Abstract
The objective of the present study was to examine the role of the endothelial selectins (i.e. P- and E-selectin) in leukocyte-endothelium interactions in colonic venules by use of intravital microscopy.
Balb/c mice were exposed to dextran sodium sulphate (DSS) in the drinking water for 5 days or treated intraperitoneally (i.p.) with tumour necrosis factor-α (TNF-α) for 3 h.
In DSS-treated mice, mRNA of both P- and E-selectin were expressed and leukocyte rolling and adhesion was increased to 27±3 cells min−1 and 36±8 cells mm−1, respectively. An anti-P-selectin antibody abolished DSS-induced leukocyte rolling, whereas an antibody against E-selectin had no effect. Established leukocyte adhesion was insensitive to inhibition of the selectins.
DSS markedly increased production of TNF-α in the colon. TNF-α increased leukocyte rolling to 22±3 cells min−1 and adhesion to 45±4 cells mm−1. Only inhibition of P-selectin significantly reduced (>94%) leukocyte rolling provoked by TNF-α. Leukocyte adhesion was not changed by late anti-P-selectin antibody treatment. In contrast, pretreatment with the anti-P-selectin antibody not only abolished leukocyte rolling but also completely inhibited firm adhesion in response to TNF-α.
This study demonstrates that P-selectin plays an important role in leukocyte rolling in colonic venules, both in experimental colitis and when stimulated with TNF-α. Moreover, P-selectin-dependent leukocyte rolling was found to be a precondition for TNF-α-induced firm adhesion. Thus, these findings suggest that P-selectin may be a key target to reduce pathological recruitment of inflammatory cells in the colon.
Keywords: Adhesion, colon, intravital microscopy, leukocyte, microcirculation, P-selectin, rolling, TNF-α
Introduction
Inflammatory bowel diseases (IBD), i.e. ulcerative colitis and Crohn's disease, are characterized by release of pro-inflammatory cytokines, such as tumour necrosis factor-α (TNF-α) and interleukin-8 (IL-8), and by increased infiltration of leukocytes into the intestinal tissue (Deventer, 1997). Tissue accumulation of circulating leukocytes is mediated by a coordinated expression of specific adhesion molecules regulating the interactions between leukocytes and endothelial cells in the microcirculation (Springer, 1994; Vestweber & Blanks, 1999). The extravasation process of leukocytes is a multistep process, involving a reversible rolling adhesive interaction, firm adhesion to the endothelium and transmigration (Springer, 1994). Leukocyte rolling is considered to be principally mediated by the selectin family of adhesion molecules (P-, E- and L-selectin) although subsets of integrins have been reported to support rolling under certain conditions (Carlos & Harlan, 1994; Vestweber & Blanks, 1999). On the other hand, firm adhesion of leukocytes has been shown to be mediated by integrins on leukocytes which bind to members of the immunoglobulin gene superfamily, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on endothelial cells (Carlos & Harlan, 1994; Springer, 1994) and thereby promoting a strong adhesive interaction and arrest of rolling cells.
Substantial effort has been devoted to studies in experimental animal models of colitis in order to improve the current medical treatment of IBD. One of these models is based on oral administration of dextran sodium sulphate (DSS) in the drinking water of mice, which results in an acute colitis with several morphological and pathophysiological features in common with human ulcerative colitis, including superficial ulceration, mucosal damage and leukocyte infiltration (Okayasu et al., 1990; Cooper et al., 1993; Elson et al., 1995). In a recent study by Soriano et al. (2000), it was demonstrated that leukocyte adhesion in colonic venules is supported by VCAM-1 and MAdCAM-1 in response to DSS challenge in mice. On the other hand, the adhesive mechanisms behind leukocyte rolling in colonic venules in response to DSS is not known. We have recently found that the polysaccharide fucoidan, which inhibits P- and L-selectin, markedly reduces DSS-induced leukocyte recruitment in the colon (Zhang et al., 2001). Nonetheless, the exact role of the individual selectins in leukocyte – endothelium interactions in DSS-induced colitis remains to be determined.
Although the clinical correlate of experimental models of colitis remains uncertain, there is convincing evidence in the literature showing that TNF-α plays an important role in the pathophysiology of human IBD. For example, numerous clinical trials have demonstrated marked improvement in patients with Crohn's disease treated with high-affinity chimeric and humanized monoclonal antibody directed against TNF-α (van Dullemen et al., 1995; Stack et al., 1997; Targan et al., 1997). However, the adhesive pathways in TNF-α-induced leukocyte – endothelium interactions in the colonic microcirculation have not been specifically addressed previously. Thus, the individual role of the endothelial selectins, i.e. P- and E-selectin in TNF-α induced leukocyte rolling in colonic venules is unknown and it is not known whether such a selectin-mediated rolling adhesive interaction may be a prerequisite for the subsequent firm leukocyte adhesion in response to TNF-α challenge.
Based on the considerations above, the aim of this study was to define the molecular mechanisms behind leukocyte rolling in colonic venules by selectively inhibiting the function of P- and E-selectin. Moreover, we wanted to determine whether initial leukocyte rolling is a necessary precondition for subsequent firm adhesion in the colonic microcirculation. For these purposes, we used intravital fluorescence microscopy in the mouse colon exposed to DSS and TNF-α.
Methods
Animals
Male Balb/c mice weighing 21 – 26 g were maintained on 12-h dark diagonal 12-h light cycles and given food and water ad libitum. The animal experiments were approved by the Regional Ethical Committee for Animal Experimentation.
