyrosine-induced amnesia. Psychopharmacology. 1981;74:129–131. doi: 10.1007/BF00432678. [DOI] [PubMed] [Google Scholar]
- DENOBLE V.J., REPETTI S.J., GELPKE L.W., WOOD L.M., KEIM K.L. Vinpocetine: nootropic effects on scopolamine-induced and hypoxia-induced retrieval deficits of a step-through passive avoidance response in rats. Pharmacol. Biochem. Behav. 1986;24:1123–1128. doi: 10.1016/0091-3057(86)90465-x. [DOI] [PubMed] [Google Scholar]
- DIMATTIA B.D., KESNER R.P. Spatial cognitive maps: differential role of parietal cortex and hippocampal formation. Behav. Neurosci. 1988;102:471–480. doi: 10.1037//0735-7044.102.4.471. [DOI] [PubMed] [Google Scholar]
- FURLOW T.W., BASS N.H. Arachidonate-induced cerebrovascular occlusion in the rat. Neurology. 1976;26:297–304. doi: 10.1212/wnl.26.4.297. [DOI] [PubMed] [Google Scholar]
- GLOWINSKI J., IVERSEN L.L. Regional studies of catecholamines in the rat brain-I. J. Neurochem. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- GOTO M., DEMURA N., SAKAGUCHI T. Effects of propentofylline on disorder of learning and memory in rodents. Japan J. Pharmacol. 1987;45:373–378. doi: 10.1254/jjp.45.373. [DOI] [PubMed] [Google Scholar]
- GUZOWSKI J.F., MCGAUGH J.L. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABENER J.F. Cyclic AMP response element binding proteins: a cornucopia of transcription factors. Mol. Endocrinol. 1990;4:1087–1094. doi: 10.1210/mend-4-8-1087. [DOI] [PubMed] [Google Scholar]
- HAGIWARA M., BRINDLE P., HAROOTUNIAN A., ARMSTRONG R., RIVIER J., VALE W., TSIEN R., MONTMINY M.R. Coupling of hormonal stimulation and transcription via the cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol. Cell Biol. 1993;13:4852–4859. doi: 10.1128/mcb.13.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMANISHI T., SAWA A., ICHIMARU Y., MIYASHIRO M., KATO S., YAMAMOTO T., UEKI S. Ameliorating effects of rolipram on experimentally induced impairments of learning and memory in rodents. Eur. J. Pharmacol. 1997;321:273–278. doi: 10.1016/s0014-2999(96)00969-7. [DOI] [PubMed] [Google Scholar]
- JARRARD L.E. On the role of the hippocampus in learning and memory in the rat. Behav. Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- JONES D.J., MEDINA M.A., ROSS D.H., STAVINOHA W.B. Rate of inactivation of adenyl cyclase and phosphodiesterase: determinants of brain cyclic AMP. Life Sci. 1974;14:1577–1585. doi: 10.1016/0024-3205(74)90168-4. [DOI] [PubMed] [Google Scholar]
- KATO H., ARAKI T., ITOYAMA Y., KOGURE K. Rolipram, a cyclic AMP-selective phosphodiesterase inhibitor, reduces neuronal damage following cerebral ischemia in the gerbil. Eur. J. Pharmacol. 1995;272:107–110. doi: 10.1016/0014-2999(94)00694-3. [DOI] [PubMed] [Google Scholar]
- KOGAN J.H., FRANKLAND P.W., BLENDY J.A., COBLENTZ J., MAROWITZ Z., SCHÜTZ G., SILVA A.J. Spaced training induces normal long-term memory in CREB mutant mice. Curr. Biol. 1996;7:1–11. doi: 10.1016/s0960-9822(06)00022-4. [DOI] [PubMed] [Google Scholar]
- KORNHAUSER J.M., NELSON D.E., MAYO K.E., TAKAHASHI J.S. Regulation of jun-B messenger RNA and AP-1 activity by light and a circadian clock. Science. 1992;255:1581–1584. doi: 10.1126/science.1549784. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- LYDEN P.D., ZIVIN J.A., CHABOLLA D.R., JACOBS M.A., GAGE F.H. Quantitative effects of cerebral infarction on spatial learning in rats. Exp. Neurol. 1992;116:122–132. doi: 10.1016/0014-4886(92)90160-r. [DOI] [PubMed] [Google Scholar]
- MCGRAW C.P. Experimental cerebral infarction effects of pentobarbital in mongolian gerbils. Arch. Neurol. 1977;34:334–336. doi: 10.1001/archneur.1977.00500180028006. [DOI] [PubMed] [Google Scholar]
- MIYAKE K., TAKEO S., KAJIHARA H. Sustained decrease in brain regional blood flow after microsphere embolism in rats. Stroke. 1993;24:415–420. doi: 10.1161/01.str.24.3.415. [DOI] [PubMed] [Google Scholar]
- MORRIS R.G.M. Spatial localization does not require the presence of local cues. Learn. Motiv. 1981;12:239–260. [Google Scholar]
- MÜLLER U., CAREW T.J. Serotonin induces temporally and mechanistically distinct phases of persistent PKA activity in Aplysia sensory neurons. Neuron. 1998;21:1423–1434. doi: 10.1016/s0896-6273(00)80660-1. [DOI] [PubMed] [Google Scholar]
