Abstract
This paper reports on a primary cell culture system that predominately expresses native norepinephrine (NE) transporters (NETs), and is amenable to biophysical as well as biochemical analyses.
Previous research has identified human and rat placentas as rich sources of NET. We have exploited this to develop primary cultures of rat placental trophoblasts. NE uptake in these cultures is about 10 times higher when compared to 5HT uptake. The presence of NET protein is revealed by immunoblot analysis, while there is no detectable SERT protein.
NE transport in rat trophoblasts is sensitive to NET-specific antagonists, desipramine (DS) and nisoxetine (NX), but not to the dopamine-transporter (DAT) specific antagonist, GBR12909 or to the serotonin (5HT) transporter (SERT) specific antagonist paroxetine (PX). Drugs of abuse such as cocaine and amphetamine also inhibit NE transport in these cells. Together these results suggest that rat placental trophoblasts predominately express NET over other monoamine transporters.
Patch-clamp analysis reveals that NETs in intact rat trophoblasts are electrogenic. Comparison of NE uptake with NE-induced currents suggests that these two modes of transporter activity are differentially regulated.
Keywords: Rat placental trophoblasts, native norepinephrine transporter, uptake, binding, desipramine, cocaine, patch clamp, norepinephrine-induced current
Introduction
The catecholamines dopamine, norepinephrine, and epinephrine function both as neurotransmitters and hormones in their regulation of dopaminergic and adrenergic systems. Specific transport proteins, such as the NET, regulate catecholamine signalling and serve as the main clearance mechanism in central and peripheral nervous systems (Axelrod and Kopin, 1969; Iversen, 1978). NET malfunction underlies certain patho-physiological conditions, including diabetic cardiomyopathy, congestive heart failure, ischemia-induced arrhythmia and orthostatic intolerance (Ganguly et al., 1986; Meredith et al., 1993; Seyfarth et al., 1996; Shannon et al., 2000). Insulin, angiotensin II, atrial natriuretic peptide, and nitric oxide also regulate NET function (Figlewicz et al., 1993; Kaye et al., 1997; Sumners & Raizada, 1986; Vatta et al., 1993), implying an intricate connection between this transporter and other cellular complexes. Previous studies have shown that human placenta expresses both NET and SERT (Jayanthi et al., 1993; Ramamoorthy et al., 1993; 1995). Ramamoorthy et al. showed that NE uptake in brush border membrane vesicles prepared from human term placenta is more sensitive to the NET-specific inhibitor DS than DAT-specific inhibitor GBR 12909 (Ramamoorthy et al., 1993). In addition, Shearman & Meyer (1998) reported no saturable binding with the DAT-selective ligand [3H]-GBR 12935. Both of these studies suggest that NET rather than DAT might be responsible for NE transport in placenta. NE transport plays numerous roles in placental physiology and antidepressants, and drugs of abuse such as cocaine and amphetamines, that block placental NETs may adversely affect pregnancy and foetal development (Ramamoorthy et al., 1993; 1995).
Bzoskie et al. (1995) demonstrated that the placenta plays an important role in NE clearance from the foetal circulation. Furthermore, studies by Shearman et al. (Shearman & Meyer, 1998; Shearman et al., 1998) showed a greater abundance of NE transporters compared to 5HT transporters in normal rat placenta, using [3H]-NX and [3H]-PX as selective NET and SERT ligands, respectively. These studies reported a Bmax for [3H]-NX binding of 1.24 pmoles mg−1 of protein, and a Bmax for [3H]-PX binding of 96 fmoles mg−1 of protein, nearly 10 – 15 times more NX binding sites than PX binding sites in rat placenta. Autoradiographic localization also demonstrates high-affinity, radioligand binding sites for NE transporters in the placenta, and NET binding sites are up-regulated by administration of cocaine to the pregnant rat (Shearman & Meyer, 1999). The placenta elaborates NE transporters to shield the placenta from maternal catecholamines (Ramamoorthy et al., 1993; 1995; Thomas et al., 1995; Zhou et al., 1995). Lastly, genetic defects in dopamine β-hydroxylase, the enzyme that synthesizes NE from dopamine, lowers the number of viable pups, implicating trans-placental amine transport for normal foetal development (Thomas et al., 1995). These data suggest a dominant role of NE transporters in the mammalian placenta, and strongly implicate NETs in human diseases and malformations.
Complete cDNA sequences for NET have been cloned from human (Pacholczyk et al., 1991), bovine (Lingen et al., 1994), mouse (Fritz et al., 1998), and rat (Kitayama et al., 1999) sources. NETs belong to the monoamine subfamily, part of a family of neurotransmitter transporters whose original member is GAT1, a transporter for GABA (Barker & Blakely, 1995; Shafqat et al., 1993). In 1997, Padbury et al., 1997 cloned bovine placental SERT and NET and investigated the physiological significance of placental catecholamine clearance from the foetal circulation. For our studies we chose rat placenta, which belongs to a haemochorionic group similar to human placenta. To address NET function and regulation, heterologous expression systems (Apparsundaram et al., 1998a, 1998b; Galli et al., 1995; 1996) synaptosomes (Tatsumi et al., 1997), and re-sealed membrane preparations (Jayanthi et al., 1993) are used extensively. Heterologous expression systems have the advantage of working with single well-defined transporter clones amenable to molecular manipulation and biophysical characterization; however, they lack a native environment. Even synaptosomes and re-sealed membrane preparations do not retain intact cellular environments for regulation, trafficking and cellular distribution studies. Therefore we sought to culture trophoblasts from the rat placenta and to establish these cells for the functional characterization of NET. Rat trophoblasts in culture take up NE with nearly 10 times the efficiency of 5HT, and the high expression of NET in trophoblasts allowed us to explore the biochemical and biophysical properties of native NETs in a more natural environment.