Antibodies
RB40.34 against murine P-selectin (rat IgG) and the isotype control antibody R3-34 (rat IgG) were from Pharmingen, San Diego, CA, U.S.A. 10E9.6 against murine E-selectin (rat IgG) were produced as described (Bosse & Vestweber, 1994). Herein, 40 μg of RB40.34 and 100 μg of 10E9.6 were used, which blocks the function of P- (Bosse & Vestweber, 1994; Johnston et al., 1997; Thorlacius et al., 1997; Kanwar et al., 1999; Mansson et al., 2000) and E-selectin (Bosse & Vestweber, 1994; Ramos et al., 1997), respectively.
Experimental protocol
All mice were non-fasting. Experimental colitis was induced by administration of 5% DSS (MW=40,000, Lot 9391A, ICN Biomedicals Inc, Aurora, Ohio, U.S.A) in the drinking water for 5 days, which results in a reproducible colitis characterized by reduced body weight, rectal bleeding, mucosal ulceration, crypt destruction and infiltration of leukocytes (Cooper et al., 1993). Induction of colitis was confirmed herein by development of diarrhoea and presence of gross macroscopic bleeding (blood around the anus or in the cage). Control mice received normal drinking water without DSS. At day 5, animals were anaesthetized and prepared for intravital microscopy. A catheter was inserted in the jugular vein for intravenous (i.v.) injection of antibodies and additional anaesthesia. In separate experiments, we studied leukocyte – endothelium interactions provoked by TNF-α (R & D Systems Europe, Ltd., Abingdon, Oxon, UK). Intraperitoneal injection of TNF-α (0.5 μg) in 0.5 ml PBS was performed 3 h prior to microscopic observation. Leukocyte rolling and adhesion were observed before and after i.v. administration of the antibodies. A different protocol was used to delineate the role of selectin-mediated rolling in the extravasation process of leukocytes, i.e. the anti-P-selectin antibody was administered immediately prior (5 min before) to i.p. treatment with TNF-α. Blood samples were taken from the tail artery after the experiment for analysis of systemic and differential leukocyte counts using a hemocytometer and a 3 cm long segment of the distal colon was resected for ELISA.
Intravital microscopy
Animals were anaesthetized with 7.5 mg ketamine hydrochloride (Hoffman-La Roche, Basel, Switzerland) and 2.5 mg xylazine (Janssen Pharmaceutica, Beerse, Belgium) per 100 g body weight intraperitoneally. The colon was prepared for intravital microscopy as described earlier (Soriano et al., 2000). In brief, a midline incision of the abdomen was made and a segment of the colon was exteriorized on a pedestal for microscopic observation of the colonic microcirculation. Great care was taken to avoid any bleeding from the margins of the abdominal wall. Observations of the colonic microcirculation were made using an Olympus microscope (IX70, Olympus Optical Co. GmbH, Hamburg, Germany) equipped with lenses (×10/NA 0.25 and ×40/NA 0.6). The microscopic image was televised using a charge-coupled device videocamera (FK 6990 Cohu, Pieper GmbH, Berlin, Germany) and recorded on videotape (Sony SVT-S3000P S-VHS recorder) for subsequent off-line analysis. After positioning under the microscope, a 10-min equilibration period preceded quantitative measurements. Analysis of leukocyte flux and leukocyte-endothelium interactions (rolling and adhesion) were made in venules (inner diameter 15 – 35 μm) with stable resting blood flow. Blood perfusion within individual microvessels was studied after contrast enhancement by i.v. administration of 0.05 ml (5 mg ml−1) fluorescein isothiocyanate (FITC)-labeled dextran (MW 150000, Sigma Chemical Co., St. Louis, MO, U.S.A.). In vivo labelling of leukocytes with rhodamine-6G (0.1 ml, 0.5 mg ml−1) enabled quantitative analysis of leukocyte flow behaviour in the colonic microcirculation. Quantification of microcirculatory parameters was performed off-line by frame-to-frame analysis of the videotaped images. Microcirculatory analysis included determination of leukocyte rolling by counting the number of rolling leukocytes passing by a reference point in the venule per 20 s and expressed as cells min−1. Firm leukocyte adhesion was measured by counting the number of cells adhering to the venular endothelium (500 – 800 μm long segments) and remained stationary during the observation period of 30 s, and is given as cells mm−1 venule length. Blood flow velocities were measured off-line by frame-to-frame analysis of the videotaped images using CapImage software (Zeintl, Heidelberg, Germany). The velocity was calculated as a mean value from 8 – 10 measurements per venule and is expressed as mm s−1. Venular wall shear rate was determined based on the Newtonian definition: wall shear rate=8((red blood cell velocity/1.6)/venular diameter) as described previously (House & Lipowsky, 1987).
Reverse-transcription polymerase chain reaction(RT – PCR)
Total RNA was extracted from mouse colon tissue using Rneasy® Mini kit (Qiagen GmbH, Hilden, Germany) and treated with RNase-free DNase (DNase 1; Amersham Pharmacia Biotech, Sollentuna, Sweden) in order to remove potential genomic DNA contaminants according to manufacturer's protocol. RNA concentrations were determined by measuring the absorbance at 260 nm spectrophotometrically. RT – PCR was performed with SuperScrip One-Step RT – PCR system (GIBCO-BRL Life Technologies, Grand Island, NY, U.S.A.). Each reaction contained 500 ng of total RNA as a template and 0.2 μM of each primer in a final volume of 50 μl. Mouse β-actin served as an internal control gene. The RT – PCR profile was 1 cycle of cDNA synthesis at 50°C for 30 min and 94°C for 2 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C and extension at 72°C for 1 min, 1 cycle of final extension at 72°C for 10 min. After RT – PCR, aliquots of the RT – PCR products were separated on 2% agarose gel containing ethidium bromide and photographed. The primers sequences of E-selectin, P-selectin and β-actin were as follows: P-selectin (f) 5′-ACG AGC TGG ACG GAC CCG-3′ ;P-selectin (r) 5′-GGC TGG CAC TCA AAT TTA CAG-3′; E-selectin (f) 5′-GGT AGT TGC ACT TTC TGC GG-3′; E-selectin (r) 5′-CCT TCT GTG GCA GCA TGT TC-3′; β-actin (f) 5′-ATG TTT GAG ACC TTC AAC ACC-3′, β-actin (r) 5′-TCT CCA GGG AGG AAG AGG AT-3′.
