Abstract
The effect of the nitro-derivative of aspirin, NCX4016, was assessed on ischaemic ventricular arrhythmias and myocardial infarct size in anaesthetized pigs in comparison to native aspirin.
Pigs were given aspirin (10 mg kg−1; n=6), low dose NCX4016 (18.4 mg kg−1; n=6) or high dose NCX4016 (60 mg kg−1; n=7) orally for 5 days prior to coronary occlusion and reperfusion. None of the interventions had any effect on baseline haemodynamics prior to coronary occlusion in comparison to control pigs (n=9). Aspirin and high dose NCX4016 both prevented the generation of thromboxane A2 from platelets activated ex vivo with A23187 (30 μM), whereas all three interventions markedly attenuated platelet aggregation in response to collagen in whole blood in comparison to controls.
None of the drug interventions had any effect on the incidence of ventricular fibrillation (VF) during myocardial ischaemia (100% in all groups). However, 60 mg kg−1 NCX4016 significantly attenuated the total number of premature ventricular beats (PVB's) (62±16 vs 273±40 in control pigs; P<0.05) during the first 30 min of occlusion. The higher dose of NCX4016 also significantly reduced myocardial infarct size (22.6±3.7% of area at risk vs 53.0±2.8% of area at risk in control pigs; P<0.05).
These results suggest that the nitro-derivative of aspirin, NCX4016, is an effective antiplatelet agent, which unlike aspirin also reduces the extent of myocardial injury following ischaemia and reperfusion.
Keywords: Myocardial ischaemia/reperfusion, aspirin, NCX4016, COX inhibitor, NO donor
Introduction
The clinical use of aspirin as a means of reducing mortality from acute myocardial infarction (AMI) is well documented. However, although platelet activation is known to occur in response to ischaemia, and the platelet release products have been implicated in the consequences of ischaemia and reperfusion (Flores et al., 1994), the favourable effects of aspirin on mortality are most likely due to prevention of re-infarction, by preventing coronary thrombosis, rather than to any decrease in either infarct size or the incidence of fatal arrhythmias (Verheugt et al., 1990; Col et al., 1995). Indeed, any beneficial effect of aspirin observed on ventricular arrhythmias in experimental models of myocardial ischaemia appears to be critically dependent upon both the dose and the time of administration (Coker et al., 1981a; Parratt et al., 1987; Wainwright & Parratt, 1991). Furthermore, when given in doses similar to those used clinically, aspirin fails to exert any significant protective effect on myocardial infarct size in experimental models (Bonow et al., 1981; Alhaddad et al., 1995). The removal of thromboxane A2 (by COX inhibition) during ischaemia/reperfusion would be expected to be of benefit, since a thromboxane mimetic can exacerbate ischaemia-induced arrhythmias (Parratt et al., 1987) and inhibitors of thromboxane synthesis reduce both arrhythmias (e.g. Wainwright & Parratt, 1988) and infarct size (Golino et al., 1993). However, these protective effects can be overcome by COX inhibition (Wainwright & Parratt, 1991; Golino et al., 1993), presumably because of the simultaneous reduction in the production of PGI2, which is both cardioprotective (Hohlfeld et al., 1993) and antiarrhythmic (Coker & Parratt, 1983).
A further mechanism by which aspirin may fail to benefit the ischaemic myocardium is through the inhibition of NO synthesis, since recent studies show that COX inhibition is associated with a reduction in endogenous NO production (through iNOS) in infarcted cardiac tissue (Kimura et al., 1998; Yamamoto et al., 1999). Endogenous NO is likely to be cardioprotective since L-NAME can exacerbate injury, while NO donors have been shown to reduce myocardial infarct size (Lefer et al., 1993a), suppress neutrophil accumulation and preserve coronary arterial function (Lefer et al., 1993b) following experimental ischaemia and reperfusion. Furthermore, NO donors also exert moderate antiarrhythmic effects during ischaemia in some (but not all) species (Wainwright & Martorana, 1993; Sun & Wainwright, 1997). The major problem with organic nitrates, however, is the frequent development of clinical tolerance and unwanted haemodynamic effects. However, a new series of non-steroidal anti-inflammatory drugs with a nitrate moiety (NO-NSAID's) has recently been described (Minuz et al., 1998). One of these compounds, NCX4016 (nitro-aspirin), has been shown to inhibit thrombus formation in vivo to the same degree as that seen with equimolar doses of aspirin, through a mechanism probably involving a combination of nitric oxide release and suppression of thromboxane synthesis (Wallace et al., 1995), without gastric toxicity. These drugs therefore provide the opportunity to combine effective preventative therapy (i.e. prevention of thrombosis through COX inhibition) with the potential to afford protection against the consequences of AMI (by repleting reduced endogenous NO levels). The aim of this study, therefore, was to determine the effects of NCX4016, in comparison with aspirin, on the consequences of myocardial ischaemia and reperfusion in anaesthetized pigs.
Methods
Animal preparation and surgical procedure
Large White/Welsh Landrace cross breed pigs (20 – 25 kg) were used in the study. Sedation prior to anaesthesia was achieved with azaperone (Stresnil®; 7 mg kg−1 i.m.) and anaesthesia induced by halothane (4% in oxygen). The pigs were intubated and artificially ventilated (16 strokes min−1) and maintained on halothane anaesthesia (1 – 1.5%) until intravenous administration of chloralose (100 mg kg−1) was possible following introduction of a venous line. The stroke volume and oxygen content of the inspired air were adjusted to maintain arterial CO2 and O2 tensions to 45 – 50 mmHg and 100 mmHg respectively. Catheters were placed in the left femoral artery, for measurement of arterial blood pressure, and vein, for administration of anaesthetic and blood sampling. A mid-sternal thoracotomy was performed, from the xyphoid cartilage to the clavicle, and the pericardium opened to gain access to the coronary vessels. The left anterior descending coronary artery (LAD) was dissected free below the second major diagonal branch (approximately half the distance from the origin to the apex of the heart) and a silk ligature placed around it. The ends of the ligature were threaded through a polyethylene tube to produce a snare. To induce coronary occlusion the snare was tightened, to reperfuse the snare was released. All surgical procedures were carried out under a project licence issued under the UK Animals (Scientific Procedures) Act (1986).
