Abstract
The abilities of muscarinic agonists (arecoline, bethanechol, carbachol, McN-A343, methacholine, pilocarpine) to inhibit isoprenaline-induced cyclic AMP production in chopped fragments (via M2 receptors), and to evoke cationic current (Icat) (via M2 receptors) or calcium store release (via M3 receptors) in enzyme-dispersed, single voltage-clamped cells from longitudinal smooth muscle of the guinea-pig small intestine were examined.
All muscarinic agonists (1 – 300 μM) examined inhibited isoprenaline (1 μM)-induced accumulation of cyclic AMP, the IC50 varying from 52 to 248 μM. However, their relative potencies to evoke this M2 effect were not significantly correlated with their ability to evoke Icat, also a M2 effect, whether or not calcium stores were depleted; pilocarpine and McN-A343 inhibited the Icat response to carbachol.
Muscarinic agonists (concentration 300 or 1000 μM), except pilocarpine and McN-A343 which were ineffective, evoked Ca2+-activated K+ current (IK-Ca) resulting from Ca2+ store release (M3 effect). Their effectiveness was tested by estimating residual stored calcium by subsequent application of caffeine (10 mM). The relative potencies to evoke Ca2+ store release (M3) and for Icat activation (M2) were closely correlated (P<0.001).
These data might be explained if M2-mediated adenylyl cyclase inhibition and Icat activation involve different G proteins, or involve different populations of M2 receptors. The observed correlation of agonist potency between Icat activation and Ca2+ store release supports the proposal (Zholos & Bolton, 1997) that M3 activation can potentiate M2-cationic channel coupling through Ca2+-independent mechanisms.
Keywords: Muscarinic agonists, M2 and M3 receptors, intestinal smooth muscle, carbachol, cyclic AMP, cationic current, Ca2+-store release
Introduction
In various gastrointestinal smooth muscles, two different muscarinic receptor types are present as the target for the neurotransmitter acetylcholine. They are defined pharmacologically as M2 and M3 corresponding to the genetically defined m2 and m3 subtypes (Hulme et al., 1990; Eglen et al., 1996). Evidence for this was obtained initially by radioligand binding studies (Doods et al., 1987; Giraldo et al., 1987; Gomez et al., 1992; Zhang et al., 1991; Michel & Whiting, 1988; 1990) and confirmed by mRNA hybridization (Maeda et al., 1988; Ford et al., 1991) and immunoprecipitation studies (Wall et al., 1991). The number of M2 receptors is greater than that of M3 receptors (4 : 1 to 5 : 1). The co-existence of M2 and M3 subtypes suggests that both would be activated by acetylcholine or other muscarinic stimulants, and would contribute to the contractile response, but the intracellular signal transduction pathways (G proteins and effectors) involved are likely to be different.
The M2 receptor subtype in intestinal smooth muscle is well documented to couple via pertussis toxin (PTX)-sensitive G proteins to inhibition of adenylyl cyclase activity. Activation of M2 receptors, therefore, results in inhibition of the increase in cyclic AMP levels elicited by forskolin, isoprenaline and other compounds that stimulate adenylyl cyclase activity (Peralta et al., 1988; Candell et al., 1990; Griffin & Ehlert, 1992; Reddy et al., 1995). M2 receptor activation has also been shown to evoke a cationic current (Icat) (Benham et al., 1985; Bolton & Zholos, 1997; Zholos & Bolton, 1997; Komori et al., 1998). The current response is blocked by PTX (Inoue & Isenberg, 1990a; Komori et al., 1992), but it is not secondary to changes in cytosolic cyclic AMP levels (Komori et al., 1998). Therefore, the M2 receptor is believed to link to adenylyl cyclase and to cationic channels via separate signalling pathways that involve PTX-sensitive family G proteins. However, the exact nature of the M2-linked signal transduction mechanisms is still elusive; for example it is unclear whether the G proteins involved in these systems are identical or not.
The M3 receptors in intestinal smooth muscle are well known to couple via PTX-insensitive G proteins to stimulation of phospholipase C (PLC) leading to formation of inositol-1,4,5-trisphosphate (InsP3) and in turn to the release of Ca2+ from intracellular stores (Prestwich & Bolton, 1995a; Komori & Bolton, 1991). There is abundant evidence to show that Ca2+ mobilized from internal stores is an intermediate link between M2 and M3 subtypes, whereby M3 activation can potentiate M2-mediated Icat because of the high Ca2+ sensitivity of cationic channel opening (Pacaud & Bolton, 1991; Komori et al., 1993, 1996; Kohda et al., 1998). Recently, the existence of another link between both the subtypes was proposed, in which M3 activation generates a Ca2+-independent message potentiating Icat at the level of receptor or G protein (Bolton & Zholos, 1997; Zholos & Bolton, 1997). The underlying mechanism is still unknown, but such functional linkage implies that without the Ca2+-induced facilitation of Icat, the ability of muscarinic agonist to evoke Icat via M2 receptors depends also on its ability to stimulate the M3 receptor (perhaps via a conformational change in the receptor or activation of associated G proteins). The hypothesis deserves to be tested, in order to validate the proposed functional link between M2 and M3 subtypes.
In this report, we have examined the ability of various muscarinic agonists to elicit a M3-mediated Ca2+-activated K+ current (IK-Ca) that occurs as a result of Ca2+ store release, and two M2-mediated responses, namely the inhibition of isoprenaline-stimulated cyclic AMP accumulation and the activation of Icat, in longitudinal smooth muscle of guinea-pig small intestine. The IK-Ca response is used to assess the M3-stimulating activity. Our data indicated that there is no significant correlation of agonist potency between the two M2-mediated responses, whereas there was a significant correlation between Icat activation and M3 receptor stimulation.
Methods
Male guinea-pigs (300 – 400 g) were killed by either stunning or cervical dislocation, followed by immediate exsanguination. The small intestine was removed and exposed in a physiological medium. The longitudinal muscle layer in the ileal region or the whole length of the small intestine except the terminal 5 cm was carefully peeled from the underlying tissue, and then subjected to procedures for the use in whole-cell current recordings or cyclic AMP measurements.
Whole-cell current recordings
Experiments were performed on single smooth muscle cells from the ileal longitudinal muscle layer obtained after treatment with a combination of collagenase (0.3 – 0.6 mg−1) and papain (0.2 – 0.3 mg−1) at 37°C for 30 min, as described previously (Komori et al., 1998).
Whole cell membrane current was recorded at room temperature with patch pipettes (5 – 7 MΩ in tip resistance) and CEZ-2300 voltage-clamp amplifier (Nihon Kohden, Tokyo, Japan). Current signals were filtered at 1 KHz and then displayed on an oscilloscope (Nihon Kohden, VC9) and a thermal array recorder (Nihon Kohden, RTA-1100). They were also saved on a digital tape using a recorder (TEAC, RD-111T, Tokyo) for later analysis and illustration.
For recording of IK-Ca, cells were bathed in a conventional physiological salt solution (PSS) that had the following composition (mM): NaCl 126, KCl 6, CaCl2, 2, MgCl2 1.2, glucose 14, HEPES 10.5, pH adjusted to 7.2 with NaOH. Patch pipettes were filled with a solution consisting of (mM): KCl 134, MgCl2 1.2, MgATP 1, Na2GTP 0.1, EGTA 0.05, glucose 14, HEPES 10.5, pH adjusted to 7.4 with KOH. Membrane potential was clamped at a level of 0 mV, close to the reversal potential for Icat in these ionic conditions, so that the IK-Ca response was not contaminated by Icat activated at the same time (Komori et al., 1992). The Icat response was recorded in an external solution consisting of (mM): CsCl 120, glucose 12, HEPES 10, pH adjusted to 7.4 with CsOH. This solution was introduced to the bath (0.5 ml) 30 – 40 s before the start of Icat recordings by exchange with PSS. The pipette solution had the following composition (mM): CsCl 80, MgATP 1, Na2GTP 1, creatine 5, glucose 20, HEPES 10, BAPTA 10, CaCl2 4.6 (calculated [Ca2+]i=100 nM), pH adjusted to 7.4 with CsOH. Under these conditions, the Icat response was neither contaminated by any K+ currents including IK-Ca, nor modulated by intracellular Ca2+ or external divalent cations (Pacaud & Bolton, 1991; Zholos & Bolton, 1995, 1997). Unless otherwise stated, Icat was measured at a holding potential of −40 mV which is suitable for receptor-cationic channel coupling (Zholos & Bolton, 1994). In some experiments, Icat was recorded where [Ca2+]i was non-clamped, by using PSS as the bath solution and pipettes filled with the following solution (mM): CsCl 134, MgCl2 1.2, MgATP 1, NaGTP 0.1, glucose 14, HEPES 10.5, EGTA 0.05, pH adjusted to 7.2 with CsOH (Komori et al., 1996).
