Abstract
The present study investigated the role of second messenger-dependent protein kinase A (PKA) and C (PKC) in the regulation of endogenous secretin receptor responsiveness in NG108-15 mouse neuroblastoma×rat glioma hybrid cells.
In whole cell cyclic AMP accumulation studies, activation of PKC either by phorbol 12-myristate 13-acetate (PMA) or by purinoceptor stimulation using uridine 5′-triphosphate (UTP) decreased secretin receptor responsiveness. PKC activation also inhibited forskolin-stimulated cyclic AMP accumulation but did not affect cyclic AMP responses mediated by the prostanoid-IP receptor agonist iloprost, or the A2 adenosine receptor agonist 5′-(N-ethylcarboxamido) adenosine (NECA).
In additivity experiments, saturating concentrations of secretin and iloprost were found to be additive in terms of cyclic AMP accumulation, whereas saturating concentrations of NECA and iloprost together were not. This suggests compartmentalization of Gs-coupling components in NG108-15 cells and possible heterologous regulation of secretin receptor responsiveness at the level of adenylyl cyclase activation.
Cells exposed to the PKA inhibitor H-89, exhibited a time-dependent increase in secretin receptor responsiveness compared to control cells. This effect was selective since cyclic AMP responses to forskolin, iloprost and NECA were not affected by H-89 treatment. Furthermore, treatment with the protein synthesis inhibitor cycloheximide produced a time-dependent increase in secretin receptor responsiveness.
Together these results indicate that endogenous secretin receptor responsiveness is regulated by PKC, PKA and protein neosynthesis in NG108-15 cells.
Keywords: Secretin receptor, protein kinase A, protein kinase C, cyclic AMP, NG108-15 cells, receptor responsiveness
Introduction
Desensitization is a ubiquitous property of G protein-coupled receptors (GPCRs) and involves loss of agonist-induced receptor responsiveness. The molecular mechansims involved in GPCR desensitization are dynamically regulated and best characterized for the class I rhodopsin/β-adrenoceptor family of GPCRs, where the importance of receptor phosphorylation has been established (Hausdorff et al., 1990). GPCR phosphorylation involves two different families of serine/threonine protein kinases, G protein receptor kinases (GRKs) and second messenger-dependent protein kinases, which dictate distinct pathways of signal termination (Bunemann & Hosey, 1999). In homologous desensitization, only the agonist-occupied receptor is phosphorylated by GRKs which facilitates the binding of arrestin proteins and endocytic internalization of the receptor (Krupnick & Benovic, 1998). In contrast, second messenger-dependent protein kinases regulate heterologous desensitization involving both active and unstimulated receptors. Protein kinases A (PKA) and C (PKC) are predominantly involved in this mode of desensitization.
The secretin receptor is a prototypic example of the structurally distinct class II subtype of GPCRs. Other members of the class II subtype include receptors for pituitary adenylate cyclase-activating polypeptide (PACAP), calcitonin, parathyroid hormone, and vasoactive intestinal hormone (Ulrich et al., 1998). There is physiological evidence that members of the secretin receptor family exhibit agonist-induced desensitization, however, the signalling processes underlying this desensitization are not clearly understood (Holtmann et al., 1996). In terms of physiological significance, the gastrointestinal hormone secretin is the principal stimulant of pancreatic and biliary bicarbonate and water secretion (Rausch et al., 1985). Secretin binding sites and secretin itself have been demonstrated in the nervous system of several mammalian species and in specific rat brain areas (Fremeau et al., 1983; Gossen et al., 1990), strongly suggesting a neuromodulatory role for secretin receptors. Secretin has also been clinically tested as a possible treatment for autism (Horvath et al., 1998). The physiological effects of secretin receptor activation are mediated through Gs and Gq-coupled signalling pathways (Ulrich et al., 1998).
Agonist-stimulated phosphorylation is likely to represent an important molecular mechanism for secretin receptor desensitization in a manner analagous to the β2-adrenoceptor (Ozcelebi et al., 1995). Both GRK- and second messenger-dependent protein kinase-mediated phosphorylation of the receptor has been observed in various recombinant systems (Shetzline et al., 1998). GRK-specific phosphorylation has been shown to correlate with rapid attenuation of secretin receptor signalling in HEK293 cells (Shetzline et al., 1998), whilst PKA-mediated phosphorylation is thought to promote agonist-stimulated internalization of secretin receptors in the same cells (Walker et al., 1999). The NG108-15 cell line endogenously expresses secretin receptors (Gossen et al., 1990) and provides a convenient system to study functional desensitization of the receptor in a physiological environment. This cell line also expresses other endogenous receptors including α2-adrenoceptors and IP-prostanoid receptors (Hamprecht, 1977). Previous studies on secretin receptor desensitization in NG108-15 cells have suggested the involvement of GRKs in homologous desensitization of the receptor. Although over-expression of GRK2 does not affect secretin receptor responsiveness (Mundell & Kelly, 1998), attenuation of secretin receptor signalling was directly related to the level of GRK6 overexpression (Willets et al., 2000). The main aim of the present study was to investigate the potential roles of PKA and PKC in heterologous desensitization of natively-expressed secretin receptors.
Methods
Materials
[8-3H]-cyclic AMP (925 Gbq mmol−1) was obtained from Amersham International plc. Cell culture medium and supplements were from GIBCO BRL. N-(2-[p-bromocinnamylamino]ethyl)-5-isoquinolinesulphonamide hydrochloride (H-89), 1-(5-isoquinolinesulphonyl)-2-methylpiperazine dihydrochloride (H-7), phorbol-12-myristate-13-acetate (PMA) and GF109203X (bisindolylmaleimide I) were obtained from Tocris Cookson. Adenosine 3′5′-cyclic monophosphorothioate, Rp-isomer (Rp-cyclic AMPS) was from Calbiochem. All other reagents and drugs were from Sigma Chemical Co.
Cell culture
NG108-15 mouse neuroblastoma×rat glioma hybrid cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 6% foetal calf serum, 100 u ml−1 penicillin and 100 μg ml−1 streptomycin. The culture medium was supplemented with 1 μM aminopterin, 100 μM hypoxanthine and 16 μM thymidine. Cells were maintained at 37°C in humidified conditions under 5% CO2.