In vivo parameters measured
Data acquisition
Blood pressures were recorded with Gould capacitance transducers linked to a Buxco haemodynamics analyser for measurement of arterial blood pressure (systolic, diastolic and mean). A Lead I ECG was measured using subcutaneous limb leads and the signal processed by a Buxco ECG analyser. The outputs from the Buxco's were then analysed using a Power Lab computer software package (AD Instruments). All data were stored on computer for subsequent analysis at a later date.
Arrhythmia analysis
Arrhythmia analysis was performed under the guidelines of the Lambeth Conventions (Walker et al., 1988). The arrhythmias following coronary artery occlusion were analysed over 1 min intervals for the first 30 min of coronary occlusion to obtain a profile of distribution and a total count of premature ventricular beats (VPB's). The percentage incidence of ventricular fibrillation (VF) and the time to onset of VF was noted. When VF occurred, the hearts were de-fibrillated (within 40 – 60 s) by direct cardioversion to allow the experiment to continue for the full duration. Animals that developed persistent episodes of VF (i.e. more than five episodes within 10 – 15 min) were excluded from the subsequent parts of the study.
Experimental protocol
Following a 15 min period of stabilization following surgery the snare around the LAD was tightened to induce ischaemia. Visual confirmation of ischaemia was achieved by observing cyanosis of the cardiac tissue distal to the occlusion. The coronary artery remained occluded for 1 h, after which the heart was reperfused by loosening of the snare. Successful reperfusion was confirmed by blushing of the previously ischaemic tissue and, in most cases, accompanied by ventricular ectopic activity. Three hours after reperfusion the pigs were euthanized by an intravenous injection of saturated potassium chloride.
Experimental groups
A total of 28 pigs were entered into the study and split into four experimental groups as follows: Controls (no drug treatment; n=9), aspirin (10 mg kg−1-1 p.o.; n=6), low dose NCX4016 (18.4 mg kg−1 p.o., n=6) and high dose NCX4016 (60 mg kg−1 p.o.; n=7). The choice of aspirin dose was made based on preliminary experiments in two pigs where this dose was found to abolish platelet aggregation in response to collagen and platelet TxA2 generation (data not shown). The two doses of NCX4016 were chosen to provide a dose equimolar with the dose of aspirin employed (18.4 mg kg−1) and a higher dose that achieved a similar degree of COX inhibition (assessed by platelet generation of TxA2). Commencing 7 days prior to experimentation, drugs were administered in a small quantity of pig meal by hand feeding once daily immediately before the morning feed. For all drug groups the last dose of drug was given 1 h prior to sedation. The duration of dosing was chosen based on published evidence of a lack of acute antiplatelet effect of NCX4016 (Wallace et al., 1995).
Measurement of myocardial infarct size
At the end of the reperfusion period the ligature around the coronary artery was re-tied and Evans Blue dye (5% w v−1; 0.5 ml kg−1) was injected slowly into the left atrium to identify the area at risk. Once a clear demarcation between ischaemic and normally perfused tissue was apparent the animal was killed rapidly by injection of a saturated solution of KCl. The heart was excised, blotted free of blood and frozen for subsequent measurement of infarct size. To measure infarct size, the hearts were partially thawed and sliced (0.5 cm slices) from apex to base. The slices were incubated in triphenyltetrazolium (1% w v−1) at 37°C for 10 min to delineate viable from infarcted tissue. The normal, ischaemic and infarcted tissue were then dissected out and weighed. The area at risk was calculated as a percentage of total left ventricular weight. The infarct size was calculated as a percentage of area at risk.
Assessment of the effects of drug treatment on platelet aggregation and TxA2 generation
Platelet aggregation, using whole blood impedance aggregometry (Chronolog Aggrometer) in response to collagen (1 – 10 μg ml−1) was determined in blood samples withdrawn from the venous catheter prior to thoracotomy. Baseline circulating TxA2 levels and TxA2 generation from platelets was determined by measuring TxB2 levels in plasma obtained from blood samples stimulated with 30 μM A23187 for 30 min at 37°C by radioimmunoassay.
Determination of leukocyte adhesion to coronary vascular endothelium
In some control animals (n=5) and the high NCX4016 group the adhesion of leukocytes to segments of coronary artery dissected from the LAD after completion of the occlusion/reperfusion protocol was assessed. Prior to thoracotomy, 40 ml venous blood samples were withdrawn into 3.8% citrate (1 : 10) for isolation of leukocytes as described previously (Demiryürek et al., 1994). The leukocytes were then labelled with 51Cr (as described previously for rabbit leukocytes; Kennedy et al., 2000). After removal of the heart at the end of coronary reperfusion, rings (3 – 4 mm in length) of LAD coronary artery were removed from sites proximal (normal) and distal (ischaemic/reperfused) to the site of the ligature. Adhesion of the leukocytes to the endothelial surface of the coronary blood vessels was measured by adding an aliquot containing 5×104 labelled leukocytes (5 μl) gently to the luminal side of each artery. Adhesion was allowed to proceed for 30 min in a humidified chamber at 37°C. The tissues were then washed with HBSS to remove non-adherent cells and adhesion quantified by duplicate counts of the vessel segments in a gamma counter. Leukocyte adhesion was expressed as the percentage of cells added that remained adherent after washing.
