Abstract
Previous studies have suggested that neuronal apoptosis is the result of an abortive attempt to re-enter the cell cycle, and more recently the cyclin-dependent (CDKs) and the mitogen-activated protein (MAP) kinases, two superfamilies of kinases that influence and control cell cycle progression, have been implicated in neuronal apoptosis.
Here, to examine whether CDK/MAPK related pathways are involved in excitotoxicity, we studied the actions of various kinase inhibitors on apoptosis induced by the ionotropic glutamate (Glu) receptor agonist, kainate (KA), in primary cultures of murine cerebellar granule cells (CGCs).
KA-mediated neurotoxicity was concentration-dependent, as determined by a cell viability assay monitoring the reduction of 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and largely apoptotic in nature, as shown by morphological examination and labelling of DNA fragmentation in situ using terminal deoxynucleotidyl transferase (TdT)-mediated dUTP digoxigenin nick-end labelling (TUNEL).
KA-mediated neurotoxicity and apoptosis was completely attenuated by the mixed CDK and MAP kinase inhibitor, olomoucine, in a concentration-dependent manner (50 – 600 μM), and partially by roscovitine (1 – 100 μM), a more selective CDK inihibitor.
The p38 MAP kinase inhibitor, SB203580 (1 – 100 μM), partially attenuated KA receptor-mediated apoptosis, as did the MAP kinase kinase inhibitors PD98509 (1 – 100 μM) and U0126 (1 – 100 μM).
These findings provide new evidence for a complex network of interacting pathways involving CDK/MAPK that control apoptosis downstream of KA receptor activation in excitotoxic neuronal cell death.
Keywords: Apoptosis, cerebellar granule cells, cyclin-dependent kinases, excitotoxicity, kainate, MAP kinases, MEK, p38 MAP kinase
Introduction
Neuronal injury mediated by overstimulation of receptors for the major excitatory transmitter, L-glutamate (Glu), termed ‘excitotoxicity', is well documented, and has been implicated in a variety of neurodegenerative conditions (Leist & Nicotera, 1998; Lipton & Rosenberg, 1994). Excitotoxic neuronal injury can occur quickly, resulting in lesions in the intact nervous system, or neurones can degenerate more slowly by an apoptotic mechanism, dependent upon the intensity of the insult (Cheung et al., 1998a; Ankarcrona et al., 1995). Exposure to neurotoxic concentrations of Glu leads to necrosis via the N-methyl-D-aspartate (NMDA) receptors (Choi et al., 1988), while overstimulation of the non-NMDA receptors, kainate (KA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), commonly produces a pattern of cell death characteristic of apoptosis (Cheung et al., 1998b; Larm et al., 1997; Portera-Cailliau et al., 1997). Apoptosis, or programmed cell death, is morphologically distinct from necrosis, with neurones losing cellular shape and appear shrunken, while the neurites break down (known as neurite blebbing). Examination of the DNA from apoptotic cells reveals oligosomal fragmentation, or DNA laddering, where the DNA is digested into fragments of approximately 180 bp (Walker & Sikorska, 1994). Biochemically, apoptosis is dependent upon macromolecular and RNA synthesis, suggesting apoptosis requires the activation of various ‘death genes' to bring about the demise of the cell (Leist & Nicotera, 1998; Oppenheim et al., 1990)
Recently, neuronal apoptosis has been suggested to result from a failed attempt to re-enter the cell cycle (Ross, 1996). Various genes and/or proteins associated with the cell cycle can promote apoptosis or demonstrate altered expression during neuronal apoptosis, the most well studied being p53 (Hughes et al., 1999). Other cell cycle genes have more recently been implicated in neuronal death, namely the cyclins and their catalytic subunits, the cyclin-dependent kinases (CDK). Freeman et al. (1994) demonstrated a selective activation of cyclin D1 in sympathetic neurones deprived of nerve growth factor. Studies from our laboratory have also found a marked increase in the expression of cyclin D1 after KA-receptor mediated apoptosis in cultured cerebellar granule cells (CGCs) (Giardina et al., 1998), although, loss of cyclin D1 expression has also been reported in neurones undergoing apoptosis mediated by staurosporine (Small et al., 1999). Overexpressing endogenous inhibitors of CDKs can attenuate apoptosis mediated by DNA damage in primary neuronal cultures (Park et al., 1998). Pharmacological evidence to suggest neuronal apoptosis is an abortive attempt to re-enter the cell cycle have come from agents that inhibit various stages of the cell cycle, including CDK inhibitors. Inhibition of the G1/S transition can attenuate apoptosis in PC12 cells and sympathetic neurones, whereas agents that block the later stages of the cell cycle are ineffective (Park et al., 1997; 1996; Kranenburg et al., 1996).
Mitogen-activated protein (MAP) kinases, in addition to their already established role in differentiation and proliferation (Fukunaga & Miyamoto, 1998), have recently been implicated in neuronal apoptosis (Maas et al., 1998; Walton et al., 1998). While evidence is confounding, the extracellular receptor kinases (ERKs) are generally thought to mediate anti-apoptotic signalling, while the stress-activated protein kinases (SAPKs; including the c-Jun NH2-terminal kinases (JNKs) and the p38 MAP kinases), are believed to mediate a pro-apoptotic signal (Xia et al., 1995). While survival of sympathetic neurones is not dependent upon ERK1 or ERK2 activation (Virdee & Tolkovsky, 1995), ERK inhibition may be essential for the execution of apoptosis (Xia et al., 1995). While c-jun has been implicated in various apoptotic paradigms (Behrens et al., 1999; Cheung et al., 1998b; Araki et al., 1998; Beer et al., 1998; Guegan et al., 1997), and c-jun dominant-negative mutants attenuate apoptosis in sympathetic neurones (Ham et al., 1995), the involvement of JNK in neuronal apoptosis is less clear. While an increase in JNK activity has been reported in growth-factor mediated apoptosis in sympathetic neurones, suppression of JNK activity is not sufficient to rescue apoptotic neurones (Virdee et al., 1997). The most convincing evidence to suggest JNK is implicated in excitotoxic neuronal death has come from studies utilizing JNK3 knockout mice, where kainate (KA)-mediated seizures in vivo failed to cause apoptosis in hippocampal neurones, coincident with the reduction of c-jun phosphorylation (Yang et al., 1997).
Activation of the NMDA receptor stimulates JNK and p38 MAP kinases in cultured CGCs (Kawasaki et al., 1997), and in hippocampal neurones AMPA and KA receptors stimulate ERKs, JNK and p38 kinase (Mukherjee et al., 1999). This mosaic of results imply the MAP kinases play an important role in mediating Glu receptor responses, possibly involving the normal physiology of Glu and associated pathophysiology. Recently MAP kinases have been associated with other neuropathological models, likely to involve excitotoxic injury. Pharmacological inhibition of p38 kinase can reduce the number of dying cells in axotomized retinal ganglion cells (Kikuchi et al., 2000; Castagne & Clarke, 1999) and neuronal apoptosis occurring in in vitro models of seizure activity is prevented by ERK kinase inhibitors (Murray et al., 1998). KA and/or AMPA receptor stimulation results in the marked activation of the ERK kinases in oligodendrocytes (Liu et al., 1999) and striatal slices (Fuller et al., 2001; Cruise et al., 2000).