Whole cell cyclic AMP accumulation
NG108-15 cells were seeded in Corning 24-well microtiter plates and used at >70% confluence on the experimental day. One hour before the experiment, the cell culture medium was replaced with 0.5 ml of fresh culture medium, and 15 min before agonist addition the phosphodiesterase inhibitor 4-(3-butoxy-4-methoxybenzyl)imidazolinidin-2-one (Ro201724; 250 μM) was added to each well to prevent degradation of the cyclic AMP generated during the incubation period. In pre-incubation studies, PKA inhibitors H-89 (10 μM) and H-7 (150 μM), cyclic AMP antagonist Rp-cyclic AMPS (50 μM), PKC inhibitor GF109203X (2 μM), PKC activator PMA (1 μM) and purinergic agonist uridine 5′-triphosphate (UTP; 100 μM) were added to wells 15 – 30 min prior to agonist addition. Unless drug concentrations are otherwise stated, secretin (100 nM), forskolin (10 μM), 5′-(N- ethylcarboxamido) adenosine (NECA; 10 μM), iloprost (a prostacyclin analogue; 1 μM) or vehicle was added to each well at time point 0 and the cell plate returned to the incubator. At various time points thereafter, 20 μl of 100% trichloroacetic acid was added to terminate the signalling reaction. To assay whole cell cyclic AMP accumulation in each well, 50 μl of the supernatant was added to 50 μl of 1 M NaOH and 200 μl of TE buffer (50 mM Tris, 4 mM EDTA, pH 7.4). 100 μl of this solution was then added to LP4 tubes containing 50 μl of TE buffer, 100 μl of [3H]-cyclic AMP in TE buffer (∼20,000 c.p.m.), and 100 μl of binding protein in TE buffer prepared from bovine adrenal cortex (final concentration of ∼750 μg of protein ml−1). Cyclic AMP concentrations were measured in a competition assay as previously described (Mundell & Kelly, 1998), protein content of cell monolayers determined (Bradford, 1976) and cyclic AMP accumulation expressed as pmol cyclic AMP min−1 mg−1 protein.
Intracellular calcium measurements
Intracellular calcium mobilization was measured in confluent monolayers of NG108-15 cells using the fluorescent Ca2+ dye fura-2 (Connor & Henderson, 1996). Briefly, cells were seeded onto poly-L-lysine-treated (0.1 mg ml−1) plastic slides and cultured in Leighton tubes. On the experimental day, the cells were washed three times with buffer (containing (mM): NaCl 130, KCl 5.5, CaCl2 2.5, MgCl2 1.25, HEPES 20, glucose 10, sucrose 40 and BSA 0.05%, pH 7.3) and then incubated with the acetyl methoxy-ester of fura-2 (fura-2 AM; 3 μM) for 1 h at 37°C. After fura-2 loading, the plastic slides were cut in half and half was placed on a specially constructed block fitted inside a quartz cuvette. The cuvette was placed in a LS-5B Perkin-Elmer spectrofluorimeter and perfused with buffer (4 ml min−1 at 37°C). Drugs were added directly to the perfusion buffer. The spectrofluorimeter was controlled by a Perkin-Elmer software package on a computer. The fura-2 loaded cells were alternately exposed to light at 340 and 380 nm and the emission of the cells at 510 nm was recorded. Results for intracellular calcium measurements were expressed as un-calibrated 340/380 nm ratios, since these particular experiments were undertaken to provide a qualitative insight into the Ca2+-signalling properties of agonist stimulation in NG108-15 cells.
Experimental design and statistics
Standard curve data and concentration-effect curves were fitted to logistic expressions (non-linear regression was used to fit a sigmoidal curve of variable slope), for single-site analysis using GraphPad Prism (GraphPad Software, San Diego, CA, U.S.A.). Time-course assays were constructed using point to point measurements. Results were expressed as either fold-stimulation over basal cyclic AMP accumulation or fraction of control agonist response. Where appropriate, statistical significance of different values were assessed by a one-sample t-test, paired t-test or two-way ANOVA using GraphPad Prism Software, statistically significant differences being assumed where P<0.05.
Results
Regulation of secretin receptor responsiveness by PKC
In whole cells, secretin elicited a significant enhancement in cyclic AMP generation with a 3 – 4 fold increase in accumulation being observed after 30 min of secretin stimulation (Figure 1A control). The secretin receptor appeared to exhibit rapid and complete agonist-mediated desensitization during continuous application of agonist, since cyclic AMP accumulation levelled off after 15 – 30 min of agonist stimulation (t½ 5 – 10 min). Agonist-mediated desensitization of the secretin receptor was also observed in agonist pretreatment studies; in cells which had been pretreated with secretin for 15 min, washed and then rechallenged with secretin for a further 15 min, cyclic AMP accumulation was only 49±8% that of the control non-desensitized response (n=8). The involvement of endogenous PKC activity in secretin receptor desensitization was investigated using GF109203X, an inhibitor of conventional and novel PKC isoforms (Toullec et al., 1991). Figure 1A shows that there was no difference in the time-course profile of secretin-mediated cyclic AMP accumulation in both control and GF109203X (2 μM)-treated responses. Hence, endogenous PKC activity does not appear to regulate secretin receptor responsiveness in NG108-15 cells.
Figure 1.
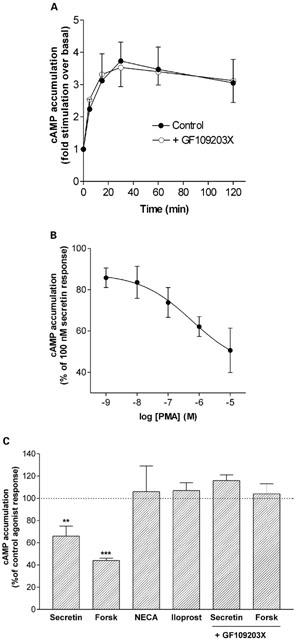
Regulation of secretin receptor responsiveness by PKC. (A) Effect of PKC inhibitor GF109203X on secretin-stimulated cyclic AMP accumulation in NG108-15 cells. Cells were incubated with or without GF109203X (2 μM) for 15 min prior to and then during addition of 100 nM secretin. (B) Concentration dependence of PMA-mediated inhibition of secretin receptor signalling. Cells were exposed to increasing concentrations of PMA for 15 min prior to and then during addition of secretin (100 nM) for 15 min. Data in parts (A) and (B) are mean±s.e.mean (bars) values from three independent experiments, each performed in triplicate. (C) Effect of PMA on cyclic AMP accumulation stimulated by secretin (100 nM), forskolin (10 μM), NECA (10 μM) and iloprost (1 μM). Cells were incubated with 1 μM PMA for 15 min prior to and then during agonist challenge at the stated concentrations for 15 min. The last two bars represent data obtained for cells which have been pretreated with 2 μM GF109203X for 15 min prior to co-addition of PMA and secretin or forskolin. Data are mean±s.e.mean (bars) from 4 – 8 independent experiments with each point performed in quadruplicate. Cyclic AMP accumulation in the presence of PMA was expressed as a per cent of the control agonist responses. **P<0.01, ***P<0.001, versus respective controls, one sample t-test. Control cyclic AMP accumulation values (pmol min−1 mg−1 protein) for basal and agonist-treated conditions were as follows: basal, 5.6±1.1; secretin, 27.7±4.3; forskolin, 157.6±12.8; NECA, 81.0±10.30; iloprost, 124.9±15.5.