ELISA
The mouse colon was removed, opened longitudinally, washed in PBS containing penicillin, streptomycin and fungizon (100 U ml−1) and then kept in cold serum-free medium (DMEM). A 3 cm long segment of the colon was cut into small pieces (0.5 cm). About 100 mg of colon tissue fragments were incubated with 1.0 ml of DMEM containing 10% serum in a well of 24-well culture plate at 37°C for 24 h. The culture medium was harvested and stored in −20°C until analysis of TNF-α by using double-antibody specific Quantikine ELISA kit using recombinant murine TNF-α as standard (R & D Systems, Europe). TNF-α production was expressed as pg mg−1 colon tissue.
Statistical analysis
Statistical evaluations were performed using Kruskal – Wallis one-way analysis of variance on ranks for unpaired samples (Dunn's method) and Wilcoxon signed rank test for paired samples. The results are presented as mean values±s.e.m. Unless stated otherwise, n represents number of animals.
Results
Leukocyte rolling in experimental colitis
After DSS treatment both P- and E-selectin mRNA were expressed in the colon tissue (Figure 1). Leukocyte – endothelium interactions were analysed in colonic venules after an equilibration time of 10 min. It was found that the level of leukocyte rolling and adhesion in control mice was 2.6±1.2 cells min−1 and 0.5±0.5 cells mm−1, respectively (Figure 2a+b, n=5). Notably, it was observed that in DSS-treated mice, the number of rolling and firmly adherent leukocytes increased significantly to 26.8±3.2 cells min−1 and 35.7±8.3 cells mm−1, respectively (Figure 2a+b, P<0.05 vs controls, n=21). In order to examine the individual role of the selectins in leukocyte – endothelium interactions in experimental colitis, we used monoclonal antibodies directed against P- and E-selectin. We found that i.v. injection of 40 μg of RB40.34, which is directed against P-selectin, completely abolished leukocyte rolling in DSS-treated animals, i.e. leukocyte rolling was reduced to zero (Figure 2a, P<0.05 vs before antibody injection, n=5). In fact, we observed also that 20 μg of RB40.34 significantly reduced the number of rolling leukocytes by 89% (data not shown). In contrast, administration of a monoclonal antibody against E-selectin (10E9.6, 100 μg, n=6) had no effect on leukocyte rolling in DSS-induced colitis (Figure 2a, P>0.05 vs before antibody injection). Moreover, an isotype-matched control antibody (R3-34, 40 μg, n=5) had no effect on the frequency of rolling leukocytes in DSS-treated mice (Figure 2a, P>0.05 vs before antibody injection, n=4). The level of leukocyte adhesion in DSS-treated mice was insensitive to inhibition of P- and E-selectin (Figure 2b, P>0.05 vs before antibody injection, n=5 – 6).
Figure 1.
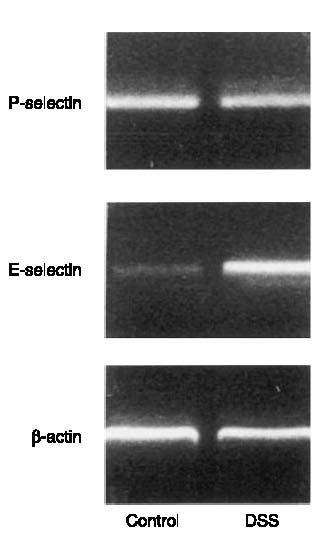
Expression of P- and E-selectin mRNA in the colon. B-actin serves as an housekeeping gene. Mice were treated with DSS (5%) for 5 days. The results presented are from one experiment, which is representative of four others performed.
Figure 2.
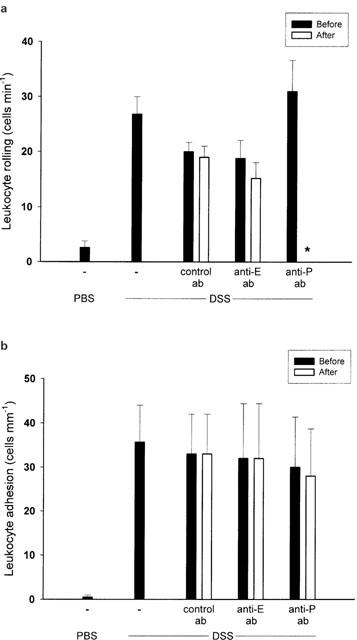
Venular (a) leukocyte rolling and (b) adhesion in the mouse colon after 5 days of administration of DSS (5%) in the drinking water. Mice were treated intravenously with a control antibody, or antibodies against E-selectin (Anti-E) and P-selectin (Anti-P). Data represents mean±s.e.m. *Indicates significant difference (P<0.05 vs before antibody injection, n=5 – 21).
