Abstract
The effects of intrathecally (i.t.) injected kinin B1 and B2 receptor agonists and antagonists were measured on mean arterial pressure (MAP) and heart rate (HR) of conscious unrestrained spontaneously hypertensive rats (SHR of 16 weeks old) and age-matched normotensive Wistar Kyoto (WKY). Quantitative in vitro autoradiographic studies were also performed on the thoracic spinal cord of both strains with specific radioligands for B2 receptors, [125I]-HPP-Hoe 140, and B1 receptors, [125I]-HPP-[des-Arg10]-Hoe140.
Bradykinin (BK) (0.81 – 810 pmol) increased MAP dose-dependently with increases or decreases of HR. The pressor response to BK was significantly greater in SHR. The cardiovascular response to 8.1 pmol BK was reversibly blocked by 81 pmol Hoe 140 (B2 antagonist) but not by 81 – 810 pmol [des-Arg10]-Hoe 140 (B1 antagonist) in both strains.
The B1 receptor agonist, des-Arg9-BK (8100 pmol) produced either no effects or increased MAP with variable effects on HR. These responses were similar in both strains and were reversibly blocked by 81 pmol Hoe 140. Inhibition with 8100 pmol [des-Arg10]-Hoe 140 was not specific to B1 agonist-mediated responses.
[125I]-HPP-Hoe 140 specific binding sites were predominantly located to superficial laminae of the dorsal horn and were significantly higher in SHR. Low levels of [125I]-HPP-[des-Arg10]-HOE 140 specific binding sites were found in all laminae of both strains.
It is concluded that the hypersensitivity of the cardiovascular response to BK is due to an increased number of B2 receptors in the spinal cord of SHR and that B1 receptors are unlikely involved in spinal cardiovascular regulation in SHR.
Keywords: Kinins, bradykinin, B1 and B2 receptors, spinal cord, blood pressure, hypertension
Introduction
Kinins refer to a family of pro-inflammatory mediators including bradykinin (BK), kallidin (KD) and their active kininase I metabolites (des-Arg9-BK and des-Arg10-KD) whose biological effects are mediated by two transmembrane G-protein-coupled receptors denoted as B2 and B1 (Regoli & Barabé, 1980; Marceau et al., 1998). Unlike the B2 receptor which is constitutively expressed in many tissues, the B1 receptor is generally absent in healthy animals or expressed in very low amount in a few tissues and cells. The B1 receptor which is activated by des-Arg9-BK and des-Arg10-KD is inducible and functionally expressed in the presence of cytokines, bacterial lipopolysaccharides and following tissue injury (Marceau et al., 1998). In addition to being involved in pain and inflammation (for review see Couture et al., 2001) ample evidence suggests a role for these peptides as neuromodulators and/or neurotransmitters in central cardiovascular regulation (Couture & Lindsey, 2000). BK produces elevation of blood pressure when injected into the cerebral ventricles (Corrêa & Graeff, 1974) or in more specific cardiovascular areas in the medulla such as the nucleus tractus solitarius and the paratrigeminal nucleus through the activation of B2 receptor (Fior et al., 1993; Lindsey et al., 1997).
In comparison to normotensive Wistar or WKY, SHR showed increased sensitivity to the pressor action of BK when injected into a lateral cerebral ventricle (Buñag & Takahashi, 1981; Lindsey et al., 1988), fourth cerebral ventricle (Lindsey et al., 1988; Martins et al., 1991; Lindsey, 1995) and the medulla (Privitera et al., 1994). This hypersensitivity of the vasopressor response to BK in SHR was correlated with increased B2 receptor binding sites in brain stem autonomic regions (Alvarez et al., 1992; Couture & Lindsey, 2000). Although pharmacological studies with agonists and antagonists have led to the conclusion that the central pressor effect to BK is mediated only by the B2 receptor, other studies suggest the implication of B1 receptor in SHR (Alvarez et al., 1992; Emanueli et al., 1999).
In the rat spinal cord, we reported that the intrathecal (i.t.) injection of BK increases arterial blood pressure via the activation of the sympatho-adrenal system and the B2 receptor (Lopes & Couture, 1992; Lopes et al., 1993). This was substantiated by autoradiographic studies which revealed the presence of B2 but not B1 receptor binding sites throughout the grey matter of the rat spinal cord (Lopes et al., 1995). A preliminary study showed a 100% increase of B2 receptor binding sites in the spinal dorsal horn of SHR when compared to WKY (Couture & Lindsey, 2000).
The present study was undertaken to test the hypothesis that SHR are hypersensitive to the spinal cardiovascular effect of BK and that both B1 and B2 receptors are over expressed in the spinal cord of SHR. This was achieved with (i) a pharmacological approach by assessing the cardiovascular effects of selective B1 and B2 receptor agonists and antagonists intrathecally injected to the T-9 spinal cord segment in SHR and WKY; (ii) an anatomical analysis of the localization and quantification of B1 and B2 receptor binding sites in the thoracic spinal cord of SHR and WKY by in vitro autoradiography.
Methods
Animal source and care
Male SHR (n=86) and Wistar Kyoto (n=60) were purchased at least 1 week prior to the study from Charles River, St-Constant, Québec, Canada or Harlan, Indianapolis, IN, U.S.A. They were housed individually in plastic cages under a 12 h light-dark cycle in a room with controlled temperature (23°C), humidity (50%) with food (Charles River Rodent) and tap water available ad libitum. The care of animals and research protocols were in compliance with the guiding principles for animal experimentation as enunciated by the Canadian Council on Animal Care and approved by the Animal Care Committee of our University.
Surgery
At the age of 15 – 16 weeks old, rats were anaesthetized with an i.p. injection of 65 mg kg−1 sodium pentobarbitone (Somnotol; M.T.C. Pharmaceuticals, Cambridge, Ontario, Canada) and a polyethylene catheter (PE-10; Intramedics, Clay Adams, NJ, U.S.A.) was inserted into the spinal subarachnoid space via an incision made in the dura at the atlanto-occipital junction and pushed to the ninth thoracic segment (T-9) as described previously (Lopes & Couture, 1992). About 20% of SHR and 40% of WKY were excluded from the study because they presented motor deficit such as partial paralysis of one posterior or anterior leg. These rats were immediately humanely killed with an overdose of pentobarbital. Thereafter, the rats were allowed to recover in individual plastic cages (40×23×20 cm) and housed in the same controlled conditions. The correct positioning of the i.t. catheter was verified by post-mortem examination at the end of experiment and the catheter was found either dorsally or laterally to the spinal cord.
Two days later, rats were re-anaesthetized with sodium pentobarbitone (65 mg kg−1, i.p.) and an intravascular siliconized (Sigmacote, Sigma, St-Louis, MO, U.S.A.) PE-50 catheter, filled with physiological saline containing 100 IU ml−1 heparin sodium salt (Sigma, St-Louis, MO, U.S.A.), was inserted into the abdominal aorta through the femoral artery for direct blood pressure recording and exteriorized at the back of the neck. Before intrathecal and vascular surgery, the animals received antibiotics Trimethoprime and Sulfadiazine (tribissen 24%, 30 mg kg−1, s.c., Schering Canada Inc., Pointe Claire, Québec, Canada). Ketoprophen was given during the first surgery only (anafen, 5 mg kg−1, s.c., Merial Canada Inc., Baie d'Urfé, Québec, Canada). Recovery from anaesthesia was monitored closely under a warming lamp to maintain the body temperature of animals. Thereafter, rats were housed individually in polyethylene cage with a top grid and returned to their resident room. Experimental protocols were initiated 24 h later, in awake and unrestrained rats.
Measurement of cardiovascular parameters
Blood pressure and heart rate were measured respectively with a Statham pressure Transducer (P23ID) and a cardiac tachometer (model 7P4) (triggered by the arterial blood pressure pulse) coupled to a Grass polygraph (model 79; Grass Instruments Co., Quincy, MA, U.S.A.). The cardiovascular response was measured 1 h after the rats were transported to the testing room. They remained in their resident cage but the top grid was removed and had no more access to the food and water for the duration of experiment. When resting blood pressure and heart rate were stable, rats received an i.t. injection of 20 μl artificial cerebrospinal fluid (aCSF). The void volume of the i.t. catheter was 10 μl. Only rats (99%) which did not show cardiovascular changes to aCSF for the 30 min period were selected in the study.