Phorbol ester treatment can induce heterologous desensitization of some GPCRs via PKC activation (Sibley et al., 1987; Hipkin et al., 2000). In the present study, NG108-15 cells treated with the potent and cell-permeable phorbol ester PMA showed a concentration-dependent decrease in secretin receptor responsiveness (Figure 1B; PMA treatment did not affect basal-stimulated cyclic AMP accumulation which was 101±9% that of the control response; n=3). The selectivity of PMA was investigated by assessing the effect of PMA on cyclic AMP responses stimulated by activation of secretin and other endogenous receptors, or directly by forskolin. Pretreatment with 1 μM PMA for 15 min significantly decreased 100 nM secretin-stimulated cyclic AMP accumulation (Figure 1C). Interestingly, forskolin-stimulated cyclic AMP accumulation was also decreased in the presence of PMA. In contrast, cyclic AMP responses to 10 μM NECA (an A2 adenosine receptor agonist) or 1 μM iloprost (an IP-prostanoid receptor agonist), were unaffected by PMA treatment. Concentration-effect curves for NECA and iloprost showed no effect of PMA over a range of agonist concentrations when compared to control responses (data not shown). Thus, PMA treatment selectively inhibited secretin and forskolin responsiveness and furthermore, this inhibition was reversed upon co-addition of GF109203X indicating that PMA-induced PKC activation is the mechansim involved (Figure 1C).
Dual signalling properties of secretin receptor activation involving cyclic AMP and PLC-coupled pathways have been reported in certain cell lines (Ulrich et al., 1998). In the present study, application of secretin (100 nM) to confluent monolayers of cells did not evoke intracellular Ca2+ mobilization when assayed using fura-2 fluorescence (Figure 2A). Likewise, forskolin alone (10 μM), did not affect intracellular Ca2+ mobilization. Gq-coupled purinoceptors which couple to PKC have been characterised in NG108-15 cells (Lustig et al., 1993; Matsuoka et al., 1995), providing a receptor system for potential cross-talk with the secretin signalling pathway. In the present study, purinergic signalling in NG108-15 cells was characterized using various purinergic agonists. Purinoceptor stimulation showed a preferential activation by trinucleophosphates since application of UTP (100 μM) and ATP (100 μM) resulted in a rapid and transient elevation of intracellular Ca2+, however, Ca2+ mobilization was not evident in response to ADP (100 μM) (Figure 2B). In order to investigate potential cross-desensitization of secretin receptor responsiveness by purinoceptor activation, cells were pretreated with UTP (100 μM; 15 min) prior to agonist challenge and cyclic AMP accumulation measured. UTP treatment did not affect basal-stimulated cyclic AMP accumulation which was 98±1% that of the control response (n=7). In a manner analagous to PMA, UTP selectively attenuated secretin- and forskolin-stimulated cyclic AMP accumulation, without affecting adenosine A2 or IP-prostanoid receptor responsiveness (Figure 2C). The ability of UTP to inhibit secretin and forskolin reponsiveness was blocked by co-addition of GF109203X (Figure 2C). Thus, activation of PKC either by phorbol ester treatment or purinoceptor stimulation heterologously regulates endogenous secretin receptor responsiveness.
Figure 2.
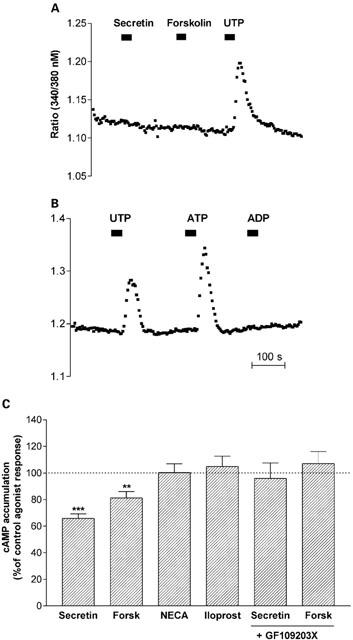
Inhibition of secretin receptor responsiveness by purinoceptor activation. (A) Secretin (100 nM) and forskolin (10 μM) do not couple to intracellular Ca2+ mobilization in NG108-15 cells. (B) Effect of purinergic agonists UTP, ATP and ADP (all at a concentration of 100 μM) on intracellular calcium mobilization. In fura-2 fluorescence studies, drugs were perfused for the duration of the bars. The data shown are representative traces from three independent experiments giving similar results, irrespective of the order of drug addition. (C) Effect of UTP (100 μM) on agonist-stimulated cyclic AMP accumulation. Cells were incubated with UTP for 15 min prior to and then during agonist challenge with secretin (100 nM), forskolin (10 μM), NECA (10 μM) or iloprost (1 μM). The last two bars represent data obtained for cells which have been pretreated with 2 μM GF109203X for 15 min prior to co-addition of UTP and secretin or forskolin. Data are mean±s.e.mean (bars) from 13 – 16 independent experiments, each performed in quadruplicate. Cyclic AMP accumulation in the presence of UTP was expressed as a per cent of the control agonist responses. **P<0.01, ***P<0.001 versus respective controls, one sample t-test.
The observation that PMA- and UTP-stimulated PKC activation selectively inhibited secretin- and forskolin- mediated responses without affecting responsiveness to adenosine A2 or IP-prostanoid receptor activation, raised the possibility that the endogenous Gs-coupled receptors may be coupled to different adenylyl cyclase isoforms. To investigate this, additivity experiments were performed whereby agonists at maximally effective concentrations were simultaneously added to cells and cyclic AMP accumulation measured. The combination of saturating concentrations of iloprost (1 μM) and NECA (80 μM) displayed no additivity in terms of cyclic AMP accumulation when compared to the control agonist responses alone (values were 27.2±2.0, 30.9±3.3 and 26.8±2.0 fold stimulation over basal for iloprost, NECA and co-addition of iloprost and NECA, respectively, n=6; Figure 3). In contrast, the action of a saturating concentration of secretin (1 μM) was significantly (P<0.05) increased in an additive fashion by co-addition of iloprost to the cells (cyclic AMP accumulation values were 10.8±2.7, 27.2±2.0 and 39±3.3 fold stimulation over basal for secretin, iloprost and co-addition of secretin and iloprost, respectively, n=6; Figure 3).
