Abstract
Dietary antioxidants are thought to be beneficial in reducing the incidence of coronary heart disease. In this study, we compared resveratrol and analogues on their antioxidation and free radical scavenging activities to their protective effects on ischaemia-reperfusion induced injuries of rat hearts.
Astringinin (3,3′,4′,5-tetrahydroxystilbene) was shown to be a more potent inhibitor than other analogues against Cu2+-induced LDL (low-density lipoprotein) oxidation, as measured by the formation of conjugated diene and TBARS (thiobarbituric acid-reactive substance) and by the electrophoretic mobility of the oxidized LDL.
Resveratrol (trans-3,4′,5-trihydroxystilbene) and astringinin scavenged the stable free radical DPPH (1,1-diphenyl-2-picryl-hydrazyl) with an IC0.200 of 7.1 and 4.3 μM, respectively.
Astringinin has a superoxide anion scavenging activity about 160 fold more potent than resveratrol.
After a 30 min global ischemia followed by 2 h reperfusion, astringinin (10 μM) significantly reduced infarct size, superoxide anion production and increased functional recovery of the coronary flow in Langendorff-perfused rat hearts.
The result showed there is a positive correlation between the anti-oxidation and cardioprotective activities among these phenolic compounds. Our finding together with the fact that astringinin is more water-soluble than resveratrol suggest that astringinin could potentially be used as an anti-oxidant and cardioprotective agent in biological systems.
Keywords: Resveratrol, antioxidant, superoxide anion, ischaemia-reperfusion injury
Introduction
Resveratrol (trans-3,4′,5-trihydroxystilbene) is a polyphenol found in various fruits and vegetables and is abundant in grapes. The root extracts of the weed polygonum cuspidatum, an important constituent of Chinese and Japanese folk medicine, is also an ample source of resveratrol (Chen et al., 2001). In plants, resveratrol functions as a phytoalexin that protects against fungal infections (Langcake & Pryce, 1977). Recently, resveratrol and astringinin have been thought to be responsible for cardiovascular benefits associated with moderate wine consumption (Puddey & Croft, 1999; Das et al., 1999). It has also been suggested that routine ingestion of antioxidants, such as phenolic components through diet supplement might reduce lipoprotein oxidation (Belguendouz et al., 1997; Fremont et al., 1999), the incidence of atherosclerosis, and the mortality of coronary heart disease (Yla-Herttuala et al., 1989; Palinski et al., 1989).
Reperfusion of ischaemic myocardium is associated with a host of pathologic derangements, including reperfusion arrhythmias, transient mechanical dysfunction, myocardial stunning, and cell death (Mao et al., 1993; Brown et al., 1988). It is generally believed that an overproduction of reactive oxygen intermediates (superoxide anion, hydroxyl radical, hydrogen peroxide, singlet oxygen) (Samaja et al., 1994; Goldhaber & Weiss, 1992; Ray et al., 1999) might be involved. Free radicals in biological tissues may be produced by xanthine oxidase (McCord, 1985), activated neutrophils (Werns et al., 1985), leakage of electrons from the electron transport system of the mitochondria (Boveris, 1977), catecholamine oxidation (Singal et al., 1982), and cyclo-oxygenase and lipoxygenase enzymes (Rowe et al., 1983). Several approaches for protection against free radical damage have been considered. Among them, the free radical scavengers such as superoxide dismutase (SOD) and antioxidants have been used to protect tissues from ischaemia-reperfusion (I/R) induced injuries (Kukreja & Hess, 1992). Whether there is a causal relationship between the overproduction of reactive oxygen derivatives and I/R injury is a question with cell physiological and medical significance. If such a relationship exists, would pretreatment of heart with antioxidant before I/R then be beneficial in decreasing the risk of CHD mortality?
In this study, the inhibitory effect of resveratrol and analogues on low-density lipoprotein (LDL) oxidation is examined. In addition, the free radical scavenging activity and I/R injury protectability of each compound are compared. We show that astringinin is about 160 fold more potent than resveratrol in free radical scavenging and is a much better anti-oxidant against copper ion-induced LDL oxidation. We also demonstrate that astringinin is a more potent cardioprotective agent than resveratrol on I/R injury in Langendorff-perfused rat hearts. Because of its higher water solubility, we suggest that astringinin could potentially be a better choice than resveratrol as a possible anti-oxidant and cardioprotective agent in biological systems.
Methods
Human LDL isolation and oxidation
LDL was prepared from fresh human plasma by ultracentrifugal flotation (Havel et al., 1965). The LDL oxidation was carried out at 37°C in the presence of 5 μM freshly prepared CuSO4. Conjugated diene formation was continuously monitored by absorbance at 234 nm. To assess the extent of lipid peroxidation, the formation of thiobarbituric acid reactive substance (TBARS) was measured and expressed as malondialdehyde (MDA) equivalents per milligram LDL protein. The change on surface charge of the LDL protein was evaluated by an increase in the electrophoretic mobility, which was determined using the Lipofilm lipoprotein electrophoresis kit (Sebia, France).
