Abstract
Inflammation may affect subpopulations of neurons of the myenteric plexus.
In the present study the effect of trinitrobenzene sulphonic acid (TNBS) induced colitis on nitrergic, purinergic and adrenergic inhibitory neurotransmission was studied as well as the consequences of the related changes on the response of 5-HT agonists using these neurotransmitters to mediate their effect.
Strips from normal and colitis rabbits (135 mg kg−1 TNBS) were subjected to electrical field stimulation (EFS, 0.3 ms, 6V, 0.5 – 32 Hz, 10 s train). The response was measured isometrically in the absence or presence of L-NAME, suramin, guanethidine, the 5-HT agonists (5-HT1/5A/7: 5-carboxamidotryptamine (5-CT), 5-HT2: α-methyl-5-HT, 5-HT3: 2-methyl-5-HT, 5-HT4: 5-methoxytryptamine (5-MeOT)) or a combination.
In normal strips L-NAME (1 – 32 Hz), suramin (0.5 – 2, 8 Hz) and guanethidine (4, 16, 32 Hz) increased the response to EFS. This effect was abolished in inflamed strips and was accompanied by a decrease in nNOS expression.
In normal strips all 5-HT agonists induced pronounced (5-CT, α-methyl-5-HT) or small (2-methyl-5-HT, 5-MeOT) inhibitory neural responses. In inflamed strips this was reversed to cholinergic excitatory responses.
The effect of inflammation on the 5-HT4 response was mimicked by preincubation of normal strips with L-NAME or suramin. Accordingly, in inflamed strips L-NAME or suramin did not affect the excitatory effects of 5-MeOT.
TNBS-colitis abolishes nitrergic, purinergic and adrenergic neurotransmission. This reverses serotonergic inhibition into excitation.
Keywords: TNBS-colitis, electrical field stimulation, inhibitory innervation, nitric oxide, 5-HT receptor subtypes
Introduction
There is evidence of structural and functional alterations in the enteric nervous system in the inflamed bowel in man and in animal models. In man, structural abnormalities include hypertrophy and hyperplasia of nerve fibers which are more characteristic for patients with Crohn's disease (CD) than for patients with ulcerative colitis (UC) (for a review see Geboes & Collins, 1998). In addition, axonal degeneration and necrosis have also been observed (Dvorak et al., 1980; Dvorak & Silen, 1985) and have been proposed as a feature helpful in the differential diagnosis of Crohn's disease from other conditions, especially ulcerative colitis (Steinhoff et al., 1988). For neuronal cell bodies the same types of abnormalities as for the nerve fibres are found. However, neuronal hypertrophy in Meissner's plexus seems more common in UC than in CD, while neuronal cell hyperplasia is more frequently reported in CD (Geboes & Collins, 1998). Immunohistochemical studies have added important information by showing that the content of noradrenaline and neuropeptides in nerve fibres and neurons can also be altered in patients with inflammatory bowel disease (IBD) (Bjorck et al., 1989; Belai et al., 1997).
Several disturbances of gut function have been demonstrated in IBD, such as changes in sensory perception (Farthing & Lennard-Jones, 1978; Rao et al., 1987) and changes in gut motility (Snape et al., 1980; Loening-Baucke et al., 1989; Koch et al., 1990; Annese et al., 1997; Chrysos et al., 2001). These findings reflect both inflammation-induced changes in the responsiveness of the smooth muscle and nerves. A bidirectional interaction between the immune system, the enteric nervous system and the smooth muscle cell layers could result in structural changes with loss of functional integrity of the bowel wall.
In animal models of inflammation some studies on the structural alterations of the enteric nervous system have been performed. Sanovic et al. (1999) reported in rats with dinitrobenzene sulphonic acid (DNBS) colitis a decrease in the number of neurons in each myenteric ganglion, but no change in the number of ganglia. Furthermore, the authors provided evidence that the structural damage is compensated by axonal proliferation. Other studies reported a decrease in nitric oxide synthase-containing neurons in the myenteric plexus of rats with trinitrobenzene sulphonic acid (TNBS) and dextran sulphate sodium (DSS) colitis (Hogaboam et al., 1995; Bossone et al., 2001; Mizuta et al., 2000).
Functional studies have shown a decrease in inhibitory neural function in the intestine of nematode-infected rats (Crosthwaite et al., 1990), however enhanced inhibitory junction potentials have been reported in rabbits with experimental terminal ileitis (Goldhill et al., 1995). Electrophysiological studies confirmed altered activity of the enteric nervous system, in both the submucous and myenteric plexus (Frieling et al., 1994; Palmer et al., 1998). In these studies hyperexcitability of enteric neurons has been the predominant finding.
Colonic peristalsis is characterized by ascending contraction and descending relaxation. Next to the adrenergic neurons (which represent a minority), it has been shown that the enteric nervous system also contains inhibitory non-adrenergic non-cholinergic (NANC) neurons which provide the main inhibitory nerve supply to the gut smooth muscle. Nitric oxide (NO), vasoactive intestinal polypeptide (VIP), and adenosine triphosphate (ATP) are postulated as the main inhibitory neurotransmitters. Impaired nitric oxide mediated relaxation has recently been reported in rat colitis induced by dextran sulphate sodium (Mizuta et al., 2000).
The aim of the present study was 2 fold. Firstly, we wanted to verify the effect of TNBS-colitis not only on the nitrergic but also on the adrenergic and purinergic innervation. Secondly, we hypothesized that a possible loss of inhibitory innervation could affect the serotonergic regulation of colonic activity. 5-hydroxytryptamine (5-HT) plays a role as neurotransmitter in inhibitory motor responses (Michel et al., 1997; Graf & Sarna, 1996) and uses NO or ATP as end neurotransmitter (Briejer et al., 1995). Furthermore 5-HT is, during inflammatory conditions, released from mast cells (Wang et al., 1995). Because it is not known which 5-HT receptor subtypes exist in the rabbit colon, we investigated the effect of 5-HT1 (5-carboxamidotryptamine), 5-HT2 (α-methyl-5-HT), 5-HT3 (2-methyl-5-HT) and 5-HT4 (5-methoxytryptamine) agonists on electrical field-induced contractions in strips from the colon of a normal and inflamed rabbit. Because 5-HT4 receptors are potential therapeutic targets in constipation and irritable bowel syndrome (IBS) (Read & Gwee, 1994), the effects of inflammation on the response of 5-methoxytryptamine and the mechanisms involved, were studied in more detail.
Methods
Induction of colitis
Adult New Zealand rabbits of either sex were anaesthetized and colitis was induced by intrarectal administration of a dialysis bag filled with 135 mg kg−1 2,4,6-trinitrobenzenesulfonic acid (TNBS) in 50% ethanol for 1 h as previously described (Depoortere et al., 1999). Rabbits were sacrificed 5 days after the induction of colitis and a piece (5 cm) of colon was removed 8 cm from the rectum. This time point was chosen as it was found in a previous study that the decrease in active tension was maximal at day 5. The extent of inflammation was determined macroscopically and histologically as previously described (Depoortere et al., 1999). All procedures were approved by the Ethical Committee for Animal Experiments of the Belgian Ministry of Agriculture (approval number LA1210225).