Experimental protocols
Dose-response curves to i.t. BK and des-Arg9-BK
Both SHR (n=16) and WKY (n=10) initially received an i.t. injection of aCSF (20 μl) followed 15 min later by a single dose of either BK (0.081, 0.81, 8.1, 81 and 810 pmol) or des-Arg9-BK (8.1, 81, 810 and 8100 pmol) to construct a complete dose-response curve. Each rat was injected randomly with one of the two agonists on the first day and the second agonist was given on the subsequent day. Increasing doses of BK or des-Arg9-BK were given at 40 – 60 min intervals. During the course of the study, a subpopulation of SHR and WKY was insensitive to des-Arg9-BK (8100 pmol). Some of these rats were therefore injected randomly at 40 – 60 min apart with two other B1 receptor agonists Lys-des-Arg9-BK (8100 pmol), Sar-[D-Phe8]-des-Arg9-BK (8100 pmol), and BK (8.1 pmol) was injected as positive control. Peptides were administered in a volume of 10 μl of vehicle. The peptide and the aCSF flush (10 μl) were given within a total period of 60 s to avoid any compression of spinal cord. Changes in MAP and HR represent the difference between recordings obtained 30 s before the start of injection and the value at the designed time after injection. Each dose was calculated in a volume of 10 μl solution injected intrathecally.
Effects of i.t. kinin receptor antagonists
SHR (n=7 – 10) and WKY (n=7 – 10) initially received either 8100 pmol des-Arg9-BK or 8.1 pmol BK and 1 h after were given i.t. one of the following antagonists: 81 pmol [des-Arg10]-Hoe 140 or 81 pmol Hoe 140, and then 3 min later was given the same agonist. If the antagonist did not block the response, a higher dose (810 and 8100 pmol) was tested against the agonist 1 h later. The agonist was re-injected alone 24 h later to assess the reversibility of any blockade observed with the antagonist on the preceding day. Only one antagonist was given to a rat.
Effect of a systemic treatment with a prostaglandin synthesis inhibitor
SHR (n=6) and WKY (n=3) initially received randomly an i.t. injection of des-Arg9-BK (8100 pmol) and BK (8.1 pmol) at 1 h apart. The same dose of each agonist was re-injected 1 h after treatment with indomethacin (5 mg kg−1, i.a.).
Tissue preparation for autoradiography
SHR (n=4) and WKY (n=4) of 16 weeks old were sacrificed by asphyxia under CO2 inhalation and subjected to a dorsal laminectomy. An incision of the dura-mater was made carefully to remove the thoracic spinal cord (T8-T11). Then, the pieces were immediately frozen in 2-methylbutane at −50°C and stored at −80°C. Matched spinal cord segments from T9 to T10 of 4 SHR and 4 WKY were separately mounted in two gelatine blocs and serially cut into 20 μm thick coronal sections with a cryostat (−11 to −13°C). Slices obtained from each grouped spinal cords were thaw-mounted on 0.2% gelatine/0.033% chromium potassium sulphate coated slides (50 slides ×8 sections rat−1). Sections were kept at −80°C until use.
In vitro receptor autoradiography
For receptor autoradiography, sections were warmed to room temperature and dipped for 30 s in 25 mM PIPES-NH4OH buffer (pH 7.4, 4°C). Incubations were conducted for 90 min at room temperature using 200 pM of [125I]-HPP-HOE 140 (B2 receptor ligand) or 150 pM of [125I]-HPP-[des-Arg10]-HOE 140 (B1 receptor ligand) in a medium containing 25 mM PIPES-NH4OH buffer (pH 7.4, 4°C), 1 mM of 1,10-phenanthroline, 1 mM of dithiothreitol, 0.014% of bacitracin, 0.1 mM of captopril, 0.2% of bovine serum albumin (protease free) and 7.5 mM of magnesium chloride. The concentrations of radioligands for the B2 receptor (Figure 1) and B1 receptor (data not shown) chosen provided maximal specific binding (Bmax) on the saturation curve in the rat spinal dorsal horn. The dissociation constant (Kd) of [125I]-HPP-HOE 140 was identical in SHR and WKY (Kd=30 pM) while that of [125I]-HPP-[des-Arg10]-HOE 140 was calculated at 27 pM in SHR. The non-specific binding was assessed in the presence of 1 μM of unlabelled peptide. To ascertain the specificity of the labelled B2 radioligand, the same concentration of unlabelled B1 ligand was added to the solution. Likewise, the same concentration of the unlabelled B2 ligand was added to the labelled B1 ligand solution. At the end of the incubation period, slides were transferred sequentially through four rinses of 4 min each in PIPES-NH4OH buffer at 4°C and rapidly dipped into cold distilled water to remove excess of salts. Sections were air-dried and juxtaposed against 3H-Hyperfilm for 2 days (B2 ligand) or 3 days (B1 ligand) along with [125I]-microscales. The films were developed in D-19 (Kodak developer) and fixed in Kodak Ektaflo.
Figure 1.
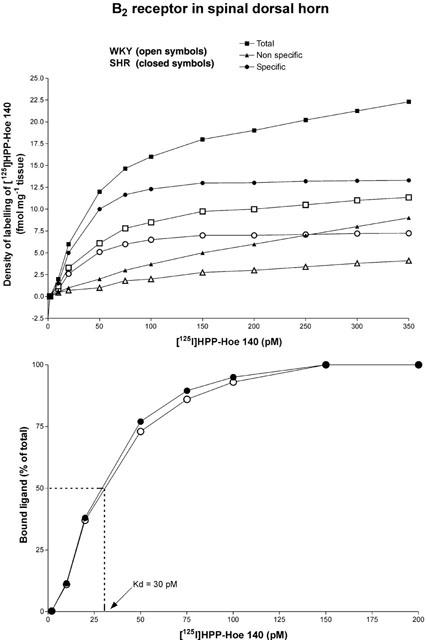
Amount of the B2 receptor radioligand [125I]-HPP-Hoe140 bound to the thoracic spinal dorsal horn of SHR (n=4) and WKY (n=4) as a function of its concentration. Quantification was performed on 96 sections (24 sections per animal) for each concentration corresponding to 1056 sections for total and non-specific binding. Specific binding is calculated as the mathematical difference between total and non-specific binding (which persists in the presence of 1 μM of unlabelled ligand) and reflects the amount of radioligand bound to specific B2 receptor binding sites (upper panel). The affinity of the binding, which is expressed as a dissociation constant (Kd), was calculated as the concentration of B2 radioligand that results in 50% of maximal specific binding (lower panel).
Quantification of receptor binding sites
The autoradiograms were quantified by densitometry using the MCID image analysis system (Imaging Research Inc., Ontario, Canada). Quantification was performed on 264 sections (saturation curves) or 400 sections (the remainder of the study) for each spinal cord on both sides. The specific binding was determined by digital subtraction of the non-specific binding from the total binding of adjacent sections except for the saturation curves the specific binding was determined by mathematical subtraction. The results represent the mean±s.e.mean of four animals per group and are expressed in fmol mg−1 tissue. The anatomical limits and nomenclature were determined in conformity with Paxinos & Watson (1998) (Figure 2).
Figure 2.
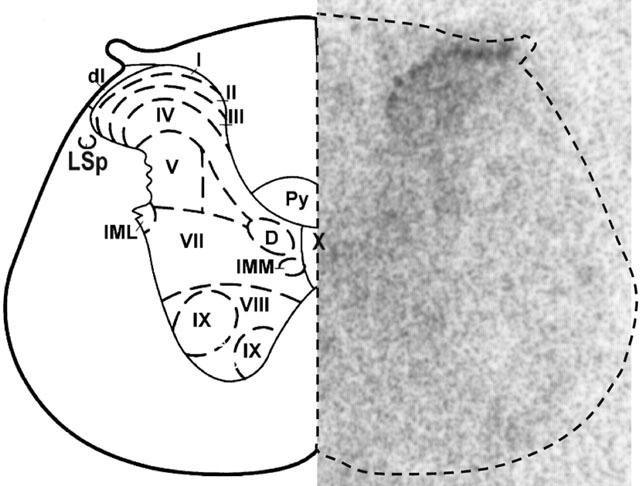
Autoradiogram representing the anatomical distribution of [125I]-HPP-Hoe 140 binding sites in the thoracic (T-10) spinal cord of SHR (right panel). Each anatomical structure of the autoradiogram was delineated according to Paxinos and Watson (1998) (left panel). This pattern was reproduced on each section with the use of a PC mouse for quantitative analysis. Abbreviations: I to X, spinal cord laminae; dl, dorsolateral fasciculus; D, dorsal nucleus; IML, intermediolateral cell column; IMM, intermediomedial cell column; LSp, lateral spinal nucleus; py, pyramidal tract.
