Abstract
The contribution of the local vascular production of angiotensin-(1-7) [Ang-(1-7)] to the control of α-adrenergic-induced contractions in the aorta of Sprague-Dawley (SD) and TGR(mRen-2)27 [mRen-2] rats was studied.
In mRen-2 rats, contractile responses to phenylephrine were diminished as compared to control SD rats in endothelium containing but not in endothelium-denuded vessels. L-NAME increased contractile responses to phenylephrine in mRen-2 rats and, after nitric oxide synthase blockade, responses to phenylephrine became comparable in both strains.
Inhibition of angiotensin-converting enzyme (ACE) by captopril potentiated contractile responses in mRen-2 rats and diminished contractile responses in SD rats, both effects being dependent on the presence of a functional endothelium. The effect of captopril in mRen-2 rats was abolished in vessels pre-incubated with Ang-(1-7).
Blockade of Ang-(1-7) and bradykinin (BK) receptors by A-779 and HOE 140 respectively, increased phenylephrine-induced contraction in mRen-2, but not in SD rats. This effect was seen only in endothelium-containing vessels.
Angiotensin II AT1 and AT2 receptor blockade by CV 11974 and PD 123319 did not affect the contractile responses to phenylephrine in aortas of transgenic animals but diminished the response in SD rats. This effect was only seen in the presence of a functional endothelium.
It is concluded that the decreased contractile responses to phenylephrine in aortas of mRen-2 rats was dependent on an intact endothelium, the local release and action of Ang-(1-7) and bradykinin.
Keywords: Renin – angiotensin system, angiotensin-(1-7), bradykinin, contractile responses, phenylephrine, endothelial receptors, nitric oxide
Introduction
The recent development of a transgenic rat expressing the mouse Ren-2 gene (Mullins et al., 1990) has provided an interesting model of experimental hypertension, which has the advantage that the genetic basis of the disease is known, and, in addition, wild-type control animals are available. The severe hypertension developed at a young age in these animals respond to treatment with angiotensin-converting enzyme (ACE) inhibitors as well as AT1 receptor antagonists indicating that angiotensin II (Ang II) is involved in this monogenic form of hypertension (Gross et al., 1995; Zhuo et al., 1999).
Because kidney and plasma renin activity is suppressed in mRen-2 rats (Mullins et al., 1990), the development and maintenance of hypertension in these animals have been attributed to the high activity of the renin – angiotensin system in extrarenal tissues, as adrenal glands, heart, brain and blood vessels (Bader et al., 1992; Campbell et al., 1995). It has been proposed that localized vascular production of components of the renin – angiotensin system could contribute to hypertension in transgenic rats. In support of this possibility, mRen-2 mRNA expression has been shown to be increased in the aorta and in the mesenteric vasculature (Hilgers et al., 1992). Furthermore, Ang II production was increased in the hindquarters vasculature and aorta of transgenic rats (Hilgers et al., 1992; Arribas et al., 1994; Campbell et al., 1995). However, in consequence of the high expression of the renin – angiotensin system in the aorta of mRen-2 rats, Ang II receptors are down-regulated (Nickenig et al., 1997).
Angiotensin-(1-7) [Ang-(1-7)] is a bioactive component of the renin – angiotensin system originated from Ang I and Ang II by the action of peptidases (for review see Santos et al., 2000). Under certain circumstances Ang-(1-7) opposes the actions of Ang II. In mRen-2 rats, cerebroventricular administration of antibodies to Ang-(1-7) produced significant elevation of blood pressure, whereas administration of a monoclonal antibody to Ang II decreased blood pressure (Moriguchi et al., 1995). Hypotensive responses to Ang-(1-7) have been reported in spontaneously hypertensive rats and dogs with renovascular hypertension (Benter et al., 1995; Nakamoto et al., 1995). Ang-(1-7) induces vasorelaxation through the release of endothelium-derived factors such as nitric oxide (NO) and prostaglandin (Nakamoto et al., 1995; Brosnihan et al., 1996; Muthalif et al., 1998; Heitsch et al., 2001) via stimulation of a specific receptor (Tallant et al., 1997) or by potentiating the vasorelaxant effect of bradykinin (BK) (Abbas et al., 1997; Gorelik et al., 1998; Fernandes et al., 2001).
In the aorta of mRen-2 rats the contractile responses to several agonists are decreased as compared to Sprague-Dawley (SD) control animals (Arnet et al., 1999). Because local production of Ang-(1-7) in the vasculature has been demonstrated (Santos et al., 1992; Chappell et al., 1994), and because it has potential for conveying cardioprotective effects by opposing the actions of Ang II, we designed this study to evaluate the role of local production of Ang-(1-7) in modulating contractile responses in the aorta of mRen-2 animals. In the present work we describe the participation of Ang-(1-7) and its interaction with BK in the endothelium- and NO-dependent reduction of contractions induced by phenylephrine in aortic rings of mRen-2.