Concentration-effect curves of muscarinic agonists for Icat activation were obtained by their application in an ascending series of six different concentrations (1 – 300 μM), with some exceptional occasions. The curves from individual cells were analysed using computor software (SPSS Inc., DeltaGraph 4.0) that fits the data directly with a logistic function, providing the EC50 value, the maximal current (Imax) and the value (h) of the Hill slope.
Cyclic AMP determination
Smooth muscle fragments were prepared and incubated as described previously (Prestwich & Bolton, 1995b). In brief, the longitudinal muscle layers from the whole length of the small intestine were chopped to 350×350 μm fragments with a McIlwain tissue chopper and placed in Krebs Ringer bicarbonate (KRB) consisting of the following composition (mM): NaCl 120, KCl 5.9, NaHCO3 15.4, NaH2PO4, 1.2, glucose 11.5, MgCl2, 1.2; CaCl2, 2.5, pH adjusted to 7.25 with NaOH. After incubation under aeration with a mixture of 95% O2 and 5% CO2 at 37°C for 15 – 20 min, the fragments were transferred to fresh KRB containing IBMX (1 mM), a phosphodiesterase inhibitor, and then divided into 300 μl aliquots in microfuge tubes and placed in a water bath at 37°C for 15 min. If necessary, both thapsigargin (1 μM), a sarcoplasmic reticulum Ca2+-ATPase inhibitor and D600 (10 μM), a voltage-dependent Ca2+ channel blocker were added to the incubation buffers when IBMX was added.
To investigate the effects, if any, of muscarinic agonists on isoprenaline-induced increases in cyclic AMP levels, the fragments were incubated for 10 min in the presence or absence of each muscarinic agonist (1 – 300 μM). After this incubation, isoprenaline was added at desired concentrations (0.1 – 100 μM), followed by further 5 min incubation. The reaction was then stopped with 10% (final concentration) trichloroacetic acid (TCA). The fragments were incubated for 15 min on ice, centrifuged for 20 min at 13,000×g, and the supernatant removed. The pellet was stored ready for protein determination (the average protein concentration was 1.10±0.02 mg/tube, n=20). TCA in the supernatant was removed by diethyl ether extraction, and then TCA-free samples were neutralized using 1 mM NaOH, and aliquots removed for cyclic AMP determination by a competition binding assay using a [3H]-cyclic AMP-labelled kit (Amersham International Ltd), as described previously (Prestwich & Bolton, 1995b).
Chemicals
Ten muscarinic agonists were used; acetylcholine chloride, McN-A343 (4-(N-[3-chlorophenyl]-carbamoyloxy)-2-butynyl-trimethylammonium chloride), oxotremorine, methacholine chloride, pilocarpine hydrochloride (purchased from Sigma, St Louis, MO, U.S.A.), carbachol chloride (from WAKO, Tokyo, Japan), arecoline HBr, bethanechol chloride, OXA-22 (cis-2-methyl-5-trimethylammoniummethyl-1,3-oxathiolane iodide), and oxotremorine-M (oxotremorine methiodide) (from Research Biochemicals, Natick, MA, U.S.A.). All the muscarinic agonists were dissolved in distilled water and stocked at −20°C in concentrations 100 times or more higher than those used in experiments.
Guanosine 5′ triphosphate (GTP, sodium salt), isobutylmethylxanthine (IBMX), thapsigargin, methoxyverapamil (D600), and 1,2-bis(2-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid (BAPTA) were obtained from Sigma, and caffeine from WAKO. All other chemicals were obtained from Sigma or WAKO.
Data analysis
Values in the text are given as means±s.e.mean of the number of experiments with smooth muscle fragments or cells (n). Student's paired or unpaired t-test was used for statistical comparison and when P<0.05, differences were considered significant. Correlationships between agonist potencies at different responses were analysed with a Pearson's correlation coefficient test.
Results
Inhibition of cyclic AMP accumulation
The ability of muscarinic agonists to inhibit isoprenaline-stimulated cyclic AMP accumulation was examined in chopped fragments of longitudinal intestinal smooth muscle. These experiments were carried out to ascertain a suitable concentration of isoprenaline such that application of muscarinic agonist might decrease or increase the cyclic AMP response. Basal cyclic AMP levels were estimated to be 22.0±4.9 pmol mg−1 protein (n=5). Incubation with isoprenaline (0.1 to 100 μM) increased cyclic AMP levels above the basal level in a concentration-dependent manner (Figure 1). The level of cyclic AMP reached in 100 μM isoprenaline was 108.0±12.6 pmol mg−1 protein (n=4), and by 1 μM, 54.8±6.3 pmol mg−1 protein (n=4), about half of the former mean value.
Figure 1.
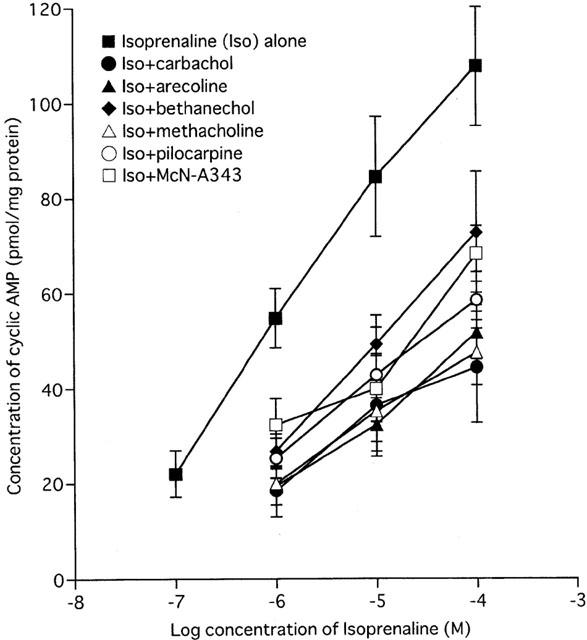
The effect of muscarinic agonists on the concentration-effect curve for isoprenaline-induced increases in cyclic AMP levels. Smooth muscle fragments were incubated with isoprenaline (0.1 – 100 μM) for 5 min in the absence or presence of each indicated muscarinic agonist (100 μM) applied 10 min before. Changes in cyclic AMP were measured in the continuous presence of 1 mM IBMX, a phosphodiesterase inhibitor, using a 3H-cyclic AMP labelled kit. Results represent the mean±s.e.mean of four experiments performed on separate occasions and are expressed as absolute pmol mg−1 protein.
Six muscarinic agonists (carbachol, arecoline, bethanechol, methacholine, pilocarpine and McN-A343) were tested; none of these up to 100 μM affected basal cyclic AMP levels. However, they all (at 100 μM) inhibited the increase of cyclic AMP elicited by isoprenaline (1 to 100 μM), although there were variations in the extent of inhibition (Figure 1). For example, the level of cyclic AMP reached by 100 μM isoprenaline (108.0±12.6 pmol mg−1 protein) was decreased to 44.5±11.7 and 73.0±12.8 pmol mg−1 protein (each n=4) by carbachol and bethanechol, respectively, and to intermediate values by other agonists. The maximum concentration used (300 μM) may not have achieved a maximum response but 1 mM or more may have had non-specific effects.
The ability of muscarinic agonists to inhibit the rise in cyclic AMP levels produced by 1 μM isoprenaline was studied. Carbachol inhibited the accumulation of cyclic AMP in a concentration-dependent manner, with an average 72.2±2.7% (n=4) inhibition at 300 μM; 52 μM carbachol (by interpolation) reduced the response to 1 μM isoprenaline to half (see open circles in Figure 2a). The other five agonists also behaved similarly (Figure 2b – f); the per cent inhibitions at 300 μM and the concentrations producing 50% inhibition of the 1 μM isoprenaline response were 64.8±7.1% and 103 μM for methacholine, 62.7±4.4% and 117 μM for arecoline, 58.5±2.0% and 127 μM for bethanechol, 66.4±5.7% and 65 μM for pilocarpine and 53.1±3.3% and 248 μM for McN-A343 (each, n=4). The potencies at 300 μM relative to carbachol ranged between 0.73 (McN-A343)and 0.92 (pilocarpine) and the rank order was carbachol> pilocarpine>methacholine>arecoline>bethanechol>McN-A343 (see Figure 7). The rank order for 50% inhibition was the same.