Statistics
All values shown are mean±s.e.m. of n observations, with the exception of the incidence of ventricular fibrillation, which is expressed as a percentage. All mean values between groups were analysed by one-way analysis of variance followed by Dunnett's multiple comparison test. Incidences of events were compared using Fischer's exact test. Leukocyte adhesion between ischaemic and non-ischaemic tissue was analysed using Student's paired t-test. Differences between groups were considered significant when P<0.05.
Results
Mortality and exclusions
Of the pigs originally entered into the study, none of the drug treated animals showed any adverse effects to oral treatment with either aspirin or NCX4016. All animals showed normal feeding and drinking patterns and weight gain. There was no evidence of adverse gastrointestinal effects (e.g. diarrhoea or presence of blood in the faeces) with either of the drugs used. Thus all 28 pigs underwent the surgical procedure. Of these, one from each group developed repetitive episodes of VF in the 30 – 60 min period after occlusion, which precluded reperfusion. These pigs were therefore excluded from subsequent infarct size analysis.
The effects of aspirin and NCX4016 on haemodynamics prior to and during coronary occlusion/reperfusion
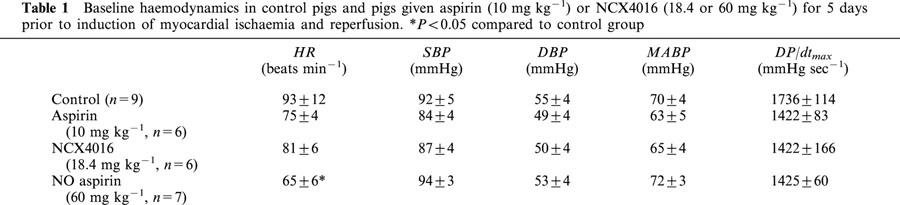
Baseline haemodynamics (i.e. during stabilization prior to coronary occlusion) did not differ significantly among groups (Table 1) with the exception that the heart rates in the pigs receiving high dose NCX4016 were significantly lower than in controls. However, by the end of coronary occlusion the heart rate in this group had increased to values similar to those seen in the other groups (85±10 b.p.m.). Coronary occlusion and subsequent reperfusion had no significant effect on either mean arterial blood pressure (MABP) or heart rate (HR) at any time point throughout the experimental period in any group.
Table 1.
Baseline haemodynamics in control pigs and pigs given aspirin (10 mg kg−1) or NCX4016 (18.4 or 60 mg kg−1) for 5 days prior to induction of myocardial ischaemia and reperfusion. *P<0.05 compared to control group
The effects of aspirin and NCX4016 on ventricular arrhythmias during coronary occlusion
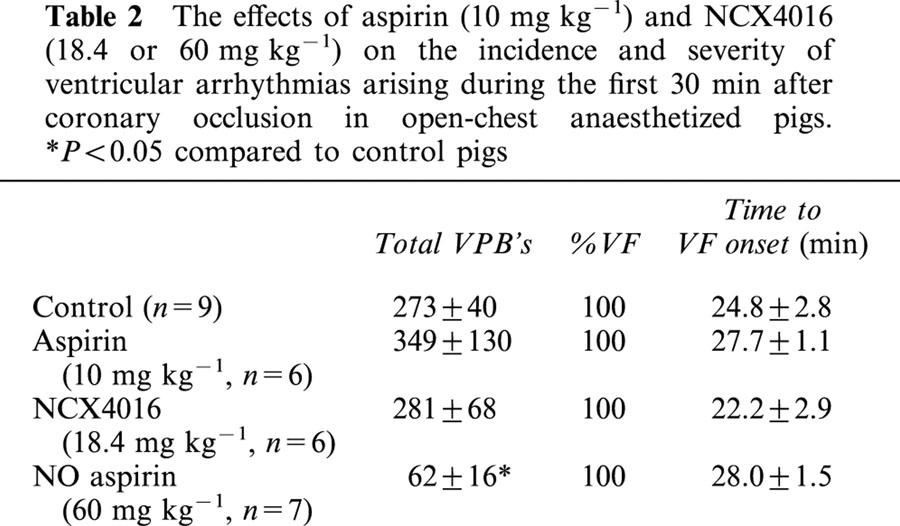
Coronary occlusion in control pigs caused the appearance of a typical bimodal distribution of ventricular premature beats within 2 – 3 min of the induction of ischaemia (Figure 1a). Ventricular fibrillation occurred in all pigs, with a mean time to onset of VF of 24.8±2.8 min after occlusion. Treatment with aspirin or low dose NCX4016 did not influence either the distribution of arrhythmias throughout the 30 min observation period (Figure 1b,c) or the total VPB count (Table 2). High dose NCX4016, however, significantly reduced total VPB count by reducing arrhythmic activity throughout the observation time (Table 2, Figure 1d). None of the drug interventions significantly affected either the total incidence of VF or the time to onset of VF (Table 2).
Figure 1.