Methods
Animals
We used 7 – 9-month-old male rats heterozygous for the mouse Ren-2 gene (TGR (mRen2)27; 431±20 g) and age-matched Hanover Sprague-Dawley (SD; 432.5±19 g) control rats. Animals were originally imported from the Max-Delbrück-Center for Molecular Medicine (Berlin-Buch, Germany) and bred in our animal facilities at the Department of Physiology and Biophysics, of the Federal University of Minas Gerais. Mean blood pressure performed by tail plethysmography was 177±7 and 111±4 mmHg for mRen-2 and SD rats, respectively. Rats were killed by a blow on the head and tissues were rapidly removed.
Rat aortic rings preparation and mounting
Rings (4 – 5 mm) from the descending thoracic aorta, free of fat and connective tissue, were set up in gassed (95% O2 and 5% CO2) Krebs-Henseleit solution (mmoll−1): NaCl 110.8, KCl 5.9, NaHCO3 25.0, MgSO4 1.07, CaCl2 2.49, NaH2PO4 2.33 and glucose 11.51, at 37°C, under a tension of 1 g, for 1 h equilibration period. After this time, two contractile responses were evoked by submaximal concentrations of phenylephrine (0.3 μM) to elicit reproducible responses. When necessary, the endothelium was removed by rubbing the intimal surface with a wooden stick. The presence of a functional endothelium was assessed by the ability of acetylcholine (1 μM) to induce more than 70% relaxation of vessels pre-contracted with phenylephrine (0.3 μM). Mechanical activity recorded isometrically by a force transducer (World Precision Instruments, Inc., Sarasota, FL, U.S.A.) was fed to an amplifier – recorder (Model TMB-4; World Precision Instruments, Inc.) and to a personal computer equipped with an analogue-to-digital converter board (AD16JR; World Precision Instruments, Inc.), using CVMS data acquisition/recording software (World Precision Instruments, Inc.).
Cumulative concentration – response curves to phenylephrine (0.001 – 30 μM) were obtained in vessels with or without a functional endothelium. When necessary, after 45 min washing, the vessels were incubated for 20 min with different drugs or vehicle, as indicated, and a second cumulative concentration – response curve for phenylephrine was constructed and compared with the first one. Ang-(1-7) was incubated at the same time as captopril, 20 min before the construction of the second curve. In control experiments (with vehicle), no difference was found between concentration – response curves (data not shown).
Drugs
Acetylcholine, captopril, NG-nitro-L-arginine-methyl-ester (L-NAME) and phenylephrine were purchased from Sigma. Angiotensin-(1-7) and A-779 (Bachem), HOE 140 (Hoechst). All drugs were dissolved in distilled water at a concentration of 10 mM and diluted to the desired concentration with Krebs-Henseleit solution just before use.
Statistical analysis
Results are expressed as means±s.e.mean. Matched two-way analysis of variance (ANOVA) was used to analyse the data, and results were considered significant when P<0.05.
Results
α-Adrenergic contractile responses in mRen-2 and SD rats
As shown in Figure 1A, contractile responses to phenylephrine in endothelium containing aorta were much smaller in transgenic animals as compared to age-matched SD rats. In endothelium denuded vessels, no difference was observed in contractile responses between the two strains (Figure 1B).
Figure 1.
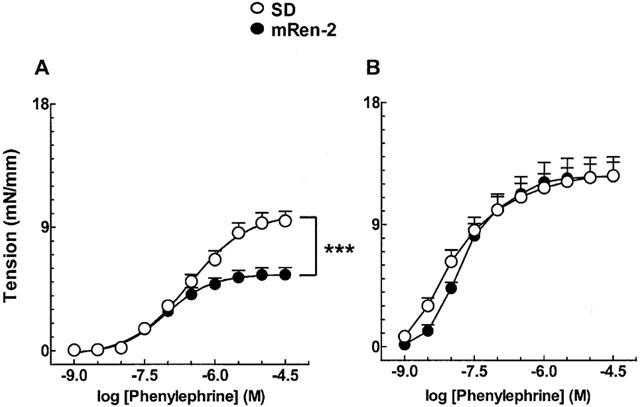
Phenylephrine-induced contractions in isolated aortic rings from control SD and mRen-2 transgenic rats. (A) Vessels containing functional endothelium. (B) Endothelium-denuded vessels. Each point represent mean±s.e.mean generated from ten separate experiments. ***P<0.001.
Inhibition of nitric oxide synthase with L-NAME (1 μM) increased contractile responses to phenylephrine in hypertensive mRen-2 (Figure 2A) and, after NO synthase blockade, contraction to phenylephrine became comparable in both strains (Figure 2B).
Figure 2.
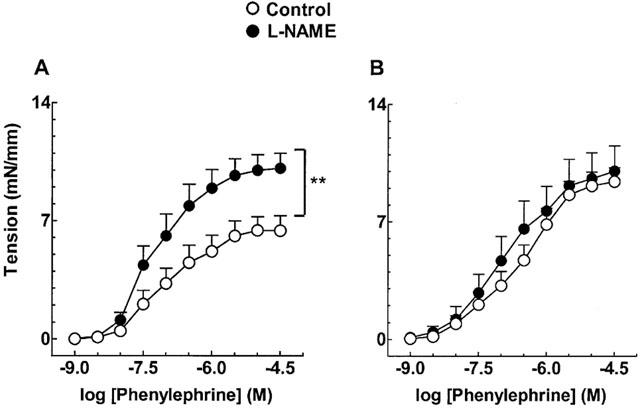
Effect of L-NAME on endothelium-containing aortic rings from mRen-2 (A) and SD rats (B). Each point represents mean±s.e.mean generated from at least four separate experiments. **P<0.01.