Figure 2.
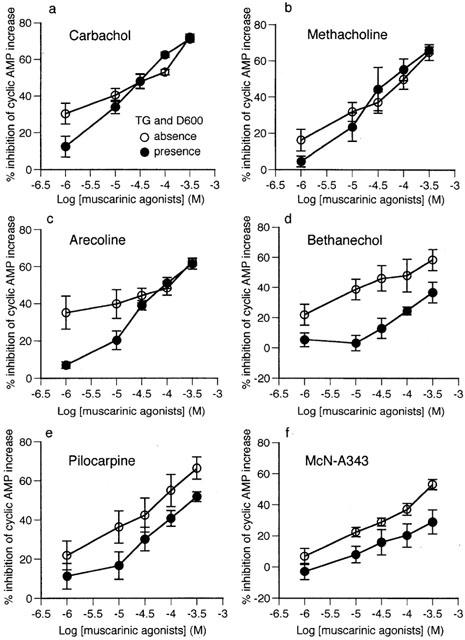
Concentration-effect curves of six muscarinic agonists (a – f) for inhibition of 1 μM isoprenaline-stimulated cyclic AMP accumulation in the absence or presence of 1 μM thapsigargin and 10 μM D600. Cyclic AMP levels were determined as described in Figure 1. Results represent the mean±s.e.mean of four experiments performed on separate occasions and are expressed as the per cent inhibition of the increase in cyclic AMP compared to control.
Figure 7.
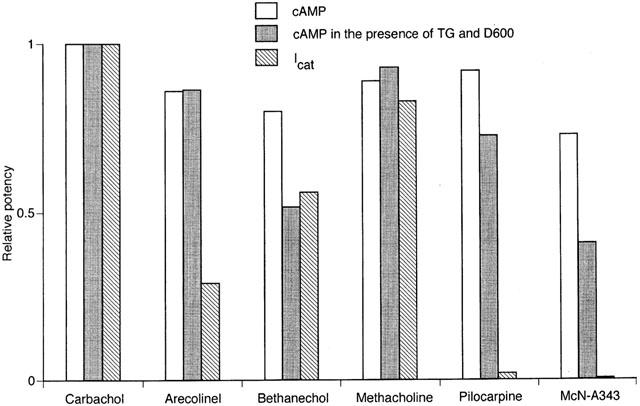
Summary of muscarinic agonist potency for cyclic AMP and Icat responses. The former potency was defined by the per cent inhibition (at 300 μM) of the isoprenaline-stimulated cyclic AMP accumulation (Figure 2) and the latter by the maximum response of Icat (the Imax in Table 1). These respective potencies were normalized by the value for carbachol. The relative potencies are shown for the cyclic AMP responses in the absence and presence of both 1 μM thapsigargin and 10 μM D600 and for the Icat response of the indicated individual agonists.
Similar experiments were carried out under conditions where internal Ca2+ stores were depleted by pretreatment with thapsigargin (1 μM) and voltage-dependent Ca2+ channel activity blocked by D600 (10 μM). Under these conditions, all six agonists were also effective in inhibiting the accumulation of cyclic AMP (see closed circles in Figure 2). For carbachol, methacholine and arecoline, the per cent inhibitions at 300 μM and concentrations reducing the 1 μM isoprenaline response to 50% were 71.0±1.6% and 36 μM, 66.0±1.9% and 58 μM and 61.5±2.8% and 91 μM, respectively (all, n=4), values which were similar to the corresponding values obtained in the absence of thapsigargin and D600. For pilocarpine, bethanechol and McN-A343, however, the per cent inhibition at 300 μM was not as great as in the absence of thapsigargin and D600, being 51.8±3.1, 36.8±7.0 and 29.1±7.7% (all, n=4), respectively. Consequently, 253 μM pilocarpine inhibited the response to isoprenaline by 50%, while neither bethanechol nor McN-A343 achieved 50% inhibition. The relative potencies of the five agonists at 300 μM relative to carbachol fell between 0.41 (McN-A343) and 0.93 (methacholine) and the apparent rank order was carbachol>methacholine> arecoline>pilocarpine>bethanechol>McN-A343 (see Figure 7). It was noted that the position of pilocarpine in the rank order shifted from the second to the fourth when thapsigargin and D600 were present.
Activation of nonselective cationic current, Icat
The above six muscarinic agonists and four others (acetylcholine, oxotremorine-M, oxotremorine and OXA-22) were studied for their ability to evoke Icat. Intracellular calcium concentration [Ca2+]i was clamped at 100 nM by the use of a BAPTA/CaCl2 combination in order to prevent Icat being modulated by changes in [Ca2+]i during muscarinic stimulation (Pacaud & Bolton, 1991; Komori et al., 1993). Five to 6 min after establishing whole cell recording mode, the solution bathing the cells was changed from PSS to Cs+-based solution in order to block K+ currents (see closed triangles in Figure 3), which resulted in the generation of an inward current, probably carried by Cs+ ion (Zholos & Bolton, 1995). The Cs+ current gradually developed to reach a steady level (up to 50 pA in size) in 20 s or so, and then each muscarinic agonist was applied in ascending concentrations of 1, 3, 10, 30, 100 and 300 μM, unless otherwise stated.
Figure 3.
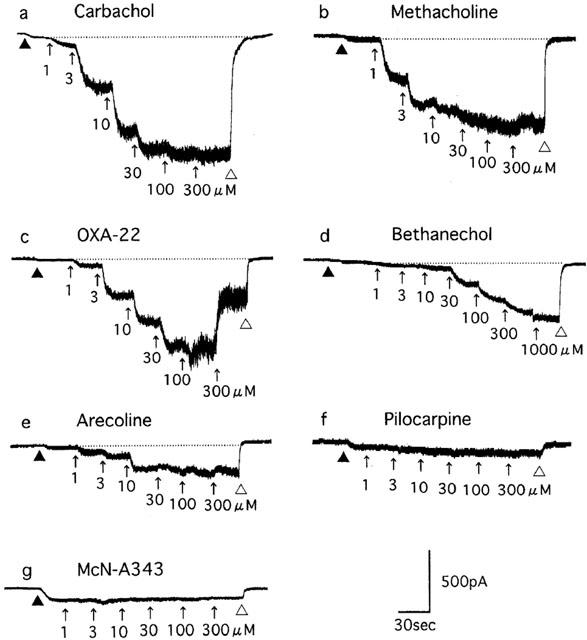
Cationic current (Icat) induced by muscarinic agonists (a – g) in single ileal smooth muscle cells. The current response was recorded at a holding potential of −40 mV and under conditions where [Ca2+]i was clampled at 100 nM with a BAPTA/CaCl2 combination. Five to 6 min after establishment of whole-cell recording mode, a physiological salt solution (PSS) bathing the cells was replaced with a Cs+-rich solution (at closed triangle) and then each indicated agonist was applied at ascending concentrations (1 to 300 μM) as indicated by the arrows. The agonists applied were washed away with PSS (at open triangle). The dotted lines indicate the levels of holding current in the Cs+-rich solution, which were used as base lines to determine the amplitude of Icat (see Figure 4). (a – g) are from different cells.
Figure 3a shows a typical Icat response to application of the ascending series of carbachol concentrations. The current response developed in a graded manner and it reached a new sustained level in 10 – 20 s after the increase in agonist concentration; maximum current was usually achieved at 100 μM. The averaged relationship between the amplitude of Icat and carbachol concentration (n=14) is shown in Figure 4a. Icat amplitudes were determined by taking the initial sustained level of current in Cs+ solution as a base line. Mean values for the Imax, EC50 and slope of the curve (h) estimated by curve fitting of a series of data from the individual cells were 1028.6±123.1 pA, 7.5±1.6 μM and 1.2±0.1 (n=14, Table 1), respectively. The EC50 value was close to that (7.6 μM) reported by Zholos & Bolton (1997).