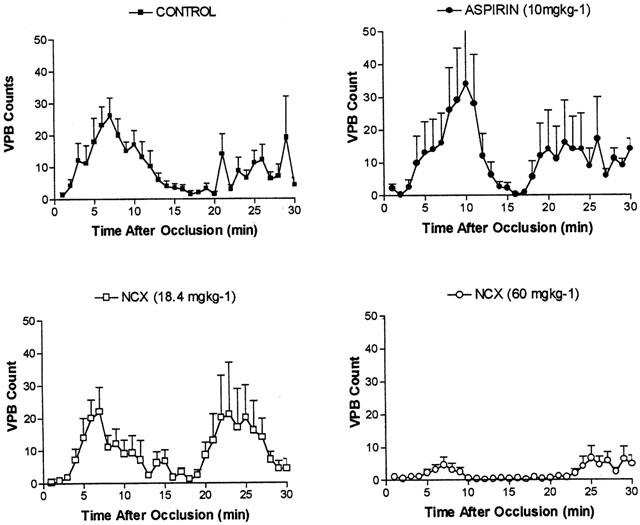
Distribution of premature ventricular beats (VPB's) during the first 30 min following coronary occlusion in anaesthetized pigs. Control pigs (n=9) are shown in the top left panel. Treated pigs were given 5 days oral dosing with either aspirin (10 mg kg−1; n=6, top right), low dose NCX4016 (18.4 mg kg−1; n=6, bottom left) or high dose NCX4016 (60 mg kg−1; n=7, bottom right).
Table 2.
The effects of aspirin (10 mg kg−1) and NCX4016 (18.4 or 60 mg kg−1) on the incidence and severity of ventricular arrhythmias arising during the first 30 min after coronary occlusion in open-chest anaesthetized pigs. *P<0.05 compared to control pigs
The effects of aspirin and NCX4016 on myocardialinfarct size
Infarct size and area at risk in all groups is shown in Figure 2. Area at risk did not differ significantly among the groups. Neither aspirin nor low dose NCX4016 had any effect on infarct size compared to controls. However, the high dose NCX4016 group had significantly smaller infarcts.
Figure 2.
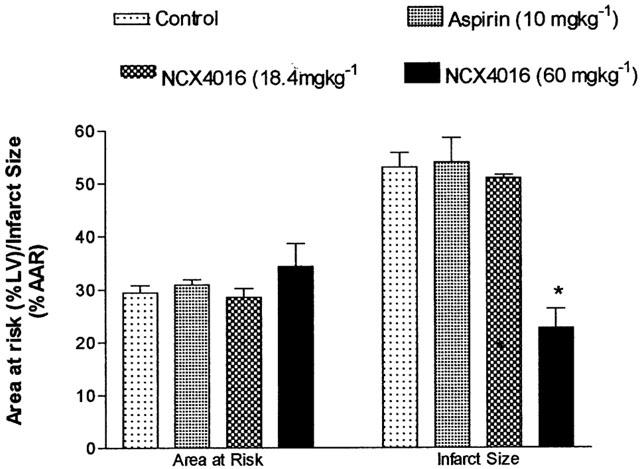
The effects of aspirin (1 mg kg−1; n=5), NCX4016 (18.4 mg kg−1; n=5, or 60 mg kg−1; n=6) on myocardial infarct size following 1 h coronary occlusion and 3 h reperfusion in anaesthetized pigs, compared to control untreated pigs (n=8). *P<0.05 compared to control group.
The influence of drug treatment on platelet aggregation and TxB2 generation from platelets
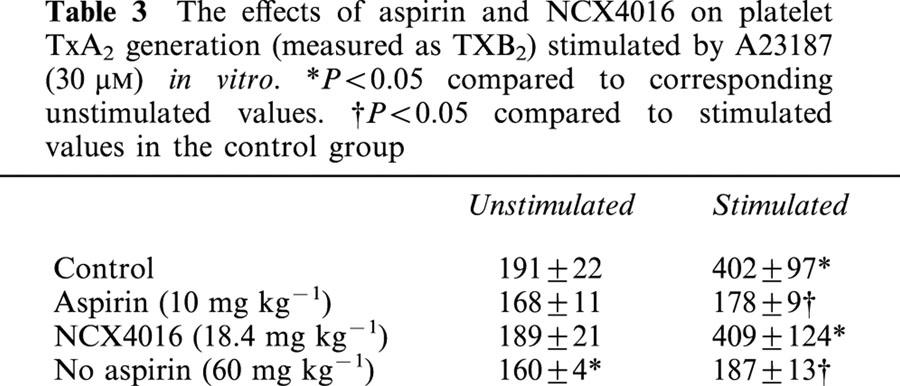
Venous blood sampled before completion of surgery exhibited a concentration-dependent aggregation in response to collagen, as determined by whole blood impedance aggregometry (Figure 3). In blood withdrawn from pigs that had received aspirin for a week prior to sampling the response to collagen was almost abolished (89% inhibition of the response to 10 μg ml−1 collagen). Both doses of NCX4016 also significantly inhibited platelet aggregation to collagen. In vitro platelet activation with A23187 resulted in a significant increase in TxB2 levels in samples taken from control animals. This was almost completely inhibited in plasma from pigs given aspirin and high dose NCX4016, whereas low dose NCX4016 did not significantly inhibit TxB2 generation from activated platelets.
Figure 3.
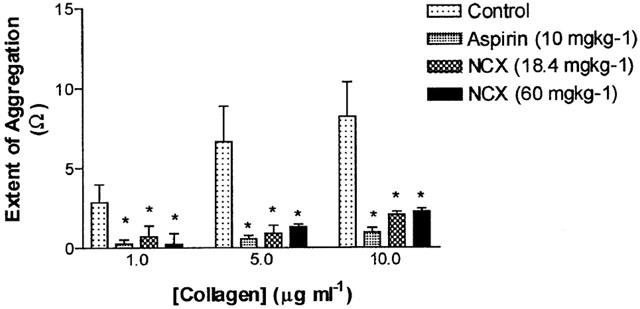
The effects of 5 days oral pretreatment with aspirin (10 mg kg−1; n=6), low dose NCX4016 (18.4 mg kg−1; n=6) or high dose NCX4016 (60 mg kg−1; n=7) on platelet aggregation in response to collagen measured ex vivo by whole blood impedence aggregometry. *P<0.05 compared to the response to the same concentration of collagen in the untreated control group.