Figure 3.
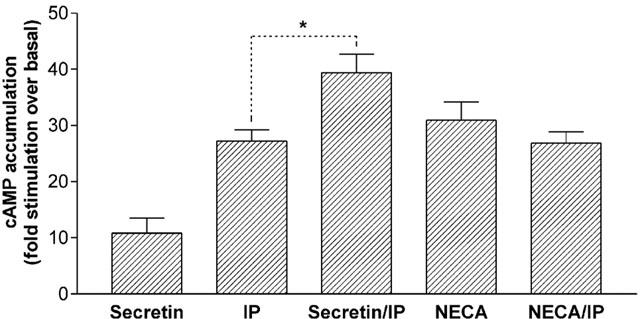
Effect of combined activation of secretin, IP-prostanoid and A2 adenosine receptors in NG108-15 cells. Cells were challenged with the indicated combinations of secretin (1 μM), iloprost (1 μM) and NECA (80 μM) for 15 min. Data are mean±s.e.mean (bars) from six separate experiments, each performed in quadruplicate. Cyclic AMP accumulation was expressed as fold-stimulation over basal. *P<0.05, indicates that co-addition of secretin and iloprost to cells results in a significantly greater cyclic AMP response compared with iloprost alone, paired t-test.
Regulation of secretin receptor responsiveness by PKA
The downstream activation of PKA arising from prolonged secretin-stimulated cyclic AMP accumulation may serve as a negative feedback mechanism to decrease receptor responsiveness. Over time, secretin-stimulated cyclic AMP accumulation was increased in the presence of the PKA inhibitor H-89 (10 μM) when compared with vehicle control (P<0.05; Figure 4A). Using a 60 min time-point of agonist stimulation, H-89 increased secretin-stimulated cyclic AMP accumulation in a concentration-dependent manner with an EC50 value of 19.8±6.1 nM (Figure 4A inset; this value is consistent with that reported for H-89-mediated inhibition of PKA activity (Chijiwa et al., 1990)). Concentration-effect curves were generated to investigate the effect of H-89 over the full range of secretin concentrations (Figure 4B). There was no difference in the EC50 value for secretin-mediated cyclic AMP accumulation between control- and H-89-treated curves (29±21 and 7±4 nM, respectively), but the maximum response to secretin was increased (P<0.05) in the presence of H-89 (Figure 4B). Treatment of the cells with another PKA inhibitor, H-7 (150 μM) and a cyclic AMP antagonist Rp-cyclic AMPS (50 μM), similarly resulted in an increase in secretin-stimulated cyclic AMP accumulation (cyclic AMP accumulation in the presence of H-7 and Rp-cyclic AMPS was 124±2 and 196±11%, that of the control secretin response, respectively; 120 min time-point, n=3 – 5).
Figure 4.
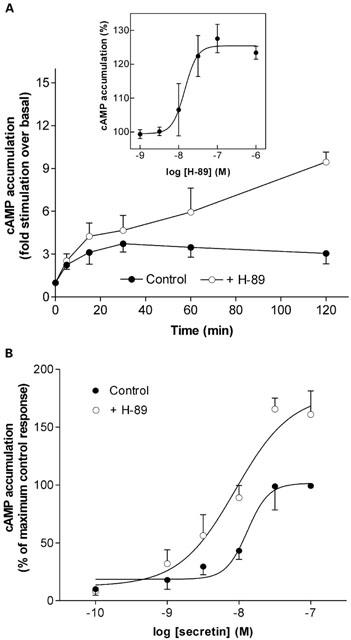
Effect of PKA inhibitor H-89 on secretin-stimulated cyclic AMP accumulation in NG108-15 cells. (A) Cells were treated with or without 10 μM H-89 for 30 min prior to and during addition of 100 nM secretin. Cyclic AMP accumulation was measured at the time-points indicated. Data are mean±s.e.mean (bars) from four separate experiments, each performed in triplicate. Over time, secretin-stimulated cyclic AMP accumulation was significantly increased in the presence of H-89 when compared with control (P=0.0003, two-way ANOVA). (Inset) Concentration-dependence of H-89-mediated increase in secretin-stimulated cyclic AMP accumulation, with calculated EC50 of 19.8±6.1 nM. Cyclic AMP accumulation was measured after 60 min of secretin stimulation and expressed as a per cent of control 100 nM secretin response. Data are mean±s.e.mean (bars) from four separate experiments, each performed in quadruplicate. (B) Cells were treated with 10 μM H-89 or vehicle for 30 min prior to co-addition of secretin at concentrations stated for 60 min. Data are mean±s.e.mean (bars) from four separate experiments, each performed in triplicate.
The selectivity of PKA in regulating adenylyl cyclase responses in NG108-15 cells was next examined. Treatment with H-89 (10 μM) enhanced secretin-stimulated cyclic AMP accumulation after 60 min of agonist stimulation to 176±21% that of the control response (Figure 5). In contrast, cyclic AMP responses stimulated by forskolin, NECA and iloprost were not affected by PKA inhibition (cyclic AMP accumulation in the presence of H-89 was 104±18, 128±15 and 118±32%, versus controls, respectively). Hence, these studies with H-89 indicate a selective role of PKA in the regulation of secretin receptor responsiveness.
Figure 5.
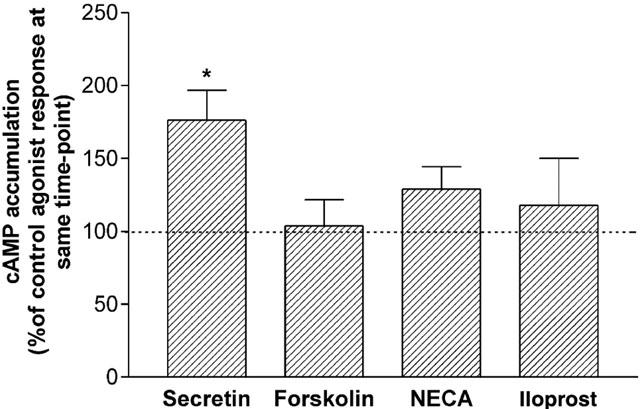
H-89 treatment selectively increases secretin receptor-mediated cyclic AMP response in NG108-15 cells. Cells were treated with 10 μM H-89 for 30 min prior to and during addition of either secretin (100 nM), forskolin, (10 μM), NECA (10 μM) or iloprost (1 μM) for 60 min. Data are mean±s.e.mean (bars) from five independent experiments, each performed in triplicate. Cyclic AMP accumulation was expressed as a per cent of the respective control agonist responses. *P<0.05, versus respective control, one sample t-test.