Inhibition of ACE by captopril (10 μM) potentiated contractile responses induced by α-adrenergic stimulation in mRen-2 rats (Figure 3A), an effect dependent on the presence of a functional endothelium (Figure 3B). In SD rats by contrast, captopril induced an endothelium-dependent diminution of the contractile response (Figure 3C,D).
Figure 3.
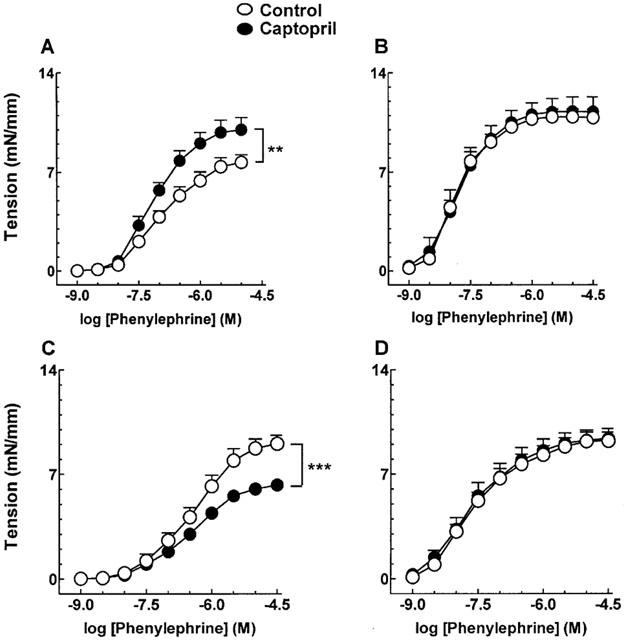
Effect of captopril (10 μM) on phenylephrine-induced contractions in aortic rings from mRen-2 (A, endothelium intact; B, endothelium denuded vessels) and SD rats (C, endothelium intact; D, endothelium denuded vessels). Each point represents mean±s.e.mean generated from at least three separate experiments. **P<0.01 and ***P<0.001.
Effect of captopril in contractile responses to phenylephrine in vessels pre-treated with Ang-(1-7)
The potentiating effect of ACE inhibition by captopril (10 μM) in contractile responses to phenylephrine (Figure 4A) was abolished when the endothelium-containing aorta of mRen-2 rats was incubated with 1 nM Ang-(1-7) (Figure 4B). Pre-treatment of vessels with Ang-(1-7) did not modify the effect of captopril in SD rats (not shown).
Figure 4.
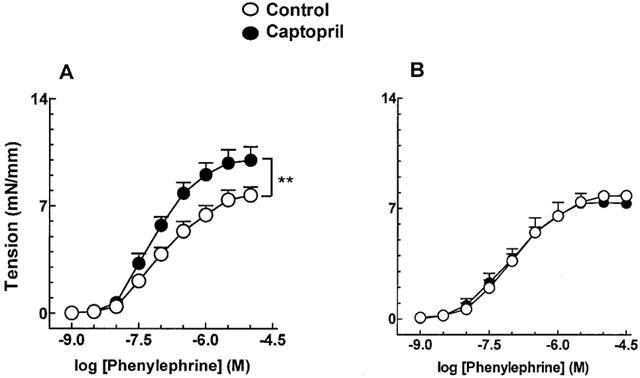
Effect of captopril (10 μM) on phenylephrine-induced contractions in endothelium-containing aortic rings from mRen-2 rats in the absence (A) and in the presence (B) of 1 nM Ang-(1-7). Each point represents mean±s.e.mean data generated from at least five separate experiments. **P<0.01.
Effect of Ang-(1-7) and BK receptors blockade in contractile responses to phenylephrine in the rat aorta
Blockade of Ang-(1-7) and BK B2 receptors by the respective selective antagonists A-779 (0.1 μM) and HOE 140 (1 μM) restored phenylephrine-induced contraction (Figure 5A,C) in the aorta of mRen-2 rats. This effect was seen only in vessels containing a functional endothelium (Figure 5B,D). The effect of blocking both receptors was not additive (not shown). A-779 and HOE 140 had no effect in contractile responses to phenylephrine in control SD rats (not shown).
Figure 5.