Figure 4.
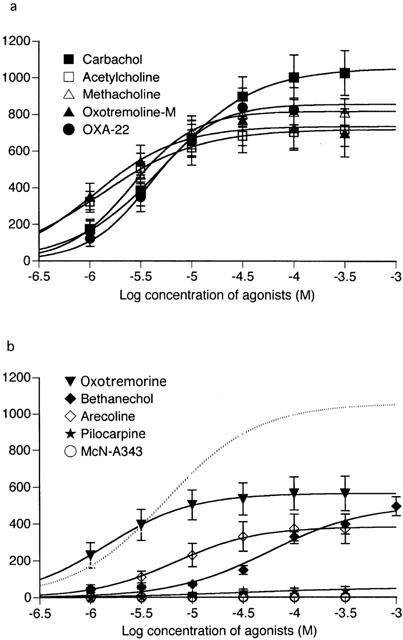
Averaged concentration-effect curves of muscarinic agonists for Icat activation. The curves were obtained from experiments as described in Figure 3. The data points for each agonist were fitted with a logistic function. The curves, depending on whether significantly different from carbachol's curve in the maximum response, are classified into the two groups; (a), not significant and (b), significant (P<0.05). The dotted curve in (b) is for carbachol. Each point indicates the mean±s.e.mean of measurements in 5 – 14 cells.
Table 1.
Maximum amplitude (Imax), half-effective concentration (EC50), and slope factor (h) in the concentration-effect curves for muscarinic agonist-evoked cationic current (Icat)
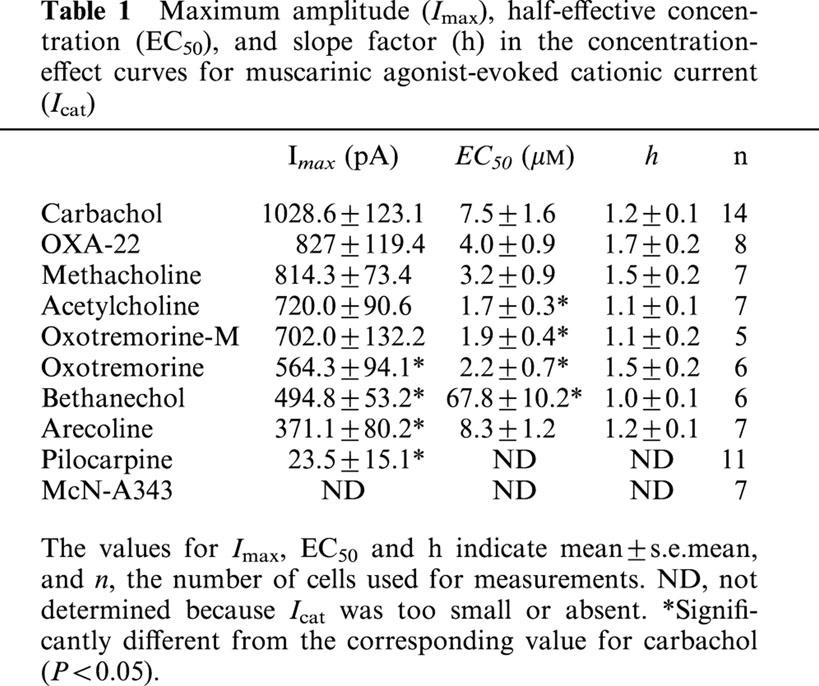
Other agonists except pilocarpine and McN-A343 were also effective in eliciting Icat. Figure 3b – e shows typical examples of Icat responses to methacholine, OXA-22, bethanechol and arecoline. The Icat response to OXA-22 often decreased when the agonist concentration was increased to 100 or 300 μM (Figure 3c). As seen from the averaged concentration-effect curves in Figure 4, all the effective agonists, except bethanechol, produced a maximal response at 30 to 300 μM. Bethanechol often required a higher concentration of 1000 μM to do so. The mean values of Imax for the seven effective agonists were all smaller than the corresponding value for carbachol, and this was statistically significant (P<0.05) for oxotremorine, bethanechol and arecoline (Table 1). The mean EC50 values for acetylcholine, oxotremorine-M and oxotremorine were 3.5 to 4.5 times lower (P<0.05) and bethanechol nine times higher (P<0.05), than carbachol. The mean h values for all agonists were not significantly differed from carbachol.
Pilocarpine had little or no effect in eliciting Icat (Figures 3f and 4b); however, when applied at 300 μM, it produced a small, noisy inward current (up to 120 pA) in five of 11 cells. The current amplitude averaged 23.5±15.1 pA (n=11), only 2% of the Imax value for carbachol (Table 1). McN-A343 was completely ineffective in all seven cells (Figures 3g and 4b). Further characterization of pilocarpine and McN-A343 was performed in different experimental conditions. Their effects on the carbachol concentration-effect curve for Icat activation were investigated. Both agonists (100 μM) shifted the curve to the right with depression of the maximum response (Figure 5); the EC50 value for carbachol was increased from 7.5±1.6 μM (Table 1) to 178.6±65.4 μM (n=5) by pilocarpine and to 40.8±6.5 μM (n=5) by McN-A343 (both P<0.05) and the Imax value decreased from 1028.6±123.1 pA (Table 1) to 635.5±101.1 pA (n=5) and 487.2±76.1 pA (n=5) by the respective agonists (both P<0.05). Their ability to induce Icat was examined over a broader range of membrane potential using a negative going ramp pulse from 40 to −80 mV over 0.5 s (Figure 6a). Current-voltage (I-V) relationships in the presence of either agonist were obtained after leakage subtraction. In two of six cells, the I-V curve for pilocarpine (300 μM) lay on the voltage axis, indicating no activation of Icat in these cells. In the remaining four cells, a detectable, but slight, current activation occurred in the voltage range between −20 and −60 mV with a maximum at some −40 mV. The I-V curve with the most clear activation is shown in Figure 6b. In this curve, current amplitude at any voltage was less than 5% of that at the corresponding voltage in a typical I-V curve for 300 μM carbachol (n=5; Figure 6a,b). The I-V curve for 300 μM McN-A343 overlapped the voltage axis in all five cells tested (Figure 6b). Finally, their Icat-eliciting activity was examined in cells immersed in PSS and internally dialyzed with a pipette solution without the BAPTA/CaCl2-buffer (Komori et al., 1996). At a holding potential of −40 or −50 mV, neither pilocarpine nor McN-A343 (100 – 1000 μM) produced an appreciable Icat (n=12 and 9, respectively), whereas in cells which had been insensitive to these agonists, carbachol (3 – 100 μM) invariably elicited a sustained or oscillatory pattern of Icat (n=8; data not shown). These results show that pilocarpine and McN-A343 have binding affinity but little or no potency at receptors mediating Icat.
Figure 5.
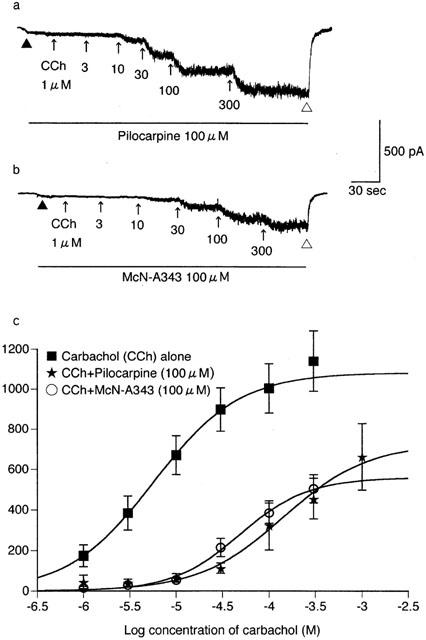
The effect of pilocarpine and McN-A343 on the carbachol concentration-effect curve for Icat activation. The current response to carbachol was recorded as described in Figure 1, except for the presence of 100 μM pilocarpine (a) or McN-A343 (b) in the bath solution as indicated by the lines. (c) The averaged curves for carbachol in the presence of pilcarpine or McN-A343 and the control curve repeated from Figure 4a. Each point for the former two curves indicates the mean±s.e.mean of measurements in five cells.
Figure 6.