Methods
Rat placental trophoblast cultures
Female Sprague-Dawley pregnant rats (Harlan, Indianapolis, Indiana, U.S.A.) of gestational period E17 were used to isolate placentas under aseptic conditions. Rats were subjected to cervical dislocation after CO2 anaesthetization to minimize stress of handling and avoid intraperitonial injections of anaesthetics, which would both stress the animal and potentially affect NET synthesis and modification in vivo. Trophoblasts were isolated from rat placentas following the protocols given for the isolation of trophoblasts from human term placentas by Kliman et al. (1986). However, several modifications were introduced in order to make the method suitable for the isolation of trophoblasts from rat placentas. Placentas were isolated from pregnant rats and the residual foetal and maternal tissues were removed from the placentas. Placental tissue was minced and digested with collagenase (Sigma, St. Louis, MO, U.S.A.) in M199 medium (GIBCO BRL Life Technologies, inc. Rockville, MD, U.S.A.) and filtered through cheesecloth. The cell suspension was centrifuged at 650×g for 5 min and the resulting pellet was resuspended in M199 medium. Trophoblasts were separated from other cells by centrifuging the cell suspension on a preformed Percoll gradient (70 to 5%) column at 1500×g for 20 min. Trophoblasts sedimented as a layer of cells at 40% Percoll were separated and cultured in Dulbeco's Modified Eagle Medium supplemented (Cellgro, Mediatech, inc. Herndon, VA, U.S.A.) with 10% foetal bovine serum, 2 mM L-glutamine and penicillin (100 u ml−1), and streptomycin (100 μg ml−1). Cells were seeded at 100,000 cells per well in 24-well cell culture plates and allowed to grow for 48 h in an atmosphere of 95% air/5% CO2 before the experiments were done. All animal procedures were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Vanderbilt University School of Medicine.
Uptake assays
NE uptake measurements were performed, as described previously (Melikian et al., 1994), in duplicate by incubating the trophoblasts for 10 min at 37°C, with [3H]-NE (Specific activity 33.0 Ci mmol−1, Amersham Pharmacia Biotech, Inc., Piscataway, New Jersey, U.S.A.) in 0.5 ml of Krebs-Ringer-HEPES (KRH) buffer pH 7.4 (mM): NaCl 120, KCl 4.7, CaCl2 2.2, HEPES 10, MgSO4 1.2, KH2PO4 1.2, Tris 5, and D-glucose 10 buffer containing ascorbic acid (100 μM), pargyline (100 μM), and U-0521 (10 μM) (Pharmacia & Upjohn: catechol-O-methyltransferase inhibitor). To examine the ion dependence of NET in trophoblasts, NE uptake was measured using [3H]-NE in three different buffers. These are (1) regular KRH buffer containing Na and Cl ions, (2) KRH buffer where all Na salts are replaced with Li salts, and (3) KRH buffer where all Cl salts are replaced with gluconate salts. [3H]-NE (50 nM) is used in all experiments except for kinetic studies, where uptake of NE was measured over a concentration of 50 nM to 5 μM NE. In kinetic experiments, concentration of radiolabelled NE was kept constant at 50 nM, and the total concentration was adjusted to desired values by adding appropriate concentrations of unlabelled NE. Specific NE uptake was measured by subtracting the NE uptake measured in the presence of 1 μM DS from total NE uptake measured in the absence of DS. Uptake of 50 nM [3H]-5HT (Specific activity 84.0 Ci mmol−1, Amersham Pharmacia Biotech, Inc., Piscataway, NJ, U.S.A.) was carried out as previously described (Jayanthi et al., 1994) using 10 nM PX to define specific 5HT uptake. Assays were terminated by removing the radiolabel and by rapid washings of cells three times with 1 ml ice-cold KRH buffer. Cells were solubilized with 1 ml of Optiphase scintillant (Wallac, Gaithersburg, MD, U.S.A.) and accumulated radioactivity quantified by direct scintillation spectrometry with a Microbeta microplate scintillation counter (Wallac). For kinetic analysis of NE uptake in trophoblasts, the values were plotted as pmoles of NE uptake versus concentration of NE and the data represent the mean±s.e.m from two experiments performed in triplicate on different batches of trophoblast cultures. Substrate Km and Vmax for NE uptake were determined by nonlinear least-square fits using the generalized Michaelis-Menten equation: V=Vmax [S]n/(Kmn+[S]n) (Kaleidagraph, Synergy Software, Reading, PA, U.S.A.), where V=transport velocity, [S]=substrate concentration (NE), and n represents the Hill coefficient fixed to 1. NE uptake was measured in the presence of DS (3 nM), NX (3 nM), GBR12909 (300 nM), PX (150 nM) and cocaine (100 nM). The above concentrations were chosen based on the previously reported IC50 values for NE uptake (Pacholczyk et al., 1991) to show that NE uptake in trophoblasts is mediated by NET and not by other monoamine transporters.