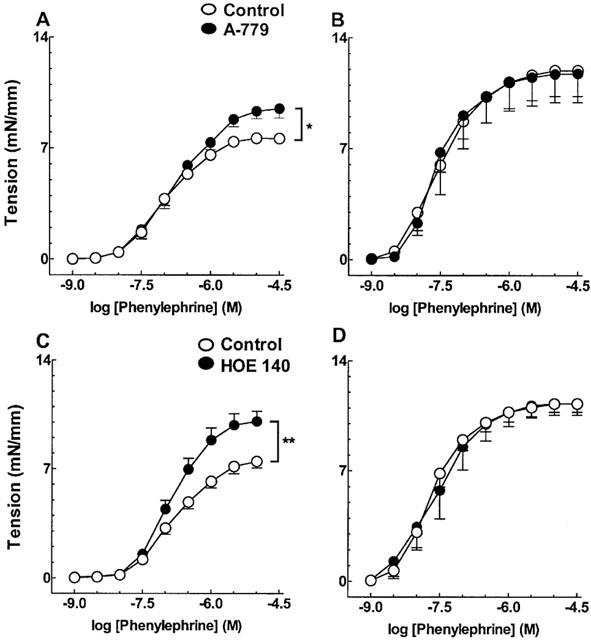
Effect of blockade of Ang-(1-7) (A,B) and BK (C,D) receptors by A-779 (0.1 μM) and HOE 140 (1 μM) on phenylephrine-induced contraction, in endothelium-containing (A,C) and -denuded (B,D) vessels from mRen-2 rats. Each point represents mean s.e.mean data generated from at least five separate experiments. *P<0.05 and **P<0.01.
In another series of experiments, Ang-(1-7) (0.1 nM – 0.3 μM) directly relaxed aorta from mRen-2 animals pre-contracted with 1 μM phenylephrine (Emax=29.59±11.15% relaxation; n=5).
Effect of AT1 and AT2 Angiotensin II receptors blockade in the contractile response to phenylephrine
Incubation of mRen-2 aortic rings with the Ang II AT1 and AT2 receptor antagonists CV 11974 (10 nM) and PD 123319 (1 μM), respectively, did not change the concentration – response curves to phenylephrine (Figure 6). In control SD rats, blockade of AT1 and AT2 receptors diminished contractile responses to phenylephrine in endothelium intact (Figure 7A,C), but not in endothelium-denuded vessels (Figure 7B,D).
Figure 6.
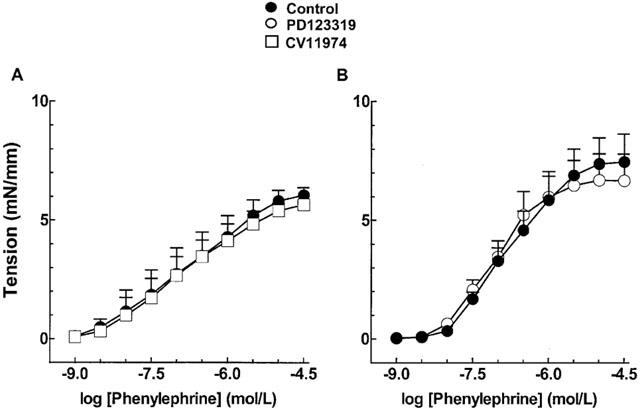
Effect of 10 nM CV 11974 (A) and 1 μM PD 123319 (B) on phenylephrine-induced contractions in endothelium-containing aortic rings from mRen-2 rats. Each point represents mean±s.e.mean generated from at least four separate experiments.
Figure 7.
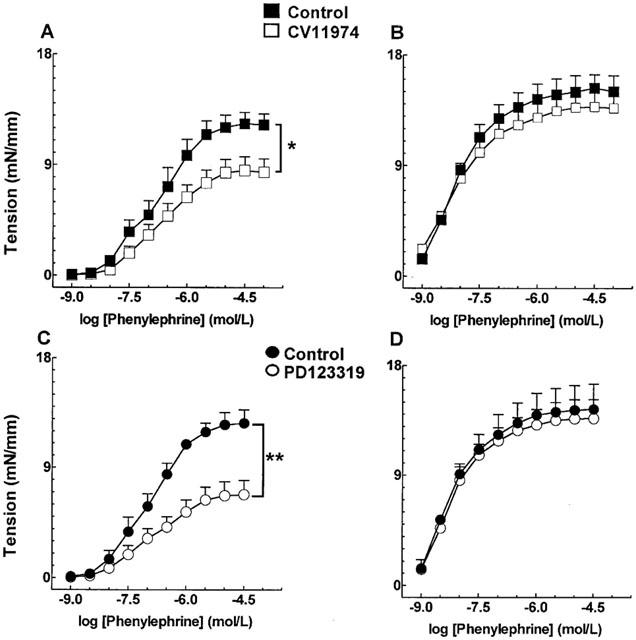
Effect of angiotensin II AT1 (A,B) and AT2 (C,D) receptors blockade by CV 11974 (10 nM) and PD 123319 (1 μM) respectively, on phenylephrine-induced contractions in endothelium-containing (A,C) or -denuded (B,D) aortic rings from Sprague-Dawley rats. Each point represents mean±s.e.mean generated from at least four separate experiments. *P<0.05 and **P<0.01.