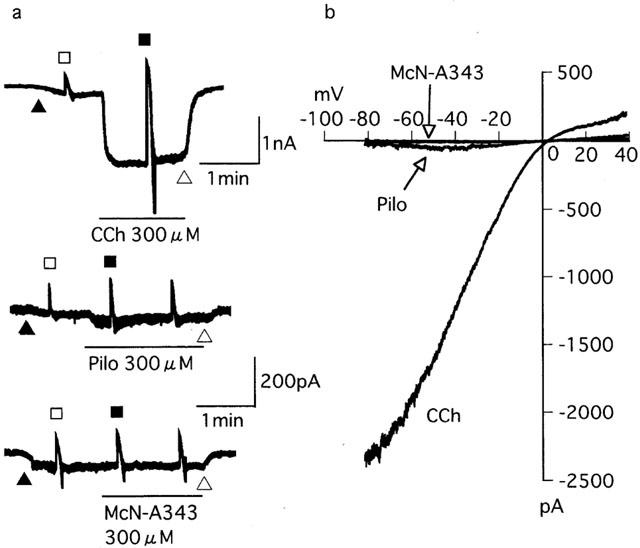
The ability of pilocarpine, McN-A343 and carbachol to activate Icat over a broad range of membrane potential. (a) Current traces from three different cells held at −40 mV to which a negative going ramp pulse from 40 to −80 mV over 0.5 s was applied before (open sqaure) and during application (closed square) of 300 μM carbachol (CCh), pilocarpine (Pilo) or McN-A343. (b) Current-voltage (I-V) curves for CCh, Pilo and McN-A343 from the respective cells in (a), which were constructed by subtracting the I-V curve before application of the agonists from that obtained during their application. See text for details.
Relationship between agonist potencies at the twoM2-mediated responses
Using the data from the group of six agonists whose activity was examined for both cyclic AMP inhibition and Icat activation, we investigated mutual relation of potencies for both responses. Potencies of the individual agonists for the cyclic AMP response and Icat activation, expressed by taking potency of carbachol as unity, are summarized in Figure 7. The relative potencies for the cyclic AMP response were estimated from the inhibition attained at 300 μM of cyclic AMP accumulation in the absence and presence of thapsigargin and D600 (Figure 2) and those for Icat activation from the maximum Icat response (Imax value in Table 1). As seen from Figure 7, the relative potency in evoking the two responses varied considerably among the different agonists, regardless of the use of thapsigargin and D600 in cyclic AMP assays; for example, arecoline and pilocarpine were as potent as methacholine for the cyclic AMP response, but obviously less potent for the Icat response. The relation between the relative potencies for both responses was statistically tested. The calculated correlation coefficient had a value of 0.582 when cyclic AMP was assayed without thapsigargin and D600, and a value of 0.679 when assayed with these drugs present. These values were smaller than the value (0.729, when n=6) for a significant correlation (P<0.05), suggesting a lack of a parallelism of agonist potency between the two effector systems linked to the M2 subtype receptor.
Activation of Ca2+-activated K+ current, IK-Ca
M3 receptor activation in this type of cell induces IK-Ca via Ca2+ release from the stores (Komori & Bolton, 1991; Komori et al., 1998). To assess potency for Ca2+ store release, we examined the ability to elicit IK-Ca of the same group of 10 agonists as tested for Icat activation. The individual agonists were applied at a maximally effective concentrations of 300 or 1000 μM and 30 – 40 s later, 10 mM caffeine, a potent Ca2+-store releaser, was routinely applied to check if releasable Ca2+ remained in the stores (Komori et al., 1998).
Application of carbachol evoked IK-Ca lasting 3 – 10 s (Figure 8a), as previously reported (Komori et al., 1998), the peak amplitude of which amounted to 3.2±0.7 nA on average (n=6). Subsequent application of caffeine in the presence of carbachol was usually without effect (Figure 8a; Table 2). All other agonists, except bethanechol, pilocarpine and McN-A343, also evoked a sizable IK-Ca (Figure 8b – d). The mean peak amplitude varied from 2.0 to 3.1 nA among the six agonists; acetylcholine, oxotremorine-M, arecoline, OXA-22, methacholine and oxotremorine (Table 2). Subsequent caffeine was without effect or evoked IK-Ca with varied amplitude, depending on the previously applied agonist and different cells stimulated with the same agonist (Table 2).
Figure 8.
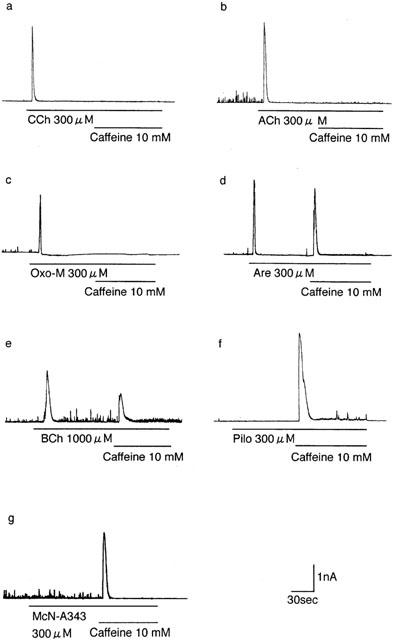
Examples of Ca2+-activated K+ current (IK-Ca) evoked by muscarinic agonists. (a – g) Current responses of different cells to (a) carbachol, (b) acetylcholine (ACh), (c) oxotremoline-M (Oxo-M), (d) arecoline (Are), (e) bethanechol (BCh), (f) pilocarpine (Pilo) and (g) McN-A343 applied at a maximally effective concentration (300 or 1000 μM) and subsequent application of 10 mM caffeine as indicated by the lines. The cells were held at 0 mV, a level close to the reversal potential for Icat and exposed to the drugs 5 min after establishment of whole-cell recording mode. Mean values for the amplitudes of agonist-evoked IK-Ca, subsequent caffeine-evoked IK-Ca and the sum of both currents are given in Table 2.
Table 2.
Ca2+-activated K+ current (IK-Ca) evoked by muscarinic agonists at maximally effective concentrations and by subsequent application of caffeine
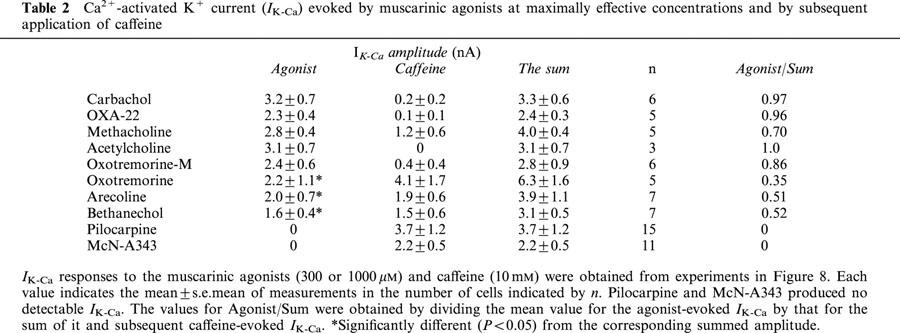
The ability of bethanechol (300 μM) varied too extensively among different cells to be reliably assessed. However, when used at a higher concentration of 1000 μM, the agonist invariably evoked IK-Ca with a peak amplitude of 1.6±0.4 nA (n=7) but subsequent caffeine also invariably evoked IK-Ca which averaged 1.5±0.6 nA (n=7) (Figure 8e). Neither pilocarpine nor McN-A343 was able to evoke an appreciable IK-Ca, but caffeine applied following the respective agonists evoked IK-Ca of 3.7±1.2 nA (n=15) and 2.2±0.5 nA (n=11). Even at 1000 μM, both agonists were without effect (n=3, respectively). When pilocarpine (100 μM) was previously added in the bath solution, carbachol evoked only a small IK-Ca of 0.1 – 0.2 nA followed by subsequent caffeine-evoked IK-Ca of some 2.0 – 3.0 nA (n=3). Substantially similar results were obtained when carbachol was applied in the presence of McN-A343 (100 μM). Therefore, pilocarpine and McN-A343 seemed to behave as antagonists rather than agonists at M3 receptors.
When the amplitude of IK-Ca evoked by a given agonist was compared with the summed amplitude of this and the subsequent caffeine current, there was a significant (P<0.05) difference for bethanechol, arecoline and oxotremoline, as well as pilocarpine and McN-A343, but not for carbachol, acetylcholine, OXA-22, methacholine and oxotremoline-M (Table 2). The results indicated that these agonists have different potencies for Ca2+ store release.