TNF-α-induced leukocyte rolling in colonic venules
We found that administration of DSS (5%) for 5 days markedly increased the colonic content of TNF-α from 0.1±0.05 to 2.3±1.1 pg mg−1 tissue (P<0.05 vs control mice, n=6). Considering that TNF-α is also an important mediator in clinical IBD, we next wanted to evaluate the mechanisms of leukocyte rolling and firm adhesion provoked by TNF-α in the colonic microcirculation. In line with DSS-treated mice, we observed that mRNA of both P- and E-selectin were expressed after challenge with TNF-α (0.5 μg, i.p.) for 3 h (Figure 3). This treatment with TNF-α increased leukocyte rolling and adhesion to 21.5±2.6 cells min−1 and 44.5±4.2 cells mM−1, respectively (Figure 4a+b, P<0.05 vs PBS, n=22). It was found that administration of the anti-P-selectin antibody (40 μg) markedly reduced TNF-α-induced leukocyte rolling by more than 94% (Figure 4a, P<0.05 vs before antibody injection, n=5), whereas treatment with the anti-E-selectin antibody (100 μg) had no effect (Figure 4a, P>0.05 vs before antibody injection, n=5 – 6). Again, leukocyte adhesion was unchanged after administration of antibodies against both selectins (Figure 4b, P>0.05 vs before antibody injection, n=5). Interestingly, in separate experiments, we found by paired observation that pretreatment with the anti-P-selectin antibody (40 μg, n=5) not only abolished leukocyte rolling in response to TNF-α challenge, but also completely inhibited firm adhesion of leukocytes to the vascular endothelium (Figure 5, P<0.05 vs control antibody, n=5).
Figure 3.
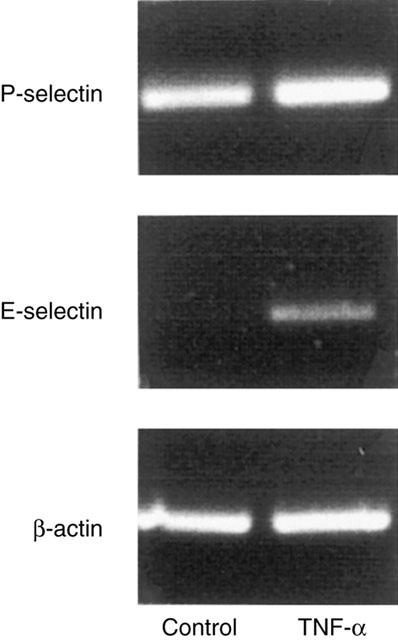
Expression of P- and E-selectin mRNA in the colon. B-actin serves as an housekeeping gene. Mice were treated with TNF-α (0.5 μg, i.p.) for 3 h. The results presented are from one experiment, which is representative of three others performed.
Figure 4.
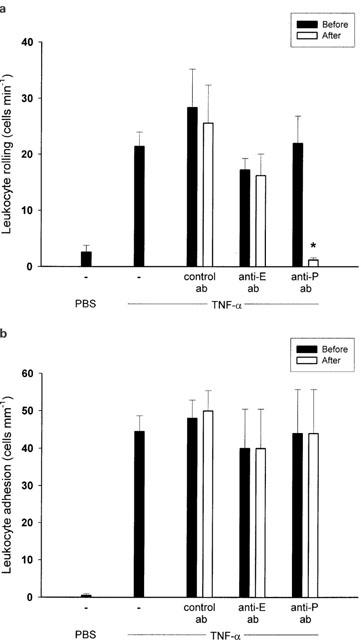
Venular (a) leukocyte rolling and (b) adhesion in the mouse colon after 3 h of treatment with TNF-α (0.5 μg, i.p.). Mice were treated intravenously with a control antibody, or antibodies against E-selectin (Anti-E) and P-selectin (Anti-P). Data represents mean±s.e.m. *Indicates significant difference (P<0.05 vs before antibody injection, n=5 – 22).
Figure 5.
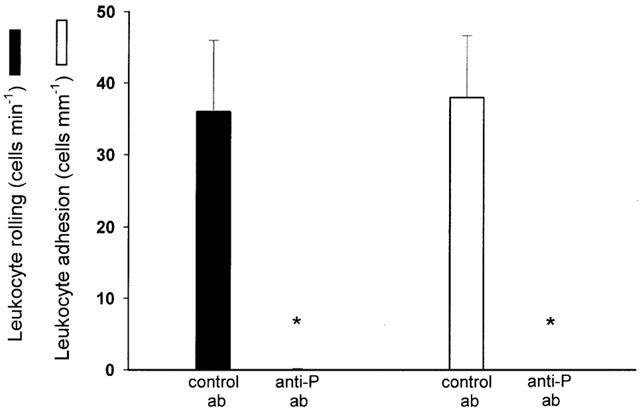
Leukocyte rolling and adhesion in the mouse colon after 3 h of treatment with TNF-α (0.5 μg, i.p.). Mice were pretreated intravenously with a control antibody or an anti-P-selectin antibody (Anti-P). Data represents mean±s.e.m. *Indicates significant difference (P<0.05 vs control antibody, n=5).
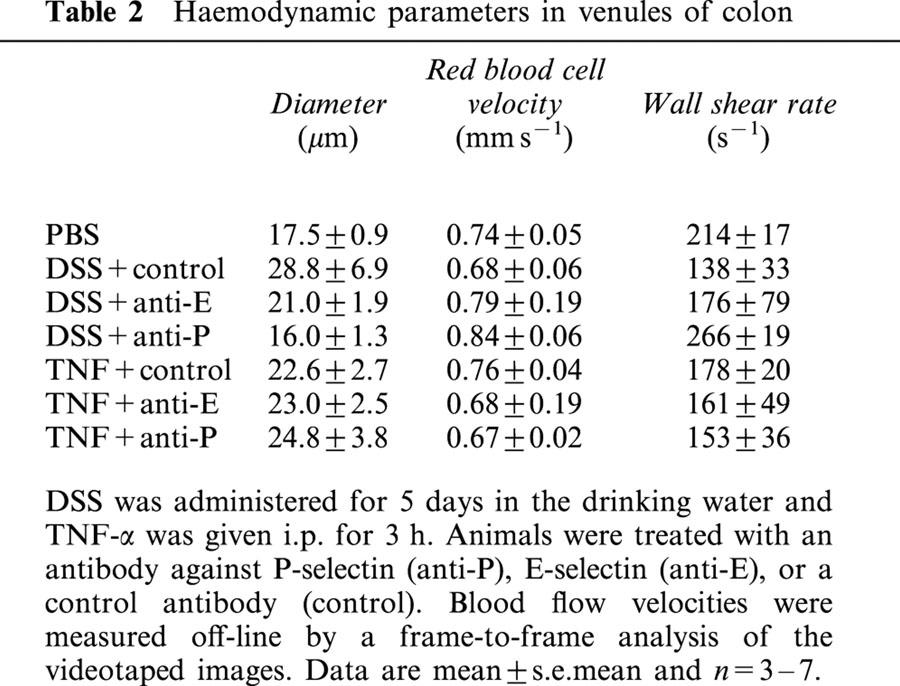
We observed no significant differences in systemic leukocyte differentials (Table 1) or haemodynamic parameters (Table 2) between the experimental groups.