1,1-Diphenyl-2-picrylhydrazyl test
An ethanolic solution of the stable nitrogen centered free radical 1,1-diphenyl-2-picrylhydrazyl (DPPH, 100 μM) was incubated with the test compounds, and the absorbance was monitored spectrophotometrically at 517 nm. The concentration (IC0.200) of the test compounds that induces a decrease of 0.200 in absorbance during a 30 min observation period was taken as the free radical-scavenging potency (Mellors & Tappel, 1966).
Superoxide scavenging activity
Superoxide anion was generated by xanthine/xanthine oxidase and measured by the nitro blue tetrazolium (NBT) reduction method (Nishikimi et al., 1972). Test compounds were incubated in 50 mM KH2PO4/K2HPO4 buffer (pH 7.4) containing K2H2-EDTA (0.3 mM), NBT (0.6 mM), xanthine (0.1 mM), and xanthine oxidase (0.02 U ml−1). The production of superoxide anion was monitored spectrophotometrically by absorbance at 560 nm. SOD (100 U ml−1) was used as a reference inhibitor.
Heart preparation
Adult male Wistar – Kyoto (WKY) rats, weighing 200 – 300 g, were anaesthetized with sodium pentobarbitone (50 mg kg−1, i.p.) and given heparin (300 units kg−1, i.p.). The Langendorff-perfused heart model with constant perfusion pressure instead of constant flow was used (Curtis & Hearse, 1989). The hearts were excised immediately and immersed in ice-cold perfusion medium. Isolated hearts were then mounted on a Langendorff apparatus via the aorta with normal Tyrode solution. The medium was gassed with 95% O2: 5% CO2 at 37°C and pH 7.4. The electrograms were recorded from a low atrial and a ventricular recording electrode and were continuously displayed on a Gould RS3400 recorder (Gould Inc., Cleveland, OH, U.S.A.) at a 5 mm s−1 chart speed and a HP oscilloscope at 100 mm s−1 sweep speed. The coronary effluent was collected for the measurement of coronary flow. Hearts were equilibrated with Tyrode solution for the first 30 min and then subjected to global ischaemia for 30 min, followed by a 90 min of reperfusion by turning off the aortic inflow line. The resveratrol and analogues were administrated in Tyrode solution at 10 μM for 15 min before I/R. The dosage used was determined from several preliminary studies shown that the stilbene is readily soluble at this concentration and is within the optimum dosage range in LDL oxidation assay.
Estimation of myocardial damage
Hearts to be used for infarct size calculations (n=7) were harvested immediately after termination of the experiment and the infarct area was determined by the triphenyl-tetrazolium chloride-Evan's blue technique (Warltier et al., 1981). The ventricular tissue was sliced into 1 mm sections and incubated in tetrazolium dye (10 mg 2,3,5-triphenyltetrazolium chloride ml−1 0.9% NaCl, pH 7.4) at 37°C for 40 min. Sections were then placed in 10% formaldehyde in saline for 2 days before infarct (white) tissue was excised. The weight of infarct tissue was expressed as the percentage of total ventricular weight.
Measurement of superoxide anion in the heart tissue
Superoxide anion production in cardiomyocytes is measured by modified lucigenin-enhanced chemiluminescence. The chemical specificity of this light-yielding reaction for superoxide anion was reported previously (Gyllenhammer, 1987). In a summary, whole heart tissue was incubated with 1.6 ml of Tyrode solution with a special chamber unit (Model No. TLU-21, Tohoku Electronic Indust. Co.), including a stainless steel cell, in an absolutely dark chamber of the Chemiluminescence Analysing System (Tohoku Electronic Industrial Co., Sendai, Japan). This system contains a photon detector (Model CLD-110), a Chemiluminescence counter (Model CLC-10), a water circulator (Model CH-20), and a 32 bit IBM personal computer system. Photon emission from heart tissue was counted at 10 s intervals at 37°C under atmospheric conditions. After 120 s, 0.4 ml of 1.25 mM lucigenin in Tyrode solution was injected into the stainless steel cell and the heart tissue was measured continuously for a total of 1200 s. Integrating the area under the curve and subtracting it from the background level calculated the total CL. The results were expressed as CL counts per 20 min per milligram of heart tissue (i.e., CL counts 20 min−1 mg−1).
Statistical analysis
Data were expressed as mean±standard error. Statistical analysis was performed using student t-test, and the significant difference was set at P<0.05. The IC50 and IC0.200 values were obtained by regression analysis.
Materials
Resveratrol (trans-3,4′,5-trihydroxystilbene) and its analogues (Figure 1) were synthesized as described (Thakkar et al., 1993), and purified by silica gel column chromatography (ethylacetate : n-hexane, 1 : 2, v/v) and recrystalization (ethylacetate : n-hexane 1 : 1, v/v). Butylated hydroxytoluene, DPPH, lucigenin, 2-thiobarbituric acid, xanthine oxidase (grade IV, from buttermilk), NBT, SOD (from bovine liver) was purchased from Sigma Chemical Company (St. Louis, MO, U.S.A.).