Relationship between agonist potency for Ca2+ store release and for Icat activation
We compared the relationship of agonist potencies for Ca2+ store release and for Icat activation, using the data obtained so far. The potency of the former was estimated from the proportional size of agonist-evoked IK-Ca to the sum of this and subsequent caffeine current and normalized by the value of 0.97 for carbachol-evoked IK-Ca (see Agonist/Sum. in Table 2). The potency for the latter determined and normalized on the basis the Imax value for carbachol. Figure 9 illustrates plots of the relative potency at Icat activation against that at Ca2+ store release for the 10 muscarinic agonists. The data points are scattered along or near the solid line drawn to indicate a positive correlation of potency between both of them. The calculated correlation coefficient of 0.923 was greater than the value (0.872, when n=10) expected for a significant correlation (P<0.001).
Figure 9.
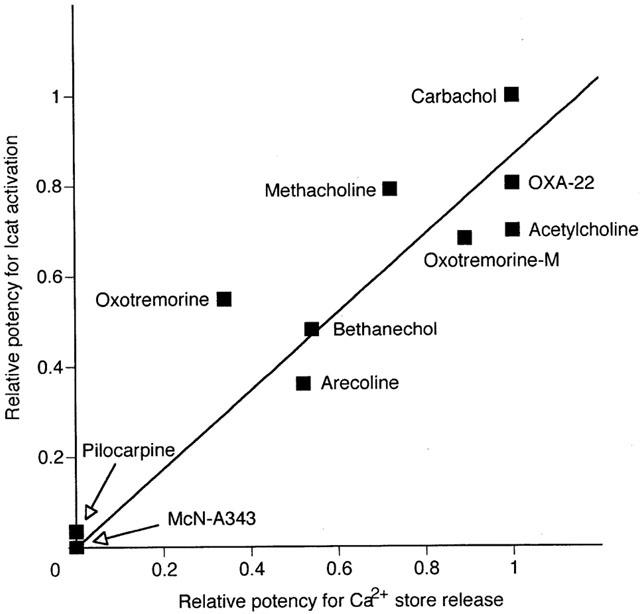
Correlation between muscarinic agonist poptency for Icat activation and for Ca2+ store release. The former potency was defined by the Imax values in Table 1 and the latter, by the proportional size of agonist-evoked IK-Ca to the sum of this and subsequent caffeine current in Table 2 (see column Sum/Agonist), and these respective potencies were normalized by the value for carbachol and plotted for Ca2+ store release on the X axis and for Icat activation on the Y axis. The solid line indicates a regression line calculated using the data points from the 10 agonists, as expressed by Y=0.865X. The obtained Pearson's correlation-coefficient of 0.923 was greater than the expected value for a significant correlation (0.872, when n=10 and P<0.001).
Discussion
Muscarinic receptors in intestinal smooth muscle mediate multiple responses including adenylyl cyclase inhibition, Icat activation, Ca2+ store release and contraction. The former two responses are mediated by M2 subtype receptors coupling to PTX-sensitive G proteins (Candell et al., 1990; Griffin & Ehlert, 1992; Zholos & Bolton, 1997; Komori et al., 1998), and the latter two by M3 subtype receptors coupling to PTX-insensitive G proteins (Komori et al., 1992, 1998; Eglen et al., 1996).
In the present study, the six agonists of carbachol, methacholine, bethanechol, arecoline, pilocarpine and McN-A343, all inhibited the isoprenaline-evoked cyclic AMP response believed to be mediated via M2 receptors. However pilocarpine and McN-A343 were virtually without ability to evoke Icat which is also believed to be evoked via M2 receptors. Moreover, the relationship of relative potencies for the two responses varied considerably among the different agonists (Figure 7) and statistical tests indicated no significant correlation between them. If M2 receptors utilize one multifunctional G protein to mediate both cyclic AMP and Icat responses, the individual agonists tested might be expected to display a parallelism in potency for the two responses. Therefore, it is possible that the G proteins involved in the individual responses are different. Based on this, it is postulated that pilocarpine and McN-A343, if they occupy M2 receptors, cannot or poorly induce the required conformational change in the receptor to activate the G protein linked to the cationic channel system. In agreement with this, both agonists acted as antagonists on the Icat response to carbachol. However, if a receptor subtype is predisposed to couple preferentially to one G protein/effector system (see Gudermann et al., 1996), then another possibility is that different populations of M2 receptors exist, one coupling to a G protein that affects adenylyl cyclase and a second to another G protein that affects cationic channels. In this case pilocarpine and McN-A343 might behave as an agonist at the former receptor, and as an antagonist at the latter.
In the present study, the two M2-mediated responses were recorded under considerably different experimental conditions. The inhibition of the cyclic AMP response was measured in chopped fragments of smooth muscle without control of [Ca2+]i and membrane potential (hence GTP was not added to the bathing solution), whereas the Icat response was recorded in single isolated cells whose [Ca2+]i and membrane potential were both clamped. These differences would mean some difficulty in comparing agonist potencies for both responses. However, when thapsigargin and D600 were used to block intracellular Ca2+ mobilization as well as electrical membrane activity in cyclic AMP assays, in an attempt to mimic the conditions used for Icat recording, the same conclusion that the agonist potencies for cyclic AMP and Icat responses did not correlate was reached. Conversely, to mimic the cyclic AMP assay system, we tried Icat recording conditions both in a physiologically relevant range of membrane potential (−80 to 40 mV) and without clamping [Ca2+]i as well as the presence of extracellular Ca2+. However, McN-A343 and pilocarpine still had little or no effect in eliciting Icat. These results confirmed the comparison of agonist potency, nevertheless measurement of cyclic AMP or adenylyl cyclase activity in conditions similar to those used for Icat recording would be of interest.
The presence of thapsigargin and D600 in assay systems resulted in reduced abilities to elicit a cyclic AMP response. This suggests the action of internal Ca2+ mobilized by Ca2+ store release and/or by an increase in voltage-dependent Ca2+ channel activity may affect the adenylyl cyclase response. In other words, the M2/adenylyl cyclase system might be under a Ca2+-dependent regulation by the M3/PLC/InsP3 system and the M2/cationic channel system which causes voltage-dependent Ca2+ channels to open by depolarizing the cell membrane. These possible interactions would be expected to have important functional implications in vivo. However, the extent to which agonist ability was affected by thapsigargin and D600 varied from one agonist to another; a relatively severe change in the ability was seen for bethanechol, pilocarpine and McN-A343 which are rather weak in their Ca2+-mobilizing activity, as judged from their weak ability to evoke IK-Ca. Therefore, beside the inhibition of intracellular Ca2+ mobilization, some nonspecific actions of thapsigargin and/or D600 (Kobayashi et al., 1991) might be involved.
The 10 muscarinic agonists showed a significant correlation for their abilities to evoke IK-Ca and Icat indicating a relationship between their ability to release Ca2+ stores and to evoke Icat activation (Figure 9). It is well known that the Icat response is strongly potentiated by a rise in [Ca2+]i due to Ca2+ store release (Pacaud & Bolton, 1991; Inoue & Isenberg, 1990b). However, this mechanism seems unlikely to be involved in the observed correlation of agonist potency, because Icat was recorded under conditions where [Ca2+]i was clamped at 100 nM with the Ca2+ buffer (10 mM BAPTA/4.6 mM CaCl2). Therefore, some other mechanism, independent of Ca2+, seems to underlie it. In this context, an idea has been proposed that M3 activation, as well as inducing Ca2+ store release, can potentiate Icat through an action at the level of receptor or G protein (Bolton & Zholos, 1997; Zholos & Bolton, 1997). This hypothesis implies that independently of Icat modulation by [Ca2+]i the ability of an agonist to elicit Icat depends on its ability to stimulate M3 receptors leading to a conformational change in receptor or activation of associated G proteins. The present observation, i.e. the close correlation of agonist potencies, substantiates this implication, corroborating the possible existence of a Ca2+-independent functional link between M2 and M3 receptors (Bolton & Zholos, 1997; Zholos & Bolton, 1997).