Table 1.
Systemic leukocyte differential counts
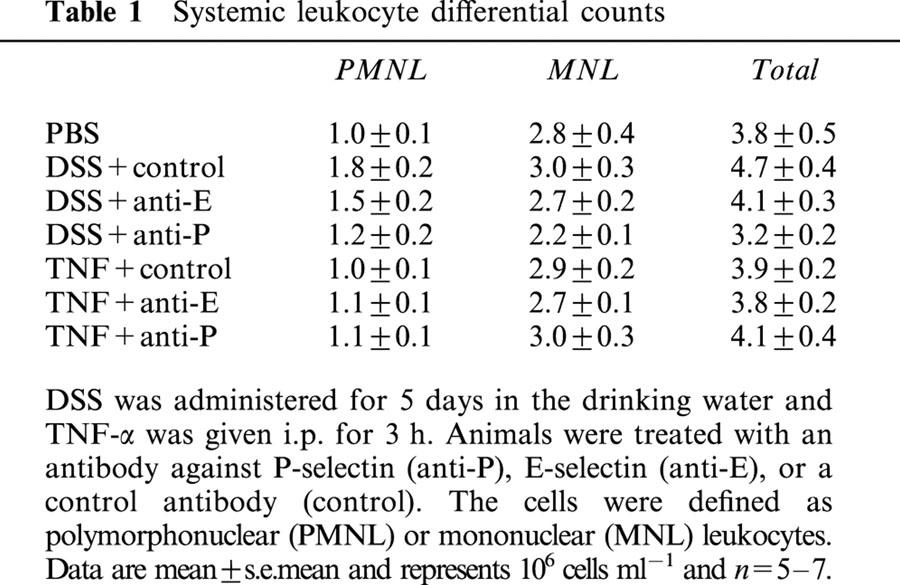
Table 2.
Haemodynamic parameters in venules of colon
Discussion
The present study demonstrates a critical function of P-selectin in mediating leukocyte rolling in colonic venules both when stimulated by exogenous TNF-α and in experimental colitis. In contrast, we observed that inhibition of E-selectin has no effect on TNF-α- and DSS-induced leukocyte rolling, indicating that P-selectin constitutes a critical and non-redundant mediator of leukocyte rolling in colonic venules. In fact, we found that P-selectin-dependent leukocyte rolling is a precondition for the subsequent TNF-α-induced firm adhesion of leukocytes to the microvascular endothelium. Taken together, these findings indicate that P-selectin plays a key role in leukocyte rolling in colonic venules in vivo and may be an important pharmacological target to treat inflammatory conditions in the large bowel.
Numerous previous studies have used intravital microscopy to delineate adhesive pathways in leukocyte – endothelium interaction in organs and tissues, such as the liver (Vollmar et al., 1995), skin (Nolte et al., 1994), mesentery (Mayadas et al., 1993) and cremaster muscle (Thorlacius et al., 1997). However, there is only a limited knowledge about microcirculatory changes in the colon, which is due to the difficulty of studying this tissue using conventional intravital microscopy. Herein, we used an inverted microscopic technique to study leukocyte – endothelium interactions in the colon. In an experimental model of colitis based on oral administration of DSS for 5 days with features in common with ulcerative colitis (Okayasu et al., 1990; Cooper et al., 1993; Elson et al., 1995), we found that inhibition of P-selectin function abolished leukocyte rolling, suggesting that P-selectin plays a dominant role in supporting leukocyte rolling in colonic venules. These findings are supported by a recent study showing that early (day 1) leukocyte rolling in TNBS-induced colitis is predominately mediated by P-selectin (Sans et al., 2001). However, in that study by Sans et al. (2001), it was found that all members of the selectin family of adhesion molecules, i.e. P-, E-, and L-selectin, significantly contributed to late (day 7) leukocyte rolling in TNBS-provoked colitis. This is in contrast to the findings in our present study showing that late (day 5) leukocyte rolling in DSS-induced colitis is completely independent of E-selectin function and critically dependent on P-selectin. The reason for this discrepancy is not known at present but may be due to the different nature of DSS- and TNBS-induced colitis. Thus, in contrast to DSS, challenge with TNBS evokes a strong lymphocyte response (Elson et al., 1995), and it is possible that at day 7 in TNBS-provoked colitis a significant proportion of rolling leukocytes is comprised of mononuclear leukocytes. Indeed, this conclusion is also supported by a previous study showing that anti-neutrophil serum, which causes depletion of circulating neutrophils, attenuates DSS-induced colitis, indicating that neutrophil accumulation plays an important role in this model of colitis (Domek et al., 1995).