Contractility studies
The segments were rinsed, cut open along the mesenteric border and the mucosa was carefully removed under a stereomicroscope. Strips with adhering submucosal and myenteric plexus (0.2×2.5 cm) were cut and suspended along their circular axis in a tissue bath filled with Krebs-buffer ((mM): NaCl, 120.9, NaH2PO4, 2.0 NaHCO3, 15.5 KCl, 5.9 CaCl2, 1.25 MgCl2, 1.2 glucose, 11.5) gassed with 95% O2/5% CO2. The muscle strips were brought at their optimal stretch using 10−4 M acetylcholine prior to administration of drugs. After equilibration for 60 min at optimal stretch with three wash-out periods, electrical field stimulation was applied via two parallel platinum rod electrodes using a Grass S88 stimulator (Grass, Guincy, MA, U.S.A.). Frequency spectra (0.5, 1, 2, 4, 8, 16, 32 Hz) were obtained by pulse trains (pulse 0.3 ms train 10 s, 6 V). Voltage was kept at 6V using a Med Lab Stimu-Splitter II (Med Lab, Loveland, CO, U.S.A.). Each consecutive pulse train was followed by a 90 s interval. Contractions were measured using an isometric force transducer/amplifier (Harvard Apparatus, South Natick, MA, U.S.A.), recorded on a multicorder and sampled for digital analysis using the Windaq data acquisition system and a DI-2000 PGH card (Dataq Instruments, Akron, Ohio, U.S.A.).
When a stable response was obtained at all frequencies, normal or inflamed strips were preincubated for 15 min, in the first set of experiments, with either TTX (10−6 M), L-NAME (3.10−4 M; Leclere & Levebvre, 1998), AMT (10−6 M; Ergun et al., 2001), suramin (10−4 M; Zagorodnyuk & Maggi, 1998), or guanethidine (3.10−6 M; Zagorodnyuk & Maggi, 1998) and in the second set of experiments with either 10−6 M (Prins et al., 1997) of the 5-HT1,5A,7 agonist (5-carboxamidotryptamine), the 5-HT2 agonist (α-methyl-5-HT), the 5-HT3 agonist (2-methyl-5-HT) or the 5-HT4 agonist (5-methoxytryptamine) before the stimulation was repeated. In the third sets of experiments the frequency spectrum in inflamed strips was first repeated in the presence of either the 5-HT1A antagonist, NAN-190 (10−6 M; Galligan, 1992), the 5-HT2 antagonist, ketanserin (10−6 M; Sevcik et al., 1996), the 5-HT3 antagonist ondansetron (10−6 M; Sevcik et al., 1996) or the 5-HT4 antagonist GR113808 (10−6 M; Gale et al., 1994) after a preincubation period of 15 min and then again repeated after preincubation for 15 min with the previous antagonists in the presence of the respective 5-HT agonists. In the fourth sets of experiments, EFS of normal or inflamed strips was first performed in the presence of L-NAME (3.10−1 M), suramin (10−4 M), L-NAME (3.10−4 M) plus atropine (5.10−6 M), suramin (10−4 M) plus atropine (5.10−6 M), or atropine (5.10−6 M) before the frequency spectrum was repeated with the 5-HT4 agonist, 5-MeOT (10−6 M).
Electrical field stimulation generated muscle twitch responses consisting of on- and off-responses. The present study was focused on the effect on the on-responses. The response was calculated as the mean response during the stimulation period (from integrating the area under the curve) and was expressed in g/mm2.
RT – PCR of nNOS and iNOS
The mucosa was separated from the muscle layer with adhering submucosa by blunted dissection under a stereomicroscope. Total RNA was prepared from the mucosa and the muscle layer of the colon using TRIzol reagent (Gibco BRL, NY, U.S.A.). Single-stranded cDNA was synthesized using a random hexameric primer (10 μM) and 200 units of Superscript™ II RNase H− reverse transcriptase (Gibco BRL, NY, U.S.A.). The obtained cDNA served as a template for the polymerase chain reaction, consisting of 30 (nNOS) or 32 (iNOS) cycles of amplification (95°C for 1′, 53°C (nNOS) or 58°C (iNOS) for 1′, 72°C for 1′ with a final extension duration of 10′ at 72°C) and using 1.5 units of Taq DNA polymerase (Pharmacia Biotech, Uppsala, Sweden). Primers for iNOS (forward: 5′-TGA CCA TCA TGG ACC ACC-3′, reverse: 5′-CAT GAG CAA AGG CRC AGA A-3′) and nNOS (forward: 5′-AGA CAG GCA AAT CRC AAG-3′, reverse: 5′-GGA GTC AGA RTA GGA GGA G-3′) were selected in conserved regions found after alignment of sequences published for human, rat and mouse iNOS or nNOS mRNA. For semi-quantitative assessment, primers of the rabbit housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward: 5′-TGA TCC ATT CAT TGA CCT CC-3′, reverse 5′-GTG GAT TCC ACC ACG TAC TC-3′) were added. PCR products were analysed on a 1.2% agarose gel, visualized with ethidium bromide and photographed. The density of the bands was determined by image analysis using the ImageMaster 1D software (Pharmacia Biotech, Uppsala, Sweden). The results were expressed as the ratio of intensity of the compound of interest to the intensity of the housekeeping gene.
Drugs
Suramin sodium was purchased from ICN (Costa Mesa, CA, U.S.A.), Nω-nitro-L-arginine methyl ester (L-NAME), atropine sulphate and guanethidine from Sigma (St. Louis, MO, U.S.A.). Tetrodotoxin (TTX) was obtained from Serva (Heidelberg, Germany) and 5-carboxamidotryptamine maleate (5-CT), α-methyl-5-hydroxytryptamine maleate (α-methyl-5-HT), 2-methyl-5-hydroxytryptamine maleate (2-methyl-5-HT), 5-methoxytryptamine hydrochloride (5-MeOT), 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine (AMT) and NAN-190 hydrobromide from RBI (Natick, MA, U.S.A.). Ondansetron hydrochloride dihydrate (GR38032F) and GR113808A maleate salt was obtained from Glaxo Wellcome Research and Development (Hertfordshire, U.K.). Ketanserin was from Janssen Pharmaceutica (Beerse, Belgium).
Statistical analysis
Data are represented as mean±standard error of the mean (s.e.m.). The modulation of the response to electrical field stimulation by pharmacological agents at the individual frequencies was analysed by Student's paired t-test. The effect of the 5-HT antagonists on the response of 5-HT agonists or of the nitrergic, purinergic or cholinergic blockers on the response of the 5-HT4 agonist was analysed by Student's unpaired t-test. A P value <0.05 was considered statistically significant.