Drugs and solutions
The composition of aCSF was (in mM) NaCl 128.6, KCl 2.6, MgCl2 2.0 and CaCl2 1.4; pH adjusted to 7.2. BK (MW: 1060.3), des-Arg9-BK (MW: 904.1) and [des-Arg10]-Hoe 140 (1146.6) were purchased from Peninsula laboratories (San Carlos, CA, U.S.A.). Lys-des-Arg9-BK (MW: 1032.2) was purchased from Bachem Bioscience Inc. (King of Prussia, PA, U.S.A.). Hoe 140 (1305.7) and Sar-[D-Phe8]-des-Arg9-BK (975.2) were obtained from Dr D. Regoli of Sherbrooke University (Sherbrooke, Québec, Canada). Heparin sodium salt (porcine, grade 1-A) and indomethacin were purchased from Sigma company (St-Louis, MO, U.S.A.). Antagonists and agonists were dissolved directly in aCSF. Indomethacin was freshly prepared in tetramethylene sulphone (Sigma) and the solution was completed with trizma base buffer (0.2 M, Sigma) (final concentration contains 10% tetramethylene sulfone). The stock solutions (10 mg ml−1) of agonists and antagonists were stored in aliquots of 100 μl at −20°C until use.
Autoradiographic [125I]-microscales (20 μm) and [3H]-Hyperfilm (single-coated, 24×30 cm) were purchased from Amersham Pharmacia Biotech Canada. Piperazine-N,N′-bis [2-ethanesulphonic-acid] (PIPES), 1,10-phenanthroline, dithiothreitol, bacitracin, captopril and bovine serum albumin were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). The previously described B2 receptor ligand HPP-Hoe 140 (3-4hydroxyphenyl-propionyl-DAng[Hyp3, Thi5, D-Tic7, Oic8]-BK) (Murone et al., 1996) was derived from the B2 antagonist Hoe 140 (Hock et al., 1991) while the B1 receptor ligand HPP-[des-Arg10]-Hoe 140 (3-4hydroxyphenyl-propionyl-des-Arg9-D-Arg[Hyp3, Thi5, D-Tic7, Oic8]-BK) was derived from the B1 antagonist [des-Arg10]-Hoe 140 (Wirth et al., 1991). Both kinin receptor ligands were synthesized in the laboratory of Dr D. Regoli (Department of Pharmacology, Sherbrooke University, Sherbrooke, Québec, Canada) and iodinated by the chloramine T method (Hunter & Greenwood, 1962) in the laboratory of Dr G. Thibault (Clinical Research Institute, Université de Montréal, Montréal, Canada). Five μg of peptide were incubated in 0.05 M phosphate buffer for 30 s in the presence of 0.5 mCi (18.5 MBq) of 125INa and 220 nmol of chloramine T in a total volume of 85 μl. The monoiodinated peptide was then immediately purified by high pressure liquid chromatography on a C4 Vydac column (0.4×250 mm) (The Separations Group, Hesperia, CA, U.S.A.) with 0.1% trifluoroacetic acid and acetonitrile as mobile phases. The specific activity of the iodinated peptides was calculated to be approximately 2000 c.p.m. fmol−1 that yields around 1212.2 Ci mmol−1.
Statistical analysis of data
Results are expressed as means±s.e. mean. Statistical significance of differences was evaluated with unpaired (between SHR and WKY) or paired (within the same group) Student's t-test. A one-way analysis of variance (ANOVA) with a post-hoc Dunnett test was used for multiple comparisons with one control group. The time course of the effects was analysed with a two-way ANOVA in conjunction with Bonferroni confidence intervals. Only probability values (P) less than 0.05 were considered to be statistically significant.
Results
Baseline MAP and HR values were significantly higher in SHR (n=86) (152.5±2.4 mmHg, P<0.001; 341±6 beats min−1, P<0.05) than in WKY (n=60) (110±3.8 mmHg; 320±10 beats min−1).
Spinal effect of BK on the cardiovascular system
The effects of four increasing doses of BK on MAP and HR in SHR and WKY are depicted in Figure 3. In SHR, BK caused two different cardiovascular responses which could not be associated to the position of the intrathecal catheter. In the first group (n=9), BK (8.1 to 810 pmol) caused triphasic changes in MAP. After an initial and marked increase of MAP, a transient drop occurred for 45 s and thereafter the pressor effect lasted about 5 min before returning gradually to baseline level during the following 10 min. These changes were accompanied by a biphasic effect on HR (Figure 3a). At 0.81 pmol, BK evoked only a pressor effect and no significant change on HR. Changes of MAP (calculated on Δ values) were statistically significant when compared to aCSF values at 0.81 pmol (15 s – 5 min, P<0.05), 8.1 pmol (15 – 30 s, 1 – 3 min, P<0.01), 81 pmol (15 s, 45 s – 6 min, P<0.05) and 810 pmol (15 s, 2 – 3 min, P<0.001). Changes of HR (calculated on Δ values) were also statistically significant at 8.1 pmol (30 s, P<0.05), 81 pmol (15 – 30 s, 2 – 3 min, P<0.05) and 810 pmol (30 s, 2 – 7 min, P<0.05).
Figure 3.
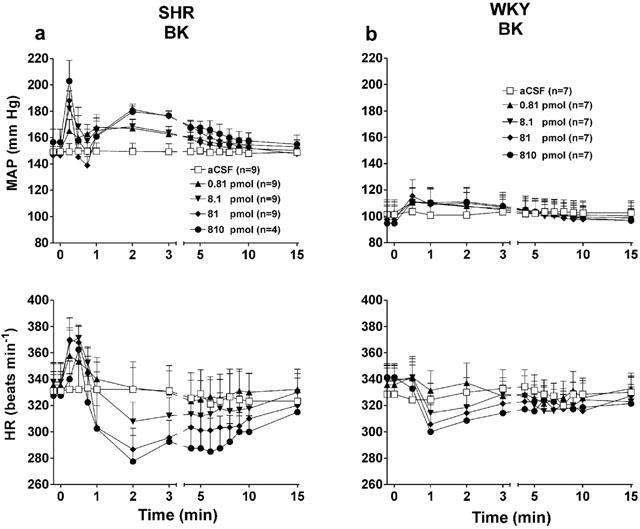
Comparison of the time-course effects on mean arterial pressure and heart rate induced by four increasing doses of BK injected to the T-9 spinal cord segment of SHR (a) and WKY (b). Each point represents the means±s.e.mean of (n) rats.
In two subgroups of SHR, BK (0.81 – 810 pmol) increased MAP (ranged from 17.7±7 mmHg to 45.9±9 mmHg, n=4; and 8.9±9 mmHg to 41.1±4 mmHg, n=3) accompanied either by a tachycardia (ranged from 30±12 to 110±18 beats min−1) or bradycardia (−13±9 to −47±9 beats min−1), respectively. Only an increase in blood pressure occurred in all these rats and no transient drop in blood pressure was seen following the initial increase.
In WKY (n=7), BK (0.81 – 810 pmol) produced short increases in MAP accompanied either by bradycardia (Figure 3b) or tachycardia in three rats (data not shown). The pressor response to BK was statistically significant when compared to aCSF values (comparison to Δ values) at 0.81 pmol (1 min, P<0.01), 8.1 pmol (0.5 – 2 min, P<0.01), 81 and 810 pmol (0.5 – 2 min, P<0.05). The maximal pressor responses evoked by BK in SHR were significantly higher than those evoked by similar doses of BK in WKY although maximal bradycardia were not significantly different at any doses between both strains (Figure 4a).
Figure 4.