Western blotting
Western blot analysis was carried out essentially as described previously (Melikian et al., 1994). Two-day cultures of trophoblasts were washed three times in PBS/Ca-Mg before lysis in Radio Immuno Precipitation Assay (RIPA) buffer (mM): Tris 10, pH 7.4, NaCl 150, EDTA 1, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate for 1 h at 4°C with constant shaking. Solutions were supplemented with protease inhibitors (1 μg ml−1 aprotinin, 1 μg ml−1 leupeptin, 1 mg ml−1 soybean trypsin inhibitor, 1 mM iodoacetamide and 250 μM PMSF). Lysates were centrifuged at 20,000×g for 30 min at 4°C and supernatant was assayed for protein content. A total of 40 μg of the lysate was separated by SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane and immunoblotted with 0.25 μg ml−1 N430 NET antibody (provided by Dr Blakely, Vanderbilt University, Nashville, TN, U.S.A.) using 1 : 3000 goat-anti-rabbit horseradish peroxidase-conjugated secondary antibody. The experiment was repeated twice using two different preparations of trophoblast cultures. In a second set of experiments, the same amount (40 μg) of trophoblast lysate was subjected to SDS – PAGE, electroblotted on to nitrocellulose membrane and probed with SERT-specific antibody SR12 (provided by Dr Ramamoorthy, Medical University of South Carolina, Charleston, SC, U.S.A.). As a control for SERT protein identification, protein extract (40 μg) from rat brain cortex was used in a parallel lane.
Whole-cell binding experiments
Binding of NX, a specific ligand for NET, was measured using [3H]-NX and intact trophoblasts as described previously (Kitayama et al., 1999). Trophoblasts were washed with ice-cold KRH buffer and incubated with increasing concentrations (0.1 – 5 nM) of [3H]-NX in KRH buffer on ice for 2 h to allow the NX binding to plasma membrane resident NET. Initial time-course studies of [3H]-NX binding in trophoblasts showed saturation of binding by 2 h incubation (data not shown). At the end of the 2 h incubations, the cells were washed, lysed and measured for radioactivity. Specific NX binding was measured by subtracting the binding measured in the presence of 10 μM unlabelled NX from the total binding measured in the absence of unlabelled NX. Bmax and Kd values were determined by nonlinear least-squares curve-fitting of specific binding of NX, a specific ligand for NET, using the saturation equation: B=Bmax [NX]n/(Kdn+[NX]n) (Kaleidagraph, Synergy Software, Reading, PA, U.S.A.), where B is the number of moles bound, Kd is the dissociation constant, n is the Hill coefficient fixed to 1, and [NX] is the concentration of the binding ligand, [3H]-NX.
Electrophysiological recordings
Standard whole-cell patch clamp experiments (Galli et al., 1995) were performed in rat trophoblasts to study the electrical properties of these cells and the biophysical properties of native NETs. Experiments were performed at room temperature (22 – 25°C). For low noise recordings, electrodes (5 – 7 M) were pulled from borosilicate glass (Garner Glass Company; Claremont, CA, U.S.A.) with a Flaming/Brown p87 programmable puller (Sutter Instrument; San Rafael, CA, U.S.A.) and polished prior to each experiment. An Axopatch 200B amplifier (Axon Instruments; Foster City, CA, U.S.A.) was used to measure NE-induced current. Data were stored digitally on a video recorder (Panasonic AG-2510) at 15 KHz and analysed on a Nicolet 4094A oscilloscope (Nicolet Instrument Corporation; Madison, WI, U.S.A.) using instrumentation and software written by WN Goolsby. Voltage-clamp recordings were digitized using Digidata 1320A interface and pClamp software (Axon Instruments). The bath solution was grounded with a silver-chloride pellet bath electrode. The bath (control) solution was (mM): NaCl 130, KH2PO4 1.3, CaCl2 1.5, MgSO4 0.5, HEPES 10, dextrose 34 and 7.35 pH, 300 mOsm. The patch electrode solution was (mM): KCl 120, CaCl2 0.1, MgCl2 2, EGTA 1.1, HEPES 10, dextrose 30 and 7.35 pH, 270 mOsm. NE was dissolved in the bath solution and applied to the cell with a puffer pipette. To prevent oxidation or degradation of NE, solutions also contained 100 μM ascorbic acid and 100 μM pargyline. To confirm the transporter specificity of the NE-induced currents, recordings were performed in the absence and presence of DS.
Results
Rat trophoblasts express NET over SERT
The typical morphologies of 2-day cultures of rat trophoblasts are shown in Figure 1A. The multinucleated giant trophoblasts along with small trophoblasts were seen along the varicosities. The cultures were assayed for both 5HT and NE uptake using 50 nM 5-hydroxy [3H]-tryptamine ([3H]-5HT) and 50 nM 1-[7,8-3H]-noradrenaline ([3H]-NE), respectively. 5HT uptake was measured in the presence or absence of 10 nM PX; NE uptake was measured in the presence or absence of 1 μM DS. Uptake measured in pmol (106 cells)−1 10 min−1 showed that NE uptake was about 10 times higher compared to 5HT uptake (Figure 1B). Immuno-blot analysis of protein extract (40 μg) from trophoblast cultures revealed the presence of NET protein as a major protein band corresponding to ∼85 kD, which corresponds to the reported human NET (hNET) protein (Galli et al., 1995) (Figure 1C). There was no detectable level of SERT protein when the same amount of protein (40 μg) from trophoblast extract was analysed using SERT-specific antibody SR-12. SR12 antibody specifically recognizes SERT protein corresponding to a ∼76 kD protein (Qian et al., 1997) from 40 μg of protein extract of rat brain cortex (Figure 1D). These results suggest that rat placental trophoblasts predominantly express NET protein over SERT.