Involvement of protein synthesis in the secretin signalling pathway
Treatment of NG108-15 cells with the protein synthesis inhibitor cycloheximide (20 μg ml−1) led to a time-dependent increase in secretin-stimulated cyclic AMP accumulation in a manner analagous to the time-course profile of secretin-stimulated cyclic AMP accumulation in the presence of H-89 (Figure 6A). At a 60 min time-point of agonist stimulation, concentration-effect curves investigating the effect of cycloheximide over a full range of secretin concentrations exhibited no difference in the EC50 value for secretin-mediated cyclic AMP accumulation between control- and cycloheximide-treated curves (15±5 and 10±3 nM, respectively; n=4). The concentration-effect curve for secretin-mediated cyclic AMP accumulation was significantly (P<0.05) increased in the presence of cycloheximide over the range of secretin concentrations tested. After 120 min of agonist stimulation, secretin-stimulated cyclic AMP accumulation in the presence of cycloheximide was greatly enhanced to 323±61% of control (Figure 6B). Cycloheximide also increased cyclic AMP accumulation mediated by forskolin, iloprost and NECA, however these increases were much smaller than that observed with secretin (at 120 min cyclic AMP accumulation was 128±24, 176±6 and 154±25%, that of the control agonist responses for forskolin, iloprost and NECA, respectively; Figure 6B).
Figure 6.
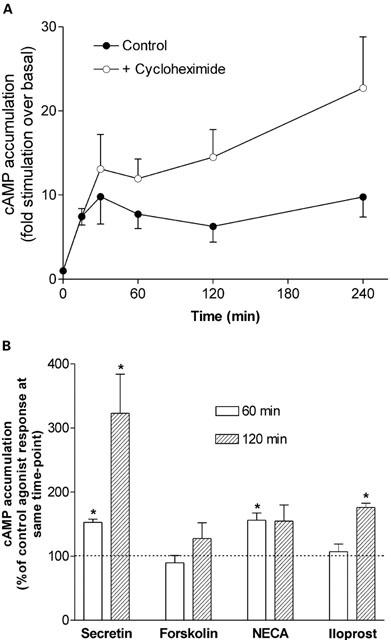
Effect of protein synthesis inhibitor cycloheximide (20 μg ml−1) on (A) time-course of secretin-stimulated (100 nM) cyclic AMP accumulation and (B) cyclic AMP accumulation stimulated by secretin (100 nM), forskolin (10 μM), NECA (10 μM) or iloprost (1 μM). Cells were treated with cycloheximide for 30 min prior to and then during agonist stimulation for the time periods specified. All data are mean±s.e.mean (bars) from at least three independent experiments, each performed in quadruplicate. (A) Over time, secretin-stimulated cyclic AMP accumulation was significantly increased in the presence of cycloheximide when compared to control (P=0.0136, two-way ANOVA). (B) Secretin-stimulated cyclic AMP accumulation was significantly enhanced at both 60 and 120 min time points of agonist stimulation in the presence of cycloheximide. *P<0.05, versus respective controls, one sample t-test.
Discussion
The present study demonstrates the involvement of both second messenger-dependent kinases PKA and PKC in distinct molecular mechanisms of endogenous secretin receptor regulation. In NG108-15 cells, the endogenous secretin receptor appears to exhibit robust agonist-induced desensitization, since secretin-stimulated cyclic AMP accumulation did not increase further after 15 min. This time frame for desensitization is consistent with that reported for the heterologously-expressed secretin receptor (Shetzline et al., 1998). However, a role for phosphodiesterase activity in contributing to the plateau in secretin receptor-stimulated cyclic AMP accumulation cannot at present be excluded. The phosphodiesterase inhibitor Ro201724 used in the experiments is a selective inhibitor of phosphodiesterase IV (IC50 2 μM; Sheppard et al., 1972), but its inclusion is necessary since it is difficult to detect secretin-stimulated cyclic AMP accumulation in the absence of the phosphodiesterase inhibitor (unpublished observations). However, phosphodiesterase activity mediated by other isoenzymes appears unlikely to play a predominant role in the waning of the secretin cyclic AMP response since we have found cyclic AMP accumulation in NG108-15 cells to increase robustly for up to an hour in the presence of forskolin and Ro201724 (Willets et al., 1999).
The potential involvement of PKC in regulating secretin receptor responsiveness was first examined using PMA, which inhibited responses to secretin and forskolin whilst having no effect on responses mediated by either NECA or iloprost. NECA is an A2 adenosine receptor agonist and PMA-induced PKC activation does not regulate A2 adenosine receptor responsiveness in NG108-15 cells (Krane et al., 1994). Instead, short-term desensitization of these receptors is thought to occur by homologous mechanisms involving GRK2 (Mundell et al., 1998). Prostanoid-IP receptors are proposed to desensitize via down-regulation of the receptor protein and Gsα from the cell surface in NG108-15 cells, without the involvement of PKC (Krane et al., 1994; Williams & Kelly, 1994). In the present study, the lack of effect of PKC activation on desensitization of A2 adenosine and prostanoid-IP receptors was therefore consistent with previous reports. The PMA-induced inhibition of the secretin signalling observed in the present study may be due to direct phosphorylation of the receptor by PKC. Phosphorylation of secretin receptors expressed in HEK293 cells following PKC activation has been reported (Shetzline et al., 1998), consistent with the existence of three consensus sites for phosphorylation by PKC within the intracellular loops of the receptor (Ulrich et al., 1998). Studies with other class II GPCRs show that PKC activation is involved in heterologous regulation of glucagon, parathyroid and PACAP-stimulated adenylyl cyclase responses (Murphy et al., 1987; Blind et al., 1996; Muller et al., 1998). In the present study, the forskolin-mediated adenylyl cyclase response was also decreased following PMA treatment suggesting that secretin receptor signalling may also be heterologously regulated by PKC-induced changes in adenylyl cyclase activity in NG108-15 cells. These cells are thought to possess predominantly the Ca2+ - inhibitable AC type VI isoform (Matsuoka et al., 1995) which can be phosphorylated and inhibited by PKC (Lai et al., 1997; 1999). PKC activation has been shown to inhibit AC type VI activity during desensitization of the A2A adenosine receptor-mediated cyclic AMP response in PC12 cells (Lai et al., 1997). That only the secretin- and forskolin-mediated responses were inhibited by PKC activation is intriguing, and suggests that the endogenous receptors may display preferential coupling to adenylyl cyclase isoforms in NG108-15 cells. Secretin- and forskolin-stimulated responses may be mediated partly through a subset of PKC-sensitive adenylyl cyclase isoforms, whereas A2 adenosine and prostanoid-IP receptor activation may signal through a pool of PKC-insensitive adenylyl cyclase isoforms (which would also be sensitive to forskolin). This possibility was supported by the additivity experiments in which the actions of secretin and iloprost were addititive in terms of cyclic AMP accumulation. Compartmentalization of Gs-coupling components has been proposed as a possible explanation for the association of adenylyl cyclase isoforms with a specific receptor in certain systems (Hanoune & Defer, 2001). Indeed recently the presence of hormone-specific subtypes of adenylyl cyclase in the rat liver, with the secretin receptor linked only to a Ca2+-calmodulin-sensitive adenylyl cyclase has been reported (Yamatani et al., 2001). Hence the PMA-induced desensitization of secretin receptor responsiveness may be due to direct phosphorylation of the receptor by PKC and/or heterologous regulation by a cognate adenylyl cyclase isoform.