Apoptosis in cultured CGCs has previously been reported to be independent of p38 kinase and JNK (Gunnmoore & Tavare, 1998), although other studies have reported confounding results (Ikeuchi et al., 1998). The ERK pathway mediates the neuroprotective role of pituitary adenylate cyclase-activating polypeptides (PACAP) in potassium-deprived CGCs (Villalba et al., 1997), whereas low potassium-induced c-jun phosphorylation seems to be governed by p38 MAPK (Yamagishi et al., 2001). These differing observations suggest that in CGCs the recruitment of MAPKs is stimulus-dependent and, here we have examined the effects of CDK, p38 and MAP kinase kinase (MEK) 1/2 inhibitors in these primary neuronal cultures undergoing KA receptor-mediated apoptosis. CGCs provide a unique model system as they are a homogenous preparation with a negligible glial population, when cultured under defined conditions in a chemically defined medium (Giardina et al., 1998), and have permitted us to examine whether the cell cycle and the stress kinase cascades contribute to KA-induced neuronal injury. This model system contains no functional AMPA receptors (Giardina & Beart, 2001), and has allowed us to describe for the first time the involvement of MAPKs downstream of KA receptor-mediated apoptosis.
Methods
Materials
KA was purchased from Tocris Cookson (Bristol, U.K.). NeurobasalTM medium (NBM), B27 nutrients, N2 supplements and Ca2+-free-Hank's balanced salt solution (HBSS) were purchased from GibcoBRL Life Technologies (Melbourne, Australia). All other reagents were purchased from Sigma or Boehringer Mannheim (Sydney, Australia) and were of cell culture or molecular biology grade. Olomoucine, iso-olomoucine, SB203580, PD98059, and roscovitine were purchased from Alexis Biochemicals (CA, U.S.A.) or Calbiochem (Sydney, Australia), and U0126 was purchased from Calbiochem (Sydney, Australia).
Experiments were performed in accordance with the ethical code of the National Health and Medical Research Council (Australia) with permission from the Standing Committee for Ethics in Animal Experimentation (Monash University).
Cell culture
CGCs were prepared from 7-day-old Swiss-White mice and cultured as previously described (Cheung et al., 1998b; Giardina et al., 1998). CGCs were grown in NBM containing B27 components (Brewer et al., 1993), 25.4 mM K+, 500 μM L-glutamine and 100 u ml−1 penicillin – streptomycin and exposed to 10% dialysed foetal calf serum for the first 24 h and left in serum-free conditions from day 1 in vitro (div). Cells were seeded at a cell density of 0.3×106 cells cm−2 in 24-well NUNCTM plates (Denmark) precoated with poly-D-lysine (50 μg ml−1). Aphidicolin (2 μg ml−1) was added to the medium 18 – 24 h after plating to inhibit non-neuronal cell proliferation (Giardina et al., 1998; Miller & Johnson, 1996). Immunocytochemistry previously established that >95% of the cells were neurones (Cheung et al., 1998b) and express KA receptors (Giardina & Beart, 2001).
Agonist exposure and cell viability assays
Initial investigations were carried out to examine the effects of the kinase inhibitors themselves on the viability of the cultures. A range of concentrations were used, according to those used in previous studies (Maas et al., 1998; Kawasaki et al., 1997; Park et al., 1996) to ensure no change in cell viability was evident. Optimal survival of the cultures, with no evidence of loss of cell viability, was determined to be a 4 h exposure time and therefore KA and the kinase inhibitors were incubated for 4 h before being left in drug-free media overnight. Studies in our laboratory have previously demonstrated that KA (10 – 1000 μM) induces apoptosis, coincident with the activation of c-jun (Cheung et al., 1998b) and cyclin D1 (Giardina et al., 1998).
Cultures were exposed to KA (10 – 1000 μM) alone or in the presence of olomoucine (50 – 600 μM), iso-olomoucine (50 – 600 μM), roscovitine (1 – 100 μM), SB203580 (1 – 100 μM), PD98059 (1 – 100 μM) or U0126 (1 – 100 μM) for 4 h at 8 div in N2 supplemented NBM containing 100 u ml−1 penicillin – streptomycin, 0.25% BSA, 83 μM D(+) galactose, 16 μM ethanolamine, 6 μM L-carnitine, 0.4 μM biotin and 25.4 mM K+ (Bottenstein & Sato, 1979). On the basis of previous experiments (Giardina et al., 1998) this injury time would to produce a maximal injury representing approximately a 50% reduction in cellular viability. After 4 h the drug containing medium was aspirated and cultures were left in fresh, drug-free N2 media overnight (for approximately 20 h). Cellular viability was determined at 24 h by the reduction of 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Cheung et al., 1998b). MTT was incubated with the cells for 30 min at 37°C and the reduced formazan product was lysed from the cells in 20% sodium dodecyl sulphate and 40% dimethylformamide and absorbance was subsequently measured at 590 nm (Ceres UV900c microplate reader; Biotek Instruments, U.S.A.). Cultures grown in 5.4 mM K+ from div 1 were taken as 100% apoptotic cell death and the results were expressed as percentage of control determined by the following formula: (cellular viability −100% apoptotic cell death)/(average control treatments −100% apoptotic cell death) ×100. Vehicle controls were conducted for all agents and included the media they were dissolved in, plus direct exposure to the inhibitors themselves to identify any inherent neuroprotective or neurotoxic actions.
After drug exposure (18 – 24 h) cultures were examined by phase contrast microscopy for morphological changes consistent with apoptosis (cellular shrinkage, neurite blebbing), necrosis (loss of cellular density and the presence of cellular debris) or neuroprotection (relative to control cultures with the preservation of neurites and cellular shape). All morphological changes induced by KA and the pharmacological inhibitors were visualized by phase contrast microscopy with an Olympus inverted microscope (Olympus, IMT-2).
In situ labelling of nuclear DNA fragments
Apoptosis was analysed by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP digoxigenin nick-end labelling (TUNEL) as previously described (Cheung et al., 1998b). After the above treatments, CGCs were fixed overnight in 4% paraformaldehyde and permeabilized with 2% Triton X-100 (TX-100) in Tris buffered saline (TBS; 50 mM Tris, 0.9% NaCl; pH 7.6). Cultures were subsequently washed in TBS and blocked overnight at 4°C in solution with 10% normal goat serum and 0.1% TX-100 in TBS and then incubated with TdT reaction mixture as previously described (Cheung et al., 1998b) for 3 h at 37°C. Digoxigenin labelled dUTP was detected by anti-DIG alkaline phosphatase (AP; 1 : 1000 dilution) in solution with 2% normal goat serum and 0.1% TX-100 and TBS. TUNEL-positive cells were detected using AP substrate solution (0.4-mg ml−1 5-bromo-4-chloro-3-indolyl-phosphate; 0.6 mg ml−1 nitroblue tetrazolium chloride, 100 mM Tris-HCl, 0.5 mM MgCl2; pH 9). Control cultures included the above treatment with the omission of TDT. Cells were visualized under bright field microscopy and random and representative fields photographed. Random cell counts were taken from 3 – 6 fields of view and unstandarized data were expressed as percentage of the total number of cells.