Results
Effect of inflammation on electrically induced muscle contractions
In circular strips from the colon of normal rabbits electrically-evoked muscle twitch responses were frequency dependent and consisted of on- and off-responses which increased in amplitude with increasing frequency: from 0.70±0.02 g mm2 (0.5 Hz) to 3.65±0.11 g mm2 (32 Hz) for the on-responses and from 0.29±0.01 g mm2 (0.5 Hz) to 6.35±0.12 g mm2 (32 Hz) for the off-responses (Figures 1 and 2). Tetrodotoxin (1 μM) abolished all contractions over the entire frequency spectrum, demonstrating that all responses were neurogenic. In preparations from colitic rabbits, on-responses were almost completely abolished between 0.5 and 1 Hz, but increased between 8 and 16 Hz (Figures 1 and 2). Spontaneous activity was recorded in 47% of the control strips but in none of the inflamed strips.
Figure 1.
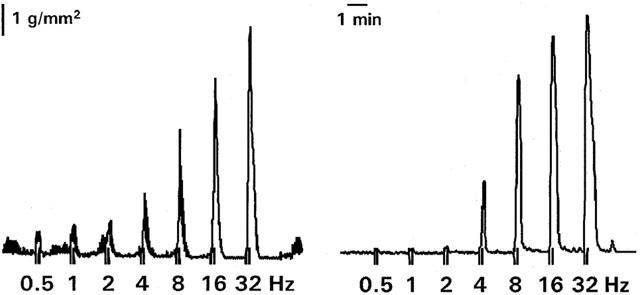
Representative tracing of muscle contractions induced by electrical stimulation (6V, 0.3 ms, 10 s) at increasing frequencies (0.5 – 32 Hz) in a strip from the colon of a normal rabbit (left) and a rabbit with TNBS-colitis (right).
Figure 2.
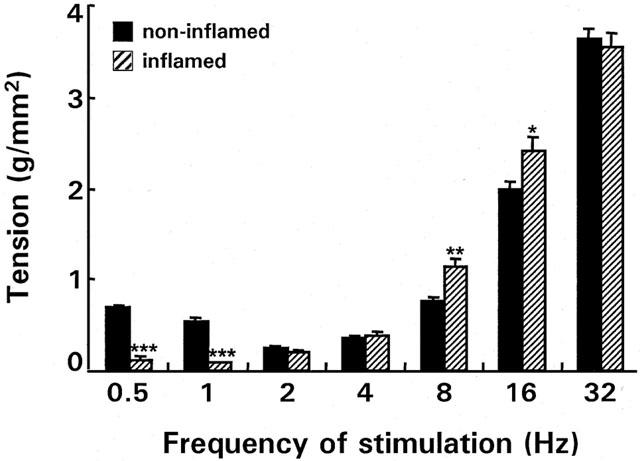
EFS-induced on-responses at different frequencies in normal and inflamed circular muscle strips from rabbit colon. The tension is expressed in (g/mm2) and is the mean (±s.e.m.) of 667 (n rabbits=71) control and 170 (n rabbits=18) inflamed strips. *P<0.05, **P<0.001, ***P<0.0001 vs control strips.
Effect of inflammation on electrically induced responses during blockade of inhibitory innervation
Preincubation of normal strips with the NO synthase blocker, L-NAME, significantly increased the EFS-induced on-responses between 1 and 32 Hz from 0.43±0.05 to 0.63±0.09 g mm2 (1 Hz) and from 2.87±0.37 to 5.81±0.50 g mm2 (32 Hz). In contrast, in inflamed strips the response was not affected by preincubation with L-NAME, indicating loss of nitrergic innervation (Figure 3).
Figure 3.
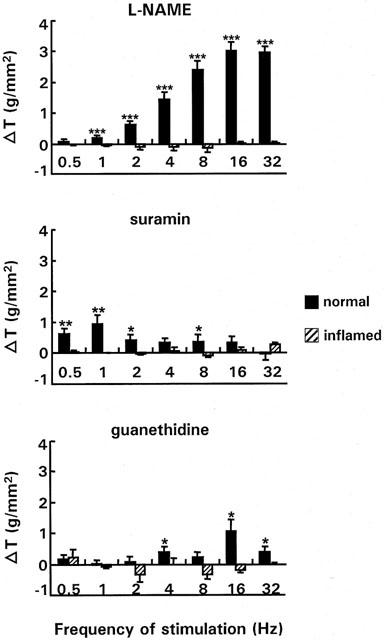
Effect of nitrergic (top), purinergic (middle) and adrenergic (bottom) blockade on the response to EFS at different frequencies in normal and inflamed strips. The strips were preincubated with either L-NAME (3.10−4 M), suramin (10−4 M) or guanethidine (3.10−6 M). Results show the change in tension (△ T) induced by the respective blockers/antagonists and are expressed in g/mm2. For L-NAME the result is the mean of 56 normal strips (n rabbits=13) and 36 inflamed strips (n rabbits=9), for suramin and guanethidine the number of strips was respectively 50 (n rabbits=18) and 31 (n rabbits=10), and 10 (n rabbits=8) and 9 (n rabbits=6). *P<0.05, **P<0.005, ***P<0.0001 indicate significant changes in tension compared to the response in the absence of antagonists/blockers.
To evaluate which isoform of NO synthase is involved, the effect of the non-selective NOS inhibitor, L-NAME, was compared with the effect of the selective iNOS inhibitor, AMT. In control strips no significant effect of AMT was observed on EFS-induced on-contractions. However in inflamed strips AMT induced a small but significant increase of the on-responses from 0.74±0.12 to 0.96±0.13 g/mm2 (P<0.005) at 16 Hz and from 1.11±0.16 to1.51±0.20 g/mm2 (P<0.0005) at 32 Hz (n=22).
In normal strips, the P2-purine receptor antagonist, suramin increased EFS-induced responses only at low frequencies (0.5-2 Hz; 8 Hz) and the effect was more pronounced than with L-NAME at 0.5 and 1 Hz. TNBS-colitis also abolished the effect of suramin (Figure 3).
In normal strips the adrenergic blocker, guanethidine, increased the EFS-response at higher frequencies (4, 16 and 32 Hz), where the effect was more pronounced than with suramin, but much smaller than with L-NAME. This effect was again abolished by inflammation (Figure 3).
nNOS and iNOS mRNA expression
To evaluate whether the loss of nitrergic innervation was due to changes in nNOS expression, semi-quantitative RT – PCR studies were performed. Figure 4 shows a representative ethidium bromide staining of the analysis of cDNA amplified for nNOS by agarose gel electrophoresis. The expression of nNOS was significantly (P<0.005) decreased with 50% (ratio nNOS/GAPDH: 0.340±0.041 (normal) (n=10) vs 0.171±0.035 (TNBS) (n=9)) in the colonic muscle layer of rabbits with TNBS-colitis. nNOS mRNA expression was not detected in the mucosa (normal: 0±0 (n=4) vs inflamed: 0.037±0.022 (n=6)). For iNOS, the ratio of the intensity of the iNOS band compared to the GAPDH band was not significantly increased in the colonic mucosa (normal: 0.197±0.039 (n=6) vs inflamed: 0.165±0.039 (n=9)) nor in the colonic muscle layer (normal: 0.079±0.028 (n=9) vs inflamed: 0.067±0.022 (n=10)).