The potential involvement of PKC activation in cross-desensitization of secretin receptor responsiveness was also investigated following purinoceptor activation. The Gq-coupled purinoceptors characterized in the fura-2 fluorescence studies in the present study, fit a P2-Y2-like receptor profile, consistent with previous reports on purinoceptor signalling in NG108-15 cells (Lustig et al., 1993; Matsuoka et al., 1995). In a manner analagous to PMA, the purinergic agonist UTP selectively desensitized secretin and forskolin-mediated responses, without affecting A2 adenosine or IP-prostanoid responsiveness. The cross talk between purinergic and secretin receptor signalling observed in the present study could have physiological relevance in tissues such as rat pancreatic ducts where both receptors are known to be co-expressed (Christoffersen et al., 1998).
The regulation of secretin receptor responsiveness by PKC was only evident upon exogenous stimulation of PKC by PMA or UTP, as treatment with GF109203X did not affect the profile of secretin receptor stimulation of cyclic AMP accumulation. Relative to cyclic AMP stimulation, high concentrations of secretin have been shown to stimulate inositol trisphosphate (IP3) production with subsequent activation of PKC (Trimble et al., 1987). The findings from the present study suggest that this Gq-mediated pathway is not involved in secretin signalling in NG108-15 cells, perhaps because of the relatively low levels of endogenous secretin receptor expression (Gossen et al., 1990).
In contrast to PKC, PKA appears to play an important role in modulating secretin receptor responsiveness following secretin receptor activation in NG108-15 cells. Pretreatment with the PKA inhibitors H-89 or H-7, or the cyclic AMP antagonist Rp-cyclic AMPS, resulted in enhanced cyclic AMP responses to secretin after longer time periods of agonist stimulation. As H-7 and H-89 are both isoquinoline-based PKA inhibitors, it is unclear as to why the effect of H-7 was less than that of H-89 in potentiating cyclic AMP responses to secretin. The Ki of H-7 for PKA inhibition is less than that of H-89 (3 μM and 0.0048 μM, respectively; Chijiwa et al., 1990; Quick et al., 1992), therefore we used a higher concentration of H-7 than H-89 in the present study (150 and 10 μM, respectively). One possibility is that the higher concentration of H-7 may have led to other effects on secretin receptor signalling, perhaps involving the activity of other protein kinases. The results concerning PKA inhibition differ from previous findings in HEK293 cells in which PKA did not affect secretin receptor signalling, but was instead proposed to be involved in receptor internalization (Shetzline et al., 1998; Walker et al., 1999). However, these studies used heterologously expressed secretin receptors and the authors only measured acute effects (<30 min) of H-89 treatment on secretin receptor responsiveness. In NG108-15 cells, the downstream activation of PKA arising from secretin-stimulated cyclic AMP accumulation may serve as a mechanism of negative feedback control to prevent over-stimulation of secretin signalling pathways. Feedback regulation by second-messenger kinases activated by a particular receptor is an established mechanism for desensitizing GPCRs, as first documented for the β2-adrenoceptor (Lefkowitz, 1998). It is feasible that PKA-mediated phosphorylation of the secretin receptor itself is involved in receptor desensitization since PKA-mediated phosphorylation of the secretin receptor has been demonstrated in HEK293 cells (Shetzline et al., 1998), consistent with the existence of two putative sites for phosphorylation by PKA located within the intracellular regions of the receptor (Walker et al., 1999). Also, the present study suggests that PKA is selectively regulating secretin receptor signalling at the level of the receptor itself, as adenylyl cyclase responses to forskolin, iloprost and NECA were unaffected by PKA inhibition. It has been reported that forskolin-mediated PKA activation can heterologously regulate A2 adenosine and prostanoid IP receptor desensitization, however, these effects were only observed after 17 h of forskolin treatment and were consistent with a decrease in receptor expression (Keen et al., 1992; Krane et al., 1994). As secretin partly exerts its physiological effects via stimulation of adenylyl cyclase with subsequent activation of PKA (Ulrich et al., 1998), feedback inhibition by PKA is likely to play an important role in physiological regulation of secretin receptor responsiveness.
From the present study, the precise role of PKA in regulating secretin receptor desensitization in NG108-15 cells remains unclear. Receptor desensitization by PKA may arise from direct uncoupling of the receptor-effector signalling pathway by receptor phosphorylation, promotion of receptor internalization (Walker et al., 1999), and/or an involvement of PKA in vesicular transport and resensitization (Goretzki & Mueller, 1997). As the effect of H-89 in decreasing receptor desensitization was only observed at longer time periods (>30 min) of agonist stimulation, PKA-mediated changes in protein synthesis was considered as a potential mechanism. Interestingly, treatment with the protein synthesis inhibitor cycloheximide enhanced secretin receptor responsiveness with a profile somewhat similar to that observed with PKA inhibition. This data could be interpreted to indicate that newly synthesized protein(s) may act as accessory molecules to regulate secretin receptor signalling in a PKA-dependent manner. A similar mechanism has been demonstrated for the class II PACAP receptor which undergoes agonist-mediated desensitization by processes involving PKA activation and protein neosynthesis in CATH.a cells (Muller et al., 1998). In the present study, cycloheximide also enhanced cyclic AMP responses stimulated by NECA and iloprost, but the increases were small in extent when compared to the effects of cycloheximide on secretin responsiveness. The increase in NECA and iloprost responses may reflect the effects of protein synthesis inhibition at the level of the receptors or G-proteins, since forskolin responsiveness was unchanged by cycloheximide pretreatment.