Data analyses
Data are given as mean±s.e.mean from at least quadruplicate experiments across 4 – 6 independent cultures and concentration – response curves were generated by non-linear regression using computer-assisted curve fitting (GraphPad PrismTM). Statistical significance (P<0.05) of data was examined by two-way ANOVA with a Bonferroni post hoc test to compare individual treatments.
Results
Kainate neurotoxicity: preliminary observations
KA exposure resulted in a reduction in cellular viability, with characteristics of apoptosis (see below), and was concentration-dependent (F(4,67)=9.18, P<0.05). KA-mediated toxicity (EC50=43 μM) was completely attenuated by the non-NMDA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 50 μM, Figure 1) suggesting a KA-receptor mediated mechanism as AMPA receptor-mediated toxicity is absent in CGCs when cultured under the present conditions (Giardina & Beart, 2001). Morphological changes induced by KA were not evident 1 – 2 h after stimulation, in particular cellular swelling was not present, implying negligible necrosis (data not shown). Examination of the cultures by phase contrast microscopy 20 – 24 h after KA exposure revealed morphological characteristics consistent with apoptosis, with cellular shrinkage and neurite blebbing evident (Figure 3B) as previously observed (Cheung et al., 1998b; Giardina et al., 1998). However, longer exposure times to higher concentrations of U0126 resulted in cellular loss (c.f. Figure 3C) and therefore a 4 h exposure time was employed, where most of the inhibitors alone produced no significant neuronal loss (data not shown). Therefore for routine experimentation, cultures were exposed to KA for 4 h in the absence and presence of kinase inhibitors, before the medium containing the drugs was completely aspirated and left in drug free medium overnight.
Figure 1.
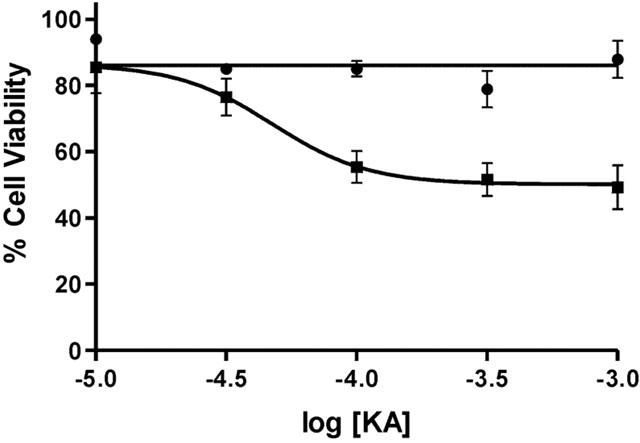
KA receptor mediated neurotoxicity in cultured cerebellar granule cells was determined using the MTT cell viability assay. At div 8 cultures were exposed for 4 h to KA (10 – 1000 μM; closed squares) and subsequently left in drug-free medium for a further 18 h. Cellular injury was concentration-dependent and completely attenuated by CNQX (50 μM; closed circles). Data were standardized relative to vehicle treated (100% cell viability) and low K+ treated cultures (100% apoptotic cell death) and are plotted as mean±s.e.mean and were from 4 – 6 replicate cultures. Further details are given in the text.
Figure 3.
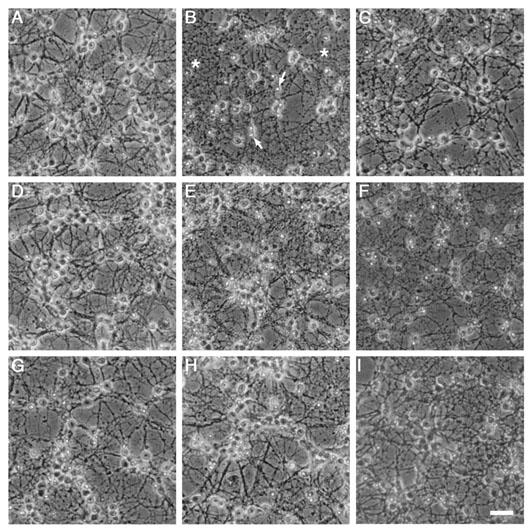
Morphological analyses of KA receptor-mediated neurotoxicity in cultured cerebellar granule cells 24 h after treatment. On div 8, cultured cells were exposed to KA in the presence and absence of various CDK and MAPK inhibitors for 4 h and then left in drug free media overnight. Phase-contrast microscopy demonstrated KA exposure (100 μM; B) induced cellular shrinkage (arrows) and neurite blebbing (asterix) when compared to control sister cultures (A). Treatment with olomoucine (200 μM; D) completely abolished any morphology changes induced by KA exposure, however, treatment with the inactive form, iso-olomoucine (200 μM; E), produced no significant neuroprotection. Roscovitine (100 μM; F) reduced the morphological changes induced by KA as did SB203580 (100 μM; G), but not completely with some evidence of shrunken cells and neurite blebbing still evident. The two ERK inhibitors, PD98059 (100 μM; H) and U0126 (10 μM; I) both slightly attenuated characteristics of KA receptor mediated apoptosis. U0126 (50 μM; C) treatment alone induced some toxicity in cultures, coinciding with MTT data. Scale bar represents 5 μM.
Kinase inhibitors and kainate neurotoxicity: cell viability studies
In biochemical investigations olomoucine produced a trend towards increasing cellular viability in cells not treated with KA, although this action was not statistically significant (F(5,43)=0.165, P>0.05). Olomoucine (200 – 600 μM; Figure 2A) exerted a concentration-dependent neuroprotection against KA-mediated neurotoxicity (F(5,246)=27.56, P<0.0001), with cellular viability returning to 100% and higher. Iso-olomoucine (50 – 600 μM; Figure 2B), the inactive form of olomoucine (Havlicek et al., 1997; Park et al., 1996; 1997), had no significant effect on KA-mediated toxicity (F(5,128)=4.10, P=0.539), confirming the selective actions of olomoucine. Roscovitine partially, but significantly, attenuated KA-induced neurotoxicity (F(5,426)=4.21, P<0.05), but not as effectively as olomoucine, and was completely ineffective at 100 μM (Figure 2C). SB203580 significantly attenuated KA receptor-mediated neurotoxicity, particularly at lower concentrations of KA ((5,276)=5.85, P<0.0001) (Figure 2D). The MEK inhibitor PD98059 effectively attenuated KA receptor-mediated toxicity (F(5,276)=7.24, P<0.0001), although its inhibitory action was not concentration-dependent, with most attenuation occurring at lower concentrations of KA (Figure 2E). The pharmacological profile of U0126 was the most complex and cellular viability showed more variability when compared to those of the other inhibitors. U0126 was effective at attenuating KA-receptor mediated neurotoxicity at concentrations 1, 5 and 10 μM (F(5,206)=17.1, P<0.0001; Figure 2F). Treatment with 50 and 100 μM U0126 resulted in a marked reduction in cellular viability, exacerbating KA-induced toxicity (Figure 2F). While previous studies have demonstrated neuroprotection against a variety of apoptotic insults using the cell cycle inhibitors mimosine, silymarin, ciclopirox and desferrioxamine (Park et al., 1997; Farinelli & Greene, 1996), the present study did not find any significant neuroprotection against KA-mediated neurotoxicity using these agents (data not shown).