Figure 4.
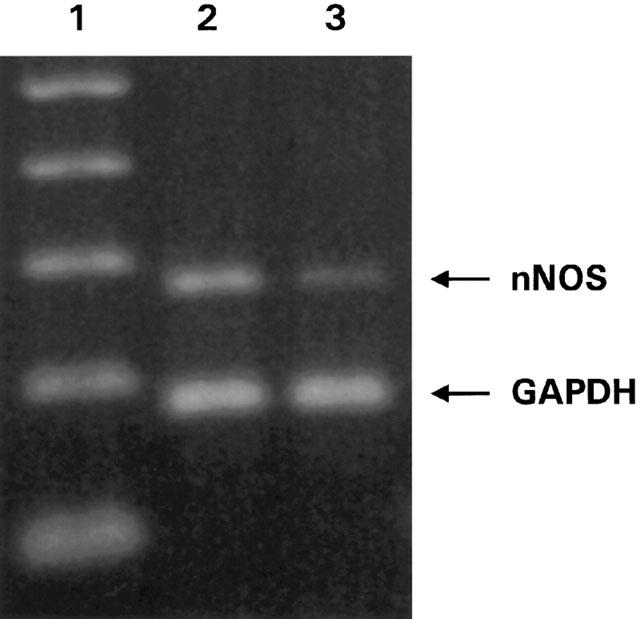
Ethidium bromide staining of agarose gel electrophoresis of the amplification products of cDNA prepared from the colonic muscle of normal (lane 2) and inflamed (lane 3) rabbits using specific primers for nNOS (277 bp) and the housekeeping gene GAPDH (195 bp). Lane 1, molecular weight marker giving bands of 100 bp spacing.
Response of 5-HT agonists in normal and inflamed strips
5-HT1/5A/7 agonist: 5-CT
The response of normal strips was increased by 5-CT at 0.5 and 1 Hz, but reduced at the higher frequencies (2 – 16 Hz). In inflamed strips 5-CT had no effect from 0.5 till 2 Hz. However at higher frequencies the responses were increased, the opposite effect of the one observed in normal strips (Figure 5). 5-CT activates a 5-HT1A receptor subtype as preincubation of inflamed strips with NAN-190 blocked completely these excitatory effects at 4, 8, and 16 Hz and strongly reduced it at 32 Hz.
Figure 5.
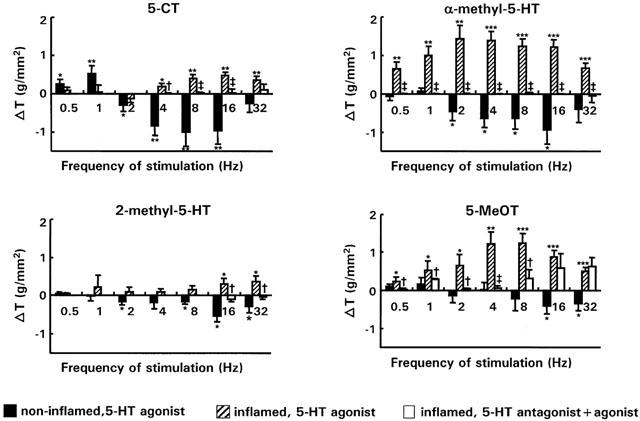
Comparison of the changes of the on-response (g/mm2) induced in normal (n strips=19; n rabbits=12) and inflamed (n strips=14; n rabbits=6) strips by the 5-HT1/5A/7 agonist (5-CT) (10−6 M), the 5-HT2 agonist (α-methyl-5-HT) (10−6 M), the 5-HT3 agonist (2-methyl-5-HT) (10−6 M) and the 5-HT4 agonist (5-MeOT) (10−6 M) over the entire frequency spectrum of stimulation. In addition the effect of the 5-HT1A antagonist NAN-190, the 5-HT2A/2C antagonist ketanserin, the 5-HT3 antagonist ondansetron and the 5-HT4 antagonist GR113808A on the response of respectively 5-CT, α-methyl-5-HT, 2-methyl-5-HT and 5-MeOT in inflamed strips (empty bars, n strips=7; n rabbits=3) is also shown. *P<0.05, **P<0.005, ***P<0.0001 versus the response in the absence of 5-HT agonists †P<0.05, ‡P<0.005 versus the response of the 5-HT agonists in inflamed strips.
5-HT2 agonist: α-methyl-5-HT
A similar, and even more pronounced phenomenon, was observed with the 5-HT2 agonist: α-methyl-5-HT. In normal strips, α-methyl-5-HT significantly reduced EFS-induced responses between 2 and 16 Hz (Figure 5), but in inflamed strips there was a marked increase over the entire frequency spectrum. The stimulatory effect of α-methyl-5-HT in inflamed strips could be blocked by the 5-HT2A/2C antagonist ketanserin.
5-HT3 agonist: 2-methyl-5-HT
The 5-HT3 agonist, 2-methyl-5-HT, had rather small effects. Still it induced small but significant inhibitory effects at 2, 8, 16 and 32 Hz in normal strips (Figure 5) and significant excitation at 16 and 32 Hz in inflamed strips, which could be blocked by the 5-HT3 antagonist, ondansetron.
5-HT4 agonist: 5-MeOT
Finally, as is shown in Figure 5, the 5-HT4 agonist 5-MeOT was, apart from some small inhibitory effects at 16 and 32 Hz, also rather ineffective in normal strips. However, in strips from rabbits with TNBS-colitis, 5-MeOT induced marked excitatory effects over the entire frequency spectrum which could be blocked by the 5-HT4 antagonist GR113808A between 0.5-8 Hz, but not at 16 and 32 Hz. This indicates that the effect of 5-MeOT at high frequencies is not attributed to interaction with 5-HT4 receptors.
Mechanism of action of the 5-HT4 agonist in normal and in inflamed strips
Preincubation of normal strips with L-NAME or suramin unmasked a pronounced excitatory effect of 5-MeOT between respectively 1 and 32 Hz and between 0.5 and 8 Hz, mimicking the effect in inflamed strips (Figure 6). Pretreatment with atropine blocked the excitatory effects of 5-MeOT observed in the presence of L-NAME between 2 and 32 Hz and of suramin between 0.5 and 8 Hz, suggesting the existence of 5-HT4 responsive neurons on cholinergic nerves (Figure 7).
Figure 6.