Discussion
There is strong evidence to suggest that, besides the circulating renin – angiotensin system of renal origin, the vascular expression of this system also contributes to the control of the cardiovascular homeostasis by autocrine and/or paracrine regulation (Unger et al., 1991; Weishaar et al., 1991; Dzau, 1993; Ganten, 1993).
The transgenic mRen-2 rat represents a monogenic model of hypertension for the study of the functional significance of local renin – angiotensin system. The high activity of the renin – angiotensin system in extrarenal tissues, including from vascular origin, promotes the development of hypertension in these animals and provides strong evidence for the importance of tissue angiotensin in the pathogenesis of hypertension (Bader et al., 1992; Hilgers et al., 1992; Arribas et al., 1994; Campbell et al., 1995; Lee et al., 1995). Here we present evidence for a functional endothelium-dependent role for Ang-(1-7), a bioactive peptide derived from the renin – angiotensin system, in the control of a-adrenergic receptors stimulation and vascular tone in the aorta of mRen-2 rats.
Contractile responses to phenylephrine in endothelium-containing aorta were reduced in mRen-2 rats as compared to SD rats. However, the reactivity of smooth muscle cells of the transgenic rats for phenylephrine was not altered since phenylephrine evoked similar contractions in both strains after removal of endothelium. Thus, it appears that the supra-expression of an endothelium-derived relaxant factor in basal conditions is accounting for the reduction in the contractile response to phenylephrine in mRen-2 rats. Our results are in accordance with previous reports in the literature showing that, although contractile properties of smooth muscle of the rat aorta are not altered, contractions to norepinephrine are diminished in endothelium-containing aortic rings from mRen-2 rats (Arnet et al., 1999).
Low concentration of L-NAME, an inhibitor of NO synthesis, which did not change contractile responses to phenylephrine in SD rats, potently increased phenylephrine-induced contractions in mRen-2 rats. After NO synthase blockade responses to phenylephrine became comparable in both strains. These results suggest that an increased basal release of NO in transgenic rats reduces contractions to phenylephrine. Another possible explanation is that smooth muscle cells of mRen-2 rats are more sensitive to NO. Very recently, vascular soluble guanylyl cyclase has been reported to be less sensitive to NO in mRen-2 rats compared to SD rats (Jacke et al., 2000). Thus, it is unlikely that similar basal release of NO is reducing contraction to phenylephrine to a greater extent in transgenic than in control rats.
Inhibition of ACE by captopril also restored contractile responses to phenylephrine in mRen-2 rats. This effect was dependent on the presence of a functional endothelium. These results strongly suggest that a local overactive renin-angiotesin system is contributing to diminish contraction to phenylephrine in mRen-2 aorta, via a NO-dependent mechanism.
In contrast, captopril reduced contractile responses to phenylephrine in control SD rats. Thus, as pointed out above, a functional renin-angiotesin system seems to potentiate contractions to phenylephrine in the aorta of SD rats. This is in accordance with several reports in the literature showing that Ang II increases vascular reactivity to α-adrenergic stimulation (Purdy & Weber. 1988; Arribas et al., 1994). The question then arose as to how inhibition of the renin – angiotensin system up-regulates α-adrenergic contractions in SD animals and down-regulates the same effect in mRen-2 transgenic rats. Blockade of Ang II AT1 receptors in SD rats diminished maximal contractile responses to phenylephrine. Surprisingly, blockade of AT2 receptors provoked a much stronger inhibition of phenylephrine-induced contractions in the aorta of SD rats. Both effects were dependent on the presence of a functional endothelium. Together, our results provide evidence to suggest that tissue formation of Ang II is potentiating α-adrenergic contractile responses in the aorta of SD rats via the release of an endothelium contractile factor. More importantly, they strongly suggest a functional role for endothelial Ang II AT2 receptors in the control of vascular tonus by potentiating α-adrenergic contractions in the rat aorta. Accordingly, vascular endothelial Ang II AT1 and AT2 receptors are already described (Pueyo & Michel, 1997) and an endothelium-dependent role for AT2 receptors has been attributed in rat renal vasculature for the potentiation of Ang II-induced constriction by NO blockade (Muller et al., 1998).
In mRen-2 rats, blockade of AT1 and AT2 angiotensin receptors had no effect on the contractile responses to phenylephrine. Thus, these results suggest that, in addition to the already proposed down regulation of AT1 receptors in the rat aorta of mRen-2 rats (Nickenig et al., 1997; Arnet et al., 1999), AT2 receptors may also be down regulated. The results also partially explain the differences found between strains in the modulation of α-adrenergic contraction by the vascular renin – angiotensin system. Because Ang II AT1 and AT2 receptors are down-regulated in the rat aorta of mRen-2 rats, the endothelium-dependent effect of other derivatives of the renin – angiotensin system should predominate.