Figure 2.
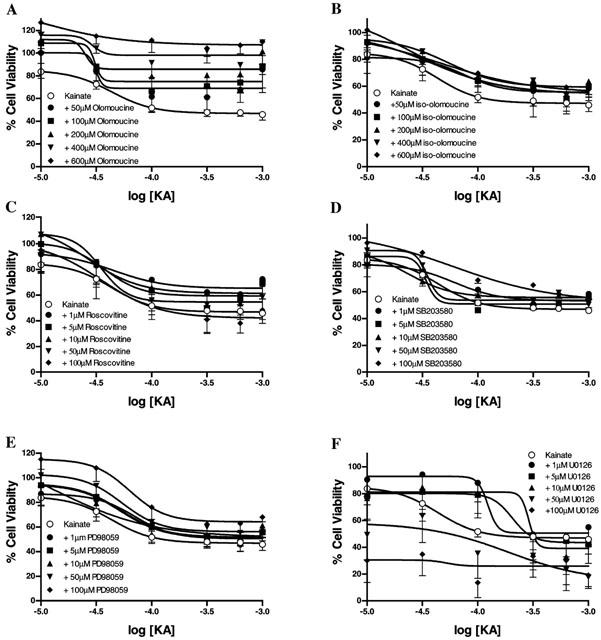
KA receptor mediated neurotoxicity in cultured cerebellar granule cells and the effect of the various kinase inhibitors was determined using the MTT cell viability assay. At div 8 cultures were exposed for 4 h to KA (10 – 1000 μM) in the absence and presence of the CDK and MAPK inhibitors. Olomoucine produced a clear concentration-dependent neuroprotection against KA-mediated toxicity (50 – 600 μM; A), whereas iso-olomoucine (50 – 600 μM; B) produced no significant neuroprotection. Roscovitine (1 – 100 μM; C) produced some neuroprotection against KA-mediated insult, however this effect was not concentration-dependent. SB203580 (1 – 100 μM; D) significantly reduced KA-mediated neurotoxicity but was more effective at lower concentrations of KA (>100 μM). The MEK inhibitors PD98059 (1 – 100 μM; E) and U0126 (1 – 100 μM; F) produced differing levels of neuroprotection. PD98059 produced a significant neuroprotection against KA-mediated toxicity without demonstrating any toxicity of its own, U0126 was extremely toxic to cultures at higher concentrations (<50 μM). Data were standardized relative to vehicle treated (100% cell viability) and low K+ treated cultures (100% apoptotic cell death) and are plotted as mean±s.e.mean and were from 4 – 6 replicate cultures. Further details are given in the text.
Kinase inhibitors and kainate neurotoxicity: morphological observations
Morphological changes induced by KA were consistent with apoptosis, where cells demonstrated shrunken cell bodies and the degeneration of neurites (Figure 3B). Treatment with olomoucine (50 – 600 μM; Figure 3D) completely attenuated, in an apparently concentration – dependent manner, all morphological changes induced by KA (Figure 3B). Iso-olomoucine (50 – 600 μM; Figure 3E), the inactive isomer used widely as a control treatment (Havlicek et al., 1997; Park et al., 1996; 1997), produced no changes when compared to cultures treated with KA alone. The other compounds demonstrated more complex actions on the cultures and observations. Roscovitine, surprisingly did not attenuate KA-induced neurotoxicity to the same extent as olomoucine, observations in aggreement with the biochemical findings. Roscovitine has previously been shown to potently attenuate neuronal apoptosis (Maas et al., 1998). While the present study showed that the neurones treated with roscovitine demonstrated less morphological changes indicative of apoptosis, unlike sister cultures treated with olomoucine, some cellular damage was still evident (Figure 3F). The p38 MAP kinase inhibitor, SB203580 (1 – 100 μM), slightly improved the cellular viability of the cultures, particularly at higher concentrations (50 – 100 μM) and in cultures treated with >100 μM KA (Figure 3G), with some preservation of neurites and cellular shape. Interestingly, the MEK inhibitors PD98059 and U0126, differed in their ability to attenuate KA-mediated neurotoxicity. While PD98059 effectively attenuated morphological changes induced by KA (10 – 100 μM), without inducing toxicity of its own (Figure 3H), concentrations of U0126 (>50 μM) induced cellular loss (Figure 3C). Lower concentrations of U0126, did attenuate cellular shrinkage and neurite blebbing associated with KA receptor-mediated cell death (Figure 3I). Exposure of CGCs to the vehicles, either 1% DMSO or 2% ethanol, had no effect on the morphological integrity of the cultures (Figure 3A; 1% DMSO).
Kinase inhibitors and kainate receptor-mediated apoptosis
KA receptor-mediated neurotoxicity under the present experimental conditions occurs almost exclusively by apoptosis (Cheung et al., 1998b; Giardina et al., 1998) and to determine whether the protective effects of the kinase inhibitors were reflected by attenuation of apoptosis TUNEL labelling was employed. TUNEL labelling revealed extensive TUNEL-positive cells in KA treated cells (300 μM; Figure 4B) when compared to vehicle treated cultures (Figure 4A), consistent with observations made under phase-contrast microscopy. Treatment with olomoucine (400 μM; Figure 4C) completely attenuated apoptotic labelling, while the inactive form iso-olomoucine did not reduce TUNEL labelling (Figure 4D). Roscovitine treatment in the presence of KA resulted in some decrease of the incidence of TUNEL labelling (Figure 4E), however when compared to control some apoptotic labelling was still evident. SB203580 (100 μM; Figure 4F) partially attenuated apoptotic labelling, except at the higher concentrations of KA (1 mM; data not shown). PD98509, like SB203580, decreased KA-mediated TUNEL labelling, particularly at the lower concentrations of KA, but not completely (Figure 4G). U0126, while decreasing TUNEL labelling at certain concentrations (1 – 10 μM), failed to exert a major effect on KA-mediated apoptosis and many TUNEL-positive cells were still evident (Figure 4H).