In summary, we show that endogenous secretin receptor responsiveness is modulated by PKA, PKC and protein neosynthesis. Furthermore, we present evidence that compartmentalization of GPCR-cyclic AMP signalling occurs in NG108-15 cells. Our future studies will seek to identify the molecular mechanisms underlying second messenger-dependent regulation of secretin receptor responsiveness, and in particular whether the receptor itself is subject to direct regulation by these kinases.
Acknowledgments
This work was supported by the U.K. Medical Research Council.
Abbreviations
- fura-2 AM
acetyl methoxy-ester of fura-2
- GF109203X
bisindolylmaleimide I
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- H-7
1-(5-isoquinolinesulphonyl)-2-methylpiperazine dihydrochloride
- H-89
N-(2-[p-bromocinnamylamino]ethyl)-5-isoquinolinesulphonamide hydrochloride
- NECA
5′-(N-ethylcarboxamido) adenosine
- PKA
protein kinase A
- PKC
protein kinase C
- PMA
phorbol-12-myristate-13-acetate
- Rp-cyclic AMPS
adenosine 3′5′-cyclic monophosphorothioate, Rp-isomer
- UTP
uridine 5′-triphosphate
References
- BLINDE E., BAMBINO T., HUANG Z., BLIZIOTES M., NISSENSON R.A. Phosphorylation of the cytoplasmic tail of the PTH/PTHrP receptor. J. Bone Miner. Res. 1996;11:578–586. doi: 10.1002/jbmr.5650110505. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BUNEMANN M., HOSEY M.M. G-protein coupled receptor kinases as modulators of G-protein signalling J. Physiol. 19995175–23.(Pt 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIJIWA T., MISHIMA A., HAGIWARA M., SANO M., HAYASHI K., INOUE T., NAITO K., TOSHIOKA T., HIDAKA H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- CHRISTOFFERSEN B.C., HUG M.J., NOVAK I. Different purinergic receptors lead to intracellular calcium increases in pancreatic ducts. Pflügers Arch. 1998;436:33–39. doi: 10.1007/s004240050601. [DOI] [PubMed] [Google Scholar]
- CONNOR M., HENDERSON G. Delta- and mu-opioid receptor mobilization of intracellular calcium in SH-SY5Y human neuroblastoma cells. Br. J. Pharmacol. 1996;117:333–340. doi: 10.1111/j.1476-5381.1996.tb15195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREMEAU R.T., Jr, JENSEN R.T., CHARLTON C.G., MILLER R.L., O'DONOHUE T.L., MOODY T.W. Secretin: specific binding to rat brain membranes. J. Neurosci. 1983;3:1620–1625. doi: 10.1523/JNEUROSCI.03-08-01620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORETZKI L., MUELLER B.M. Receptor-mediated endocytosis of urokinase-type plasminogen activator is regulated by cAMP-dependent protein kinase J. Cell Sci. 19971101395–1402.(Pt 12) [DOI] [PubMed] [Google Scholar]
- GOSSEN D., TASTENOY M., ROBBERECHT P., CHRISTOPHE J. Secretin receptors in the neuroglioma hybrid cell line NG108-15. Characterization and regulation of their expression. Eur. J. Biochem. 1990;193:149–154. doi: 10.1111/j.1432-1033.1990.tb19316.x. [DOI] [PubMed] [Google Scholar]
- HAMPRECHT B. Structural, electrophysiological, biochemical, and pharmacological properties of neuroblastoma-glioma cell hybrids in cell culture. Int. Rev. Cytol. 1977;49:99–170. doi: 10.1016/s0074-7696(08)61948-8. [DOI] [PubMed] [Google Scholar]
- HANOUNE J., DEFER N. Regulation and role of adenylyl cyclase isoforms. Annu. Rev. Pharmacol. Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- HAUSDORFF W.P., CARON M.G., LEFKOWITZ R.J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- HIPKIN R.W., WANG Y., SCHONBRUNN A. Protein kinase C activation stimulates the phosphorylation and internalization of the sst2A somatostatin receptor. J. Biol. Chem. 2000;275:5591–5599. doi: 10.1074/jbc.275.8.5591. [DOI] [PubMed] [Google Scholar]
- HOLTMANN M.H., ROETTGER B.F., PINON D.I., MILLER L.J. Role of receptor phosphorylation in desensitization and internalization of the secretin receptor. J. Biol. Chem. 1996;271:23566–23571. doi: 10.1074/jbc.271.38.23566. [DOI] [PubMed] [Google Scholar]
- HORVATH K., STEFANATOS G., SOKOLSKI K.N., WACHTEL R., NABORS L., TILDON J.T. Improved social and language skills after secretin administration in patients with autistic spectrum disorders. J. Assoc. Acad. Minor. Phys. 1998;9:9–15. [PubMed] [Google Scholar]
- KEEN M., KELLY E., KRANE A., AUSTIN A., WILTSHIRE R., TAYLOR N., DOCHERTY K., MACDERMOT J. Cyclic AMP produces desensitization of prostacyclin and adenosine A2 receptors in hybrid cell lines but does not affect Gs function. Biochim. Biophys. Acta. 1992;1134:157–163. doi: 10.1016/0167-4889(92)90039-e. [DOI] [PubMed] [Google Scholar]
- KRANE A., MALKHANDI J., MERCY L., KEEN M. The role of protein kinase A and protein kinase C in prostanoid IP receptor desensitization in NG108-15 cells. Biochim. Biophys. Acta. 1994;1206:203–207. doi: 10.1016/0167-4838(94)90209-7. [DOI] [PubMed] [Google Scholar]
- KRUPNICK J.G., BENOVIC J.L. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu. Rev. Pharmacol. Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- LAI H.L., LIN T.H., KAO Y.Y., LIN W.J., HWANG M.J., CHERN Y. The N terminus domain of type VI adenylyl cyclase mediates its inhibition by protein kinase C. Mol. Pharmacol. 1999;56:644–650. doi: 10.1124/mol.56.3.644. [DOI] [PubMed] [Google Scholar]
- LAI H.L., YANG T.H., MESSING R.O., CHING Y.H., LIN S.C., CHERN Y. Protein kinase C inhibits adenylyl cyclase type VI activity during desensitization of the A2a-adenosine receptor-mediated cAMP response. J. Biol. Chem. 1997;272:4970–4977. doi: 10.1074/jbc.272.8.4970. [DOI] [PubMed] [Google Scholar]