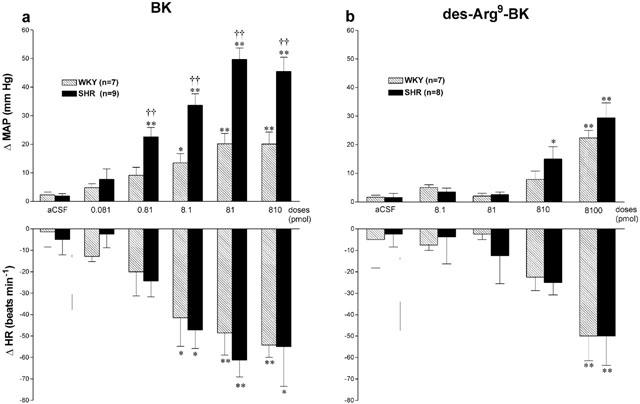
Maximal changes in mean arterial pressure (ΔMAP) and heart rate (ΔHR) elicited by increasing doses of BK (a) and des-Arg9-BK (b) injected to the T-9 spinal cord segment of SHR and WKY. All values represent the means±s.e.mean of (n) rats. Statistical comparison to aCSF (*) or WKY (†) values is indicated by *P<0.05; **,††P<0.01.
Spinal effect of des-Arg9-BK on the cardiovascular system
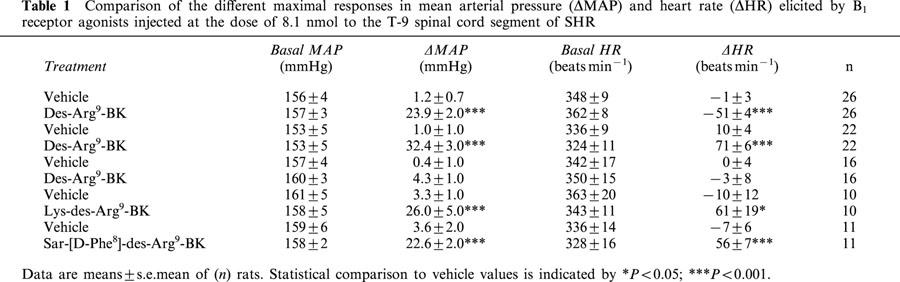
Des-Arg9-BK caused inconsistent cardiovascular responses throughout the study which could not be attributed to the position of the intrathecal catheter. Whereas it increased significantly MAP at 810 and 8100 pmol in SHR (n=8) and at the highest dose in WKY (n=7) with a similar bradycardia in both strains (Figure 4b), des-Arg9-BK also produced tachycardic responses or no cardiovascular changes in several SHR and WKY even if BK (8.1 pmol) had a cardiovascular effect in all tested animals. Thus we pooled all the rats which were tested with des-Arg9-BK in the different protocols to better highlight the variability of the B1-induced cardiovascular response (Tables 1 and 2). The two other B1 agonists induced consistent increases in MAP and HR in SHR which failed to be activated by des-Arg9-BK (Table 1). In WKY which were insensitive to des-Arg9-BK, the B1 agonists were either inactive (Lys-des-Arg9-BK) or less potent (Sar-[D-Phe8]-des-Arg9-BK) to increase MAP, yet they did not affect HR (Table 2).
Table 2.
Comparison of the different maximal responses in mean arterial pressure (ΔMAP) and heart rate (ΔHR) elicited by B1 receptor agonists injected at the dose of 8.1 nmol to the T-9 spinal cord segment of WKY
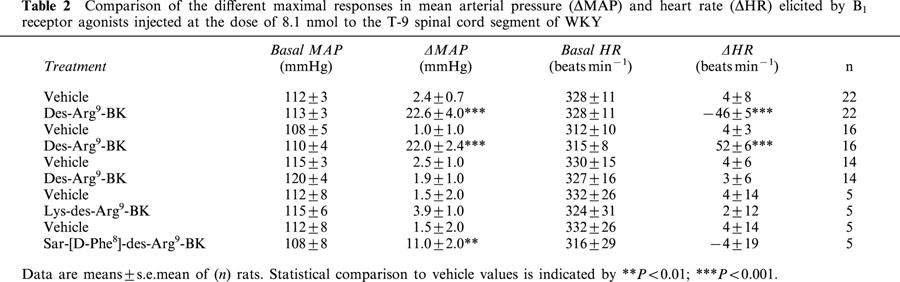
Table 1.
Comparison of the different maximal responses in mean arterial pressure (ΔMAP) and heart rate (ΔHR) elicited by B1 receptor agonists injected at the dose of 8.1 nmol to the T-9 spinal cord segment of SHR
Effects of agonists under B2 receptor blockade in SHR and WKY
The influence of B2 receptor blockade with Hoe 140 was tested against the cardiovascular changes induced by 8100 pmol des-Arg9-BK and 8.1 pmol BK in SHR and WKY. The changes in MAP and HR evoked by BK were completely blocked by the prior i.t. injection of Hoe 140 (81 pmol, 5 min earlier) in both strains (Figure 5a). Whereas the changes in MAP and HR evoked by des-Arg9-BK were also completely blocked by the prior i.t. injection of Hoe 140 (81 pmol) in WKY, a larger dose of Hoe 140 (8100 pmol) was required to abolish the pressor response to the B1 agonist in SHR (Figure 5b). Nevertheless, the des-Arg9-BK-induced bradycardia was abolished by the lowest dose of Hoe 140 in SHR (Figure 5b). Hoe 140 was devoid of any direct effect on MAP and HR (data not shown).
Figure 5.
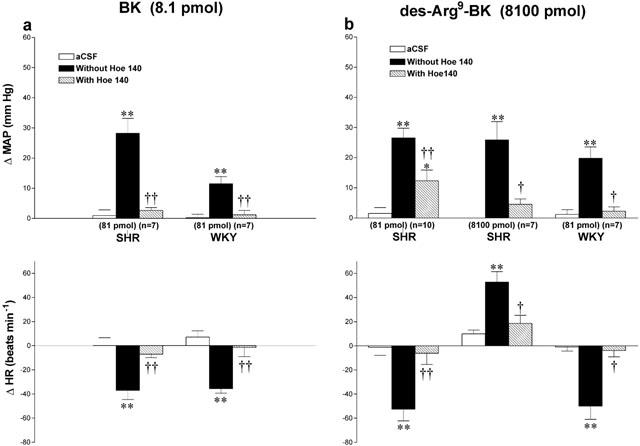
Maximal changes in mean arterial pressure (ΔMAP) and heart rate (ΔHR) induced by 8.1 pmol BK (a) and 8100 pmol des-Arg9-BK (b) injected to the T-9 spinal cord segment of SHR and WKY without or with Hoe 140 at the dose indicated at the bottom of each column. Data are means±s.e.mean of (n) rats. Statistical comparison to aCSF values (*) or to the agonist without Hoe 140 (†) is indicated by *,†P<0.05; **,††P<0.01. Basal MAP and HR in each group were: (a) SHR: 149±3.9 mmHg and 369±11 beats min−1; WKY: 113±6.7 mmHg and 323±13 beats min−1; (b) SHR (81 pmol): 148±5.3 mmHg and 368±18 beats min−1; SHR (8100 pmol): 173±7.4 mmHg and 334±17 beats min−1; WKY: 102±9 mmHg and 310±25 beats min−1.
Effects of agonists under B1 receptor blockade in SHR and WKY
Effects of the B1 receptor antagonist [des-Arg10]-Hoe 140 on the cardiovascular response to 8100 pmol des-Arg9-BK or 8.1 pmol BK in SHR and WKY are shown in Figures 6 and 7. The cardiovascular changes to both agonists were significantly reduced by [des-Arg10]-Hoe 140 (8100 pmol i.t., 5 min beforehand) in both strains. All inhibitions were over when the agonists were re-injected alone 24 h later (data not shown). Lower doses of the antagonist had no significant effect on BK and des-Arg9-BK mediated responses except at the dose of 810 pmol [des-Arg10]-Hoe 140 caused a small reduction of the pressor response to BK in SHR. However, the highest dose of [des-Arg10]-Hoe 140 did not affect the increases in MAP evoked by 810 pmol BK in SHR (Δ MAP before: 40±4 mmHg, and after: 46±2 mmHg, n=4). The highest dose of [des-Arg10]-Hoe 140 had no direct effects on MAP and HR for a period of 30 min when injected alone (data not shown).
Figure 6.