Figure 1.
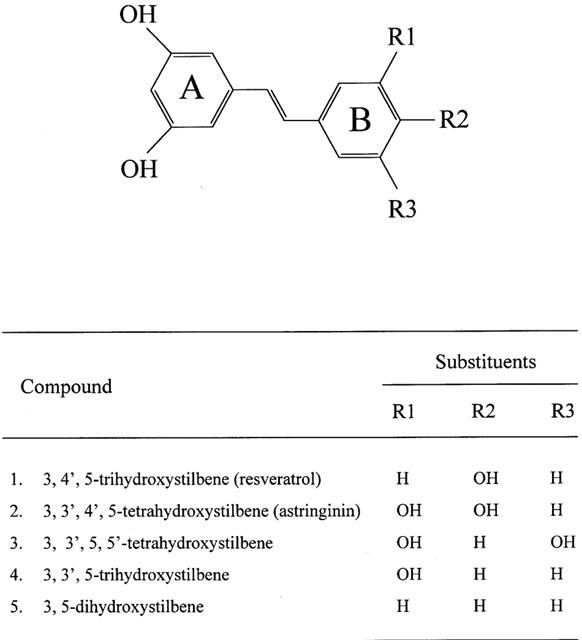
Chemical structures of resveratrol and analogues used in this study. The substituents are hydroxy (OH) groups.
Results
Effect of resveratrol and analogues on LDL oxidation
The inhibitory effect of resveratrol and analogues on Cu2+-induced LDL oxidation is evaluated by conjugated dienes and TBARS formation and the electrophoretic mobility of the oxidized LDL. Figure 2 shows that resveratrol and astringinin are more potent in prolonging the lag phase of Cu2+-induced conjugated diene formation in the LDL than other resveratrol analogues. The LDL oxidation-elicited TBARS formation in the presence and absence of resveratrol or analogues is also examined (Figure 3). A 50% inhibition (IC50) of the TBARS formation by resveratrol and astringinin is obtained at 7.8 and 3.1 μM, respectively. Other resveratrol analogues, 3,3′,5,5′-tetrahydroxystilbene; 3,3′,5-trihydroxystilbene; and 3,5-dihydroxystilbene exhibit no significant inhibitory effect. The oxidation of PUFA (polyunsaturated fatty acids) generates reactive aldehydes; these may then react with the lysine residue of apoB. The reaction increases the negativity of the LDL particle and can be measured by an increased electrophoretic mobility. As shown in Figure 4, compared with the unoxidized LDL, fully oxidized (in the absence of anti-oxidant) LDL exhibits a relative gel mobility (RM) of 1.417. In the presence of resveratrol or astringinin, the RM is reduced to 1.125. Resveratrol analogues 3, 4 and 5 of the Figure 1 exhibited little anti-oxidation activity; in the presence of each of the above compounds under the same conditions, the LDL is oxidized to have a RM of about 1.375.
Figure 2.
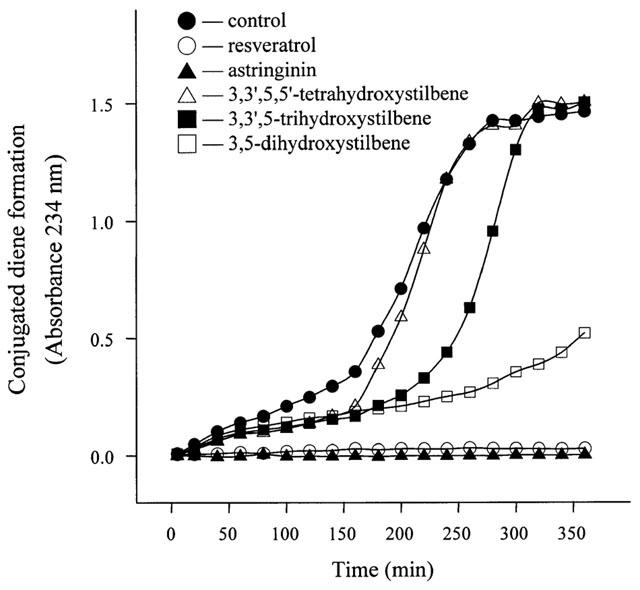
Conjugated diene formation in CuSO4-induced LDL oxidation in the absence or presence of resveratrol and analogues. LDL protein (50 μg ml−1) was incubated with 1.2 μM CuSO4 for the indicated time in the absence or presence of 10 μM resveratrol or analogues. Conjugated dienes were monitored at 20 min intervals by absorbance at 234 nm. Each data point shown is from a single experiment with four independent incubations.
Figure 3.