- LEFKOWITZ R.J. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J. Biol. Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- LUSTIG K.D., SHIAU A.K., BRAKE A.J., JULIUS D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUOKA I., ZHOU Q., ISHIMOTO H., NAKANISHI H. Extracellular ATP stimulates adenylyl cyclase and phospholipase C through distinct purinoceptors in NG108-15 cells. Mol. Pharmacol. 1995;47:855–862. [PubMed] [Google Scholar]
- MULLER A., LUTZ-BUCHER B., KIENLEN-CAMPARD P., KOCH B., LOEFFLER J.P. Continuous activation of pituitary adenylate cyclase-activating polypeptide receptors elicits antipodal effects on cyclic AMP and inositol phospholipid signalling pathways in CATH.a cells: role of protein synthesis and protein kinases. J. Neurochem. 1998;70:1431–1440. doi: 10.1046/j.1471-4159.1998.70041431.x. [DOI] [PubMed] [Google Scholar]
- MUNDELL S.J., KELLY E. The effect of inhibitors of receptor internalization on the desensitization and resensitization of three Gs-coupled receptor responses. Br. J. Pharmacol. 1998;125:1594–1600. doi: 10.1038/sj.bjp.0702234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNDELL S.J., LUTY J.S., WILLETS J., BENOVIC J.L., KELLY E. Enhanced expression of G protein-coupled receptor kinase 2 selectively increases the sensitivity of A2A adenosine receptors to agonist-induced desensitization. Br. J. Pharmacol. 1998;125:347–356. doi: 10.1038/sj.bjp.0702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY G.J., HRUBY V.J., TRIVEDI D., WAKELAM M.J., HOUSLAY M.D. The rapid desensitization of glucagon-stimulated adenylate cyclase is a cyclic AMP-independent process that can be mimicked by hormones which stimulate inositol phospholipid metabolism. Biochem. J. 1987;243:39–46. doi: 10.1042/bj2430039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZCELEBI F., HOLTMANN M.H., RENTSCH R.U., RAO R., MILLER L.J. Agonist-stimulated phosphorylation of the carboxyl-terminal tail of the secretin receptor. Mol. Pharmacol. 1995;48:818–824. [PubMed] [Google Scholar]
- QUICK J., WARE J.A., DRIEDGER P.E. The structure and biological activities of the widely used protein kinase inhibitor, H7, differ depending on the commercial source. Biochem. Biophys. Res. Commun. 1992;187:657–663. doi: 10.1016/0006-291x(92)91245-l. [DOI] [PubMed] [Google Scholar]
- RAUSCH U., VASILOUDES P., RUDIGER K., KERN H.F. In-vivo stimulation of rat pancreatic acinar cells by infusion of secretin. I. Changes in enzyme content, pancreatic fine structure and total rate of protein synthesis. Cell Tissue Res. 1985;242:633–639. doi: 10.1007/BF00225430. [DOI] [PubMed] [Google Scholar]
- SHEPPARD H., WIGGAN G., TSIEN W.H. Structure-activity relationships for inhibitors of phosphodiesterases from erthyrocytes and other tissues. Adv. Cyclic Nucleotide Res. 1972;1:103–112. [PubMed] [Google Scholar]
- SHETZLINE M.A., PREMONT R.T., WALKER J.K., VIGNA S.R., CARON M.G. A role for receptor kinases in the regulation of class II G protein-coupled receptors. Phosphorylation and desensitization of the secretin receptor. J. Biol. Chem. 1998;273:6756–6762. doi: 10.1074/jbc.273.12.6756. [DOI] [PubMed] [Google Scholar]
- SIBLEY D.R., BENOVIC J.L., CARON M.G., LEFKOWITZ R.J. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987;48:913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- TOULLEC D., PIANETTI P., BELLEVERGUE P., GRAND-PERRET T., AJAKANE M., BAUDET V., BOISSIN P., BOURSIER E., LORIOLLE F., DUHAMEL L., CHARON D., KIRILIVSKY J. The bisindoylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- TRIMBLE E.R., BRUZZONE R., BIDEN T.J., MEEHAN C.J., ANDREU D., MERRIFIELD R.B. Secretin stimulates cyclic AMP and inositol trisphosphate production in rat pancreatic acinar tissue by two fully independent mechanisms. Proc. Natl. Acad. Sci. U.S.A. 1987;84:3146–3150. doi: 10.1073/pnas.84.10.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ULRICH C.D., HOLTMANN M., MILLER L.J. Secretin and vasoactive intestinal peptide receptors: members of a unique family of G protein-coupled receptors. Gastroenterology. 1998;114:382–397. doi: 10.1016/s0016-5085(98)70491-3. [DOI] [PubMed] [Google Scholar]
- WALKER J.K., PREMONT R.T., BARAK L.S., CARON M.G., SHETZLINE M.A. Properties of secretin receptor internalization differ from those of the beta(2)-adrenergic receptor. J. Biol. Chem. 1999;274:31515–31523. doi: 10.1074/jbc.274.44.31515. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R.J., KELLY E. Gs alpha-dependent and -independent desensitisation of prostanoid IP receptor-activated adenylyl cyclase in NG108-15 cells. Eur. J. Pharmacol. 1994;268:177–186. doi: 10.1016/0922-4106(94)90187-2. [DOI] [PubMed] [Google Scholar]
- WILLETS J.M., BENOVIC J.L., KELLY E. GRK6 selectively regulates secretin receptor responsiveness. Br. J. Pharmacol. 2000;129:92P. doi: 10.1038/sj.bjp.0705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLETS J.M., PARENT J.-L., BENOVIC J.L., KELLY E. Selective reduction in A2 adenosine receptor desensitization following antisense-induced suppression of G protein-coupled receptor kinase 2 expression. J. Neurochem. 1999;73:1781–1789. doi: 10.1046/j.1471-4159.1999.0731781.x. [DOI] [PubMed] [Google Scholar]
- YAMATANI K., SAITO K., TAKAHASHI K., OHNUMA H., MANAKA H., SASAKI H. Hormone-specific combinations of isoforms of adenylyl cyclase and phosphodiesterase in the rat liver. Regul. Pept. 2001;99:45–52. doi: 10.1016/s0167-0115(01)00228-2. [DOI] [PubMed] [Google Scholar]