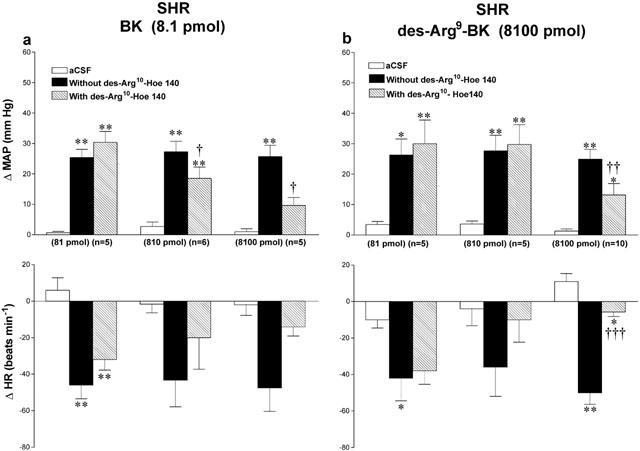
Maximal changes in mean arterial pressure (ΔMAP) and heart rate (ΔHR) induced by 8.1 pmol BK (a) and 8100 pmol des-Arg9-BK (b) injected to the T-9 spinal cord segment of SHR without or with [des-Arg10]-Hoe 140 at the dose indicated at the bottom of each column. Data are means±s.e.mean of (n) rats. Statistical comparison to aCSF values (*) or to the agonist without [des-Arg10]-Hoe 140 (†) is indicated by *,†P<0.05; **,††P<0.01; †††P<0.001. Basal MAP and HR in each group were: (a) 81 pmol: 156±6.9 mmHg and 340±17 beats min−1; 810 pmol: 162±3.8 mmHg and 370±20 beats min−1; 8100 pmol: 149±10 mmHg and 358±18 beats min−1; (b) 81 pmol: 147±7.4 mmHg and 386±38 beats min−1; 810 pmol: 144±8.3 mmHg and 384±39 beats min−1; 8100 pmol: 162±6.6 mmHg and 352±15 beats min−1.
Figure 7.
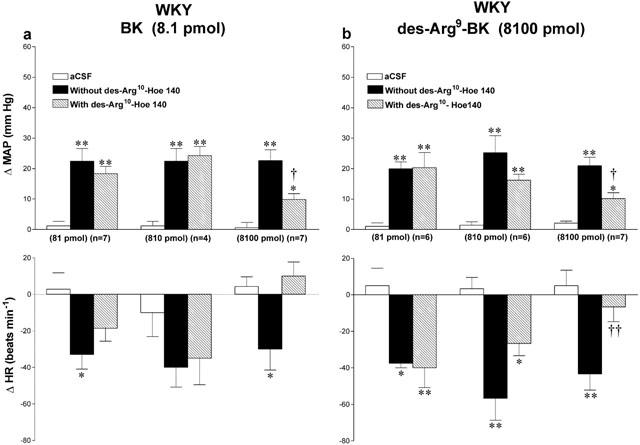
Maximal changes in mean arterial pressure (ΔMAP) and heart rate (ΔHR) induced by 8.1 pmol BK (a) and 8100 pmol des-Arg9-BK (b) injected to the T-9 spinal cord segment of WKY without or with [des-Arg10]-Hoe 140 at the dose indicated at the bottom of each column. Data are means±s.e.mean of (n) rats. Statistical comparison to aCSF values (*) or to the agonist without [des-Arg10]-Hoe 140 (†) is indicated by *,†P< 0.05; **,††P<0.01. Basal MAP and HR in each group were: (a) 81 pmol: 125±7.4 mmHg and 310±9 beats min−1; 810 pmol: 116±3.4 mmHg and 300±21 beats min−1; 8100 pmol: 104±7.4 mmHg and 299±9 beats min−1; (b) 81 pmol: 103±6.7 mmHg and 293±12 beats min−1; 810 pmol: 123±3.6 mmHg and 322±21 beats min−1; 8100 pmol: 112±8.2 mmHg and 317±20 beats min−1.
Effect of indomethacin in SHR and WKY
The cyclo-oxygenase inhibitor, indomethacin (5 mg kg−1, i.a. 1 h earlier), did not affect the increases in MAP and HR evoked by 8100 pmol des-Arg9-BK or 8.1 pmol BK in both SHR (Figure 8) and WKY (data not shown). Moreover, indomethacin had no direct effect on baseline values (data not shown).
Figure 8.
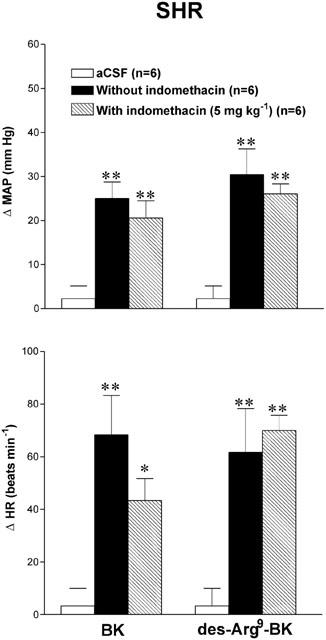
Maximal changes in mean arterial pressure (ΔMAP) and heart rate (ΔHR) induced by 8.1 pmol BK and 8100 pmol des-Arg9-BK injected to the T-9 spinal cord segment of SHR without or with indomethacin. Data are means±s.e.mean of (n) rats. Statistical comparison to aCSF values is indicated by *P<0.05; **P<0.01. There is no statistical difference between values before and after indomethacin. Basal MAP and HR were: 145±9.3 mmHg and 357±17 beats min−1.
Behavioural responses to kinins
Concomitant with the cardiovascular effects, i.t. injection of BK and des-Arg9-BK induced behavioural responses in SHR and WKY. For a period that lasted up to 1 min after injection of agonists, the rat became restless and showed exploratory activity at all doses which produced cardiovascular effects. A lateral rocking motion on the posterior limbs was also observed in most of the rats. Behavioural responses induced by des-Arg9-BK were less striking than those elicited by BK. The initial behavioural excitation induced by BK was followed by a period of sedation which lasted for more than 15 min. Whereas vocalization was observed at 810 pmol BK in some WKY, all SHR displayed a sharp episode of vocalization and jumping during the first 30 s. Behavioural responses induced by des-Arg9-BK and BK were prevented by Hoe 140 and also by the highest dose of [des-Arg10]-Hoe 140.
Autoradiographic distribution of kinin receptors in thoracic spinal cord of SHR and WKY
Quantitative in vitro autoradiography was performed to analyse the distribution of B2 and B1 receptors in the thoracic spinal cord (T9-T10) of 16 weeks old SHR (n=4) and age-matched WKY (n=4). The overall anatomical distribution of both kinin receptor binding sites is illustrated in Figure 9. Analysis of the autoradiograms from WKY showed that the [125I]-HPP-Hoe140 specific binding was found predominantly in superficial laminae of the dorsal horn (DH) with very few binding sites in other laminae (IV-X), intermediolateral (IML) and intermediomedial (IMM) cell column, dorsal nucleus (D), and pyramidal tract (PY) with values ranging from 0.33 – 9.90 fmol mg−1 tissue (Figure 10a). Levels of specific B2 receptor binding sites were significantly higher in SHR in most laminae with values ranging from 0.63 – 47.12 fmol mg−1 tissue, particularly in laminae I and II of the spinal dorsal horn (Figure 10a).
Figure 9.
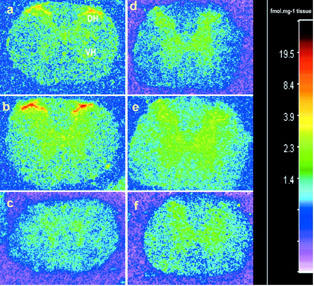
Autoradiographic distribution of [125I]-HPP-Hoe 140 (a,b,c) and [125I]-HPP-[des-Arg10]-Hoe 140 (d,e,f) binding sites in the thoracic spinal cord of WKY (a,d) and SHR (b,c,e,f). Note the high level of specific B2 receptor binding sites in the dorsal horn (DH) of both strains and the greater density of labelling in SHR (b). A weak density of B1 receptor binding sites is found in the grey matter of WKY (d) and SHR (e). Non specific binding in the presence of 1 μM of HPP-Hoe 140 and HPP-[des-Arg10]-Hoe 140 are shown in (c) and (f), respectively.
Figure 10.