The inhibitory effects of resveratrol and analogues on lipid peroxidation in LDL induced by copper ion. LDL (100 μg ml−1) was preincubated with DMSO alone (0.1% final) or with same concentration of DMSO containing appropriate amount of either resveratrol or analogues (10 μM final) at 37°C for 10 min, copper sulphate (5 μM) was then added, and further incubated for 12 h. Data are mean±s.e.mean (n=4). *P<0.05, as compared with the control.
Figure 4.
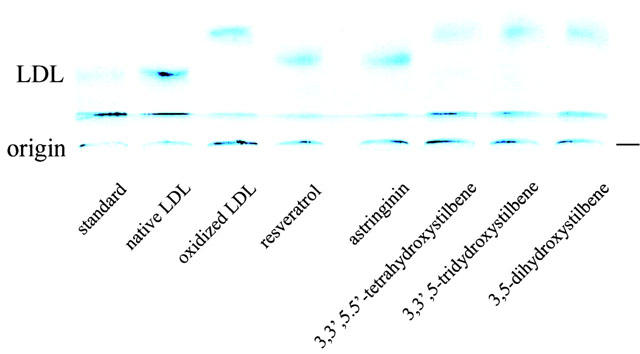
Effect of resveratrol and analogues on the electrophoretic mobility of LDL after coincubation with CuSO4. LDL (200 μg ml−1) was incubated with CuSO4 (5 μM) in phosphate-buffered saline at 37°C for 12 h in the absence or presence of either resveratrol or analogues (10 μM). The reaction was stopped by the addition of 50 μM BHT, and the samples were subjected to electrophoretic analysis.
DPPH radical scavenging activity
The DPPH radical scavenging activity of resveratrol and its analogues was expressed as IC0.200. The decrease in optical absorbance at 517 nm after addition of resveratrol or its analogues is monitored following the trapping of the unpaired electron of DPPH. As shown in Figure 5, resveratrol and astringinin scavenge DPPH in a concentration-dependent manner with an IC0.200 of 7.1 and 4.3 μM, respectively.
Figure 5.
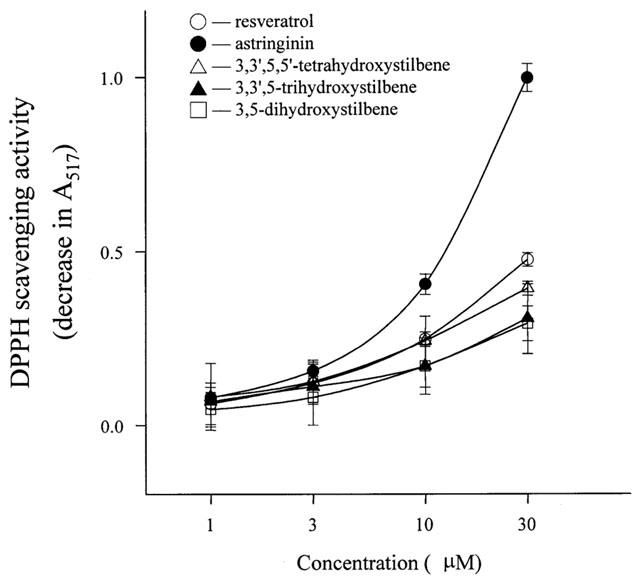
Dose-responsive curves of resveratrol and analogues in scavenging DPPH radical. After incubation with various concentrations of resveratrol and analogues at room temperature (25°C) for 30 min, the decrease in the absorbance at 517 nm was measured. Data are mean±s.e.mean (n=4).
Superoxide anion scavenging activity
Superoxide anion generated by the xanthine/xanthine oxidase system is monitored by the reduction of NBT (Table 1). The initial rate of NBT reduction (0.19±0.01 OD/min, n=6) is almost completely inhibited by superoxide dismutase (100 U ml−1, 0.03±0.01 OD/min, n=6). As shown in Table 1, addition of resveratrol or its analogues inhibit the NBT reduction; a 50% scavenging (EC50) of the superoxide anion produced is observed at a resveratrol concentration of 245.1±1.8 μM. In the same assay system, astringinin and 3,3′,5,5′-tetrahydroxystilbene are about 160 and 16 fold more potent than resveratrol. Compounds 4 and 5 (Figure 1) also exhibited much stronger superoxide anion scavenging activity than resveratrol.
Table 1.