Figure 4.
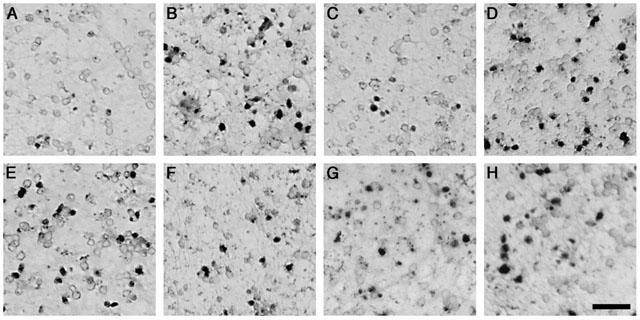
The mode of cell death induced by KA primary cultures of cerebellar granule cells was determined using the TUNEL technique which detects the presence of DNA fragmentation, indicative of apoptosis. Bright field photomicrographs from representative fields of neurones exposed to KA (300 μM) revealed a marked increase in TUNEL positive profiles (B) when compared to vehicle treated controls (A). Olomoucine (400 μM) attenuated TUNEL labelling induced by KA treatment (C), however iso-olomoucine (400 μM) was ineffective (D). The other kinase inhibitors only partially attenuated KA-mediated TUNEL labelling, while some apoptotic cells are still evident fewer TUNEL positive cells are present when compared to KA treated alone cultures, including roscovitine (100 μM; E), SB203580 (100 μM; F), PD98059 (100 μM; G) and U0126 (50 μM; H). Scale bar represents 5 μM.
Cell counts for TUNEL-labelling generally followed the trends seen in MTT data. The best correlation among the various kinase inhibitors was seen with olomoucine, which produced the greatest attenuation of TUNEL-positive staining induced by KA (F(5,83)=85.48, P<0.0001) and essentially blocked all KA-mediated apoptosis even at low concentrations (Figure 5A). While roscovitine significantly attenuated TUNEL-labelling, it was less effective than olomoucine (F(5,83)=13.52, P<0.0001) (Figure 5B). SB203580 significantly decreased KA-mediated TUNEL labelling (F(5,77)=10.106, P<0.001), however closer examination of individual KA treated groups revealed only some concentrations of SB203580 were actually statistically significant when compared to cultures treated with KA alone (Figure 5C). PD98509 and U0126 significantly reduced TUNEL labelling (F(5,65)=10.875, P<0.0001) and (F(5,72)=7.39, P<0.0001), respectively (Figure 5D and E, respectively). TUNEL counts did not correlate with MTT data, with no significant attenuation at 100 μM of KA in the presence of the MEK inhibitors observed for TUNEL counts, despite a marked increase in cell viability as determined by MTT.
Figure 5.
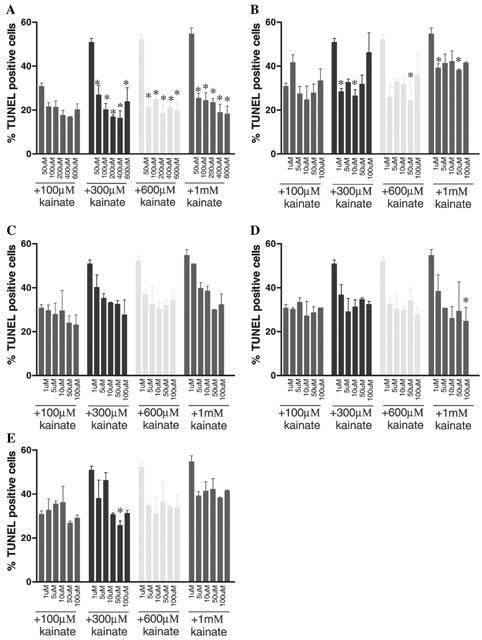
Primary cultures of cerebellar granule cells were exposed to KA on div 8. Apoptosis was assessed using the TUNEL technique, which labels the nick-end DNA fragments. Increasing concentrations of KA increased the number of TUNEL positive cells, implying KA concentration-dependently induces apoptosis. Olomoucine (A) significantly attenuated KA-mediated TUNEL labelling, whereas the other inhibitors demonstrated more complex actions on KA receptor-mediated apoptosis. Roscovitine (B) produced a definite trend towards neuroprotection, however not all concentrations were statistically significant. SB203580 (C) produced a strong trend towards neuroprotection, and this was statistically significant when analysed by two-way ANOVA, however no points were significant when compared to their own treatment group by one-way ANOVA. The MEK inhibitors PD98059 (D) and U0126 (E) while both produced a trend toward attenuating apoptotic labelling, like SB203580 few individual points were significant when compared to KA alone treatment group. Data are plotted as mean±s.e.mean and were from 2 – 3 replicate cultures. Further details are given in the text. * Represents statistical significance, P<0.05, one-way ANOVA.
Discussion
Using cultures of primary murine CGCs and pharmacological inhibitors of CDK and MAPK we have demonstrated for the first time an involvement of these intracellular signalling cascades in KA-receptor mediated apoptosis. Recently, we fully documented the pharmacological profile of KA excitotoxicity in the current model system where functional AMPA receptors are not present (Giardina & Beart, 2001), and here confirm that KA treated CGCs demonstrate the morphological characteristics of apoptosis, with shrunken cell bodies and the degeneration of neurites. TUNEL analysis revealed a marked increase in positive-labelled cells in KA-treated CGCs when compared to vehicle control as previously reported (Cheung et al., 1998b; Giardina et al., 1998). To our knowledge there has been no studies conducted to examine the effects of pharmacologically inhibiting p38, p44 and p42 MAP kinases and CDKs on excitotoxic neuronal death mediated by the KA receptor, without influences from the AMPA receptor, or other cell types such as glia.
KA exposure has previously been shown to activate p42 and p44 MAP kinases in oligodendrocyte progenitors, an action thought to be mediated through the activation of the AMPA receptors (Liu et al., 1999), however in striatal organotypic slices activation of ERK occurs through activation of KA and NMDA, but not AMPA receptors (Fuller et al., 2001). Activation of JNK, p38 and the ERK kinases have been reported after NMDA receptor stimulation in hippocampal neuronal cultures (Mukherjee et al., 1999).