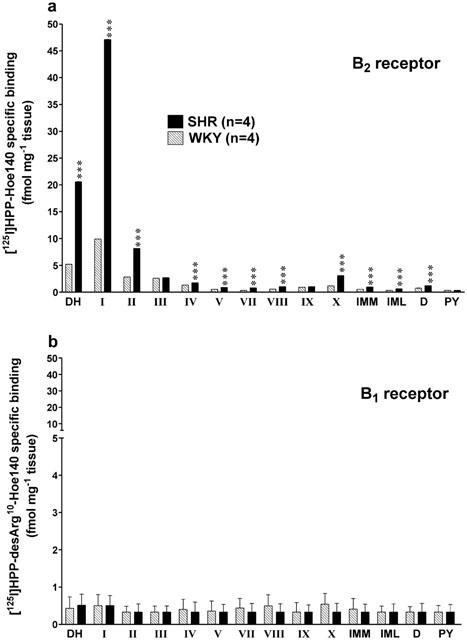
Quantification of specific binding sites with [125I]-HPP-Hoe 140 (a) and [125I]-HPP-[des-Arg10]-Hoe 140 (b) throughout the laminae of thoracic spinal cord of WKY and SHR. Values represent the means±s.e.mean of four rats in each strain. S.e.means are too small to be depicted in the upper panel. Statistical comparison between SHR and WKY is indicated by ***P<0.001. Abreviations: DH=dorsal horn; IMM=intermediomedial cell column; IML=intermediolateral cell column; number of lamina is from I to X; PY=pyramidal tract; D=dorsal nucleus.
Low levels of [125I]-HPP-[des-Arg10]-Hoe140 specific binding were observed in all laminae of the thoracic spinal cord. Values varied from 0.33 – 0.54 fmol mg−1 tissue in WKY and from 0.33 – 0.51 fmol mg−1 tissue in SHR. No statistical differences were observed among the laminae or between WKY and SHR (Figure 10b).
Discussion
Spontaneously hypertensive rats are more sensitive than normotensive rats to the pressor action of BK injected either intracerebrally or in specific cardiovascular centres of the brain stem (for review see Couture & Lindsey, 2000). In the present study, we show that the cardiovascular response to BK is also greater at the level of the spinal cord in SHR. This hypersensitivity is characterized by enhanced amplitude and duration of the vasopressor effect induced by intrathecal BK in SHR as compared to WKY. Moreover, the threshold dose for BK is 10 fold lower in SHR. Data with B2 receptor antagonist Hoe 140 confirm that the cardiovascular response mediated by BK in the spinal cord of SHR and WKY is mediated by B2 receptors as already reported in Wistar rats and streptozotocin (STZ)-diabetic rats (Lopes et al., 1993; Cloutier & Couture, 2000).
Since the spinal action of BK on blood pressure was attributable to the release of catecholamines from sympathetic fibers and the adrenal medulla in Wistar rats (Lopes & Couture, 1992), it is likely that the elevation of blood pressure in SHR is also due to the release of peripheral catecholamines. The activity and reactivity of the sympathetic nervous system is well documented in SHR (de champlain, 1990). Thus, it is likely that the greater vasopressor effects mediated by spinal BK derive from an exaggerated sympatho-adrenal medullary response. The mechanism underlying the rapid offset of the pressor response has not been investigated in this study but could derive from the activation of vascular β2-adrenoceptors similarly to the compensatory skeletal muscle vasodilatory mechanism occurring during the defence response in SHR (Kirby & Johnson, 1990; Kirby et al., 1991). This hypothesis is also predicted by the presence of a β-adrenoceptor component in the spinal vasodepressor response to BK under systemic α1-adrenoceptor inhibition (Lopes & Couture, 1992). Hence, the activation of β-adrenoceptors in the skeletal muscle vasculature by circulating adrenaline may blunt large pressor responses to spinal BK in SHR.
Previous study in Wistar rats has shown that the bradycardia produced by i.t. injection of BK is due to a vagal reflex involving sensory C-fibres and a spinobulbar pathway (Lopes & Couture, 1992). Whereas most WKY responded to BK by a bradycardia, SHR displayed either a biphasic effect on HR or a tachycardia under stimulation with BK. Since atropine converted the BK-induced bradycardia into a tachycardia in Wistar rats (Lopes & Couture, 1992), it is likely that the sympathetic drive to the heart predominates over the parasympathetic component in SHR. This would mean that the baroreceptor reflex which normally occurs under increases of blood pressure is impaired in SHR and is substituted by a greater sympathetic drive (Nosaka & Okamoto, 1970; Gonzalez et al., 1983).
Site of action of intrathecal kinins
The cardiovascular responses induced by BK and B1 agonists had a rapid onset, suggesting an action directly at the segment of injection in the spinal cord. Indeed, the biological half-life of BK after i.t. injection is thought to be as short as that reported (<30 s) after intracerebroventricular (i.c.v.) administration in conscious rats (Kariya et al., 1982). Although the biological half-life of des-Arg9-BK in the CSF remains unknown, this fragment of BK is also a substrate for kininase II (Décarie et al., 1996), the major metabolic pathway for kinins. Peripheral leakage of the injected peptide into systemic circulation cannot account for the cardiovascular responses to kinins because BK produces vasodepressor responses in the periphery contrary to its spinal vasopressor effect (Regoli & Barabé, 1980). Previous studies using dyes and radiotracers have documented that drugs and peptides injected to rats by an i.t. catheter do not reach supraspinal structures within the time frame of the experiments (Yaksh & Rudy, 1976; Cridland et al., 1987).
Autoradiographic distribution of BK receptor binding sites in the thoracic spinal cord
Increases in the density of B2 receptor binding sites in SHR are likely due to increases in the number of receptors and not to changes in receptor affinity since Kd values for [125I]-HPP-Hoe 140 were similar between strains and 200 pM of the B2 receptor radioligand provided complete saturation of total specific receptor binding sites. In the present study, the laminar distribution of [125I]-HPP-Hoe 140 binding sites in the thoracic spinal cord was similar in WKY and SHR and was found to be predominantly located to the dorsal horn as previously reported in Wistar rats (Lopes et al., 1995). It is worth noting that in Wistar rats, the highest density of B2 receptor binding sites was located in lamina II when using [125I]-Tyr8-BK (Lopes et al., 1995) or [125I]-HPP-Hoe 140 (data not shown) as radioligands. Surprisingly, the highest density of [125I]-HPP-Hoe 140 binding sites was found in lamina I instead of lamina II in SHR and WKY. Notwithstanding that differential laminar distribution between strains, the presence of B2 receptor binding sites on primary sensory terminals in the superficial layers of the dorsal horn correlates well with the putative role of BK in the mediation of nociceptive information (Couture et al., 2001). In the present study, BK induced higher nociceptive behavioural excitation in SHR which is consistent with the higher density of [125I]-HPP-Hoe 140 binding sites in laminae I and II of the spinal dorsal horn in SHR. This may appear to be in disagreement with the increased response threshold to noxious stimuli and hypoalgesia reported in human and animals affected with chronic hypertension (Ghione, 1996). However, the activation of B2 receptor in the rat spinal horn also produces antinociceptive responses through the activation of bulbospinal noradrenergic inhibitory fibres (Laneuville et al., 1989) which is congruent with the period of sedation evoked by intrathecal BK in the present study. Further studies are however needed to address the relative contribution of the dual effects of kinins on nociception in hypertension.
A moderate density of [125I]-Tyr8-BK binding sites was also reported in the IML (Lopes et al., 1995). This region corresponds to the site of origin of the pre-ganglionic sympathetic neurons which provide an anatomical substrate for the spinal effect of BK on the cardiovascular system. Whereas SHR and WKY exhibited low densities of [125I]-HPP-Hoe 140 binding sites in the IML, the number of binding sites was slightly higher in SHR and could contribute to the hypersensitivity of the pressor effect induced by BK. However, whether BK activates sympathetic pre-ganglionic fibres directly or indirectly through spinal interneurons is presently unknown.
The possibility that the enhanced density of B2 receptor binding sites seen in SHR is linked to a strain difference or to hypertension will require further studies with other models of hypertension. Nevertheless, we found in a recent study that B2 receptor binding sites were significantly increased in the spinal cord of SHR from the age of 8 to 16 weeks when compared to age-matched WKY and this difference between strains was not affected by preventing the development of hypertension following treatment with an anti-hypertensive agent (losartan, antagonist of angiotensin AT1 receptor) from the age of 4 to 16 weeks (Ongali et al., 2001). This finding suggests that the up-regulation of B2 receptors in SHR is not secondary to hypertension but to a genetic feature. Thus, one cannot exclude the possibility that spinal kinins and B2 receptors are involved somehow in the development of hypertension.