Scavenging activities of resveratrol and analogues on superoxide anion
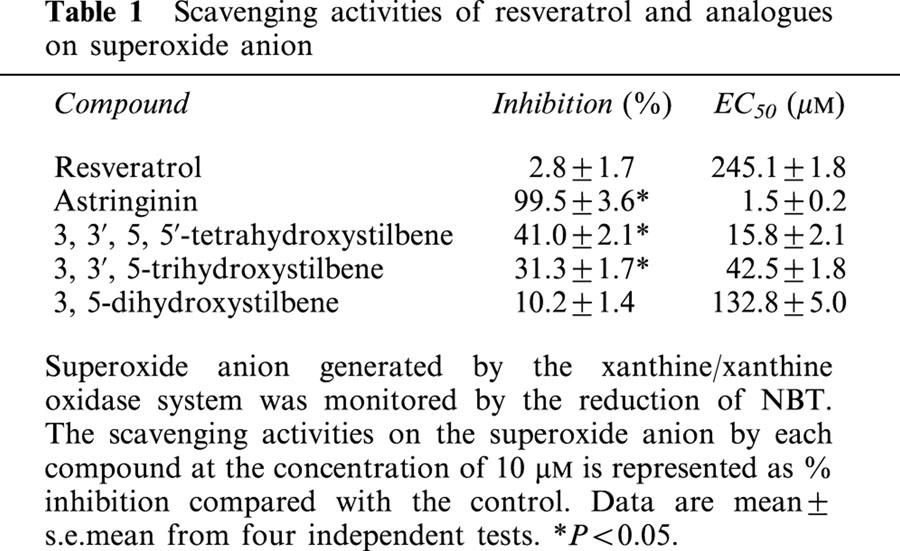
Effects on infarct size in ischaemia-reperfused rat hearts
As shown in Figure 6, the infarct volume and infarct size (per cent of the infarcted tissue of the ventricle) are noticeably smaller in the astringinin (10 μM) pretreated group compared to the vehicle group (6.5±2.0% vs 52.5±7.5%) and other experimental groups.
Figure 6.
Effects of resveratrol and analogues on infarct size in isolated rat hearts subjected to ischaemia (30 min) – reperfusion (120 min). Resveratrol and analogues (10 μM) were administrated 15 min before global ischaemia. Results are expressed as mean±s.e.mean from seven rats per group. *P<0.05 compared with vehicle.
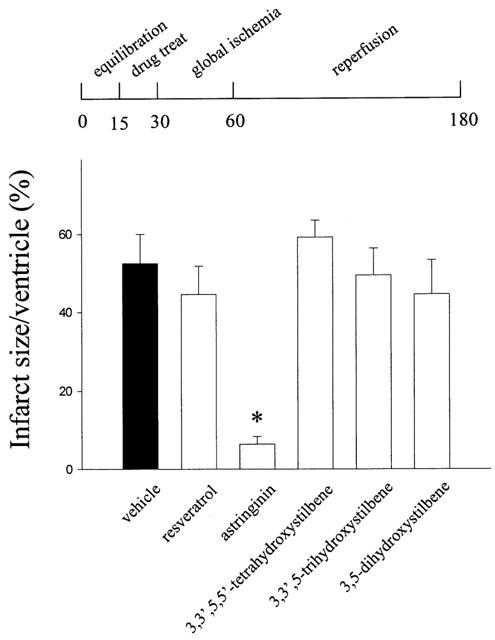
Effects on coronary flow in ischaemia-reperfusion
There is no difference in the coronary flow in rats with and without pretreatment with resveratrol and analogues (10 μM) (Figure 7a). As is expected, the coronary flow was decreased upon reperfusion as compared with the respective baseline values. Animals pretreated with astringinin display a significant recovery of post-ischaemic myocardial coronary flow (Figure 7b). No significant improvements of the post-ischaemic coronary flow were observed in other treated groups.
Figure 7.
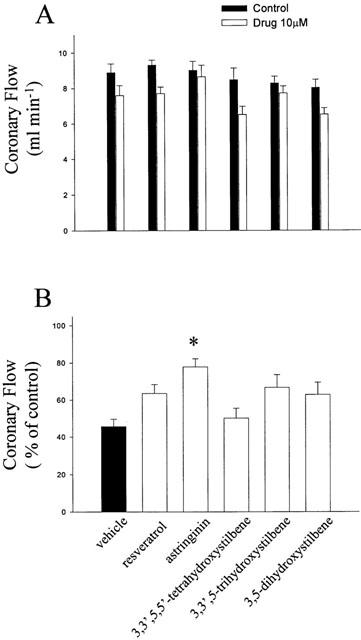
Effects of resveratrol and analogues on the coronary flow before (a) and after (b) global ischaemia (30 min) – reperfusion (120 min). Desired test compounds were administrated 15 min before global ischemia. Values are mean±s.e.mean (n=7 in each group). *P<0.05 compared with vehicle.
Superoxide anion production
Myocardial superoxide anion production was measured in sham, vehicle and resveratrol or astringinin (10 μM)-treated groups after 30 min ischaemia/2 h reperfusion. As shown in Figure 8, pretreatment with 10 μM astringinin reduced the superoxide anion in the myocardium after I/R from 543±23 to 308±41 CL counts mg−1 tissue) (P<0.05).
Figure 8.