Figure 1.
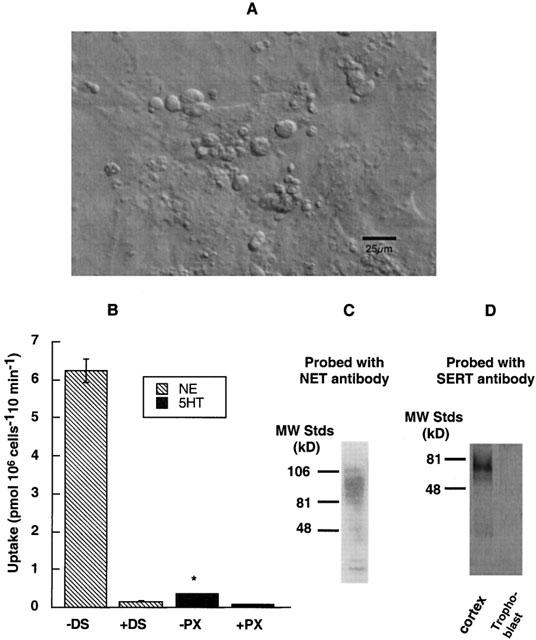
Trophoblast cultures and norepinephrine transport (A) Images of trophoblasts in tissue culture: The live cell images of 2-day cultures of rat placental trophoblasts are captured using Zeiss MC 100 35-mm camera system. The multinucleated giant trophoblasts along with small trophoblasts are seen along the varicosities. Bar, 2 μm. (B) Uptake of NE and 5HT by trophoblasts: Uptake of 50 nM [3H]-NE in the presence or absence of 1 μM DS, and uptake of 50 nM [3H]-5HT in the presence or absence of 10 nM paroxetine were measured during a 10 min incubation time in KRH buffer at 37°C. Specific NE was measured by subtracting NE uptake in the presence of 1 μM DS from total uptake in the absence of DS. Specific 5HT uptake was obtained in the same way using 10 nM PX. Results are given in pmoles of NE or 5HT pmol (106 cells)−1 10 min. Specific NE uptake by trophoblasts is ∼10 times higher than specific 5HT uptake. The data represent the mean±s.e.m. from two experiments carried out in triplicate using different trophoblast cultures. Asterisk denotes significant difference of PX-sensitive 5HT uptake as compared to DS-sensitive NE uptake, P<0.05 (Student's t-test and ANOVA). (C) Identification of NET protein: A heavily glycosylated protein band (∼85 kD) corresponding to NET is observed when trophoblast lysates (40 μg of protein) were run on SDS – PAGE and probed with NET-specific antibody N430. (D) Absence of SERT protein: There was no detectable level of SERT protein when same amount of protein (40 μg) from trophoblast lysate was analysed using SERT-specific antibody SR-12. SR12 antibody specifically recognizes a ∼76 kD protein band corresponding to SERT from 40 μg of protein extract of rat brain cortex.
NE uptake in trophoblasts depends on Na and Cl and is sensitive to NET-specific antagonists
NE uptake showed a monotonic increase for 30 min and started to plateau after this time point (Figure 2A). DS-sensitive NE uptake was Na- and Cl-dependent; removal of either ion abolished NE transport (Figure 2B). In Figure 2C, uptake was measured using 50 nM [3H]-NE as the substrate in the presence/absence of indicated drugs in KRH buffer. Two experiments carried out in triplicate are represented as percentage of NE uptake measured in the absence of transporter inhibitors. NET-specific antagonists, DS and NX were potent inhibitors of NE uptake in trophoblasts. At 3 nM concentration both DS and NX inhibited more than 50% of normal NE uptake. DAT-specific antagonist GBR12909 and SERT-specific antagonist PX were less potent inhibitors of NE uptake in trophoblasts. GBR12909 at 300 nM and PX at 150 nM inhibited 50% of NE uptake in trophoblasts. Cocaine at 100 nM inhibited ∼50% of NE uptake. These data suggest that NE uptake in rat trophoblasts is NET-specific.
Figure 2.