Lack of evidence for B1 receptor in the spinal action of kinins in SHR and WKY
In SHR and WKY, des-Arg9-BK increased blood pressure and caused either bradycardia or tachycardia. This cardiovascular response appears to be related to the activation of B2 receptors in both strains. First, des-Arg9-BK was 1000 fold less potent than BK in producing changes in HR and MAP. This is consistent with the fact that this B1 agonist has less than 1% the affinity of BK on peripheral B2 receptor (Regoli & Barabé, 1980). Second, the spinal effects of des-Arg9-BK were abolished by the B2 receptor antagonist, Hoe 140 (Hock et al., 1991), at relatively low doses (81 pmol). This blockade cannot be due to the metabolic transformation of Hoe 140 into [des-Arg10]-Hoe 140 (B1 receptor antagonist, Wirth et al., 1991) because 81 pmol of the latter B1 antagonist did not block the effects of des-Arg9-BK. Indeed, [des-Arg10]-Hoe 140 inhibited partially the spinal effects of the B1 agonist in both strains only at a fairly high dose (8100 pmol) which also blocked the cardiovascular response induced by 8.1 pmol BK. Although [des-Arg10]-Hoe 140 has been described as B1 receptor antagonist with little affinity for B2 receptor (Wirth et al., 1991), it can block the response mediated by BK in the nanomolar range (Seabrook et al., 1997). It is also unlikely that BK is converted into des-Arg9-BK following its degradation by kininase I since BK was active at doses (0.81 – 8.1 pmol) where des-Arg9-BK was inactive. Third, very low densities of specific [125I]-HPP-[des-Arg10]-Hoe 140 binding sites were found throughout the grey matter of the spinal cord in WKY and SHR, and no differences were found between the two strains. Nevertheless, a small population of binding sites appears to belong to B1 receptor since these sites were not displaced by unlabelled HPP-Hoe 140 (B2 radioligand even at 10,000 fold greater concentration) but only by HPP-[des-Arg10]-Hoe 140. The physiological significance of these sites remains to be established. To sum up, the present study highlights the need to carry out a critical pharmacological analysis of any B1 receptor mediated responses in vivo as des-Arg9-BK and des-Arg10-Hoe 140 are not highly selective agonist and antagonist at the B1 receptor when used at high doses (nmol range). However, the two radioligands which were used to probe B1 and B2 receptors displayed a high selectivity when employed in the pmol range.
The cardiovascular response to des-Arg9-BK displayed a large variability among animals as several SHR and WKY were insensitive to this agonist despite responding to a low dose of BK (8.1 pmol). The more consistent cardiovascular effects seen with Lys-des-Arg9-BK and Sar-[D-Phe8]-des-Arg9-BK in rats which failed to respond to des-Arg9-BK could be explained by the metabolism since Lys-des-Arg9-BK is believed to be more resistant to aminopeptidase while Sar-[D-Phe8]-des-Arg9-BK is a stable peptide agonist protected by angiotensin-1 converting enzyme and aminopeptidase M (Drapeau et al., 1991). Although the biological half-life of des-Arg9-BK in the CSF remains unknown, this fragment of BK is also a substrate for kininase II (Décarie et al., 1996).
Pharmacological evidence indicates that the B1 receptor is up-regulated in the spinal cord of STZ-diabetic rats (Cloutier & Couture, 2000). In this model, [des-Arg10]-Hoe 140 was tenfold more potent in inhibiting the cardiovascular response induced by des-Arg9-BK in contrast to SHR and WKY. Moreover, the cardiovascular response induced by i.t. injection of B1 and B2 agonists was dissociated by indomethacin in STZ-diabetic rats. While the spinal effect of des-Arg9-BK was abolished by indomethacin, this treatment was without any effect against the spinal effect of BK, suggesting that prostaglandins are involved in the activation of B1 but not B2 receptors in the spinal cord of STZ-diabetic rats. In SHR and WKY, indomethacin failed to modify the spinal effects of des-Arg9-BK and BK. Thus, in contrast to STZ-diabetic rats, prostaglandins are not involved in the spinal action of kinins in SHR and WKY. This finding provides an indirect evidence that B1 receptors are not involved in the cardiovascular response to B1 agonists in SHR.
Because antagonists of the B1 receptor ([des-Arg10]-Hoe 140) and B2 receptor (Hoe 140) tested in this study had no direct effect on blood pressure and heart rate upon their i.t. administration, it is unlikely that these receptors are involved in the tonic control of blood pressure at the level of the spinal cord in SHR and WKY. A similar conclusion was drawn in Wistar rats (Lopes et al., 1993) and STZ-diabetic rats (Cloutier & Couture, 2000). However, it is feasible that kinins act as neuromodulators of spinal autonomic functions.
Conclusion
The hypersensitivity of the pressor response to BK and the higher densities of B2 receptor binding sites provide evidence for an up-regulation of B2 receptors in the spinal cord of SHR. Also this pharmacologic and quantitative autoradiographic study makes unlikely a role for B1 receptor in spinal autonomic control of blood pressure in SHR and WKY.
Acknowledgments
The authors acknowledge Dr D. Regoli (Sherbrooke University, Sherbrooke, Canada) for the donation of kinin receptor analogues and ligands, and Dr G. Thibault (Clinical Research Institute, Université de Montréal, Montréal, Canada) for the iodination of kinin receptor ligands. This work was supported by a grant-in-aid from the Canadian Institutes of Health Research. F. Cloutier and B. Ongali hold Studentships from the Fonds de la recherche en santé du Québec and The Republic of Gabon, respectively. Dr H. S. Buck was a Post-doctoral Fellow from Fundação de Amparo a Pesquisa no Estado de São Paulo, Brazil (99/10064-7).
Abbreviations
- aCSF
artificial cerebrospinal fluid
- HR
heart rate
- i.t.
intrathecal
- MAP
mean arterial blood pressure
- SHR
spontaneously hypertensive rat
- WKY
Wistar Kyoto rat
References
- ALVAREZ A.L., DELORENZI A., SANTAJULIANA D., FINKIELMAN S., NAHMOD V.E., PIROLA C.J. Central bradykininergic system in normotensive and hypertensive rats. Clin. Sci. 1992;82:513–519. doi: 10.1042/cs0820513. [DOI] [PubMed] [Google Scholar]
- BUÑAG R.D., TAKAHASHI H. Exaggerated sympathetic responses to bradykinin in spontaneously hypertensive rats. Hypertension. 1981;3:433–440. doi: 10.1161/01.hyp.3.4.433. [DOI] [PubMed] [Google Scholar]
- CLOUTIER F., COUTURE R. Pharmacological characterization of the cardiovascular responses elicited by kinin B1 and B2 receptor agonists in the spinal cord of streptozotocin-diabetic rats. Br. J. Pharmacol. 2000;130:375–385. doi: 10.1038/sj.bjp.0703319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORRÊA F.M.A., GRAEFF F.G. Central mechanisms of the hypertensive action of intraventricular bradykinin in the unanaesthetized rat. Neuropharmacology. 1974;13:65–75. doi: 10.1016/0028-3908(74)90008-2. [DOI] [PubMed] [Google Scholar]
- COUTURE R., HARRISSON M., VIANNA R.M., CLOUTIER F. Kinin receptors in pain and inflammation. Eur. J. Pharmacol. 2001;429:161–176. doi: 10.1016/s0014-2999(01)01318-8. [DOI] [PubMed] [Google Scholar]
- COUTURE R., LINDSEY C.J. Brain kallikrein-kinin system: from receptors to neuronal pathways and physiological functions Handbook of Chemical Neuroanatomy 2000Vol. 16Amsterdam: Elsevier Science B.V; 241–300.eds. Quirion, R., Björklund, A., Hökfelt, TPart I, Peptide Receptors. pp [Google Scholar]