The feasibility of targeting adhesion molecules in IBD has gained support in studies on experimental colitis and observations documenting increased expression of adhesion molecules in colonic tissues from patients with IBD. For example, inhibition of ICAM-1, VCAM-1, and MadCAM-1 has been reported to ameliorate induction of colitis (Hamamoto et al., 1999; Sans et al., 1999; Taniguchi et al., 1998; Bennett et al., 1997; Wong et al., 1995). The specific role of P-selectin has recently been addressed in two studies, however, reporting contradictory results. In one study by Yoshida et al. (2001) it was shown that immunoneutralization of P-selectin protected against TNBS-induced colitis whereas Sans et al. (2001) could not confirm such a role of P-selectin in colitis provoked by TNBS. The reason for these discrepancies are not clear at present. We have recently shown that the polysaccharide fucoidan, which inhibits P- and L-selectin, reduces colonic accumulation of neutrophils and protects against DSS-induced colitis, which support a role of P- and/or L-selectin in this model of experimental colitis (Zhang et al., 2001). This notion is also supported by the observation that P-selectin is increased in active clinical colitis (Schürmann et al., 1995).
TNF-α has been demonstrated to be important in the pathophysiology of IBD and inhibition of TNF-α has turned out to be a successful approach in the treatment of IBD (van Dullemen et al., 1995; Stack et al., 1997; Targan et al., 1997). Although, DSS-induced colitis displays several features in common with human IBD, clinical extrapolations based on findings in experimental models of colitis should be cautious. We found an increased production of TNF-α in the colonic tissue of DSS-treated mice, suggesting that this DSS-induced colitis in mice also exhibits molecular features in common with human IBD. However, in this context, it is important to note that the literature on the adhesive pathways of leukocyte recruitment in response to TNF-α is complex and partly contradictory. For example, one previous report has suggested that P- and E-selectin may have overlapping functions, and that inhibition of both P- and E-selectin is required in order to reduce the recruitment of leukocytes in TNF-α activated tissues (Ley et al., 1995). In contrast, others have reported that interference with P-selectin alone is sufficient to attenuate lipopolysaccharide- and cytokine-induced leukocyte recruitment (Frenette et al., 1996; Johnston et al., 1997, Mansson et al., 2000). In the present study, we found that although both P- and E-selectin mRNA were concomitantly expressed, interference of P-selectin by use of a monoclonal antibody completely inhibited leukocyte rolling in colonic venules in response to TNF-α challenge, whereas inhibition of E-selectin had no effect. These results are compatible with a previous study demonstrating that TNF-α-induced leukocyte rolling is intact in E-selectin-deficient mice, whereas the number of firmly adherent leukocytes was reduced (Milstone et al., 1998). Together with other findings, it may be suggested that E-selectin is not primarily involved in supporting the rolling adhesive interaction, but may rather facilitate downstream events such as activation and adhesion of rolling leukocytes (Milstone et al., 1998; Simon et al., 2000). This notion is also supported by a previous study, which demonstrated that this anti-E-selectin antibody (10E9.6) markedly reduced peritoneal recruitment of neutrophils but had no effect on TNF-α-induced leukocyte rolling (Ramos et al., 1997). Collectively, these results suggest that TNF-α-induced leukocyte rolling is exclusively dependent on P-selectin and excludes the possibility of redundancy of selectins in supporting leukocyte rolling in the colon. This notion is in line with a recent study showing that P-selectin plays a critical role in TNF-α-induced leukocyte rolling in the striated muscle (Mansson et al., 2000).
Leukocyte extravasation is a multistep process, in which selectin-mediated rolling is generally considered to be a precondition for the subsequent adhesion and transendothelial migration of leukocytes out into the extravascular space. This paradigm has been challenged by studies reporting that leukocyte rolling may be supported by α4-integrins (Johnston et al., 1997), and some reports suggesting that leukocyte rolling is not a prerequisite for firm adhesion to the endothelial surface in certain organs such as the lung (Burns et al., 2001). In order to evaluate the role of the selectin-mediated rolling in the leukocyte response to TNF-α stimulation, we used i.v. pretreatment with the monoclonal antibody against P-selectin. It was found that inhibition of P-selectin function not only abolished leukocyte rolling but concomitantly completely inhibited TNF-α-induced adhesion of leukocytes, indicating that P-selectin-dependent rolling is indeed a precondition for TNF-α-induced leukocyte adhesion in the colonic venules. This notion is in line with a previous study showing that P-selectin plays a critical role in TNF-α-provoked tissue recruitment of leukocytes in the cremaster muscle (Mansson et al., 2000). In this context, it is noteworthy that dislodgement of activated cells by inhibition of firm adhesion and transmigration (i.e. events subsequent to leukocyte activation) in an inflamed tissue could cause distant damage in the lung. Therefore, it is tempting to stipulate that leukocyte rolling, which occurs prior to leukocyte activation, may be an attractive step to target in pathological inflammation. In addition, integrins are widely expressed in all tissues, whereas expression of P-selectin is limited to within the vasculature, and inhibitors of P-selectin would potentially have fewer side effects compared with substances directed against integrins. In fact, this notion is supported by a previous study demonstrating that inhibition of neutrophil recruitment by immunoneutralization of CD18 at the site of infection (peritonitis) increased neutrophil accumulation in the lung and liver and tissue injury in liver (Mercer-Jones et al., 1997).
Taken together, this study provides in vivo evidence that leukocyte rolling in the colon is mainly supported by P-selectin both in experimental colitis and in response to TNF-α stimulation. On the other hand, E-selectin did not contribute to leukocyte rolling, indicating that P-selectin exclusively support rolling adhesive interactions in the colon. In addition, this P-selectin-dependent leukocyte rolling is a precondition for TNF-α-induced firm adhesion in colonic venules. Finally, it may be suggested that P-selectin may be a central target to interfere with leukocyte accumulation in pathological inflammation in the large bowel.
Acknowledgments
This study was supported by Swedish Medical Research Council (K98-27I-11610-03, K2000-04P-13411-01A, K2002-73-X-14273-01A), Cancerfonden (4265-B99-01XAB), Crafoordska stiftelsen (20010968), the Franke and Margareta Bergqvists stiftelse för främjande av cancerforskning, Ruth och Rickard Julins stiftelse, Svenska Läkaresällskapet (2001-907), Allmäna sjukhusets i Malmö stiftelse för bekämpande av cancer, MAS fonder. Amjid Ali Riaz is supported by a European Surgical Research Grant from the Royal College of Surgeons England, Malmö University Hospital and Lund University.