- NARITOMI H. Experimental basis of multi-infarct dementia: memory impairments in rodent models of ischemia. Alzheimer Dis. Assoc. Disord. 1991;5:103–111. doi: 10.1097/00002093-199100520-00007. [DOI] [PubMed] [Google Scholar]
- O'KEEFE J., NADEL L. The hippocampus as a cognitive map. Oxford: Oxford University Press; 1978. [Google Scholar]
- RANDT C.T., JUDGE M.E., BONNET K.A., QUARTERMAIN D. Brain cyclic AMP and memory in mice. Pharmacol. Biochem. Behav. 1982;17:677–680. doi: 10.1016/0091-3057(82)90344-6. [DOI] [PubMed] [Google Scholar]
- SAVE E., POUCET B., FOREMAN N., BUHOT M.-C. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav. Neurosci. 1992;106:447–456. [PubMed] [Google Scholar]
- SCHNEIDER H.H. Brain cAMP response to phosphodiesterase inhibitors in rats killed by microwave irradiation or decapitation. Biochem. Pharmacol. 1984;33:1690–1693. doi: 10.1016/0006-2952(84)90295-8. [DOI] [PubMed] [Google Scholar]
- SCHREIBER E., MATTHIAS P., MÜLLER M.M., SCHAFFNER W. Rapid detection of octamer binding proteins with ‘mini-extracts', prepared from a small number of cells. Nucleic. Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUBHAN Z., HINDMARCH I. Psychopharmacological effects of vinpocetine in normal healthy volunteers. Eur. J. Clin. Pharmacol. 1985;28:567–571. doi: 10.1007/BF00544068. [DOI] [PubMed] [Google Scholar]
- SILVA A.J., KOGAN J.H., FRANKLAND P.W., KIDA S. CREB and memory. Annu. Rev. Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- TAKAGI N., MIYAKE K., TAGUCHI T., TAMADA H., TAKAGI K., SUGITA N., TAKEO S. Failure in learning task and loss of cortical cholinergic fibers in microsphere-embolized rats. Exp. Brain Res. 1997;114:279–287. doi: 10.1007/pl00005636. [DOI] [PubMed] [Google Scholar]
- VENTRA C., PORCELLINI A., FELICIELLO A., GALLO A., PAOLILLO M., MELE E., AVVEDIMENTO V.E., SCHETTINI G. The differential response of protein kinase A to cyclic AMP in discrete brain areas correlates with the abundance of regulatory subunit II. J. Neurochem. 1996;66:1752–1761. doi: 10.1046/j.1471-4159.1996.66041752.x. [DOI] [PubMed] [Google Scholar]
- VILLIGER J.W., DUNN A.J. Phosphodiesterase inhibitors facilitate memory for passive avoidance conditioning. Behav. Neural Biol. 1981;31:354–359. doi: 10.1016/s0163-1047(81)91424-2. [DOI] [PubMed] [Google Scholar]
- WACHTEL H. Potential antidepressant activity of rolipram and other selective cyclic adenosine 3′,5′-monophosphate phosphodiesterase inhibitors. Neuropharmacology. 1983;22:267–272. doi: 10.1016/0028-3908(83)90239-3. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO M., OZAWA H., SAITO T., FRÖLICH L., RIEDERER P., TAKAHATA N. Reduced immunoreactivity of adenylyl cyclase in dementia of the Alzheimer type. NeuroReport. 1996;7:2965–2970. doi: 10.1097/00001756-199611250-00033. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO M., OZAWA H., SAITO T., RÖSLER M., RIEDERER P. Impaired phosphorylation of cyclic AMP response element binding protein in the hippocampus of dementia of the Alzheimer type. Brain Res. 1999;824:300–303. doi: 10.1016/s0006-8993(99)01220-2. [DOI] [PubMed] [Google Scholar]
- YONEMORI F., YAMAGUCHI T., YAMADA H., TAMURA A. Spatial cognitive performance after chronic focal cerebral ischemia in rats. J. Cereb. Blood Flow Met. 1999;19:483–494. doi: 10.1097/00004647-199905000-00002. [DOI] [PubMed] [Google Scholar]
- ZHANG H.-T., CRISSMAN A.M., DORAIRAJ N.R., CHANDLER L.J., O'DONNELL J.M. Inhibition of cyclic AMP phosphodiesterase (PDE4) reverses memory deficits associated with NMDA receptor antagonism. Neuropsychopharmacol. 2000;23:198–204. doi: 10.1016/S0893-133X(00)00108-1. [DOI] [PubMed] [Google Scholar]
- ZHANG H.-T., O'DONNELL J.M. Effects of rolipram on scopolamine-induced impairment of working and reference memory in the radial-arm maze tests in rats. Psychopharmacol. 2000;150:311–316. doi: 10.1007/s002130000414. [DOI] [PubMed] [Google Scholar]
- ZHAO W.Q., POLYA G.M., WANG B.H., GIBBS M.E., SEDMAN G.L., NG K.T. Inhibitors of cAMP-dependent protein kinase impair long-term memory formation in day-old chicks. Neurobiol. Learn. Mem. 1995;64:106–118. doi: 10.1006/nlme.1995.1049. [DOI] [PubMed] [Google Scholar]