Changes in leukocyte adhesion to coronary endothelium following ischaemic/reperfusion injury
Baseline leukocyte adhesion to a segment of normal coronary artery (dissected from the portion of LAD distal to the site of occlusion) in control pigs was 19.7±0.38% of leukocytes added to the vessel segment. Adhesion of leukocytes to a segment of vessel sampled from the coronary artery distal to the site of occlusion (i.e. subjected to ischaemia/reperfusion) was enhanced to 24.9±1.8% (P=0.07). Leukocyte adhesion in pigs pre-treated with high dose NCX4016 to normal artery (19.0±0.7%) was similar to controls and was also increased in artery segments from the ischaemic reperfused area (25.6±0.8; P<0.05). Thus NCX4016 had no effect on either baseline leukocyte adhesion or on the ischaemia/reperfusion induced increase in adhesion.
Discussion
The results of the present study demonstrate that the novel nitrated non-steroidal antiinflammatory drug, NCX4016, significantly reduces infarct size following combined ischaemia and reperfusion and has a moderate antiarrhythmic effect during ischaemia in an animal model with a similar cardiac anatomy to humans. In contrast, native aspirin had no effect on either infarct size or arrhythmias, a finding that is consistent with the large literature in both experimental models and patients. Low dose NCX4016 (18.4 mg kg−1) did not modify either the incidence of arrhythmias, infarct size or TxA2 generation, despite having a significant effect on platelet aggregation measured ex vivo. The anti-platelet effect at this dose, therefore, is most likely to result from a combination of both COX inhibition and NO donation (Wallace et al., 1995). It is not entirely clear why an equimolar dose of NCX4016 did not inhibit TxA2 generation to the same extent as that seen with aspirin, although it may be related to the amount of free salicylate in the plasma, since we have found in recent studies in rats that the plasma salicylate levels are lower with equimolar doses of NCX4016 compared to native aspirin (unpublished data). However, the higher dose of NCX4016 (60 mg kg−1) attenuated the consequences of ischaemia/reperfusion in a dose that prevented agonist-stimulated generation of TxA2 (measured as the stable metabolite TxB2), as well as inhibiting platelet aggregation. Thus it would appear that significant inhibition of platelet TxA2 generation by NCX4016 is required for the cardioprotective effect. However, this is unlikely to be solely responsible for the observed cardioprotective effect, since aspirin similarly prevented TxA2 generation and inhibited platelet aggregation but did not reduce arrhythmias or infarct size.
The most likely explanation for the cardioprotective effect of NCX4016 is the presence of the nitrate moiety in addition to COX inhibition. Nitric oxide donors have been shown previously to possess both modest antiarrhythmic properties (Wainwright & Martorana, 1993) and to reduce myocardial infarct size (Lefer et al., 1993a). Indeed, the antiarrhythmic profile of NCX4016 observed in the present study was identical to that seen with the NO donor, pirsidomine, in a previous study in pigs (Wainwright & Martorana, 1993), that is a reduction in the less severe types of arrhythmias but no antifibrillatory effect. Interestingly, a similar profile of protection against arrhythmias was also observed with NCX4016 in a very recent study in rats, where a clear dose-dependent suppression of ischaemia- and reperfusion-induced VPB's was observed, with no significant reduction in ventricular fibrillation (Rossoni et al., 2001). Furthermore, in those studies the antiarrhythmic effect of NCX4016 correlated with an increase in cardiac cyclic GMP levels, indicating an activation of cardiac guanylate cyclase by NO. The mechanism underlying the antiarrhythmic effect of NO is not immediately obvious, since there was no evidence in the present study of any haemodynamic effects that could contribute to a reduction in the severity of ischaemia (such as a reduced afterload), and thus the severity of arrhythmias. However, the decrease in heart rate in this group, possibly mediated by an effect of increased cyclic GMP levels on the SA node, may contribute to an antiarrhythmic effect. Furthermore, in this model, an effect on coronary flow to the ischaemic region to reduce the severity of ischaemia and thus arrhythmias by decreasing tissue injury is unlikely, since the pig heart has very little coronary collateral reserve. However, it is possible that coronary flow reserve during reperfusion is increased by NCX4016. This is clearly an avenue that requires further investigation. It is also possible that the modest antiarrhythmic effect of NCX4016 could be due to its inhibitory effects on platelet activation during ischaemia/reperfusion, mediated by a combination of COX inhibition and NO donation. Local activation of platelets in the region of ischaemia has been demonstrated to be an arrhythmogenic stimulus (Flores et al., 1994) and the extent of local TxA2 generation has been directly correlated with arrhythmia severity (Coker et al., 1981b). Aspirin similarly prevented the rise in systemic levels of TxB2 without having an antiarrhythmic effect, lending support to a role for the NO moiety in the antiarrhythmic effect of NCX4016. One other possibility is a direct electrophysiological effect of NO generated from NCX4016, although the evidence surrounding this property of NO remains controversial (Pabla & Curtis, 1995; Barnes & Coker, 1995). Furthermore, the observation that both phase 1a and 1b arrhythmias were attenuated by NCX4016 would argue against this, since there is some evidence that different electrophysiological mechanisms underlie these two phases of arrhythmias in the porcine heart (Smith et al., 1995).