Olomoucine was most effective at attenuating KA-receptor mediated apoptosis in the current model system, but importantly iso-olomoucine, considered an internal control for non-specific effects of the olomoucine chemical moiety (Havlicek et al., 1997; Park et al., 1996; 1997), was ineffective. The concentrations employed in the current study have previously been shown to inhibit CDC2/cyclin B1, CDK2/cyclin A, CDK5/p35, CDK6/cyclin D3, ERK, JNK and p38 kinase activity and are consistent with literature reports on its potency (see Maas et al., 1998 for summary). Therefore, to further elucidate the kinases implicated in KA receptor-mediated apoptosis we used more selective compounds. Roscovitine is a selective CDC2, CDK2 and CDK5 inhibitor (Meijer et al., 1997), and under the present conditions only inhibited some 20% of neuronal death and apoptosis, suggesting that the CDKs only play a minor role in mediated excitotoxic apoptotic death. Roscovitine has previously been shown to be less potent at protecting retinal neurons against serum-withdrawal-mediated injury than olomoucine (Maas et al., 1998), consistent with findings presented here. SB203580 at concentrations used in the present study is a selective p38 MAP kinase inhibitor with little effects on the CDKs (Cuenda et al., 1995). SB203580 was effective at attenuating KA-receptor mediated neuronal loss, particularly at the lower concentrations of KA (10 – 100 μM), with little effect at 1 mM KA. TUNEL labelling was slightly reduced at 300 – 1000 μM KA treatment after SB2032580 exposure, however TUNEL counts revealed no significant change at 100 μM KA, although a trend towards neuroprotection was evident. p38 MAP kinases have previously been shown not to be activated in after KA receptor activation in superfused striatal slices (Fuller et al., 2001). Surprisingly, PD98059 and U0126 produced differing levels of protection implying that while these compounds are directed at the same kinase (Favata et al., 1998), they possess different pharmacological and toxicological profiles.
While MTT data and TUNEL cell counts generally followed the same pattern, less neuroprotection was noted for the kinase inhibitors as reflected by the number of TUNEL positive cells than was expected from the MTT data. This difference may have been attributable to technical considerations or it is possible while these inhibitors are increasing cell viability, they are not attenuating, but rather delaying apoptosis, resulting in a relative increase in MTT reduction but only a slight reduction of TUNEL labelling. Alternatively, since cellular viability measured by MTT reduction reflects mitochondrial function, the diminution seen here may reflect upstream changes that are not totally predictive of the extent of final downstream DNA fragmentation (Budd et al., 2000). The delay in apoptosis may explain the good correlation between the MTT and TUNEL data for olomoucine, as this inhibitor blocks both the CDK and MAP kinases, indicating the therapeutic benefits of inhibiting concurrently these two mechanisms for the execution of apoptosis mediated by the KA receptor. By inhibiting one specific kinase the apoptotic pathway may proceed down an alternative pathway still allowing cell death. Excitotoxicity has previously been shown to involve the cell cycle (Giardina et al., 1998; Uberti et al., 2000) and MAP kinases (Murray et al., 1998; Yang et al., 1997), and it is therefore likely that not just one pathway is essential for the execution of apoptosis. While many intracellular pathways are intertwined, the activation of either the cell cycle or the MAP kinases may in fact result in neuronal death, through the activation of a common downstream pathway, perhaps involving the Bcl-2 family and caspases. However, if both pathways are inhibited, as demonstrated by olomoucine, the apoptotic pathways are completely blocked and apoptosis cannot occur.
While the exact mechanism by which these kinases mediate neuronal apoptosis and/or neuronal survival is still to be elucidated, our data add to the growing body of evidence to suggest they play a central role in neuronal apoptosis. In addition, the MAP kinases and CDKs seem to be central to a complex mosaic of pathways, influencing various intracellular mediators including nuclear factor κβ, AKT (protein kinase B), and the Bcl-2 family, which have all been implicated in neuronal apoptosis (Thomson et al., 1999; Fukunaga & Miyamoto, 1998). Unfortunately, no kinase inhibitors commercially available are specific for one kinase, and it is not presently possible to elucidate whether one kinase per se is responsible for the execution of apoptosis. As the activities of these kinase inhibitors directly on the KA receptor itself has not, to our knowledge, been determined, we can not rule out the possibility these kinases act directly on the KA receptor, or one of its domains, rather than act downstream of receptor activation. We however believe this is unlikely, as no neuroprotection is evident in the inactive isoform, iso-olomoucine. In conclusion, our results support the growing body of evidence involving the cell cycle and MAP kinases in excitotoxicity, and provide the first demonstration of the involvement of MAPKs in KA receptor-mediated neuronal injury.
Acknowledgments
Supported by the National Health and Medical Research Council (Australia), of which P.M. Beart is a Senior Principal Research Fellow, and by grants from the Ramaciotti, Rebecca Cooper and William Buckland Foundations, and Perpetual Trustees.
Abbreviations
- CDK
cyclin-dependent kinase
- CGCs
cerebellar granule cells
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- div
days in vitro. Glu, glutamate
- KA
kainate
- MAP
kinase, mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase kinase
- MTT
3-94,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide
- TUNEL
terminal deoxynucleotidyl transferase (TdT)-mediated dUTP digoxigenin nick-end labelling
References
- ANKARCRONA M., DYPBUKT J.M., BONFOCO E., ZHIVOTOVSKY B., ORRENIUS S., LIPTON S.A., NICOTERA P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- ARAKI T., ENOKIDO Y., INAMURA N., AIZAWA S., REED J.C., HATANAKA H. Changes in c-Jun but not Bcl-2 family proteins in p53-dependent apoptosis of mouse cerebellar granule neurons induced by DNA damaging agent bleomycin. Brain Res. 1998;794:239–247. doi: 10.1016/s0006-8993(98)00231-5. [DOI] [PubMed] [Google Scholar]