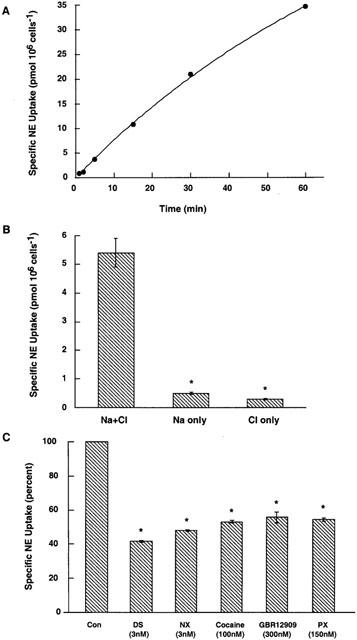
NE uptake in trophoblasts is NET mediated. (A) Time-dependent uptake: Uptake of 50 nM [3H]-NE in the presence or absence of 1 μM DS was measured at different time periods for 60 min. Specific NE uptake was measured by subtracting NE uptake measured in the presence of 1 μM DS from NE uptake measured in the absence of DS. Data are represented in pmol (106 cells)−1. Specific NE uptake plotted against time showed a monotonic relationship. The data represent the mean±s.e.m. from two experiments carried out in triplicate using different trophoblast cultures. (B) Ion-dependent uptake: NE uptake, given as pmol (106 cells)−1 10 min was measured in the presence of Na and Cl ions, or in the presence of Na only or Cl only as described in panel A. Transport of NE by trophoblasts requires both Na and Cl ions. The data represent the mean±s.e.m. from two different experiments carried out in triplicate on different trophoblast cultures. Asterisks denote significant difference of DS-sensitive NE uptake in the absence of Na and Cl ions as compared to DS-sensitive NE uptake in the presence of both the ions, P<0.05 (Student's t-test and ANOVA). (C) Inhibitor sensitivity of NE uptake: Inhibitor potencies were estimated in uptake assays performed using 50 nM of [3H]-NE with indicated concentrations of inhibitors for monoamine transporters. We chose concentrations of inhibitors near their IC50 values reported previously for NET. Data are expressed as percentage of NE accumulated in the absence of inhibitors. The data represent the mean±s.e.m. from two different experiments carried out in triplicate using different trophoblast cultures. Asterisks denote significant difference of DS-sensitive NE uptake in the presence of inhibitors as compared to control NE uptake in their absence, P<0.05 (Student's t-test and ANOVA).
NE uptake in rat trophoblasts is [NE] dependent
Nonlinear least-squares curve-fitting of saturation data was achieved for the generalized Michaelis – Menten equation. NE transport in trophoblasts obeyed saturation kinetics with a Km of 2.4±0.3 μM and a Vmax of 279.20±18.11 pmol (106 cells)−1 10 min−1 (R=0.999) (Figure 3A). We obtained a Hill coefficient of 0.803 when data were fit to either a single-site or a two-site model. Linear fitting of the uptake data was consistent with a single population of NE uptake sites (R=0.980) (Figure 3B).
Figure 3.
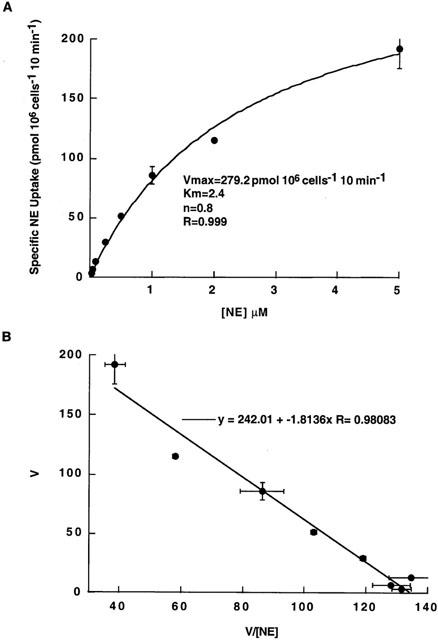
NE uptake kinetics in trophoblasts. (A) Concentration-dependent uptake: NE uptake was measured from 0.05 to 5 μM during 10 min incubation in KRH buffer at 37°C. We kept the concentration of radiolabelled NE constant at 50 nM and adjusted the total concentration by adding unlabelled NE. Specific NE uptake was calculated by subtracting NE uptake measured in the presence of 1 μM DS from NE uptake measured in the absence of DS. NE transport in trophoblasts is saturable with a Km=2.4±0.3 μM and Vmax=279.20±18.11 pmol (106 cells)−1 10 min. The R value (0.999) shows the goodness-of fit. We obtained a Hill coefficient of 0.803 when data were fit to either a single-site or a two-site model. (B) Eadie – Hofstee plot: The data from panel A are plotted as NE uptake velocity (V) versus V/[NE]. The Eadie – Hofstee plot was linear and the R value (0.980) shows the goodness-of fit. The data represent the mean±s.e.m. from two experiments carried out in triplicate using different trophoblast cultures.
Equilibrium binding of NX to trophoblast NETs
Nonlinear least-squares curve-fitting of binding data was achieved for the saturation equation. NX binding saturated with a Kd value of 1.15±0.2 nM and a Bmax of 0.43±0.03 pmol (106 cells)−1 (R=0.995) (Figure 4A). We obtained a Hill coefficient of 0.789 when binding data were fit to either a single-site or a two-site model. Linear fit to a Rosenthal transformation of the binding data was consistent with a single population of NX binding sites (R=0.942) (Figure 4B).
Figure 4.