BK and Ang-(1-7) are described in the literature as putative regulators of vascular tonus via an NO-dependent mechanism (Mombouli & Vanhoutte, 1999; Santos et al., 2000). Here, we found that endothelium-dependent contraction to phenylephrine was increased in the aorta of mRen-2 rats after blockade of Ang-(1-7) and BK receptors. Interesting, treatment of mRen-2 aorta with Ang-(1-7) abolished the endothelium-dependent potentiation of phenylephrine contractile responses induced by captopril. In addition, Ang-(1-7) had a direct relaxing effect on the aorta of mRen-2 animals pre-contracted with phenylephrine. Therefore, our results point to a participation of Ang-(1-7), in the modulation of α-adrenergic-induced contractions. The observation that blockade of BK B2 receptors also produced potentiation of α-adrenergic-induced contraction and the absence of additive effects of simultaneous blockade of Ang-(1-7) and BK B2 receptors suggest that Ang-(1-7) exerts its modulator effect also through receptor-mediated release and/or potentiation of BK. Evidence for the involvement of kinins in vascular action of Ang-(1-7) has been provided by several studies (Brosnihan et al., 1996; Abbas et al., 1997; Heitsch et al., 2001). However, kinins are not always mediators of the vascular action of Ang-(1-7), as demonstrated in rat mesenteric microvessels (Oliveira et al., 1999).
Ang-(1-7) is formed directly from Angiotensin I by peptidases neprilysin, thimet oligopeptidase and prolyl-endopeptidase, as well as by hydrolysis of the Pro-Phe bound in Ang II by enzymes such as prolyl-endopeptidase and prolyl-carboxypeptidases (Santos et al., 2000). In addition, Ang-(1-7) can be formed from Ang I or Ang II by a pathway including ACE2 (Donoghue et al., 2000). Our observation that captopril enhanced the vasoconstriction produced by phenylephrine, and that this potentiating effect was abolished in the presence of Ang-(1-7), suggests that in rat aorta of mRen-2 rats, Ang-(1-7) might be formed mainly from Ang II by an ACE-dependent pathway. Significant contribution of Ang II for Ang-(1-7) formation has been previously described in the isolated rat heart (Neves et al., 1995). In addition, in the presence of losartan production of Ang-(1-7) via Ang II is increased (Yamada et al., 1999). Therefore, the accumulation of Ang II in the aorta of mRen-2 rats in consequence of down-regulation of its receptors (Nickenig et al., 1997) would contribute to the increased formation of Ang-(1-7) through Ang II.
Captopril was less effective, as compared to L-NAME, to potentiate the effects of phenylephrine. Among other possibilities, this could be a consequence of decreased degradation of BK by ACE inhibition or alternatively due to Ang-(1-7) derived from other pathways (Santos et al., 2000), which could induce NO release. By contrast, L-NAME would block all NO derived from the activation of Ang-(1-7) and BK receptors and, thus, be more effective.
In conclusion, our results support the participation of Ang-(1-7) as the active component of the renin – angiotensin system in the endothelial modulation of α-adrenergic-induced tonus in aortic rings of mRen-2 rats. The interaction of Ang-(1-7) with BK is also pointed-out. Finally, our results also suggest that the local production of Ang-(1-7) plays an important role in the control of the vascular reactivity in mRen-2 rats.
Acknowledgments
V.S. Lemos, S.F. Côrtes, M.J. Campagnole-Santos and R.A.S. Santos received financial support from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
Abbreviations
- ACE
angiotensin-converting enzyme
- ACh
acetylcholine
- Ang II
angiotensin II
- Ang-(1-7)
angiotensin-(1-7)
- ANOVA
two-way analysis of variance
- BK
bradykinin
- L-NAME
NG-Nitro-L-arginine Methyl Ester
- mRen-2
transgenic (mRen-2)27 rat
- NO
nitric oxide
- SD
Sprague-Dawley
References
- ABBAS A., GORELIK G., CARBINI L.A., SCICLIA A.G. Angiotensin-(1-7) induces bradykinin-mediated hypotensive responses in anesthetized rats. Hypertension. 1997;30:217–221. doi: 10.1161/01.hyp.30.2.217. [DOI] [PubMed] [Google Scholar]