In studies using several muscarinic agonists there was a close correlation between their abilities to evoke contraction of longitudinal muscle from isolated guinea pig ileum and phosphoinositide hydrolysis in CHO cells with expressed m3 receptor (Ehlert et al., 1999). The rank orders of potencies for the agonists to cause contraction or phosphoinositide hydrolysis were very similar to the results presented in this study supporting a link between M3 receptor activation, Ca store release and contraction. However Mc-N-343 and pilocarpine were antagonists or ineefective in our studies on single cells whereas they were weakly effective in the experiements of Ehlert et al. (1999).
The present and other results lead us to suggest possible arrangements of the functional expression of muscarinic receptors in intestinal smooth muscle. One possible relationship consists of an M2 and an M3 subtype receptor, the former coupling to two different PTX-sensitive G proteins that affect either adenylyl cyclase or the cationic channel (see above), and the latter coupling to PTX-insensitive G proteins, potentially Gq/G11, linked to the PLC/InsP3 system. In this scheme, it would be predicted that the Ca2+-independent potentiation of Icat by M3 activation would arise through associated G proteins or some downstream factors other than Ca2+. The prediction, however, contradicts our recent observation that anti-Gq/G11 protein antibodies had no significant effect on the Icat response to carbachol, but blocked the agonist-evoked IK-Ca (Yan et al., 2000). Thus, the M3 effect on M2 activation is likely to be upstream of the Gq/G11 protein or a separate system.
Another possibility is the existence of two different types of M2 receptors, a population of independent M2 receptors and a second population of M2/M3 receptor complexes (Zholos & Bolton, 1997) which would be consistent with the fact that M2 binding sites are much more abundant than M3 binding sites (4 : 1 to 5 : 1; Giraldo et al., 1987). The discrete M2 receptor couples via a PTX-sensitive G protein to the adenylyl cyclase system, while the M2 part of the M2/M3 complex couples via another PTX-sensitive G protein to a cationic channel system, and the M3 part, via Gq/G11 proteins, to a PLC/InsP3 system. When an agonist occupies the M2/M3 complex, the resulting conformational change in the M3 receptor generates a Ca2+/G protein-independent message to potentiate M2-mediated Icat, and also activates the PLC/InsP3 system via Gq/G11, leading to Ca2+ store release and in turn Ca2+-induced potentiation of Icat. Our recent experiments using antibodies against various G proteins provided evidence favorable for involvement of Go proteins in the activation of Icat (Yan, Zholos, Unno, Komori & Bolton; unpublished data), as suggested in guinea-pig gastric myocytes (Kim et al., 1998). Together with the general belief that adenylyl cyclase inhibition involves Gi proteins, it may be speculated that the major G proteins coupling to the discrete and complex-type M2 receptors are a Gi and a Go G-protein, respectively. The model discussed here is a working hypothesis amenable to various molecular biological and pharmacological tests such as have been made in the opioid system, where it has been demonstrated that G protein-coupled opioid δ and κ subtypes dimerize to form a functional receptor (Jordan & Devi, 1999).
In the present study, pilocarpine and McN-A343 failed to evoke IK-Ca, but were able to inhibit the IK-Ca-evoking activity of carbachol, indicating their antagonist behaviour at M3 receptors. Such properties are also indicated from other studies in which their effects on phosphoinositide turnover and Ca2+ store-dependent changes in [Ca2+]i or tension were examined (Gardner & Mitchelson, 1988; Hishinuma et al., 1997; Morel et al., 1997; Wang et al., 1992). Both agonists also shifted the carbachol concentration-effect curve for Icat activation to the right with depression of the maximum response (Figure 5). Similar changes in the carbachol curve are seen with certain muscarinic antagonists, and it is suggested that the rightward shift is due to M2 blockade and depression of the maximum, due to M3 blockade (Zholos & Bolton, 1997). These properties of pilocarpine and McN-A343 combined with their obvious potency for the cyclic AMP response can be readily explained by the expression pattern we postulate for the M2 receptor and M2/M3 complexes.
In conclusion, the present study shows that in longitudinal smooth muscle of guinea-pig small intestine, there was no significant correlation of muscarinic agonist potency between the M2/adenylyl cyclase and the M2/Icat system, while a significant correlation exists between the potencies of the M2/Icat and M3/PLC/InsP3 systems. These results combined with others provide a hypothetical model for the functional expression pattern of muscarinic receptors. However, further evaluation of this model will require investigation to clarify the precise muscarinic signalling mechanisms involved.
Acknowledgments
This work was supported by The Wellcome Trust (TB Bolton, SA Prestwich) grant number 051162 and by a Grain-in-Aid Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 13460141).
Abbreviations
- BAPTA
1, 2-bis (2-aminophenoxy) ethan-N,N′ N′-tetraacetic acid
- [Ca2+]i
intracellular calcium concentration
- D600
methoxyverapamil
- EGTA
ethyleneglycol-bis (β-aminoethyl ether) N,N,N′,N′-tetraacetic acid
- G protein
GTP binding protein
- IBMX
isobutylmethylxanthine
- Icat
nonselective cationic current
- IC50
concentration required to produce 50% inhibition
- IK-Ca
Ca2+-activated K+ current
- Imax
maximum current
- InsP3
inositol-1,4,5-triphosphate
- McN-A343
4-(N-[3-chlorophenyl]-carbamoyloxy)-2-butynyl-trimethylammonium chloride
- OXA-22
cis-2-methyl-5-trimethylammoniummethyl-1,3-oxathiolane iodide
- PLC
phospholipase C
- PTX
petrussis toxin
References
- BENHAM C.D., BOLTON T.B., LANG R.J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985;316:345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- BOLTON T.B., ZHOLOS A.V. Activation of M2 muscarinic receptors in guinea-pig ileum opens cationic channels modulated by M3 muscarinic receptors. Life Sci. 1997;60:1121–1128. doi: 10.1016/s0024-3205(97)00056-8. [DOI] [PubMed] [Google Scholar]
- CANDELL L.M., YUN S.H., TRAN L.L., EHLERT F.J. Differential coupling of subtypes of the muscarinic receptor to adenylate cyclase and phosphoinositide hydrolysis in the longitudinal muscle of the rat ileum. Mol. Pharmacol. 1990;38:689–697. [PubMed] [Google Scholar]
- DOODS H.N., MATHY M.J., DAVIDESKO D., VAN CHARLDORP K.J., DE JONGE A., VAN ZWIETEN P.A. Selectivity of muscarinic antagonists in radioligand and in vivo experiments for the putative M1, M2 and M3 receptors. J. Pharmacol. Exp. Ther. 1987;242:257–262. [PubMed] [Google Scholar]
- EGLEN R.M., HEGDE S.S., WATSON N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- EHLERT F.J., GRIFFIN M.T., SAWYER G.W., BAILON R. A simple method for estimation of agonist activity at receptor subtypes: Comparison of native and cloned M3 muscarinic receptors in guinea pig ileum and transfected cells. J. Pharmacol. Exptl Therap. 1999;289:981–992. [PubMed] [Google Scholar]
- FORD A.P.D.W., LEVINE W.B., BAXTER G.S., HARRIS G.C., EGLEN R.M., WHITING R.L. Pharmacological, biochemical and molecular characterization of muscarinic receptors in the guinea-pig ileum: a multidisciplinary study. Mol. Neuropharmacol. 1991;1:117–127. [Google Scholar]
- GARDNER L.K., MITCHELSON F. Comparison of the effects of some muscarinic agonists on smooth muscle function and phosphatidylinositol turnover in the guinea-pig taenia caeci. Br. J. Pharmacol. 1988;94:199–211. doi: 10.1111/j.1476-5381.1988.tb11516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRALDO E., MONFERINI E., LADINSKY H., HAMMER R. Muscarinic receptor heterogeneity in guinea pig intestinal smooth muscle: binding studies with AF-DX 116. Eur. J. Pharmacol. 1987;141:475–477. doi: 10.1016/0014-2999(87)90568-1. [DOI] [PubMed] [Google Scholar]