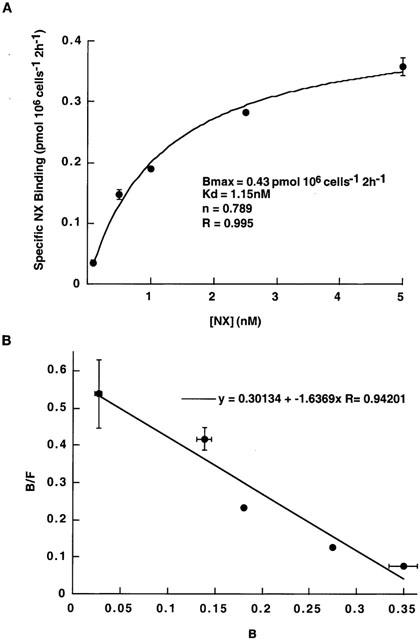
NX binding kinetics in trophoblasts. (A) Equilibrium binding of [3H]-NX: Binding of [3H]-NX was done by incubating intact trophoblasts with increasing concentrations (0.1 – 5 nM) of [3H]-NX in KRH buffer on ice for 2 h. Nonspecific binding was obtained using 10 μM unlabelled NX, and this was subtracted from total binding. NX binding shows saturable kinetics with a Kd=1.15±0.2 nM and a Bmax=0.43±0.03 pmol (106 cells)−1. The R value (0.995) shows the goodness-of fit. We obtained a Hill coefficient of 0.789 when binding data were fit to either a single-site or a two-site model. (B) Scatchard analysis of NX binding: A linear fit to the Rosenthal transformation of the binding shows a single population of NX binding sites. The R value (0.942) shows the goodness-of fit. The data represent the mean±s.e.m. from two experiments performed in triplicate on different batches of trophoblast cultures.
NE-induced currents in placental trophoblasts
To study the electrical properties of rat trophoblasts, we recorded the current – voltage relationships and compared I(V)s in cells perfused with the control solution (physiological saline), NE-containing solutions, or NE plus DS. The current (I) at the test potential (V) was obtained at the end of 500 ms test voltages. The I – V relationships varied within the same batch and fell into two categories: approximately linear I(V) curves with a reversal potential near −50 mV (Figure 5A), and monotonically increasing I(V) curves with a reversal near −10 mV (Figure 5B). Although we were able to record pharmacologically defined NE-induced currents in 15% of the cells in a particular dish, there was no pattern for the prevalence of type A or B. Other cells may have similar currents below background, which we were unable to detect. To study NET-associated currents, the membrane potential was stepped from −40 to −80 or −120 mV for 500 ms. For each cell, 3 – 5 such protocols were performed in control solutions and then the solution was changed to one containing 30 μM NE and the protocols were repeated. Following these recordings, we repeated the experiment in the presence of 30 μM NE+2 or 30 μM DS. Only cells that had control currents less than 50 pA at −40 mV and showed no change during the course of the control experiment before adding NE were analysed (n=26). Of this set, NE-induced currents >1 pA at −40 mV were observed in four cells. Figure 5C shows the effect of NE at −80 and −120 mV. Compared to control, 30 μM NE-induced 5 – 9 pA at −80 and −120 mV, respectively. The NE-induced current was blocked by 30 μM DS. For the cells that responded, we normalized to control at −40 mV and averaged the NE and NE+DS response (Figure 5D). On average, NE application induced a 7% increase in the current magnitude at −80 mV and a 12% increase at −120 mV. Inhibition of NET with DS blocked 75% of the NE-induced increase in current amplitude at −120 mV (Figure 5D). These data indicate that activation of NET in rat trophoblasts is electrogenic, but that the expression of measurable NE-induced current occurs in only one in six cells on average.
Figure 5.
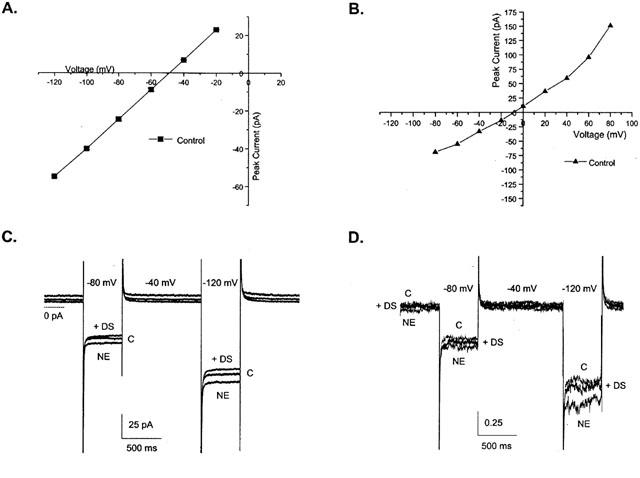
NE induces currents mediated by NET in rat trophoblast cells. (A) I(V) relationship for rat trophoblast perfused with control solution. The current is obtained at the end of each 500 ms test potential between −120 and −20 mV. The I(V) curve shows a reversal potential of −49 mV (type A). (B) I(V) relationship for a second category of rat trophoblast: The current is obtained at the end of each of the 500 ms test potentials. The I(V) curve between −80 and +80 mV for a second category of trophoblast shows a reversal potential of −9 mV (type B). (C) NE-induced currents in rat trophoblasts: Compared to control, 30 μM NE induces a current 8 or 5 pA at −120 and −80 mV respectively. The NE-induced current was blocked by concomitant application of 30 μM NE plus 30 μM DS, indicating that NET mediated the NE-induced currents observed. (D) Average effect of NE on the current response obtained from three trophoblasts. For cells that showed NE-induced currents >1 pA, the responses in the presence of NE or NE+DS were normalized to the leak at −40 mV under control conditions and averaged. On average, NE induced a 5, 7 and 12% increase in the current magnitude at −40, −80 and −120 mV respectively. Specific inhibition of NET with DS blocked 60, 57 and 75% of the NE-induced increase in current amplitude at −40, −80 and −120 mV, respectively.