The infarct-size reducing effect of NCX4016 may also be explained by one of a number of potential mechanisms. COX inhibition alone is unlikely to account for the reduced infarct size, since aspirin and other non-steroidal anti-inflammatory drugs (NSAID's) fail to reduce infarct size (Bonow et al., 1981; Such et al., 1981). However, selective inhibitors of TxA2 synthesis have been shown to reduce infarct size by an aspirin-sensitive mechanism (Mullane & Fornabaio, 1988; Golino et al., 1993), suggesting that the failure of NSAID's to reduce infarct size is due to the simultaneous inhibition of TxA2 and PGI2 synthesis. Thus the effect of NCX4016 to reduce TxA2 synthesis could play a part in the infarct reducing effect, while the presence of the NO moiety, which shares many physiological actions with PGI2, may compensate for the detrimental loss of PGI2 production. Furthermore, although it is not clear whether the protective effect of the NO moiety is dependent upon its presence during ischaemia, the apparent failure of nitrates, given at the time of clinical thrombolysis, to reduce mortality (reviewed in Morris & Cowan, 1995) suggests that it is. Clearly additional studies are required to determine fully the role of the NO moiety in the cardioprotective effect, for example by determining the effects of NCX4016 given either immediately prior to the onset of ischaemia or at the time of reperfusion. This would exclude any contribution from COX inhibition, which requires several days to develop (Wallace et al., 1995).
The cardioprotective effect of nitric oxide donors has previously been demonstrated to be associated with both a reduction in inflammatory cell accumulation in the ischaemic/reperfused myocardium (Lefer et al., 1993b) and to reduced adherence of polymorphonuclear leukocytes to ischaemic/reperfused vasculature (Lefer et al., 1993a). Here we also studied the effects of NCX4016 on ischaemic/reperfusion injury-induced leukocyte adherence to coronary endothelium and found that there was no inhibition of this by NCX4016, suggesting that the reduced infarct size is not due to a failure of inflammatory cells to adhere to the microvascular endothelium prior to transmigration. However, the situation may be different in patients where significant atherosclerosis is likely to be present in the coronary arteries. NCX4016 has been shown to reduce cardiac myeloperoxidase activity associated with a reduction in infarct size in rats (Rossoni et al., 2001), suggesting that NCX4016 may interfere with the inflammatory process at some other stage in the sequence of events. Indeed, NO possesses a number of antiinflammatory actions, such as suppression of cytokine release (De Caterina et al., 1995) and inhibition of NF-κB activation (Spiecker et al., 1997), which would interfere with the cardiac inflammatory response. Finally, increased levels of NO may result in the increased generation of peroxynitrite as a result of interaction with superoxide. Although a free radical, peroxynitrite has been shown to be cardioprotective against both arrhythmias and infarct size (Nossuli et al., 1997; Altug et al., 1999) when administered to isolated heart preparations.
In summary, NCX4016 appears to act as a stable carrier for nitric oxide that is orally active and produces no untoward haemodynamic effect. Under conditions of acute myocardial ischaemia and reperfusion, NCX4016 exerts a significant protection against the development of myocardial injury, with a concomitant modest suppression of ventricular arrhythmias, although the exact mechanism underlying this effect remains to be fully determined. Since NCX4016 is an equally effective anti-thrombotic agent to aspirin (Wallace et al., 1995), which is devoid of the gastric toxicity often associated with prolonged aspirin use, it may serve as a viable alternative to native aspirin in secondary prevention of thrombotic occlusion since it would provide the added benefit that it could confer cardioprotection against tissue injury should thrombosis occur. Its modest antiarrhythmic profile, however, suggests that it would not protect against the serious malignant arrhythmias (e.g. ventricular fibrillation) that occur in the early stages of ischaemia.
Table 3.
The effects of aspirin and NCX4016 on platelet TxA2 generation (measured as TXB2) stimulated by A23187 (30 μM) in vitro. *P<0.05 compared to corresponding unstimulated values. †P<0.05 compared to stimulated values in the control group
Acknowledgments
We are grateful to Professor Tim Warner, William Harvey Research Institute, London, for performing the TxB2 assays.