- BEER J., MIELKE K., ZIPP M., ZIMMERMANN M., HERDEGEN T. Expression of c-jun, junB, c-fos, fra-1 and fra-2 mRNA in the rat brain following seizure activity and axotomy. Brain Res. 1998;794:255–266. doi: 10.1016/s0006-8993(98)00233-9. [DOI] [PubMed] [Google Scholar]
- BEHRENS A., SIBILIA M., WAGNER E.F. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nature Genetics. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- BOTTENSTEIN J.E., SATO G.H. Growth of a rat neuroblastoma cell line in serum free supplemented medium. Proc. Natl. Acad. Sci., USA. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREWER G.J., TORRICELLI J.R., EVERGE E.K., PRICE P.J. Optimised survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- BUDD S.L., TENNERTI L., LISHNAK T., LIPTON S.A. Mitochondrial and extramitochondrial apoptotic signaling pathways in cerebrocortical neurons. Proc. Natl. Acad. Sci., USA. 2000;97:6161–6166. doi: 10.1073/pnas.100121097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTAGNE V., CLARKE P.G.H. Inhibitors of mitogen-activated protein kinases protect axotomized developing neurons. Brain Res. 1999;842:215–219. doi: 10.1016/s0006-8993(99)01823-5. [DOI] [PubMed] [Google Scholar]
- CHEUNG N.S., PASCOE C.J., GIARDINA S.F., JOHN C.A., BEART P.M. Micromolar L-glutamate induces extensive apoptosis in an apoptotic-necrotic continuum of insult-dependent, excitotoxic injury in cultured cortical neurones. Neuropharmacology. 1998a;37:1419–1429. doi: 10.1016/s0028-3908(98)00123-3. [DOI] [PubMed] [Google Scholar]
- CHEUNG N.S., CARROLL F.Y., LARM J.A., BEART P.M., GIARDINA S.F. Kainate-induced apoptosis correlates with c-Jun activation in cultured cerebellar granule cells. J. Neurosci. Res. 1998b;52:69–82. doi: 10.1002/(SICI)1097-4547(19980401)52:1<69::AID-JNR7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- CHOI D.W., KOH J.Y., PETERS S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J. Neurosci. 1988;8:185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRUISE L., HO L.K., VEITCH K., FULLER G., MORRIS B.J. Kainate receptors activate NF-κβ via MAP kinase in striatal neurones. Neuroreport. 2000;11:395–398. doi: 10.1097/00001756-200002070-00034. [DOI] [PubMed] [Google Scholar]
- CUENDA A., ROUSE J., DOZA Y.N., MEIER R., COHEN P., GALLAGHER T.F., YOUNG P.R., LEE J.C. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- FARINELLI S.E., GREENE L.A. Cell cycle blockers mimosine, ciclopirox, and deferoxamine prevent the death of PC12 cells and postmitotic sympathetic neurons after removal of trophic support. J. Neurosci. 1996;16:1150–1162. doi: 10.1523/JNEUROSCI.16-03-01150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAVATA M.F., HORIUCHI K.Y., MANOS E.J., DAULERIO A.J., STRADLEY D.A., FEESER W.S., VAN DYK D.E., PITTS W.J., EARL R.A., HOBBS F., COPELAND R.A., MAGOLDA R.L., SCHERLE P.A., TRZASKOS J.M. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- FREEMAN R.S., ESTUS S., JOHNSON E.M. , JR Analysis of cell cycle-related gene expression in postmitotic neurones: selective induction of cyclin D1 during programmed cell death. Neuron. 1994;12:343–355. doi: 10.1016/0896-6273(94)90276-3. [DOI] [PubMed] [Google Scholar]
- FUKUNAGA K., MIYAMOTO E. Role of MAP kinase in neurons. Mol. Neurobiol. 1998;16:79–95. doi: 10.1007/BF02740604. [DOI] [PubMed] [Google Scholar]
- FULLER G., VEITCH K., HO L.K., CRUISE L., MORRIS B.J. Activation of p44/p42 MAP kinase in striatal neurons via kainate receptors and PI3 kinase. Mol. Brain Res. 2001;89:126–132. doi: 10.1016/s0169-328x(01)00071-7. [DOI] [PubMed] [Google Scholar]
- GIARDINA S.F., CHEUNG N.S., REID M.T., BEART P.M. Kainate-induced apoptosis in cultured murine cerebellar granule cells elevates expression of the cell cycle gene cyclin D1. J. Neurochem. 1998;71:1325–1328. doi: 10.1046/j.1471-4159.1998.71031325.x. [DOI] [PubMed] [Google Scholar]
- GIARDINA S.F., BEART P.M. Excitotoxic profiles of novel, low-affinity kainate receptor agonists in primary cultures of murine cerebellar granule cells. Neuropharmacology. 2001;41:421–432. doi: 10.1016/s0028-3908(01)00086-7. [DOI] [PubMed] [Google Scholar]
- GUEGAN C., LEVY V., DAVID J.P., AJCHENBAUM-CYMBALISTA F., SOLA B. c-Jun and cyclin D1 proteins as mediators of neuronal death after a focal ischaemic insult. Neuroreport. 1997;8:1003–1007. doi: 10.1097/00001756-199703030-00037. [DOI] [PubMed] [Google Scholar]
- GUNNMOORE F.J., TAVARE J.M. Apoptosis of Cerebellar Granule Cells Induced By Serum Withdrawal, Glutamate or Beta-Amyloid, Is Independent of Jun Kinase or P38 Mitogen Activated Protein Kinase Activation. Neurosci. Lett. 1998;250:53–56. doi: 10.1016/s0304-3940(98)00438-8. [DOI] [PubMed] [Google Scholar]
- HAM J., BABIJ C., WHITFIELD J., PFARR C.M., LALLEMAND D., YANIV M., RUBIN L.L. A c-jun dominant-negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- HAVLICEK L., HANUS J., VESELY J., LECLERC S., MEIJER L., SHAW G., STRNAD M. Cytokinin-derived cyclin-dependent kinase inhibitors: synthesis and cdc2 inhibitory activity of olomoucine and related compounds. J. Med. Chem. 1997;40:408–412. doi: 10.1021/jm960666x. [DOI] [PubMed] [Google Scholar]
- HUGHES P.E., ALEXI T., WALTON M., WILLIAMS C.E., DRAGUNOW M., CLARK R.G., GLUCKMAN P.D. Activity and injury-dependent expression of inducible transcription factors, growth factors and apoptosis-related genes within the central nervous system. Prog. Neurobiol. 1999;57:421–450. doi: 10.1016/s0301-0082(98)00057-4. [DOI] [PubMed] [Google Scholar]
- IKEUCHI T., SHIMOKE K., KUBO T., YAMADA M., HATANAKA H. Apoptosis-inducing and -preventing signal transduction pathways in cultured cerebellar granule neurons. Human Cell. 1998;11:125–140. [PubMed] [Google Scholar]
- KAWASAKI H., MOROOKA T., SHIMOHAMA S., KIMURA J., HIRANO T., GOTOH Y., NISHIDA E. Activation and involvement of p38 mitogen-activated protein kinase in glutamate-induced apoptosis in rat cerebellar granule cells. J. Biol. Chem. 1997;272:18518–18521. doi: 10.1074/jbc.272.30.18518. [DOI] [PubMed] [Google Scholar]
- KIKUCHI M., TENNETI L., LIPTON S.A. Role of p38 mitogen-activated protein kinase in axotomy-induced apoptosis of rat retinal ganglion cells. J. Neurosci. 2000;13:5037–5044. doi: 10.1523/JNEUROSCI.20-13-05037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRANENBURG O., VAN DER EB A.J., ZANTEMA A. Cyclin D1 is an essential mediator of apoptotic neuronal cell death. EMBO J. 1996;15:46–54. [PMC free article] [PubMed] [Google Scholar]