Discussion
Since the cloning of human NET (Pacholczyk et al., 1991), hNET has been studied extensively using heterologously expression systems (Apparsundaram et al., 1998a, 1998b; Galli et al., 1995; 1996; 1998). Kitayama et al. (1999) recently cloned and characterized rNET from rat brain. We (Jayanthi et al., 1993; Ramamoorthy et al., 1995), and other investigators (Ramamoorthy et al., 1993; Shearman & Meyer, 1998; Shearman et al., 1998) have demonstrated the presence of SERT and NET in human and rat placentas. In the present study, we developed a method to culture trophoblasts from rat placenta, and to characterize these cells with regard to NE uptake, nisoxetine binding, and electrogenic properties. Trophoblast cultures exhibit DS-sensitive NE uptake many times higher than PX-sensitive 5HT uptake, therefore providing us a suitable model to study NET in a native membrane. Immuno-blots revealed abundant expression of rNET protein, which corresponds to the reported hNET protein in transfected cells. NE transport in trophoblasts increases over time and requires both Na and Cl ions, which is a hallmark for NE transport mediated by NET. NET-specific inhibitors, both DS and NX were very potent in inhibiting NE uptake in trophoblasts. DAT-specific antagonist GBR12909 and SERT-specific antagonist PX were less potent inhibitors of NE uptake in trophoblasts. Inhibitor sensitivity reported for NE transport system in human placental brush border membrane vesicles (Ramamoorthy et al., 1993) also indicates that NET-specific inhibitor DS is more potent (IC50=0.45 μM) than DAT-specific inhibitor GBR 12909 (10 μM). However, the reported IC50 values for NE uptake in membrane vesicles (Ramamoorthy et al., 1993) are higher (in higher nM to μM) compared to intact cellular systems (Pacholczyk et al., 1991). Kinetic analysis revealed Km and Vmax values similar to those reported by Kitayama et al. (1999). The kinetic parameters from NX binding experiments reveal a high-affinity single binding site for NET. When our data were fitted to a single binding site, we obtained a Bmax value of 0.43 pmol (106 cells)−1 for rat placental NET.
The present study suggests the transporter turnover rate:
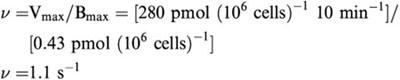 |
Assuming NET stoichiometry of 1NE+: 1Na+: 1Cl− would yield a 1 electronic charge unit (e) per turnover and the net current cell−1
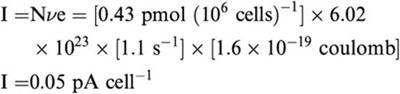 |
The rNET currents reported here in a minority of cells (∼10 pA for one in six cells) are thus orders of magnitude larger than expected from this simple model of transport. We do not report cells with currents less that 1 pA, but it is possible that in these cells, too, NE-induced currents exceed the predicted 0.05 pA. Because we are dealing with average Vmax and Bmax values, it remains to be ascertained whether the large currents observed in a minority of cells are due to expression levels or differential extrinsic regulation of uptake and current. These calculations suggest that NE-induced currents with channel-like properties are similar to those observed in HEK-293 cells expressing hNET (∼50 pA). In both the heterologously expressed and native NET preparations, NE-induced currents are blocked by DS and have similar I(V) curves, suggesting that currents in trophoblasts (Figure 5) are similar to HEK-293 cells expressing hNET. These data provide the first evidence for the NET-associated currents in a native cell, and they characterize placental trophoblasts as a useful model system to study the biochemical and biophysical properties of native NETs.
Acknowledgments
This work was supported by NARSAD Young Investigator award and Medical University of South Carolina (L.D. Jayanthi), a Ford Foundation Award (G. Vargas), and a National Institutes of Health Grant NS-34075. We wish to thank Dr Randy Blakely at Vanderbilt University, Nashville, TN, U.S.A. for providing the NET antibody for immunoblotting and for useful discussions through this work. We also thank Dr Sammanda Ramamoorthy at Medical University of South Carolina, Charleston, SC, U.S.A. for providing SERT antibody.