Abbreviations
- AMI
acute myocardial infarction
- COX
cyclo-oxygenase
- HR
heart rate
- INOS
inducible nitric oxide synthase
- LAD
left anterior descending coronary artery
- MABP
mean arterial blood pressure
- NO
nitric oxide
- NO-NSAIDs
nitrate non-steroidal anti-inflammatory drugs
- PGI2
prostacyclin
- TxA2
thromboxane A2
- VF
ventricular fibrillation
- VPB
ventricular premature beat
References
- ALHADDAD I.A., TKACZEVSKI L., SIDDIQUI F., MIR R., BROWN E.J. Aspirin enhances the benefits of late reperfusion on infarct shape: A possible mechanism of the beneficial effects of aspirin on survival after acute myocardial infarction. Circulation. 1995;91:2819–2823. doi: 10.1161/01.cir.91.11.2819. [DOI] [PubMed] [Google Scholar]
- ALTUG S., DEMIRYÜREK A.T., CAKICI I., KANZIK I. The beneficial effect of peroxynitrite on ischaemic preconditioning in rat isolated hearts. Eur. J. Pharmacol. 1999;384:157–162. doi: 10.1016/s0014-2999(99)00682-2. [DOI] [PubMed] [Google Scholar]
- BARNES C.S., COKER S.J. Failure of nitric oxide donors to alter arrhythmias induced by acute myocardial ischaemia or reperfusion in anaesthetized rats. Br. J. Pharmacol. 1995;114:349–356. doi: 10.1111/j.1476-5381.1995.tb13233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONOW R.O., LIPSON L.C., SHEEHAN F.H., CAPURRO N.L., ISNER J.M., ROBERTS W.C., GOLDSTEIN R.E., EPSTEIN S.E. Lack of effect of aspirin on myocardial infarct size in the dog. Amer. J. Cardiol. 1981;47:258–264. doi: 10.1016/0002-9149(81)90395-7. [DOI] [PubMed] [Google Scholar]
- COKER S.J., LEDINGHAM I. McA., PARRATT J.R., ZEITLIN I.J. Aspirin inhibits the early myocardial release of thromboxane B2 and ventricular ectopic activity following acute coronary occlusion in dogs. Br. J. Pharmacol. 1981a;72:593–595. doi: 10.1111/j.1476-5381.1981.tb09138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COKER S.J., PARRATT J.R. Prostacyclin – antiarrhythmic or arrhythmogenic? Comparison of the effects of intravenous and intracoronary prostacyclin and ZK 36374 during coronary artery occlusion and reperfusion in anaesthetised greyhounds. J. Cardiovasc. Pharmacol. 1983;5:557–567. doi: 10.1097/00005344-198307000-00008. [DOI] [PubMed] [Google Scholar]
- COKER S.J., PARRATT J.R., LEDINGHAM I. McA., ZEITLIN I.J. Thromboxane and prostacyclin release from the ischaemic myocardium in relation to arrhythmias. Nature. 1981b;291:323–324. doi: 10.1038/291323a0. [DOI] [PubMed] [Google Scholar]
- COL N.F., YARZEBSKI J., GORE J.M., ALPERT J.S., GOLDBERG R.J. Does aspirin consumption affect the presentation or severity of acute myocardial infarction. Arch. Int. Med. 1995;155:1386–1389. [PubMed] [Google Scholar]
- DE CATERINA R., LIBBY P., PENG H.B., THANNICKAL V.J., RAJAVASHTISH T.B., GIMBRONE M., SHIN W.S., LIAO J.K. Nitric oxide decreases cytokine-induced endothelial activation: nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J. Clin. Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMIRYÜREK A.T., WAINWRIGHT C.L., WADSWORTH R.M., KANE K.A. Characterization of a method for the detection of drugs with free radical scavenging activity using porcine activated leukocytes. J. Pharmacol. Meth. 1994;32:35–40. doi: 10.1016/1056-8719(94)90015-9. [DOI] [PubMed] [Google Scholar]
- FLORES N.A., GOULIELMOS N.V., SEGHATCHIAN M.J., SHERIDAN D.J. Myocardial ischaemia induces platelet activation with adverse electrophysiological and arrhythmogenic effects. Cardiovasc. Res. 1994;28:1662–1671. doi: 10.1093/cvr/28.11.1662. [DOI] [PubMed] [Google Scholar]
- GOLINO P., AMBROSIO G., VILLARI B., RAGNI M., FOCACCIO A., PACE L., DE CLERK F., CONDORELLI M., CHIARIELLO M. Endogenous prostacyclin endoperoxides may alter infarct size in the presence of thromboxane synthetase inhibition: Studies in a rabbit model of coronary artery occlusion-reperfusion. J. Amer. Coll. Cardiol. 1993;21:493–501. doi: 10.1016/0735-1097(93)90694-v. [DOI] [PubMed] [Google Scholar]
- HOHLFELD T., STROBACH H., SCHROR K. Stimulation of endogenous prostacyclin protects the reperfused pig myocardium from ischemic injury. J. Pharmacol. Exp. Ther. 1993;264:397–405. [PubMed] [Google Scholar]
- KENNEDY S., WADSWORTH R.M., MCPHADEN A.R., WAINWRIGHT C.L. A rapid, quantitative method for measuring leukocyte adhesion to normal and balloon-injured arteries in vitro. J. Imm. Meth. 2000;244:153–162. doi: 10.1016/s0022-1759(00)00266-0. [DOI] [PubMed] [Google Scholar]
- KIMURA A., ROSETO J., SUH K.-Y., COHEN A.M., BING R.J. Effect of acetylsalicylic acid on nitric oxide production in infarcted heart in situ. Biochem. Biophys. Res. Comm. 1998;251:874–878. doi: 10.1006/bbrc.1998.9551. [DOI] [PubMed] [Google Scholar]
- LEFER A.M., SIEGFRIED M.R., MA X.-L. Protection of ischemia-reperfusion injury by sydnonimine donors via inhibition of neutrophil-endothelium interaction. J. Cardiovasc. Pharmacol. 1993a;22 Suppl 7:S27–S33. [PubMed] [Google Scholar]