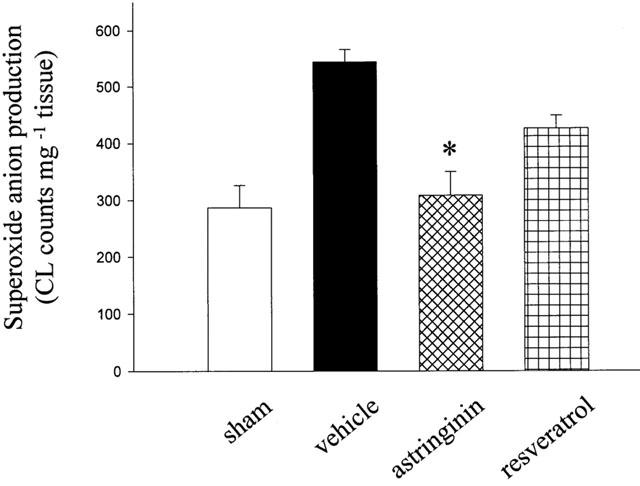
Effect of resveratrol and astringinin on the superoxide anion production in Langendorff perfused rat hearts after a 30 min ischemia followed by 2 h reperfusion. Values are expressed as mean±s.e.mean (n=4 in each group). *Denotes P<0.05 vs the vehicle value.
Discussion
Polyphenolic compounds produced by grapes and found in red wine have the potential to donate hydrogen or react with superoxide anion (Afanas'ev et al., 1989), hydroxyl radicals (Hanasaki et al., 1994) and lipid peroxyl radicals (Cao et al., 1997). All of these free radicals can cause lipid peroxidation in vivo, which is detrimental to the cardiovascular system. If potent anti-oxidants, such as phenolic components, are routinely ingested through diet supplement, they may collectively reduce oxidation of lipoproteins (Ursini et al., 1994); reduce the incidence of atherosclerosis, and the mortality of coronary heart disease (Goldberg et al., 1995). In this study, the anti-oxidant activity and the protective effect on the rat hearts against I/R injury of resveratrol and analogues are compared. We found that resveratrol and astringinin (3,3′, 4′, 5-tetrahydroxystilbene) were more potent inhibitors than other analogues against Cu2+-induced oxidation of LDL. Astringinin was 2 to 2.5 fold more potent than resveratrol in TBARS and DPPH assays. However, astringinin was about 160 fold more potent than resveratrol in superoxide anion scavenging. These results showed the possible structural criteria important for the antioxidant activities of these polyphenolic compounds. Deletion of the hydroxyl group at B-4 of resveratrol reduces its antioxidant activity. In contrast, the presence of ortho-dihydroxy structure in ring B (astringinin) enhanced its activity to inhibit LDL peroxidation and free radical trapping, especially superoxide anion. The effects of astringinin on LDL oxidation and DPPH scavenging observed here is consistent with that reported by Fauconneau et al. (1997). However, why astringinin exhibited such a great discrepancy in its efficacy in scavenging superoxide anion vs DPPH is currently not clear.
Several epidemiological studies have suggested that the mortality rate of coronary heart disease can be decreased with moderate consumption of alcohol, particularly red wines (Renaud & De Lorgeril, 1992). Resveratrol and astringinin are polyphenolic natural antioxidants relatively abundant in red wine; they have been thought to be the major components in the red wine responsible for such epidemiological observations (Renaud et al., 1998). Recently, we showed that resveratrol could serve as a protective agent against some of the acute scenarios of the I/R injury of the rat heart. However, resveratrol was not effective in preventing arrhythmias nor in reducing the mortality rate in sustained coronary artery occlusion (Hung et al., 2000). We further showed that pre-infusion of astringinin not only effectively suppressed ischaemia (30 min, occlusion) or I/R (5 min, occlusion)-induced arrhythmias but also reduced the infarct size caused by prolonged coronary artery occlusion (240 min) (Hung et al., 2001). However, why astringinin is a better protective drug than resveratrol was not known. The concept that oxygen free radicals play a role in the I/R injuries of the heart and anti-oxidants might be beneficial in the treatment of these diseases has long been proposed (Terada et al., 1991; Kukreja & Hess, 1992). In this study, we showed that astringinin exhibits a much stronger anti-oxidant activity than resveratrol and is more effective in reducing superoxide anion production and infarct size in isolated rat hearts. Moreover, astringinin is also shown to be more effective than resveratrol in promoting the functional recovery of the coronary flow in the same system. Thus, the protective effect of these compounds against I/R injury is correlated with their anti-oxidation activity. These results indicate that phenolic compounds (resveratrol, astringinin) exhibit interesting properties, which may account in part for the so-called ‘French paradox' and may be a potential therapeutic drug in treating acute scenarios of the I/R injury.
Acknowledgments
The authors thank Dr S.E. Chow for technical assistance. This work was supported by a grant from the National Science Council, Taiwan (NSC 89-2320-B-182-058).