- ARNET U.A., NOVOSEL D., BARTON M., NOLL G., GANTEN D., LÜCHER T.F. Endothelium dysfunction in the aorta of transgenic rats harboring the mouse Ren-2 gene. Endothelium. 1999;6:175–184. doi: 10.3109/10623329909053408. [DOI] [PubMed] [Google Scholar]
- ARRIBAS S., SANCHEZ-FERRER C.F., PIERO C., PONTE A., SALAICES M., MARIN J. Functional vascular renin-angiotensin system in hypertensive transgenic rats for the mouse renin gene Ren-2. Gen. Pharmacol. 1994;25:1163–1170. doi: 10.1016/0306-3623(94)90133-3. [DOI] [PubMed] [Google Scholar]
- BADER M., ZHAO Y.I., SANDER M., LEE M.A., BACHMANN J., BÖHM M., DJAVIDANI B., PETERS J., MULLINS J.J., GANTEN D. Role of tissue renin in the pathophysiology of hypertension in TGR(mREN2)27 rats. Hypertension. 1992;19:681–686. doi: 10.1161/01.hyp.19.6.681. [DOI] [PubMed] [Google Scholar]
- BENTER I.F., FERRARIO C.M., MORRIS M., DIZ D.I. Antihypertensive actions of angiotensin-(1-7) in spontaneously hypertensive rats. Am. J. Physiol. 1995;269:H313–H319. doi: 10.1152/ajpheart.1995.269.1.H313. [DOI] [PubMed] [Google Scholar]
- BROSNIHAN K.B, , LI P., FERRARIO C.M. Angiotensin-(1-7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension. 1996;27:523–528. doi: 10.1161/01.hyp.27.3.523. [DOI] [PubMed] [Google Scholar]
- CAMPBELL D.J., RONG P., KLADIS A., REES B., GANTEN D., SKINNER S.L. Angiotensin and BK peptides in the TGR(mRen-2)27 rat. Hypertension. 1995;25:1014–1020. doi: 10.1161/01.hyp.25.5.1014. [DOI] [PubMed] [Google Scholar]
- CHAPPELL M.C., TALLANT E.A., BROSNIHAN K.B., FERRARIO C.M. Conversion of angiotensin I to angiotensin-(1-7) by thimet oligopeptidase (EC 3.4.24.15) in vascular smooth muscle cells. J. Vasc. Med. Biol. 1994;5:129–137. [Google Scholar]
- DONOGHUE M., HSIEH F., BARONAS E., GODBOUT K., GOSSELIN M., STAGLIANO N., DONOVAN M., WOOLF B., ROBISON K., JEYASEELAN R., BREITBART R.E., ACTON S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- DZAU V.J. Local expression and pathophysiological role of renin-angiotensin in the blood vessels and heart. Basic Res. Cardiol. 1993;88:1–14. doi: 10.1007/978-3-642-72497-8_1. [DOI] [PubMed] [Google Scholar]
- FERNANDES L., FORTES Z.B., NIGRO D., TOSTES R.C.A., SANTOS R.A.S., CARVALHO M.H.C. Potentiation of bradykinin by angiotensin-(1-7) on arterioles of spontaneously hypertensive rats studied in vivo. Hypertension. 2001;37:703–709. doi: 10.1161/01.hyp.37.2.703. [DOI] [PubMed] [Google Scholar]
- GANTEN D. The transition from experimental to clinical pharmacology: the renin-angiotensin paradigm. J. Cardiovasc. Pharmacol. 1993;22:S14–S29. [PubMed] [Google Scholar]
- GORELIK G., CARBINI L.A, SCICLI A.G. Angiotensin 1-7 induces bradykinin-mediated relaxation in porcine coronary artery. J. Pharmacol. Exp. Ther. 1998;286:403–410. [PubMed] [Google Scholar]
- GROSS V., LIPPOLDT A., SCHNEIDER W., LUFT F.C. Effect of captopril and angiotensin II receptor blockade on pressure natriuresis in transgenic TGR(mRen-2)27 rats. Hypertension. 1995;26:471–479. doi: 10.1161/01.hyp.26.3.471. [DOI] [PubMed] [Google Scholar]
- HEITSCH H., BROVKOVYCH S., MALINSKI T., WIEMER G. Angiotensin-(1-7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension. 2001;37:72–76. doi: 10.1161/01.hyp.37.1.72. [DOI] [PubMed] [Google Scholar]
- HILGERS K.F., PETERS J., VEELKEN R., SOMMER M., RUPPRECHT G., GANTEN D., LUFT F.C., MANN J.F.E. Increased vascular angiotensin formation in female rats harboring the mouse Ren-2 gene. Hypertension. 1992;19:687–691. doi: 10.1161/01.hyp.19.6.687. [DOI] [PubMed] [Google Scholar]
- JACKE K., WITTE K., HUSER L., BEHRENDS S., LEMMER B. Contribution of the renin-angiotensin system to subsensitivity of soluble guanylyl cyclase in TGR(mREN2)27 rats. Eur. J. Pharmacol. 2000;403:27–35. doi: 10.1016/s0014-2999(00)00577-x. [DOI] [PubMed] [Google Scholar]
- LEE M.A., BÖHM M., KIM S., BACHMANN S., BACHMANN J., BADER M., GANTEN D. Differential gene expression of renin and angiotensinogen in the TGR(mREN-2)27 transgenic rat. Hypertension. 1995;25:570–580. doi: 10.1161/01.hyp.25.4.570. [DOI] [PubMed] [Google Scholar]