- CRIDLAND R.A., YASHPAL K., ROMITA V.V., GAUTHIER S., HENRY J.L. Distribution of label after intrathecal administration of 125I-subtances P in the rat. Peptides. 1987;8:213–221. doi: 10.1016/0196-9781(87)90092-1. [DOI] [PubMed] [Google Scholar]
- DÉCARIE A., RAYMOND P., GERVAIS N., COUTURE R., ADAM A. Serum interspecies differences in metabolic pathways of bradykinin and [des-Arg9]BK: Influence of enalaprilat. Am. J. Physiol. 1996;270:H1340–H1347. doi: 10.1152/ajpheart.1996.271.4.H1340. [DOI] [PubMed] [Google Scholar]
- DE CHAMPLAIN J. Pre- and postsynaptic adrenergic dysfunctions in hypertension. J. Hypertens. 1990;8 Suppl.7:S77–S85. [PubMed] [Google Scholar]
- DRAPEAU G., DEBLOIS D., MARCEAU F. Hypotensive effects of Lys-des-Arg9-Bradykinin and metabolically protected agonists of B1 receptors for kinins. J. Pharmacol. Exp. Ther. 1991;259:997–1003. [PubMed] [Google Scholar]
- EMANUELI C., CHAO J., REGOLI D., CHAO L., NI A., MADEDDU P. The bradykinin B1 receptor and the central regulation of blood pressure in spontaneously hypertensive rats. Br. J. Pharmacol. 1999;126:1769–1776. doi: 10.1038/sj.bjp.0702527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIOR D.R., MARTINS D.T.O., LINDSEY C.J. Localization of central pressor action of bradykinin in medulla oblongata. Am. J. Physiol. 1993;265:H1000–H1006. doi: 10.1152/ajpheart.1993.265.3.H1000. [DOI] [PubMed] [Google Scholar]
- GHIONE S. Hypertension-associated hypalgesia. Evidence in Experimental animals and humans, pathological mechanisms, and potential clinical consequences. Hypertension. 1996;28:494–504. doi: 10.1161/01.hyp.28.3.494. [DOI] [PubMed] [Google Scholar]
- GONZALEZ E.R., KRIEGER A.J., SAPRU H.N. Central resetting of baroreflex in the spontaneously hypertensive rat. Hypertension. 1983;5:346–352. doi: 10.1161/01.hyp.5.3.346. [DOI] [PubMed] [Google Scholar]
- HOCK F.J., WIRTH K., ALBUS U., LINZ W., GERHARDS H.J., WIEMER G., HENKE St., BREIPOHL G., KÖNIG W., KNOLLE J., SCHÖLKENS B.A. Hoe 140 a new potent and long acting bradykinin-antagonist: in vitro studies. Br. J. Pharmacol. 1991;102:769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W.M., GREENWOOD F.C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- KARIYA K., YAMAUCHI A., HATTORI S., TSUDA Y., OKADA Y. The disappearance rate of intraventricular bradykinin in the brain of the conscious rat. Biochem. Biophys. Res. Commun. 1982;107:1461–1466. doi: 10.1016/s0006-291x(82)80163-0. [DOI] [PubMed] [Google Scholar]
- KIRBY R.F., JOHNSON A.K. Role of β2-adrenoreceptors in cardiovascular response of rats to acute stressors Am. J. Physiol. 1990258H683–H688.(Heart Circ. Physio. 27) [DOI] [PubMed] [Google Scholar]
- KIRBY R.F., WOODWORTH C.H., WOODWORTH G.G., JOHNSON A.K. Beta-2 adrenoceptor mediated vasodilation: Role in cardiovascular responses to acute stressors in spontaneously hypertensive rats. Clin. Exper. Hyper.–Theory and Practice. 1991;A13:1059–1068. doi: 10.3109/10641969109042112. [DOI] [PubMed] [Google Scholar]
- LANEUVILLE O., READER T.A., COUTURE R. Intrathecal bradykinin acts presynaptically on spinal noradrenergic terminals to produce antinociception in the rat. Eur. J. Pharmacol. 1989;159:273–283. doi: 10.1016/0014-2999(89)90158-1. [DOI] [PubMed] [Google Scholar]
- LINDSEY C.J. Central bradykinin receptors in the SHR and blood pressure. Prog. Hypertens. 1995;3:109–125. [Google Scholar]
- LINDSEY C.J., BUCK H.S., FIOR-CHADI D.R., LAPA R.C.R.S. Pressor effect mediated by bradykinin in the paratrigeminal nucleus of the rat. J. Physiol. 1997;502:119–129. doi: 10.1111/j.1469-7793.1997.119bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDSEY C.J., FUJITA K., MARTINS T.O. The central pressor effect of bradykinin in normotensive and hypertensive rats. Hypertension. 1988;11 suppl I:I126–I129. doi: 10.1161/01.hyp.11.2_pt_2.i126. [DOI] [PubMed] [Google Scholar]
- LOPES P., COUTURE R. Cardiovascular responses elicited by intrathecal kinins in the conscious rat. Eur. J. Pharmacol. 1992;210:137–147. doi: 10.1016/0014-2999(92)90664-p. [DOI] [PubMed] [Google Scholar]
- LOPES P., KAR S., CHRÉTIEN L., REGOLI D., QUIRION R., COUTURE R. Quantitative autoradiographic localization of [125I-TYR8]Bradykinin receptor binding sites in the rat spinal cord: effects of neonatal capsaicin, noradrenergic deafferentation, dorsal rhizotomy and peripheral axotomy. Neuroscience. 1995;68:867–881. doi: 10.1016/0306-4522(95)00161-b. [DOI] [PubMed] [Google Scholar]
- LOPES P., REGOLI D., COUTURE R. Cardiovascular effects of intrathecally administered bradykinin in the rat: characterization of receptors with antagonists. Br. J. Pharmacol. 1993;110:1369–1374. doi: 10.1111/j.1476-5381.1993.tb13971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCEAU F., HESS J.F., BACHVAROV D.R. The B1 receptors for kinins. Pharmacol. Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- MARTINS D.T.O., FIOR D.R., NAKAIE C.R., LINDSEY C.J. Kinin receptors of the central nervous system of spontaneously hypertensive rats related to the pressor response to bradykinin. Br. J. Pharmacol. 1991;103:1851–1856. doi: 10.1111/j.1476-5381.1991.tb12341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURONE C., PERICH R.B., SCHLAWE I., CHAI S.Y., CASLEY D., MACGREGOR D.P., MÜLLER-ESTERL W., MENDELSOHN F.A.O. Characterization and localization of bradykinin B2 receptors in the guinea pig using a radioiodinated HOE 140 analogue. Eur. J. Pharmacol. 1996;306:237–247. doi: 10.1016/0014-2999(96)00190-2. [DOI] [PubMed] [Google Scholar]
- NOSAKA S., OKAMOTO K. Modified characteristics of the aortic baroreceptor activities in the spontaneously hypertensive rat. Jap. Cir. J. 1970;34:685–693. doi: 10.1253/jcj.34.685. [DOI] [PubMed] [Google Scholar]
- ONGALI B., BUCK H.S., CLOUTIER F., LEGAULT F., REGOLI D., LAMBERT C., THIBAULT G., COUTURE R. Up-regulation of spinal B2 kinin receptors by ACE inhibitors in spontaneously hypertensive rat (SHR) 2001. P 52. International symposium on Peptide Receptors, Canada, Montreal, July 29–August 2, 2001
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates 1998California, USA: Academic Press, San Diego; 4th edition [Google Scholar]
- PRIVITERA P.J., THIBODEAUX H., YATES P. Rostral ventrolateral medulla as a site for the central hypertensive action of kinins. Hypertension. 1994;23:52–58. doi: 10.1161/01.hyp.23.1.52. [DOI] [PubMed] [Google Scholar]
- REGOLI D., BARABÉ J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- SEABROOK G.R., BOWERY B.J., HEAVENS R., BROWN N., FORD H., SIRINATHSINGHI D.J.S., BORKOWSKI J.A., HESS J.F., STRADER C.D., HILL R.G. Expression of B1 and B2 bradykinin receptor mRNA and their functional roles in sympathetic ganglia and sensory dorsal root ganglia neurones from wild-type and B2 receptor knockout mice. Neuropharmacology. 1997;36:1009–1017. doi: 10.1016/s0028-3908(97)00065-8. [DOI] [PubMed] [Google Scholar]
- WIRTH K., BREIPOHL G., STECHL J., KNOLLE J., HENKE S., SCHÖLKENS B.A. Des-Arg9-D-Arg-[Hyp3,Thi5,DTic7,Oic8]-BK (des-Arg10-[Hoe140]) is a potent bradykinin B1 receptor antagonist. Eur. J. Pharmacol. 1991;205:217–218. doi: 10.1016/0014-2999(91)90824-a. [DOI] [PubMed] [Google Scholar]
- YAKSH T.L., RUDY T.A. Chronic catheterization of the spinal subarachnoid space. Physiol. Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]