Abbreviations
- DPPH
1,1-diphenyl-2-picryl-hydrazyl
- I/R
ischaemia reperfusion
- LDL
low-density lipoprotein
- MDA
malondialdehyde
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid reactive substance
References
- AFANAS'EV I.B., DOROZHKO A.I., BRODSKII A.V., KOSTYUK V.A., POTAPOVITCH A.I. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem. Pharmacol. 1989;38:1763–1769. doi: 10.1016/0006-2952(89)90410-3. [DOI] [PubMed] [Google Scholar]
- BELGUENDOUZ L., FREMONT L., LINARD A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochem. Pharmacol. 1997;53:1347–1355. doi: 10.1016/s0006-2952(96)00820-9. [DOI] [PubMed] [Google Scholar]
- BOVERIS A. Mitochondrial production of superoxide radical and hydrogen peroxide. Adv. Exp. Med. Biol. 1977;78:67–82. doi: 10.1007/978-1-4615-9035-4_5. [DOI] [PubMed] [Google Scholar]
- BROWN J.M., GROSSO M.A., WHITMAN G.J., TERADA L.S., REPINE J.E., HARKEN A.H. Cardiac oxidase systems mediate oxygen metabolite reperfusion injury. Surgery. 1988;104:266–271. [PubMed] [Google Scholar]
- CAO G., SOFIC E., PRIOR R.L. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic. Biol. Med. 1997;22:749–760. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- CHEN L., HAN Y., YANG F., ZHANG T. High-speed counter-current chromatography separation and purification of resveratrol and piceid from polygonum cuspidatum. J. Chromatogr. 2001;907:343–346. doi: 10.1016/s0021-9673(00)00960-2. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., HEARSE D.J. Ischaemia-induced and reperfusion-induced arrhythmias differ in their sensitivity to potassium: implications for mechanisms of initiation and maintenance of ventricular fibrillation. J. Mol. Cell. Cardiol. 1989;21:21–40. doi: 10.1016/0022-2828(89)91490-9. [DOI] [PubMed] [Google Scholar]
- DAS D.K., SATO M., RAY P.S., MAULIK G., ENGELMAN R.M., BERTELLI A.A., BERTELLI A. Cardioprotection of red wine: role of polyphenolic antioxidants. Drug Exp. Clin. Res. 1999;25:115–120. [PubMed] [Google Scholar]
- FAUCONNEAU B., WAFFO-TEGUO P., HUGUET F., BARRIER L., DECENDIT A., MERILLON J.M. Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci. 1997;61:2103–2110. doi: 10.1016/s0024-3205(97)00883-7. [DOI] [PubMed] [Google Scholar]
- FREMONT L., BELGUENDOUZ L., DELPAL S. Antioxidant activity of resveratrol and alcohol-free wine polyphenols related to LDL oxidation and polyunsaturated fatty acids. Life Sci. 1999;64:2511–2521. doi: 10.1016/s0024-3205(99)00209-x. [DOI] [PubMed] [Google Scholar]
- GOLDBERG D.M., HAHN S.E., PARKES J.G. Beyond alcohol: beverage consumption and cardiovascular mortality. Clin. Chim. Acta. 1995;237:155–187. doi: 10.1016/0009-8981(95)06069-p. [DOI] [PubMed] [Google Scholar]
- GOLDHABER J.I., WEISS J.N. Oxygen free radicals and cardiac reperfusion abnormalities. Hypertension. 1992;20:118–127. doi: 10.1161/01.hyp.20.1.118. [DOI] [PubMed] [Google Scholar]
- GYLLENHAMMER H. Lucigenin chemiluminescence in the assessment of neutrophil superoxide production. J. Immunol. Methods. 1987;97:209–213. doi: 10.1016/0022-1759(87)90461-3. [DOI] [PubMed] [Google Scholar]
- HANASAKI Y., OGAWA S., FUKUI S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994;16:845–850. doi: 10.1016/0891-5849(94)90202-x. [DOI] [PubMed] [Google Scholar]
- HAVEL R.J., EDER H.A., BRADGON L.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 1965;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNG L.M., CHEN J.K., HUANG S.S., LEE R.S., SU M.J. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc. Res. 2000;47:549–555. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- HUNG L.M., CHEN J.K., LEE R.S., LIANG H.C., SU M.J. Beneficial effects of astringinin, a resveratrol analogue, on the ischemia and reperfusion damage in rat heart. Free Radic. Biol. Med. 2001;30:877–883. doi: 10.1016/s0891-5849(01)00474-9. [DOI] [PubMed] [Google Scholar]
- KUKREJA R.C., HESS M.L. The oxygen free radical system: from equations through membrane-protein interactions to cardiovascular injury and protection. Cardiovasc. Res. 1992;26:641–655. doi: 10.1093/cvr/26.7.641. [DOI] [PubMed] [Google Scholar]