- LARM J.A., CHEUNG N.S., BEART P.M. Apoptosis induced via AMPA-selective glutamate receptors in cultured murine cortical neurons. J. Neurochem. 1997;69:617–622. doi: 10.1046/j.1471-4159.1997.69020617.x. [DOI] [PubMed] [Google Scholar]
- LEIST M., NICOTERA P. Apoptosis, excitotoxicity, and neuropathology. Experimental Cell Research. 1998;239:183–201. doi: 10.1006/excr.1997.4026. [DOI] [PubMed] [Google Scholar]
- LIPTON S.A., ROSENBERG P.A. Excitatory amino acids as a final common pathway for neurologic disorders. New Engl. J. Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- LIU H.N., LAROCCA J.N., ALMAZAN G. Molecular pathways mediating activation by kainate of mitogen-activated protein kinase in oligodendrocyte progenitors. Mol. Brain Res. 1999;66:50–61. doi: 10.1016/s0169-328x(99)00009-1. [DOI] [PubMed] [Google Scholar]
- MAAS J.W., HORSTMANN S., BORASIO G.D., ANNESER J.M.H., SHOOTER E.M., KAHLE P.J. Apoptosis of central and peripheral neurons can be prevented with cyclin-dependent kinase mitogen-activated protein kinase inhibitors. J. Neurochem. 1998;70:1401–1410. doi: 10.1046/j.1471-4159.1998.70041401.x. [DOI] [PubMed] [Google Scholar]
- MEIJER L., BORGNE A., MULNER O., CHONG J.P., BLOW J.J., INAGAKI N., INAGAKI M., DELCROS J.G., MOULINOUX J.P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- MILLER T.M., JOHNSON E.M. , JR Metabolic and genetic analyses of apoptosis in potassium/serum-deprived rat cerebellar granule cells. J. Neurosci. 1996;16:7487–7495. doi: 10.1523/JNEUROSCI.16-23-07487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUKHERJEE P.K., DECOSTER M.A., CAMPBELL F.Z., DAVIS R.J., BAZAN N.G. Glutamate receptor signaling interplay modulates stress-sensitive mitogen-activated protein kinases and neuronal cell death. J. Biol. Chem. 1999;274:6493–6498. doi: 10.1074/jbc.274.10.6493. [DOI] [PubMed] [Google Scholar]
- MURRAY B., ALESSANDRINI A., COLE A.J., YEE A.G., FURSHPAN E.J. Inhibition of the p44/42 MAP kinase pathway protects hippocampal neurons in a cell-culture model of seizure activity. Proc. Natl. Acad. Sci., USA. 1998;95:11975–11980. doi: 10.1073/pnas.95.20.11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPPENHEIM R.W., PREVETTE D., TYTELL M., HOMMA S. Naturally occuring and induced neuronal death in the chick embryo in vivo requires protein and RNA synthesis: evidence for the role of cell death genes. Dev. Biol. 1990;138:104–113. doi: 10.1016/0012-1606(90)90180-q. [DOI] [PubMed] [Google Scholar]
- PARK D.S., FARINELLI S.E., GREENE L.A. Inhibitors of cyclin-dependent kinases promote survival of post-mitotic neuronally differentiated PC12 cells and sympathetic neurons. J. Biol. Chem. 1996;271:8161–8169. doi: 10.1074/jbc.271.14.8161. [DOI] [PubMed] [Google Scholar]
- PARK D.S., MORRIS E.J., GREENE L.A., GELLER H.M. G1/S cell cycle blockers and inhibitors of cyclin-dependent kinases suppress camptothecin-induced neuronal apoptosis. J. Neurosci. 1997;17:1256–1270. doi: 10.1523/JNEUROSCI.17-04-01256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK D.S., MORRIS E.J., PADMANABHAN J., SHELANSKI M.L., GELLER H.M., GREENE L.A. Cyclin-dependent kinases participate in death of neurons evoked by DNA-damaging agents. J. Cell Biol. 1998;143:457–467. doi: 10.1083/jcb.143.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTERA-CAILLIAU C., PRICE D.L., MARTIN L.J. Non-NMDA and NMDA receptor-mediated excitotoxic neuronal deaths in adult brain are morphologically distinct: further evidence for an apoptosis-necrosis continuum. J. Comp. Neurol. 1997;378:88–104. [PubMed] [Google Scholar]
- ROSS M.E. Cell division and the nervous system: regulating the cycle from neural differentiation to death. Trends Neurosci. 1996;19:62–68. doi: 10.1016/0166-2236(96)89622-6. [DOI] [PubMed] [Google Scholar]
- SMALL D.L., MONETTE R., COMAS T., FOURIER M.C., MORLEY P. Loss of cyclin D1 in necrotic and apoptotic models of cortical neuronal degeneration. Brain Res. 1999;842:376–383. doi: 10.1016/s0006-8993(99)01852-1. [DOI] [PubMed] [Google Scholar]
- THOMSON S., MAHADEVAN L.C., CLAYTON A.L. MAP kinase-mediated signalling to nucleosomes and immediate-early gene induction. Sem. Cell Dev. Biol. 1999;10:205–214. doi: 10.1006/scdb.1999.0302. [DOI] [PubMed] [Google Scholar]
- UBERTI D., GRILLI M., MEMO M. Contribution of NF-kappaB and p53 in the glutamate-induced apoptosis. Int. J. Dev. Neurosci. 2000;18:447–454. doi: 10.1016/s0736-5748(00)00018-6. [DOI] [PubMed] [Google Scholar]
- VILLALBA M., BOCKAERT J., JOURNOT L. Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J. Neurosci. 1997;17:83–90. doi: 10.1523/JNEUROSCI.17-01-00083.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIRDEE K., BANNISTER A.J., HUNT S.P., TOLKOVSKY A.M. Comparison between the timing of JNK activation, c-Jun phosphorylation, and onset of death commitment in sympathetic neurones. J. Neurochem. 1997;69:550–561. doi: 10.1046/j.1471-4159.1997.69020550.x. [DOI] [PubMed] [Google Scholar]
- VIRDEE K., TOLKOVSKY A.M. Activation of p44 and p42 MAP kinases is not essential for the survival of rat sympathetic neurons. Eur. J. Neurosci. 1995;7:2159–2169. doi: 10.1111/j.1460-9568.1995.tb00637.x. [DOI] [PubMed] [Google Scholar]
- WALKER P.R., SIKORSKA M. Endoplasmic activities, chromatin structure and DNA degradation in apoptosis. Biochem. Cell Biol. 1994;264:9000–9003. doi: 10.1139/o94-081. [DOI] [PubMed] [Google Scholar]
- WALTON K.M., DIROCCO R., BARTLETT B.A., KOURY E., MARCY V.R., JARVIS B., SCHAEFER E.M., BHAT R.V. Activation of p38MAPK in microglia after ischemia. J. Neurochem. 1998;70:1764–1767. doi: 10.1046/j.1471-4159.1998.70041764.x. [DOI] [PubMed] [Google Scholar]
- XIA Z., DICKENS M., RAINGEAUD J., DAVIS R.J., GREENBERG M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- YAMAGISHI S., YAMADA M., ISHIKAWA Y., MATSUMOTO T., IKEUCHI T., HATANAKA H. p38 mitogen-activated protein kinase regulates low potassium-induced c-jun phosphorylation and apoptosis in cultured cerebellar granule cells. J. Biol. Chem. 2001;276:5129–5133. doi: 10.1074/jbc.M007258200. [DOI] [PubMed] [Google Scholar]
- YANG D.D., KUAN C.Y., WHITMARSH A.J., RINCON M., ZHENG T.S., DAVIS R.J., RAKIC P., FLAVELL R.A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]