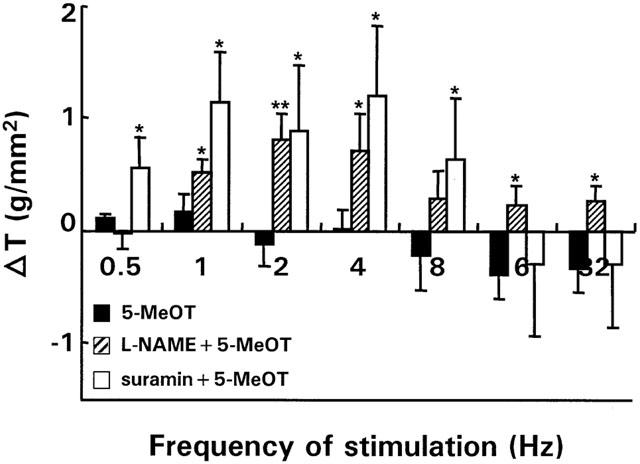
Effect of L-NAME (3.10−4 M, n strips=10; n rabbits=7) and suramin (10−4 M, n strips=9; n rabbits=6) on the on-response of normal strips in the presence of the 5-HT4 agonist, 5-MeOT (10−6 M). For comparison, the control response of 5-MeOT, which is the same as those in Figure 5 has been redrawn. Results show the change in tension (△ T) induced by the respective blockers and are expressed in g/mm2. *P<0.05, **P<0.005 versus the response of 5-MeOT in normal strips in the absence of blockers.
Figure 7.
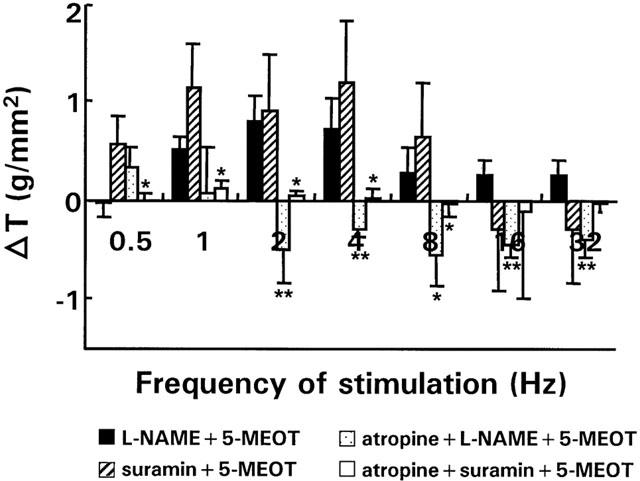
Effect of atropine (5.10−6 M, n strips=9; n rabbits=5) on the effect of 5-MeOT in the presence of 3.10−4 M L-NAME or 10−4 M suramin on EFS-induced on-responses in normal strips over the entire frequency spectrum. For comparison the effect of 5-MeOT on EFS-induced on-responses in the absence of atropine but in the presence of L-NAME or suramin is shown as well. Results show the change in tension (△ T) induced by the respective antagonists/blockers and are expressed in g/mm2. *P<0.05, **P<0.005 versus the response to 5-MeOT in the absence of atropine but in the presence of L-NAME or suramin respectively.
In accordance with the loss of inhibitory innervation observed in inflamed strips, pretreatment of inflamed strips with L-NAME or suramin did not affect the excitatory effects of 5-MeOT (Figure 8). The contractions observed with 5-MeOT in inflamed strips were cholinergic in origin as they could be blocked by atropine (Figure 8).
Figure 8.
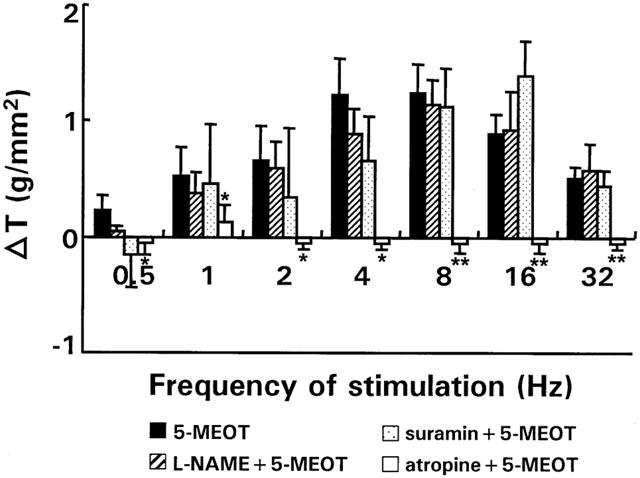
Effect of L-NAME (3.10−4 M, n strips=7; n rabbits=4), suramin (10−4 M, n strips=8; n rabbits=4) and atropine (5.10−6 M, n strips=5; n rabbits=4) on the on-response of inflamed strips in the presence of the 5-HT4 agonist, 5-MeOT (10−6 M) over the entire frequency spectrum. For comparison the effect of 5-MeOT on EFS-induced on-responses in inflamed strips in the absence of blockers is shown as well. Results show the change in tension (△ T) induced by the respective antagonists/blockers and are expressed in g/mm2. *P<0.05, **P<0.005 versus the response of 5-MeOT in inflamed strips in the absence of blockers.
Discussion
This study for the first time provides evidence that TNBS-colitis is accompanied by a generalized loss of inhibitory (nitrergic, purinergic, adrenergic) innervation. We also documented the presence of functional neural 5-HT1A, 5-HT2A/2C, 5-HT3 and 5-HT4 receptors in the rabbit colon and found that their activation produced an inhibitory effect in the normal colon. After inflammation however, the inhibitory response was reversed into excitation. For the 5-HT4 agonist, 5-MeOT, it was shown that in non-inflamed strips the cholinergic excitatory response was masked by NO and ATP. Consequently, the reversal to an excitatory response by 5-MeOT in inflamed strips could be mimicked by incubating normal strips with L-NAME or suramin. Accordingly loss of inhibitory innervation resulted in an excitatory response of 5-MeOT in inflamed strips as was confirmed by the loss of effectiveness of L-NAME and suramin.
Our study confirms earlier observations of the loss of nitrergic innervation by inflammation, and extends it to adrenergic and purinergic pathways. A reduction in inhibitory innervation was reported in the jejunum from rats infected with N. Brasiliensis (Crosthwaite et al., 1990), and loss of the nitrergic modulation of tachykinergic neutrotransmission was also reported in rabbits with ricin-induced ileitis (Goldhill et al., 1997). Recently, evidence for an impaired nitrergic innervation has been reported in rats with dextran sulphate sodium-colitis and TNBS colitis (Mizuta et al., 2000; Bossone et al., 2001). In man, it was found that the intrinsic inhibitory neural input to circular smooth muscle is reduced in Crohn's colitis and it was hypothesized that this involves alterations of intrinsic VIP-containing nerves (Koch et al., 1990). It should be mentioned that all these observations are at variance with other functional and immunohistochemical studies. Indeed Tomita et al. (1998) reported that the colon from patients with UC was more strongly innervated by NANC inhibitory nerves, with NO as major neurotransmitter, than was the normal colon and Belai et al. (1997) observed increased VIP, NOS and PACAP immunoreactivity in the myenteric plexus and nerve fibres of the circular muscle layer of the afflicted segment of the ileum of patients with Crohn's disease. The reason for this discrepancy is unclear but it would be of interest to study the role of VIP-ergic neurotransmission in our model.