- GOMEZ A., MARTOS F., BELLIDO I., MARQUEZ E., GARCIA A.J., PAVIA J., SANCHEZ DE LA CUESTA F. Muscarinic receptor subtypes in human and rat colon smooth muscle. Biochem. Pharmacol. 1992;43:2413–2419. doi: 10.1016/0006-2952(92)90321-9. [DOI] [PubMed] [Google Scholar]
- GRIFFIN M.T., EHLERT F.J. Specific inhibition of isoproterenol-stimulated cyclic AMP accumulation by M2 muscarinic receptors in rat intestinal smooth muscle. J. Pharmacol. Exp. Ther. 1992;263:221–225. [PubMed] [Google Scholar]
- GUDERMANN T., KALKBREMER F., SCHULTZ G. Diversity and selectivity of receptor-G protein interaction. Annu. Rev. Pharmacol. Toxicol. 1996;36:429–459. doi: 10.1146/annurev.pa.36.040196.002241. [DOI] [PubMed] [Google Scholar]
- HISHINUMA S., HONGO I., MATSUMOTO Y., NARITA F., KUROKAWA M. Contrasting effects of carbachol, McN-A-343 and AHR-602 on Ca2+-mobilization and Ca2+-influx pathways in taenia caeci. Br. J. Pharmacol. 1997;122:985–992. doi: 10.1038/sj.bjp.0701467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULME E.C., BIRDSALL N.J., BUCKLEY N.J. Muscarinic receptor subtypes. Annu. Rev. Pharmacol. Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- INOUE R., ISENBERG G. Acetylcholine activates nonselective cation channels in guinea pig ileum through a G protein. Am. J. Physiol. 1990a;258:C1173–C1178. doi: 10.1152/ajpcell.1990.258.6.C1173. [DOI] [PubMed] [Google Scholar]
- INOUE R., ISENBERG G. Intracellular calcium ions modulate acetylcholine-induced inward current in guinea-pig ileum. J. Physiol. 1990b;424:73–92. doi: 10.1113/jphysiol.1990.sp018056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN B.A., DEVI L.A. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM Y.C., KIM S.J., SIM J.H., CHO C.H., JUHNN Y.S., SUH S.H., SO I., KIM K.W. Suppression of the carbachol-activated nonselective cationic current by antibody against α subunit of Go protein in guinea-pig gastric myocytes. Pflügers Arch. 1998;436:494–496. doi: 10.1007/s004240050663. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI S., GONG M.C., SOMLYO A.V., SOMLYO A.P. Ca2+ channel blockers distinguish between G protein-coupled pharmacomechanical Ca2+ release and Ca2+ sensitization. Am. J. Physiol. 1991;260:C364–C370. doi: 10.1152/ajpcell.1991.260.2.C364. [DOI] [PubMed] [Google Scholar]
- KOHDA M., KOMORI S., UNNO T., OHASHI H. Carbachol-induced oscillations in membrane potential and [Ca2+]i in guinea-pig ileal smooth muscle cells. J. Physiol. 1998;511:559–571. doi: 10.1111/j.1469-7793.1998.559bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., BOLTON T.B. Calcium release induced by inositol 1,4,5-trisphosphate in single rabbit intestinal smooth muscle cells. J. Physiol. 1991;433:495–517. doi: 10.1113/jphysiol.1991.sp018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., IWATA M., UNNO T., OHASHI H. Modulation of carbachol-induced [Ca2+]i oscillations by Ca2+ influx in single intestinal smooth muscle cells. Br. J. Pharmacol. 1996;119:245–252. doi: 10.1111/j.1476-5381.1996.tb15978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., KAWAI M., PACAUD P., OHASHI H., BOLTON T.B. Oscillations of receptor-operated cationic current and internal calcium in single guinea-pig ileal smooth muscle cells. Pflügers Arch. 1993;424:431–438. doi: 10.1007/BF00374905. [DOI] [PubMed] [Google Scholar]
- KOMORI S., KAWAI M., TAKEWAKI T., OHASHI H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J. Physiol. 1992;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., UNNO T., NAKAYAMA T., OHASHI H. M2 and M3 muscarinic receptors couple, respectively, with activation of nonselective cationic channels and potassium channels in intestinal smooth muscle cells. Jpn. J. Pharmacol. 1998;76:213–218. doi: 10.1254/jjp.76.213. [DOI] [PubMed] [Google Scholar]
- MAEDA A., KUBO T., MISHIMA M., NUMA S. Tissue distribution of mRNAs encoding muscarinic acetylcholine receptor subtypes. FEBS Lett. 1988;239:339–342. doi: 10.1016/0014-5793(88)80947-5. [DOI] [PubMed] [Google Scholar]
- MICHEL A.D., WHITING R.L. Methoctramine reveals heterogeneity of M2 muscarinic receptors in longitudinal ileal smooth muscle membranes. Eur. J. Pharmacol. 1988;145:305–311. doi: 10.1016/0014-2999(88)90434-7. [DOI] [PubMed] [Google Scholar]
- MICHEL A.D., WHITING R.L. The binding of [3H]4-diphenylacetoxy-N-methylpiperidine methiodide to longitudinal ileal smooth muscle muscarinic receptors. Eur. J. Pharmacol. 1990;176:197–205. doi: 10.1016/0014-2999(90)90528-e. [DOI] [PubMed] [Google Scholar]
- MOREL J.L., MACREZ N., MIRONNEAU J. Specific Gq protein involvement in muscarinic M3 receptor-induced phoaphatidylinositol hydrolysis and Ca2+ release in mouse duodenal myocytes. Br. J. Pharmacol. 1997;121:451–458. doi: 10.1038/sj.bjp.0701157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACAUD P., BOLTON T.B. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J. Physiol. 1991;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERALTA E.G., ASHKENAZI A., WINSLOW J.W., RAMACHANDRAN J., CAPON D.J. Differential regulation of PI hydrolysis and adenylyl cyclase by muscarinic receptor subtypes. Nature. 1988;334:434–437. doi: 10.1038/334434a0. [DOI] [PubMed] [Google Scholar]
- PRESTWICH S.A., BOLTON T.B. G-protein involvement in muscarinic receptor-stimulation of inositol phosphates in longitudinal smooth muscle from the small intestine of the guinea-pig. Br. J. Pharmacol. 1995a;114:119–126. doi: 10.1111/j.1476-5381.1995.tb14915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESTWICH S.A., BOLTON T.B. Inhibition of muscarinic receptor-induced inositol phospholipid hydrolysis by caffeine, β-adrenoceptors and protein kinase C in intestinal smooth muscle. Br. J. Pharmacol. 1995b;114:602–611. doi: 10.1111/j.1476-5381.1995.tb17182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDY H., WATSON N., FORD A.P., EGLEN R.M. Characterization of the interaction between muscarinic M2 receptors and β-adrenoceptor subtypes in guinea-pig isolated ileum. Br. J. Pharmacol. 1995;114:49–56. doi: 10.1111/j.1476-5381.1995.tb14904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALL S.J., YASUDA R.P., LI M., WOLFE B.B. Development of an antiserum against m3 muscarinic receptors: distribution of m3 receptors in rat tissues and clonal cell lines. Mol. Pharmacol. 1991;40:783–789. [PubMed] [Google Scholar]
- WANG X-B., OSUGI T., UCHIDA S. Different pathways for Ca2+ influx and intracellular release of Ca2+ mediated by muscarinic receptors in ileal longitudinal smooth muscle. Jpn. J. Pharmachol. 1992;58:407–415. doi: 10.1254/jjp.58.407. [DOI] [PubMed] [Google Scholar]
- YAN H-D., KOMORI S., UNNO T., OHASHI H. Effects of anti-G protein alpha subunit antibodies on muscarinic activation of membrane currents in intestinal smooth muscle cells. Jpn. J. Pharmacol. 2000;82 Suppl. I:98. [Google Scholar]
- ZHANG L.B., HOROWITZ B., BUXTON I.L. Muscarinic receptors in canine colonic circular smooth muscle. I. Coexistence of M2 and M3 subtypes. Mol. Pharmacol. 1991;40:943–951. [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. G-protein control of voltage dependence as well as gating of muscarinic metabotropic channels in guinea-pig ileum. J. Physiol. 1994;478:195–202. doi: 10.1113/jphysiol.1994.sp020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. Effects of divalent cations on muscarinic receptor cationic current in smooth muscle from guinea-pig small intestine. J. Physiol. 1995;486:67–82. doi: 10.1113/jphysiol.1995.sp020791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. Muscarinic receptor subtypes controlling the cationic current in guinea-pig ileal smooth muscle. Br. J. Pharmacol. 1997;122:885–893. doi: 10.1038/sj.bjp.0701438. [DOI] [PMC free article] [PubMed] [Google Scholar]