- MOMBOULI J.-V., VANHOUTTE P.M. Endothelial dysfunction: from physiology to therapy. J. Mol. Cell Cardiol. 1999;31:61–74. doi: 10.1006/jmcc.1998.0844. [DOI] [PubMed] [Google Scholar]
- MORIGUCHI A., TALLANT E.A., MATSUMURA K., REILLY T.M., WALTON H., GANTEN D., FERRARIO C.M. Opposing actions of angiotensin-(1-7) and angiotensin II in the brain of transgenic hypertensive rats. Hypertension. 1995;25:1260–1265. doi: 10.1161/01.hyp.25.6.1260. [DOI] [PubMed] [Google Scholar]
- MULLER C, , ENDLICH K. , and , HELWIG JJ. Role of eicosanoids in renal angiotensin II vasoconstriction during nitric oxide blockade. Kidney Int. Suppl. 1998;67:S234–S237. doi: 10.1046/j.1523-1755.1998.06759.x. [DOI] [PubMed] [Google Scholar]
- MULLINS J.J, , PETERS J., GANTEN D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature. 1990;344:541–544. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- MUTHALIF M.M., BENTER I.F., UDDIN M.R., HARPER J.L., MALIK K.U. Signal transduction mechanisms involved in angiotensin-(1-7)-stimulated arachidonic acid release and prostanoid synthesis in rabbit aortic smooth muscle cells. J. Pharmacol. Exp. Ther. 1998;284:388–398. [PubMed] [Google Scholar]
- NAKAMOTO H., FERRARIO C.M., FULLER S.B., ROBACZEWSKI D.L., WINICOV E., DEAN R.P. Angiotensin-(1-7) and nitric oxide interaction in renovascular hypertension. Hypertension. 1995;25:796–802. doi: 10.1161/01.hyp.25.4.796. [DOI] [PubMed] [Google Scholar]
- NEVES L.A.A., ALMEIDA A.P., KHOSLA M.C., SANTOS R.A.S. Metabolism of angiotensin I in isolated rat hearts. Effect of angiotensin converting enzyme inhibitors. Biochem. Pharmacol. 1995;50:1451–1459. doi: 10.1016/0006-2952(95)02049-7. [DOI] [PubMed] [Google Scholar]
- NICKENIG G., LAUFS P., SCHNABEL P., KNORR A., PAUL M., BÖHM M. Down-regulation of aortic and cardiac AT1 receptor gene expression in transgenic (mRen-2) 27 rats. Br. J. Pharmacol. 1997;121:134–140. doi: 10.1038/sj.bjp.0701088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIVEIRA M.A., FORTES Z.B., SANTOS R.A.S., KHOSLA M.C., CARVALHO M.H.C. Synergistic effect of angiotensin-(1-7) on bradykinin arteriolar dilatation in vivo. Peptides. 1999;20:1195–1201. doi: 10.1016/s0196-9781(99)00123-0. [DOI] [PubMed] [Google Scholar]
- PUEYO M.E., MICHEL J.-B. Angiotensin II receptors in endothelial cells. Gen. Pharmac. 1997;29:691–696. doi: 10.1016/s0306-3623(97)00021-9. [DOI] [PubMed] [Google Scholar]
- PURDY R.E., WEBER M.A. Angiotensin amplification of α-adrenergic vasoconstriction: role of receptor reserve. Circ. Res. 1988;63:748–757. doi: 10.1161/01.res.63.4.748. [DOI] [PubMed] [Google Scholar]
- SANTOS R.A.S., BROSNIHANN K.B., JACOBSEN D.W., DICORLETO P.E., FERRARIO C.M. Production of angiotensin-(1-7) by human vascular endothelium. Hypertension. 1992;19:II-56–II-61. doi: 10.1161/01.hyp.19.2_suppl.ii56. [DOI] [PubMed] [Google Scholar]
- SANTOS R.A.S, , CAMPAGNOLE-SANTOS M.J., ANDRADE S.P. Angiotesin-(1-7): an update. Regul. Pept. 2000;91:45–62. doi: 10.1016/s0167-0115(00)00138-5. [DOI] [PubMed] [Google Scholar]
- TALLANT E.A., LU X., WEISS R.B., CHAPPELL M.C., FERRARIO C.M. Bovine aortic endothelial cells contain an angiotensin-(1-7) receptor. Hypertension. 1997;29:388–393. doi: 10.1161/01.hyp.29.1.388. [DOI] [PubMed] [Google Scholar]
- UNGER T.H., GOHLKE P., PAUL M., RETTIG R. Tissue renin-angiotensin systems: fact or fiction. J. Cardiovasc. Pharmac. 1991;18:S20–S25. [PubMed] [Google Scholar]
- WEISHAAR R.E., PANEK R.L., MAJOR T.C., SIMMERMAN J., RAPUNDALO S.T., TAYLOR D.G. Evidence for a functional tissue renin-angiotensin system in the rat mesenteric vasculature and its involvement in regulating blood pressure. J. Pharmacol. Exp. Ther. 1991;256:568–574. [PubMed] [Google Scholar]
- YAMADA K., IYER S.N., CHAPPELL M.C., BROSNIHAN K.B., FUKUHARA M., FERRARIO C.M. Differential response of angiotensin peptides in the urine of hypertensive animals. Regul. Pept. 1999;80:57–66. doi: 10.1016/s0167-0115(99)00005-1. [DOI] [PubMed] [Google Scholar]
- ZHUO J., OHISHI M., MENDELSOHN F.A.O. Roles of AT1 and AT2 receptors in the hypertensive Ren-2 gene transgenic rat kidney. Hypertension. 1999;33:347–353. doi: 10.1161/01.hyp.33.1.347. [DOI] [PubMed] [Google Scholar]