By semi-quantitative RT – PCR we found that the loss of neuronal nitrergic innervation was reflected in a decreased nNOS expression, but iNOS expression was unchanged. Still, studies with the selective iNOS inhibitor, AMT, indicated that after inflammation a small part of the on-responses at high frequencies was mediated by iNOS. Miampamba & Sharkey (1999) reported that in rats with TNBS-colitis some iNOS immunoreactivity was observed in the nerve fibres of the myenteric plexus at day 7 while a more pronounced staining was apparent at day 14. Conversely, iNOS immunoreactivity dramatically increased in the mucosa and submucosa at the early stages of inflammation (day 1 – 2) and was present in monocytes and macrophages. As our study was performed at day 5 after the induction of colitis it is likely that the effect of AMT may become even more pronounced during the more chronic phase of the inflammatory process. This might also explain why RT – PCR studies did not show an increase in iNOS expression in the inflamed mucosa nor muscle layer of the colon. Similarly to our observations, Mizuta et al. (2000) did not observe any iNOS-immunopositive cells and iNOS activity in the whole muscle layer in DSS-treated rats at day 7 although the number of nNOS-immunopositive cells, catalytic activity of nNOS, and nNOS synthesis in the colonic wall were significantly reduced.
In our study it is therefore unlikely that increased levels of NO, as has been shown to occur in patients with inflammatory bowel disease and in animal models of inflammation (Rachmilewitz et al., 1995; Miller et al., 1995; Singer et al., 1996) induced by iNOS, downregulate nNOS expression. Furthermore such a mechanism does not explain the loss of purinergic and adrenergic innervation. Therefore, we hypothesize that cytokine mediated effects are involved. It has been shown that LPS or the cytokine IFN-γ can decrease the expression of nNOS in rat tissues (Bandyopadhyay et al., 1997). Also the suppression of both electrical field stimulation and KCl evoked release of [3H] noradrenaline in rats infected with T spirals can be mimicked by IL-1β and TNF-α (Hurst et al., 1993; 1994).
The major source for 5-HT is the enterochromaffin(-like) cells of the mucosa, although 5-HT is also found in the myenteric plexus in descending interneurons (Gershon, 1999). In the intestine, 5-HT released from enterochromaffin cells or mast cells has been shown to activate enteric neurons and initiate the peristaltic reflex. Briejer et al. (1995) have shown that in the guinea-pig colon, 5-HT3 and 5-HT4 receptors on the cholinergic nerves and 5-HT2A receptors on the smooth muscle mediate contraction whereas a 5-HT2-like receptor mediates relaxation using nitric oxide and ATP as end neurotransmitter. In the human colon 5-HT inhibits motility via 5-HT1-like receptors on the longitudinal muscle, whereas 5-HT4 receptors mediate inhibition of spontaneous activity and relaxation of the circular muscle (Tam et al., 1994). Recently, it was shown that in the canine colon a contractile 5-HT2A receptor exist on longitudinal muscle and an inhibitory 5-HT4 receptor on circular muscle (Prins et al., 1997; 1999).
In the present study, we document for the first time 5-HT1A, 5-HT2A, 5-HT3 and 5-HT4 receptors on neurons in the normal rabbit colon. 5-CT and α-methyl-5-HT induced pronounced whereas 2-methyl-5-HT and 5-MeOT elicited small inhibitory neural responses at high frequencies. In contrast to the situation in the normal colon, in the inflamed colon all 5-HT agonists induced cholinergic excitatory responses which were more prominent for the 5-HT2 and 5-HT4 agonists than for the 5-HT1/5A/7 and 5-HT3 agonists. Studies with the 5-HT1A antagonist, NAN-190, indicated that 5-CT activates a 5-HT1A receptor subtype. Only the response induced by 5-CT at 32 Hz could not be blocked by the antagonist. The specificity of the response induced by α-methyl-5-HT and 2-methyl-5-HT over the entire frequency spectrum was confirmed by the effect of the 5-HT2A/2C antagonist ketanserin and the 5-HT3 antagonist ondansetron which blocked the respective responses over the entire frequency spectrum. The responses induced by 5-MeOT could not be significantly blocked by the 5-HT4 antagonist GR113808A at 16 and 32 Hz indicting a possible interaction with other 5-HT receptors.
Because the 5-HT4 receptor is considered to be a good therapeutic target, the mechanism of action of the 5-HT4 agonist, 5-MeOT, was investigated in detail. In the presence of L-NAME or suramin, 5-MeOT induced atropine sensitive contractions indicating that under these conditions excitatory cholinergic influences prevail over the inhibitory ones and might override the relaxant response. However, there is also evidence that L-NAME can evoke ACh release from cholinergic excitatory nerves and enhance neurally induced contractions in smooth muscle (Baccari et al., 1997). Thus another mechanism by which 5-MeOT could reduce the amplitude of the evoked contractions would be the inhibition of ACh release via the activation of 5-HT4 responsive nitrergic neurons. In fact, the excitatory effect of 5-MeOT in inflamed strips mimicked therefore the response of 5-MeOT in normal strips in the presence of blockers/antagonists of the nitrergic/purinergic innervation. Our data therefore suggest that the loss of inhibitory innervation in the inflamed colon resulted in the shift from inhibitory to excitatory neural responses of the 5-HT4 agonist and possibly also of the other 5-HT agonists.
In conclusion, TNBS-induced colitis in rabbits induces a loss of the inhibitory nitrergic, purinergic and adrenergic innervation. What is clear from the present study is that such changes are likely to affect the response of other agents, such as those of the 5-HT4 agonist, 5-MeOT, and probably also of the 5-HT1, 5-HT2 and 5-HT3 agonists. These findings should therefore be taken into consideration in therapeutical applications.
Acknowledgments
Supported by grants from the Fund for Scientific Research – Flanders (Belgium) (FWO grant number G 0109.00) and the Belgian Ministry of Science (GOA 98/011 and IUAP P4/16). I. Depoortere is a postdoctoral research fellow of the Fund for Scientific Research – Flanders (Belgium).