- LEFER D.J., NAKANISHI K., VINTEN-JOHANSEN J. Endothelial and myocardial cell protection by a cysteine-containing nitric oxide donor after myocardial ischaemia and reperfusion. J. Cardiovasc. Pharmacol. 1993b;22 Suppl 7:S34–S43. [PubMed] [Google Scholar]
- MINUZ P., LECHI C., ZULIANI V., GAINO S., TOMMASOLI R., LECHI A. NO-aspirins: Antithrombotic activity of derivatives of acetyl salicylic acid releasing nitric oxide. Cardiovasc. Drug Rev. 1998;16:31–47. [Google Scholar]
- MORRIS J.L., COWAN J.C. Nitrates in myocardial infarction: a current perspective. Can. J. Cardiol. 1995;11 Suppl B:5B–10B. [PubMed] [Google Scholar]
- MULLANE K.M., FORNABAIO D. Thromboxane synthetase inhibitors reduce infarct size by a platelet-dependent aspirin-sensitive mechanism. Circ. Res. 1988;62:668–678. doi: 10.1161/01.res.62.4.668. [DOI] [PubMed] [Google Scholar]
- NOSSULI T.O., HAYWARD R., SCALIA R., LEFER A.M. Peroxynitrite reduces myocardial infarct size and preserves coronary endothelium after ischemia and reperfusion in cats. Circulation. 1997;96:2317–2324. doi: 10.1161/01.cir.96.7.2317. [DOI] [PubMed] [Google Scholar]
- PABLA R., CURTIS M.J. Effects of NO modulation on cardiac arrhythmias in the rat isolated heart. Circ. Res. 1995;77:984–992. doi: 10.1161/01.res.77.5.984. [DOI] [PubMed] [Google Scholar]
- PARRATT J.R., COKER S.J., WAINWRIGHT C.L. Eicosanoids and susceptibility to ventricular arrhythmias during myocardial ischaemia and reperfusion. J. Mol. Cell. Cardiol. 1987;19 Suppl V:55–66. doi: 10.1016/s0022-2828(87)80610-7. [DOI] [PubMed] [Google Scholar]
- ROSSONI G., MANFREDI B., COLONNA V.D.G., BERNAREGGI M., BERTI F. The nitroderivative of aspirin, NCX4016, reduces infarct size caused by myocardial ischaemia in the anaesthetised rat. J. Pharmacol. Exp. Ther. 2001;297:380–387. [PubMed] [Google Scholar]
- SMITH W.T., FLEET W.F., JOHNSON T.A., ENGLE C.L., CASCIO W.E. The 1b phase of ventricular arrhythmias in ischaemic in situ porcine heart is related to changes in cell-to-cell electrical coupling. Circulation. 1995;92:3051–3060. doi: 10.1161/01.cir.92.10.3051. [DOI] [PubMed] [Google Scholar]
- SPIECKER M., PENG H.B., LAIO J.K. Inhibition of endothelial vascular cell adhesion molecule expression by nitric oxide involves induction and nuclear translocation of IκBα. J. Biol. Chem. 1997;271:11317–11324. doi: 10.1074/jbc.272.49.30969. [DOI] [PubMed] [Google Scholar]
- SUCH L., MORCILLO E., VINA J., ESPLUGUES J. Differential effects of anti-inflammatory agents on canine acute myocardial infarction. IRCS Med. Sci. 1981;9:574–575. [Google Scholar]
- SUN W., WAINWRIGHT C.L. The role of nitric oxide in modulating ischaemia-induced arrhythmias in rats. J. Cardiovasc. Pharmacol. 1997;29:554–562. doi: 10.1097/00005344-199704000-00018. [DOI] [PubMed] [Google Scholar]
- VERHEUGT F.W.A., VAN DER LAARSE A., FUNKE-KUPPER A.J., STERKMAN L.G.W., GALEMA T.W., ROOS J.P. Effects of early intervention with low-dose aspirin (100 mg) on infarct size, reinfarction and mortality in anterior wall acute myocardial infarction. Amer. J. Cardiol. 1990;66:267–270. doi: 10.1016/0002-9149(90)90833-m. [DOI] [PubMed] [Google Scholar]
- WAINWRIGHT C.L., MARTORANA P. Pirsidomine, a novel nitric oxide donor, suppresses ischemic arrhythmias in anaesthetized pigs. J. Cardiovasc. Pharmacol. 1993;22 Suppl 7:S44–S50. [PubMed] [Google Scholar]
- WAINWRIGHT C.L., PARRATT J.R. The effects of L655,240, a selective thromboxane and prostaglandin endoperoxide antagonist, on ischemia- and reperfusion-induced cardiac arrhythmias. J. Cardiovasc. Pharmacol. 1988;12:264–271. doi: 10.1097/00005344-198809000-00002. [DOI] [PubMed] [Google Scholar]
- WAINWRIGHT C.L., PARRATT J.R. Failure of cyclo-oxygenase inhibition to protect against arrhythmias induced by ischaemia and reperfusion: implications for the role of prostaglandins as endogenous myocardial protective substances. Cardiovasc. Res. 1991;25:93–100. doi: 10.1093/cvr/25.2.93. [DOI] [PubMed] [Google Scholar]
- WALKER M.J.A., CURTIS M.J., HEARSE D.J., CAMPBELL R.W.F., JANSE M.J., YELLON D.M., COBBE S.M., COKER S.J., HARNESS J.B., HARRON D.W.G., HIGGINS A.J., JULIAN D.G., LAB M.J., MANNING A.S., NORTHOVER B.J., PARRATT J.R., RIEMERSMA R.A., RIVA E., RUSSELL D.C. , SHERIDAN D.J., WINSLOW E., WOODWARD B. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia, reperfusion and infarction. Cardiovasc. Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MCKNIGHT W., DEL SOLDATO P., BAYDOUN A.R., BIRINO G. Anti-thrombotic effects of a nitric oxide-releasing, gastric-sparing aspirin derivative. J. Clin. Invest. 1995;96:2711–2718. doi: 10.1172/JCI118338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO T., COHEN A.M., KAKAR N.R., YAMAMOTO M., JOHNSON P.E. , CHO Y.K., BING R.J. Production of prostanoids and nitric oxide by infarcted heart in situ and the effect of aspirin. Biochem. Biophys. Res. Comm. 1999;257:488–493. doi: 10.1006/bbrc.1999.0488. [DOI] [PubMed] [Google Scholar]