- LANGCAKE P., PRYCE R.J. A new class of phytoalexins from grape-vines. Experientia. 1977;33:151–152. doi: 10.1007/BF02124034. [DOI] [PubMed] [Google Scholar]
- MAO G.D., THOMAS P.D., LOPASCHUK G.D., POZNANSKY M.J. Superoxide dismutase (SOD)-catalase conjugates. Role of hydrogen peroxide and the Fenton reaction in SOD toxicity. J. Biol. Chem. 1993;268:416–420. [PubMed] [Google Scholar]
- MCCORD J.M. Oxygen-derived free radicals in postischaemic tissue injury. N. Engl. J. Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- MELLORS A., TAPPEL A.L. The inhibition of mitochondrial peroxidation by ubiquinone and ubiquinol. J. Biol. Chem. 1966;241:4353–4356. [PubMed] [Google Scholar]
- NISHIKIMI M., APPAJI N., YAGI K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Bioph. Res. Co. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- PALINSKI W., ROSENFELD M.E., YLA-HERTTUALA S., GURTNER G.C., SOCHER S.S., BUTLER S.W., PARTHASARATHY S., CAREW T.E., STEINBERG D., WITZTUM J.L. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl. Acad. Sci. U.S.A. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUDDEY I.B., CROFT K.D. Alcohol, stroke and coronary heart disease. Are there anti-oxidants and pro-oxidants in alcoholic beverages that might influence the development of atherosclerotic cardiovascular disease. Neuroepidemiology. 1999;18:292–302. doi: 10.1159/000026224. [DOI] [PubMed] [Google Scholar]
- RAY P.S., MAULIK G., CORDIS G.A., BERTELLI A.A., BERTELLI A., DAS D.K. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic. Biol. Med. 1999;27:160–169. doi: 10.1016/s0891-5849(99)00063-5. [DOI] [PubMed] [Google Scholar]
- RENAUD S.C., DE LORGERIL M. Wine, Alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- RENAUD S.C., GUEGUEN R., SCHENKER J., D'HOUTAUD A. Alcohol and mortality in middle aged men from Eastern France. Epidemiology. 1998;9:184–188. [PubMed] [Google Scholar]
- ROWE G.T., MANSON N.H., CAPLAN M., HESS M.L. Hydrogen peroxide and hydroxyl radical mediation of activated leukocyte depression of cardiac sarcoplasmic reticulum. Participation of the cyclooxygenase pathway. Circ. Res. 1983;53:584–591. doi: 10.1161/01.res.53.5.584. [DOI] [PubMed] [Google Scholar]
- SAMAJA M., MOTTERLINI R., SANTORO F., DELL' ANTONIO G., CORNO A. Oxidative injury in reoxygenated and reperfused hearts. Free Radic. Biol. Med. 1994;16:255–262. doi: 10.1016/0891-5849(94)90150-3. [DOI] [PubMed] [Google Scholar]
- SINGAL P.K., KAPUR N., DHILLON K.S., BEAMISH R.E., DHALLA N.S. Role of free radicals in catecholamine-induced cardiomyopathy. Can. J. Physiol. Pharmacol. 1982;60:1390–1397. doi: 10.1139/y82-207. [DOI] [PubMed] [Google Scholar]
- TERADA L.S., RUBINSTEIN J.D., LESNEFSKY E.J., HORWITZ L.D., LEFF J.A., REPINE J.E. Existence and participation of xanthine oxidase in reperfusion injury of ischaemic rabbit myocardium. Am. J. Physiol. 1991;260:H805–H810. doi: 10.1152/ajpheart.1991.260.3.H805. [DOI] [PubMed] [Google Scholar]
- THAKKAR K., GEAHLEN R.L., CUSHMAN M. Synthesis and protein-tyrosine kinase inhibitory activity of polyhydroxylated stilbene analogues of piceatannol. J. Med. Chem. 1993;36:2950–2955. doi: 10.1021/jm00072a015. [DOI] [PubMed] [Google Scholar]
- URSINI F., MAIORINO M., MORAZZONI P., ROVERI A., PIFFERI G. A novel antioxidant flavonoid (IdB) affecting molecular mechanisms of cellular activation. Free Radic. Biol. Med. 1994;16:547–553. doi: 10.1016/0891-5849(94)90054-x. [DOI] [PubMed] [Google Scholar]
- WARLTIER D.C., ZYVOLOSKI M.G., GROSS G.J., HARDMAN H.F., BROOKS H.L. Determination of experimental myocardial infarct size. J. Pharmacol. Meth. 1981;6:199–210. doi: 10.1016/0160-5402(81)90109-1. [DOI] [PubMed] [Google Scholar]
- WERNS S.W., SHEA M.J., LUCCHESI B.R. Free radicals in ischaemic myocardial injury. Free Radic. Biol. Med. 1985;1:103–110. doi: 10.1016/0748-5514(85)90013-3. [DOI] [PubMed] [Google Scholar]
- YLA-HERTTUALA S., PALINSKI W., ROSENFELD M.E., PARTHASARATHY S., CAREW T.E., BUTLER S., WITZTUM J.L., STEINBERG D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J. Clin. Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]