Abbreviations
- DSS
dextran sodium sulphate
- ELISA
enzyme-linked immunosorbent assay
- IBD
inflammatory bowel diseases
- ICAM-1
intercellular adhesion molecule-1
- IL-8
interleukin-8
- i.p.
intraperitoneal
- MAdCAM-1
mucosal addressin cell adhesion molecule-1
- RT-PCR
reverse-transcription polymerase chain reaction
- TNF-α
tumour necrosis factor-α
- VCAM-1
vascular cell adhesion molecule-1
References
- BENNETT C.F., KORNBRUST D., HENRY S., STECKER K., HOWARD R., COOPER S., DUTSON S., HALL W., JACOBY H.I. An ICAM-1 antisense oligonucleotide prevents and reverses dextran sulfate sodium-induced colitis in mice. J. Pharmacol. Exp. Ther. 1997;280:988–1000. [PubMed] [Google Scholar]
- BOSSE R., VESTWEBER D. Only simultaneous blocking of L- and P-selectin completely inhibits neutrophil migration into mouse peritoneum. Eur. J. Immunol. 1994;24:3019–3024. doi: 10.1002/eji.1830241215. [DOI] [PubMed] [Google Scholar]
- BURNS J.A., ISSEKUTZ T.B., YAGITA H., ISSEKUTZ A.C. The alpha 4 beta 1 (very late antigen (VLA)-4, CD49d/CD29 and alpha 5 beta 1 (VLA-5, CD49e/CD29) integrins mediate beta 2 (CD11/CD18) integrin-independent neutrophil recruitment to endotoxin-induced lung inflammation. J. Immunol. 2001;166:4644–4649. doi: 10.4049/jimmunol.166.7.4644. [DOI] [PubMed] [Google Scholar]
- CARLOS T.M., HARLAN J.M. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- COOPER H.S., MURTHY S.N., SHAH R.S., SEDERGRAN D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 1993;9:238–249. [PubMed] [Google Scholar]
- DEVENTER S.J.H. Tumor necrosis factor and Crohn's disease. Gut. 1997;40:443–448. doi: 10.1136/gut.40.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOMEK M.J., IWATA F., BLACKMAN E.I., KAO J., BAKER M., VIDRICH A., LEUNG F.W. Anti-neutrophil serum attenuates dextran sulfate sodium-induced colonic damage in the rat. Scand. J. Gastroenterol. 1995;30:1089–1094. doi: 10.3109/00365529509101612. [DOI] [PubMed] [Google Scholar]
- ELSON C.O., SARTOR R.B., TENNYSON G.S., RIDDELL R.H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- FRENETTE P.S., MAYADAS T.N., RAYBURN H., HYNES R.O., WAGNER D.D. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectin. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- HAMAMOTO N., MAEMURA K., HIRATA I., MURANO M., SASAKI S., KATSU K. Inhibition of dextran sulphate sodium (DSS)-induced colitis in mice by intracolonically administered antibodies against adhesion molecules (endothelial leucocyte adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1 (ICAM-1)) Clin. Exp. Immunol. 1999;117:462–468. doi: 10.1046/j.1365-2249.1999.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUSE S.D., LIPOWSKY H.H. Leukocyte-endothelium adhesion: microhaemodynamics in mesentery of the cat. Microvasc. Res. 1987;34:363–379. doi: 10.1016/0026-2862(87)90068-9. [DOI] [PubMed] [Google Scholar]
- JOHNSTON B., WALTER U.M., ISSEKUTZ T.B., ANDERSON D.C., KUBES P. Differential role of selectins and the alpha4-integrin in acute, subacute, and chronic leukocyte recruitment in vivo. J. Immunol. 1997;159:4514–4523. [PubMed] [Google Scholar]
- KANWAR S., STEEBER D.A., TEDDER T.F., HICKEY M.J., KUBES P. Overlapping roles for L-selectin and P-selectin in antigen-induced immune responses in the microvasculature. J. Immunol. 1999;162:2709–2716. [PubMed] [Google Scholar]
- LEY K., BULLARD D.C., ARBONES M.L., BOSSE R., VESTWEBER D., TEDDER T.F., BEAUDET A.L. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSSON P., ZHANG X.W., JEPPSSON B., JOHNELL O., THORLACIUS H. Critical role of P-selectin-dependent rolling in tumour necrosis factor-α-induced leukocyte adhesion and extravascular recruitment in vivo. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;362:190–196. doi: 10.1007/s002100000268. [DOI] [PubMed] [Google Scholar]
- MAYADAS T.N., JOHNSON R.C., RAYBURN H., HYNES R.O., WAGNER D.D. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- MERCER-JONES M.A., HEINZELMANN M., PEYTON J.C., WICKEL D., COOK M., CHEADLE W.G. Inhibition of neutrophil migration at the site of infection increases remote organ neutrophil sequestration and injury. Shock. 1997;8:193–199. doi: 10.1097/00024382-199709000-00007. [DOI] [PubMed] [Google Scholar]
- MILSTONE D.S., FUKUMURA D., PADGETT R.C., O'DONNELL P.E., DAVIS V.M., BENAVIDEZ O.J., MONSKY W.L., MELDER R.J., JAIN R.K., GIMBRONE M.A. Mice lacking E-selectin show normal numbers of rolling leukocytes but reduced leukocyte stable arrest on cytokine-activated microvascular endothelium. Microcirculation. 1998;5:153–171. [PubMed] [Google Scholar]