Abbreviations
- α-methyl-5-HT
α-methyl-5-hydroxytryptamine maleate
- 2-methyl-5-HT
2-methyl-5-hydroxytryptamine maleate
- 5-CT
5-carboxamidotryptamine maleate
- 5-HT
5-hydroxytryptamine
- 5-MeOT
5-methoxytryptamine hydrochloride
- AMT
2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine
- EFS
electrical field stimulation
- iNOS
inducible nitric oxide synthase
- L-NAME
NG-nitro-L-arginine methyl ester
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- TNBS
2,4,6-trinitrobenzenesulphonic acid
References
- ANNESE V., BASSOTTI G., NAPOLITANO G., USAI PL., ANDRIULLI A., VANTRAPPEN G. Gastrointestinal motility disorders in patients with inactive Crohn's disease. Scand. J. Gastroenterol. 1997;32:1107–1117. doi: 10.3109/00365529709002989. [DOI] [PubMed] [Google Scholar]
- BACCARI M.C., IACOVIELLO C., CALAMAI F. Nitric oxide as modulator of cholinergic neurotransmission in gastric muscle of rabbits. Am. J. Physiol. 1997;273:G456–G463. doi: 10.1152/ajpgi.1997.273.2.G456. [DOI] [PubMed] [Google Scholar]
- BANDYOPADHYAY A., CHAKDER S., RATTAN S. Regulation of inducible and neuronal nitric oxide synthase gene expression by interferon-gamma and VIP. Am. J. Physiol. 1997;272:C1790–1797. doi: 10.1152/ajpcell.1997.272.6.C1790. [DOI] [PubMed] [Google Scholar]
- BELAI A., BOULOS P.B., ROBSON T., BURNSTOCK G. Neurochemical coding in the small intestine of patients with Crohn's disease. Gut. 1997;40:767–774. doi: 10.1136/gut.40.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BJORCK S., DAHLSTROM A., AHLMAN H. Topical treatment of ulcerative proctitis with lidocaine. Scand. J. Gastroenterol. 1989;24:1061–1072. doi: 10.3109/00365528909089256. [DOI] [PubMed] [Google Scholar]
- BOSSONE C., HOSSEINI J.M., PINEIRO-CARRERO V., SHEA-DONOHUE T. Alterations in spontaneous contractions in vitro after repeated inflammation of rat distal colon. Am. J. Physiol. 2001;280:G949–G957. doi: 10.1152/ajpgi.2001.280.5.G949. [DOI] [PubMed] [Google Scholar]
- BRIEJER M.R., AKKERMANS L.M., SCHUURKES J.A. Interactions of serotonin with multiple receptors and neurotransmitters in the guinea-pig isolated colon. Arch. Int. Pharmacodyn. Ther. 1995;329:121–133. [PubMed] [Google Scholar]
- CHRYSOS E., ATHANASAKIS E., TSIAOUSSIS J., ZORAS O., NICKOLOPOULOS A., VASSILAKIS J.S., XYNOS E. Rectoanal motility in Crohn's disease patients. Dis. Colon Rectum. 2001;44:1509–1513. doi: 10.1007/BF02234607. [DOI] [PubMed] [Google Scholar]
- CROSTHWAITE A.I., HUIZINGA J.D., FOX J.A. Jejunal circular muscle motility is decreased in nematode-infected rat. Gastroenterology. 1990;98:59–65. doi: 10.1016/0016-5085(90)91291-d. [DOI] [PubMed] [Google Scholar]
- DEPOORTERE I., VAN ASSCHE G., THIJS T., GEBOES K., PEETERS T.L. Differential changes in ACh-, motilin-, substance P-, and K(+)-induced contractility in rabbit colitis. Am. J. Physiol. 1999;277:G61–G68. doi: 10.1152/ajpgi.1999.277.1.G61. [DOI] [PubMed] [Google Scholar]
- DVORAK A.M., OSAGE J.E., MONAHAN R.A., DICKERSIN G.R. Crohn's disease: Transmission electron microscopic studies; III. Target tissues. Proliferation of and injury to smooth muscle and the autonomic nervous system. Hum. Pathol. 1980;11:620–634. doi: 10.1016/s0046-8177(80)80073-6. [DOI] [PubMed] [Google Scholar]
- DVORAK A.M., SILEN W.S. Differentiation between Crohn's disease and other inflammatory conditions by electron microscopy. Ann. Surg. 1985;201:53–63. [PMC free article] [PubMed] [Google Scholar]
- ERGUN Y., O GULENER N., DIKMEN A. Involvement of nitric oxide in non-adrenergic non-cholinergic relaxation and action of vasoactive intestinal polypeptide in circular muscle strips of the rat gastric fundus. Pharmacol. Res. 2001;4:221–228. doi: 10.1006/phrs.2001.0844. [DOI] [PubMed] [Google Scholar]
- FARTHING M.J.G., LENNARD-JONES J.E. Sensitivity of the rectum to distension and the anorectal distension reflex in ulcerative colitis. Gut. 1978;19:64–69. doi: 10.1136/gut.19.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIELING T., PALMER J.M., COOKE H.J., WOOD J.D. Neuroimmune communications in the submucous plexus of guinea pig colon after infection with Trichinella spiralis. Gastroenterology. 1994;107:1602–1609. doi: 10.1016/0016-5085(94)90798-6. [DOI] [PubMed] [Google Scholar]
- GALE J.D., GROSSMAN C.J., WHITEHEAD J.W., OXFORD A.W., BUNCE K.T., HUMPHREY P.P. GR113808: a novel, selective antagonist with high affinity at the 5-HT4 receptor. Br. J. Pharmacol. 1994;111:332–338. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLIGAN J.J. Differential inhibition of cholinergic and noncholinergic neurogenic contractions by 5-hydroxytryptamine1A receptor agonists in guinea pig ileum. J. Pharmacol. Exp. Ther. 1992;260:306–312. [PubMed] [Google Scholar]
- GEBOES K., COLLINS S. Structural abnormalities of the nervous system in Crohn's disease and ulcerative colitis. Neurogastroenterol. Motil. 1998;10:189–202. doi: 10.1046/j.1365-2982.1998.00102.x. [DOI] [PubMed] [Google Scholar]
- GERSHON M.D. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment. Pharmacol. Ther. 1999;13:15–30. [PubMed] [Google Scholar]
- GOLDHILL J.M., SANDERS K.M., SJOGREN R., SHEA-DONOHUE T. Changes in enteric neural regulation of smooth muscle in a rabbit model of small intestinal inflammation. Am. J. Physiol. 1995;268:G823–G830. doi: 10.1152/ajpgi.1995.268.5.G823. [DOI] [PubMed] [Google Scholar]
- GOLDHILL J.M., SHEA-DONOHUE T., ALI N., PINEIRO-CARRERO V.M. Tachykinergic neurotransmission is enhanced in small intestinal circular muscle in a rabbit model of inflammation. J. Pharmacol. Exp. Ther. 1997;282:1373–1378. [PubMed] [Google Scholar]
- GRAF S., SARNA S.K. 5-HT-induced jejunal motor activity: enteric locus of action and receptor subtypes. Am. J. Physiol. 1996;270:G992–G1000. doi: 10.1152/ajpgi.1996.270.6.G992. [DOI] [PubMed] [Google Scholar]
- HOGABOAM C.M., JACOBSON K., COLLINS S.M., BLENNERHASSETT M.G. The selective beneficial effects of nitric oxide inhibition in experimental colitis. Am. J. Physiol. 1995;268:G673–G684. doi: 10.1152/ajpgi.1995.268.4.G673. [DOI] [PubMed] [Google Scholar]
- HURST S., COLLINS S.M. Interleukin-1 β modulation of norepinephrine release from rat myenteric nerves. Am. J. Physiol. 1993;264:G30–G35. doi: 10.1152/ajpgi.1993.264.1.G30. [DOI] [PubMed] [Google Scholar]
- HURST S.M., COLLINS S.M. Mechanism underlying tumor necrosis factor-suppression of norepinephrine release from rat myenteric plexus. Am. J. Physiol. 1994;266:G1123–G1129. doi: 10.1152/ajpgi.1994.266.6.G1123. [DOI] [PubMed] [Google Scholar]
- KOCH T.R., CARNEY J.A., GO V.L., SZURSZEWSKI J.H. Altered inhibitory innervation of circular smooth muscle in Crohn's colitis. Association with decreased vasoactive intestinal polypeptide levels. Gastroenterology. 1990;98:1437–1444. doi: 10.1016/0016-5085(90)91073-f. [DOI] [PubMed] [Google Scholar]
- LECLERE P.G., LEFEBVRE R.A. Investigation of the interaction between cholinergic and nitrergic neurotransmission in the pig gastric fundus. Br. J. Pharmacol. 1998;125:1779–1787. doi: 10.1038/sj.bjp.0702244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOENING-BAUCKE V., METCALF A.M., SHIRAZI S. Rectosigmoid motility in patients with quiescent and active ulcerative colitis. Am. J. Gastroenterol. 1989;84:34–39. [PubMed] [Google Scholar]
- MIAMPAMBA M., SHARKEY K.A. Temporal distribution of neuronal and inducible nitric oxide synthase and nitrotyrosine during colitis in rats. Neurogastroenterol. Motil. 1999;11:193–206. doi: 10.1046/j.1365-2982.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- MICHEL K., SANN H., SCHAAF C., SCHEMANN M. Subpopulations of gastric myenteric neurons are differentially activated via distinct serotonin receptors: projection, neurochemical coding, and functional implications. J. Neurosci. 1997;17:8009–8017. doi: 10.1523/JNEUROSCI.17-20-08009.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER M.J., THOMPSON J.H., ZHANG X.J., SADOWSKA-KROWICKA H., KAKKIS J.L., MUNSHI U.K., SANDOVAL M., ROSSI J.L., ELOBY-CHILDRESS S., BECKMAN J.S., et al. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology. 1995;109:1475–1483. doi: 10.1016/0016-5085(95)90633-9. [DOI] [PubMed] [Google Scholar]
- MIZUTA Y., ISOMOTO H., TAKAHASHI T. Impaired nitrergic innervation in rat colitis induced by dextran sulfate sodium. Gastroenterology. 2000;118:714–723. doi: 10.1016/s0016-5085(00)70141-7. [DOI] [PubMed] [Google Scholar]
- PALMER J.M., WONG-RILEY M., SHARKEY K.A. Functional alterations in jejunal myenteric neurons during inflammation in nematode-infected guinea pigs. Am. J. Physiol. 1998;275:G922–G935. doi: 10.1152/ajpgi.1998.275.5.G922. [DOI] [PubMed] [Google Scholar]
- PRINS N.H., BRIEJER M.R., SCHUURKES J.A. Characterization of the contraction to 5-HT in the canine colon longitudinal muscle. Br. J. Pharmacol. 1997;120:714–720. doi: 10.1038/sj.bjp.0700954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINS N.H., VAN HASELEN J.F., LEFEBVRE R.A., BRIEJER M.R., AKKERMANS L.M., SCHUURKES J.A. Pharmacological characterization of 5-HT4 receptors mediating relaxation of canine isolated rectum circular smooth muscle. Br. J. Pharmacol. 1999;127:1431–1437. doi: 10.1038/sj.bjp.0702665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACHMILEWITZ D., STAMLER J.S., BACHWICH D., KARMELI F., ACKERMAN Z., PODOLSKY D.K. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Gut. 1995;36:718–723. doi: 10.1136/gut.36.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAO S.S.C., READ N.W., BROWN C., BRUCE C., HOLDSWORTH C.D. Studies on the mechanism of bowel disturbance in ulcerative colitis. Gastroenterology. 1987;93:934–940. doi: 10.1016/0016-5085(87)90554-3. [DOI] [PubMed] [Google Scholar]
- READ N.W., GWEE K.A. The importance of 5-hydroxytryptamine receptors in the gut. Pharmacol. Ther. 1994;62:159–173. doi: 10.1016/0163-7258(94)90009-4. [DOI] [PubMed] [Google Scholar]
- SANOVIC S., LAMB D.P., BLENNERHASSETT M.G. Damage to the enteric nervous system in experimental colitis. Am. J. Pathol. 1999;155:1051–1055. doi: 10.1016/S0002-9440(10)65207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVCIK J., RUZICKA V., SLANSKY J., MASEK K. Which type of 5-hydroxytryptamine receptor mediates relaxation of the longitudinal muscle of guinea pig proximal colon in vitro. Methods Find. Exp. Clin. Pharmacol. 1996;18:421–430. [PubMed] [Google Scholar]
- SINGER I.I., KAWKA D.W., SCOTT S., WEIDNER J.R., MUMFORD R.A., RIEHL T.E., STENSON W.F. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996;111:871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- SNAPE W.J., MATARAZZO S.A., COHEN S. Abnormal gastrocolonic response in patients with ulcerative colitis. Gut. 1980;21:392–396. doi: 10.1136/gut.21.5.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINHOFF M.M., KODNER I.J., DE SCHRIJVER-KECSKEMETI K. Axonal degeneration/necrosis. A possible ultrastructural marker for Crohn's disease. Mod. Pathol. 1988;1:182–187. [PubMed] [Google Scholar]
- TAM F.S., HILLIER K., BUNCE K.T. Characterization of the 5-hydroxytryptamine receptor type involved in inhibition of spontaneous activity of human isolated colonic circular muscle. Br. J. Pharmacol. 1994;113:143–150. doi: 10.1111/j.1476-5381.1994.tb16186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMITA R., MUNAKATA K., TANJOH K. Role of non-adrenergic non-cholinergic inhibitory nerves in the colon of patients with ulcerative colitis. J. Gastoenterol. 1998;33:48–52. doi: 10.1007/pl00009965. [DOI] [PubMed] [Google Scholar]
- WANG L., SAVEDIA S., BENJAMIN M., PERDUE M.H. The role of mast cells in intestinal immunophysiology. Adv. Exp. Med. Biol. 1995;371:287–292. doi: 10.1007/978-1-4615-1941-6_60. [DOI] [PubMed] [Google Scholar]
- ZAGORODNYUK V., MAGGI C.A. Pharmacological evidence for the existence of multiple P2 receptors in the circular muscle of guinea-pig colon. Br. J. Pharmacol. 1998;123:122–128. doi: 10.1038/sj.bjp.0701558. [DOI] [PMC free article] [PubMed] [Google Scholar]