- NOLTE D., HECHT R., BOTZLAR A., MENGER M.D., NEÜMULLER C., SINOWATZ F., VESTWEBER D., MESSMER K. Role of Mac-1 and ICAM-1 in ischemia-reperfusion injury in a microcirculation model of BALB/c-mice. Am. J. Physiol. 1994;267:H1320–H1328. doi: 10.1152/ajpheart.1994.267.4.H1320. [DOI] [PubMed] [Google Scholar]
- OKAYASU I., HATAKEYAMA S., YAMADA M., OHKUSA T., INAGAKI Y., NAKAYA R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- RAMOS C.L., KUNKEL E.J., LAWRENCE M.B., JUNG U., VESTWEBER D., BOSSE R., MCINTYRE K.W., GILLOOLY K.M., NORTON C.R., WOLITZKY B.A., LEY K. Differential effect of E-selectin antibodies on neutrophil rolling and recruitment to inflammatory sites. Blood. 1997;89:3009–3018. [PubMed] [Google Scholar]
- SANS M., PANES J., ARDITE E., ELIZALDE J.I., ARCE Y., ELENA M., PALACIN A., FERNANDEZ-CHECA J.C., ANDERSON D.C., LOBB R., PIQUE J.M. VCAM-1 and ICAM-1 mediate leukocyte-endothelial cell adhesion in rat experimental colitis. Gastroenterology. 1999;116:874–883. doi: 10.1016/s0016-5085(99)70070-3. [DOI] [PubMed] [Google Scholar]
- SANS M., SALAS A., SORIANO A., PRATS N., GIRONELLA M., PIZCUETA P., ELENA M., ANDERSON D.C., PIQUÉ J.M., PANES J. Differential role of selectins in experimental colitis. Gastroenterology. 2001;120:1162–1172. doi: 10.1053/gast.2001.23252. [DOI] [PubMed] [Google Scholar]
- SCHÜRMANN G.M., BISHOP A.E., VECCHIO M., LEE J.C., RAMPTON D.S., POLAK J.M. Increased expression of cell adhesion molecule P-selectin in active inflammatory bowel disease. Gut. 1995;36:411–418. doi: 10.1136/gut.36.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON S.I., HU Y., VESTWEBER D., SMITH C.W. Neutrophil tethering on E-selectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J. Immunol. 2000;164:4348–4358. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- SORIANO A., SALAS A., SALAS A., SANS M., GIRONELLA M., ELENA M., ANDERSON D.C., PIQUÉ J.M., PANES J. VCAM-1, but not ICAM-1 or MadCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab. Invest. 2000;80:1541–1551. doi: 10.1038/labinvest.3780164. [DOI] [PubMed] [Google Scholar]
- STACK W.A., MANN S.D., ROY A.J., HEATH P., SOPWITH M., FREEMAN J., HOLMES G., LONG R., FORBES A., KAMM M.A. Randomised controlled trial of CDP571 antibody to tumour necrosis factor-alpha in Crohn's disease. Lancet. 1997;349:521–524. doi: 10.1016/s0140-6736(97)80083-9. [DOI] [PubMed] [Google Scholar]
- SPRINGER T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- TANIGUCHI T., TSUKADA H., NAKAMURA H., KODAMA M., FUKUDA K., SAITO T., MIYASAKA M., SEINO Y. Effects of the anti-ICAM-1 monoclonal antibody on dextran sodium sulphate-induced colitis in rats. J. Gastroenterol. Hepatol. 1998;13:945–949. doi: 10.1111/j.1440-1746.1998.tb00766.x. [DOI] [PubMed] [Google Scholar]
- TARGAN S.R., HANAUER S.B., VAN DEVENTER S.J., MAYER L., PRESENT D.H., BRAAKMAN T., DEWOODY K.L., SCHAIBLE T.F., RUTGEERTS P.J. A short-term study of chimeric monoclonal antibody cA2 to tumour necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N. Engl. J. Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- THORLACIUS H., LINDBOM L., RAUD J. Cytokine-induced leukocyte rolling in mouse cremaster muscle arterioles is P-selectin-dependent. Am. J. Physiol. 1997;272:H1725–H1729. doi: 10.1152/ajpheart.1997.272.4.H1725. [DOI] [PubMed] [Google Scholar]
- VAN DULLEMEN H.M., VAN DEVENTER S.J., HOMMES D.W., BIJL H.A., JANSEN J., TYTGAT G.N., WOODY J. Treatment of Crohn's disease with anti-tumour necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- VESTWEBER D., BLANKS J.E. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- VOLLMAR B., GLASZ M.D., MENGER M.D., MESSMER K. Leukocytes contribute to hepatic ischemia/reperfusion injury via intercellular adhesion molecule-1-mediated venular adherence. Surgery. 1995;117:195–200. doi: 10.1016/s0039-6060(05)80085-6. [DOI] [PubMed] [Google Scholar]
- WONG P.Y., YUE G., YIN K., MIYASAKA M., LANE C.L., MANNING A.M., ANDERSON D.C., SUN F.F. Antibodies to intercellular adhesion molecule-1 ameliorate the inflammatory response in acetic acid-induced inflammatory bowel disease. J. Pharmacol. Exp. Ther. 1995;274:475–480. [PubMed] [Google Scholar]
- YOSHIDA N., YAMAGUCHI T., NAKAGAWA S., NAKAMURA Y., NAITO Y., YOSHIKAWA T. Role of P-selectin and intercellular adhesion molecule-1 in TNB-induced colitis in rats. Digestion. 2001;63:81–86. doi: 10.1159/000051916. [DOI] [PubMed] [Google Scholar]
- ZHANG X.W., LIU Q., THORLACIUS H. Inhibition of selectin-function and leukocyte rolling protects against dextran sodium sulfate-induced murine colitis. Scand. J. Gastroenterol. 2001;36:270–275. doi: 10.1080/003655201750074555. [DOI] [PubMed] [Google Scholar]