Abbreviations
- DAT
dopamine transporter
- DS
desipramine
- NE
norepinephrine
- NET
norepinephrine transporter
- NX
nisoxetine
- PX
paroxetine
- SERT
serotonin transporter
References
- APPARSUNDARAM S., GALLI A., DEFELICE L.J., HARTZELL H.C., BLAKELY R.D. Acute regulation of norepinephrine transport: I. PKC-linked muscarinic receptors influence transport capacity and transporter density in SK-N-SH cells. J. Pharmacol. Exp. Ther. 1998a;287:733–743. [PubMed] [Google Scholar]
- APPARSUNDARAM S., SCHROETER S., BLAKELY R.D. Acute regulation of norepinephrine transport. II. PKC-modulated surface expression of human norepinephrine transporter proteins. J. Pharmacol. Exp. Ther. 1998b;287:744–751. [PubMed] [Google Scholar]
- AXELROD J., KOPIN I.J. The uptake, storage, release, and metabolism of noradrenaline in sympathetic nerves. Prog. Brain Res. 1969;31:21–32. doi: 10.1016/S0079-6123(08)63224-0. [DOI] [PubMed] [Google Scholar]
- BARKER E.L., BLAKELY R.D. Norepinephrine and serotonin transporters: Molecular targets of antidepressant drugs Psychopharmacology: The Fourth Generation of Progress 1995New York: Raven Press; 321–333.ed. Bloom, F.E. & Kupfer, D.J. pp [Google Scholar]
- BZOSKIE L., BLOUNT L., KASHIWAI K., TSENG Y., HAY W.W., PADBURY J.F. Placental norepinephrine clearance: In vivo measurement and physiological role. The American Physiological Society. 1995. pp. E145–E149. [DOI] [PubMed]
- FIGLEWICZ D.P., SZOT P., ISRAEL P.A., PAYNE C., DORSA D.M. Insulin reduces norepinephrine transporter mRNA in vivo in rat locus coeruleus. Brain Res. 1993;602:161–164. doi: 10.1016/0006-8993(93)90258-o. [DOI] [PubMed] [Google Scholar]
- FRITZ J., LANKUPALLE J., THORESON M., BLAKELY R.D. Cloning and chromosomal mapping of the murine norepinephrine transporter. J. Neurochem. 1998;70:2241–2251. doi: 10.1046/j.1471-4159.1998.70062241.x. [DOI] [PubMed] [Google Scholar]
- GALLI A., BLAKELY R.D., DEFELICE L.J. Norepinephrine transporters have channel modes of conduction. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8671–8676. doi: 10.1073/pnas.93.16.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLI A., BLAKELY R.D., DEFELICE L.J. Patch-clamp and amperometric recordings from norepinephrine transporters: Channel activity and voltage-dependent uptake. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13260–13265. doi: 10.1073/pnas.95.22.13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLI A., DEFELICE L.J., DUKE B.J., MOORE K.R., BLAKELY R.D. Sodium-dependent norepinephrine-induced currents in norepinephrine transporter transfected HEK-293 cells blocked by cocaine and antidepressants. J. Exp. Biol. 1995;198:2197–2212. doi: 10.1242/jeb.198.10.2197. [DOI] [PubMed] [Google Scholar]
- GANGULY P.K., DHALLA K.S., INNES I.R., BEAMISH R.E., DHALL N.S. Altered norepinephrine turnover and metabolism in diabetic cardiomyopathy. Circ. Res. 1986;59:684–693. doi: 10.1161/01.res.59.6.684. [DOI] [PubMed] [Google Scholar]
- IVERSEN L.L. Uptake processes for biogenic amines Handbook of Psychopharmacology 1978New York: Prenum Press; 381–442.ed. Iversen, I. pp [Google Scholar]
- JAYANTHI L.D., PRASAD P.D., RAMAMOORTHY S., MAHESH V.B., LEIBACH F.H., GANAPATHY V. Sodium- and chloride-dependent, cocaine-sensitive, high-affinity binding of nisoxetine to the human placental norepinephrine transporter. Biochemistry. 1993;32:12178–12185. doi: 10.1021/bi00096a030. [DOI] [PubMed] [Google Scholar]
- JAYANTHI L.D., RAMAMOORTHY S., MAHESH V.B., LEIBACH F.H., GANAPATHY V. Calmodulin-dependent regulation of the catalytic function of the human serotonin transporter in placental choriocarcinoma cells. J. Biol. Chem. 1994;269:14424–14429. [PubMed] [Google Scholar]
- KAYE D.M., WIVIOTT S.D., KOBZIK L., KELLY R.A., SMITH T.W. S-nitrosothiols inhibit neuronal norepinephrine transport. Am. J. Physiol. 1997;272:H875–H883. doi: 10.1152/ajpheart.1997.272.2.H875. [DOI] [PubMed] [Google Scholar]
- KITAYAMA S., IKEDA T., MITSUHATA C., SATO T., MORITA K., DOHI T. Dominant negative isoform of rat norepinephrine transporter produced by alternative RNA splicing. J. Biol. Chem. 1999;274:10731–10736. doi: 10.1074/jbc.274.16.10731. [DOI] [PubMed] [Google Scholar]
- KLIMAN H.J., NESTLER J.E., SERMASI E., SANGER J.M., STRAUSS J.F. , III Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- LINGEN B., BRÜSS M., BÖNISCH H. Cloning and expression of the bovine sodium- and chloride-dependent noradrenaline transporter. FEBS. 1994;342:235–238. doi: 10.1016/0014-5793(94)80508-3. [DOI] [PubMed] [Google Scholar]
- MELIKIAN H.E., MCDONALD J.K., GU H., RUDNICK G., MOORE K.R., BLAKELY R.D. Human norepinephrine transporter: Biosynthetic studies using a site-directed polyclonal antibody. J. Biol. Chem. 1994;269:12290–12297. [PubMed] [Google Scholar]
- MEREDITH I.T., EISENHOFER G., LAMBERT G.W., DEWAR E.M., JENNINGS G.L., ESLER M.D. Cardiac sympathetic nervous activity in congestive heart failure. Evidence for increased neuronal norepinephrine release and preserved neuronal uptake. Circulation. 1993;88:136–145. doi: 10.1161/01.cir.88.1.136. [DOI] [PubMed] [Google Scholar]
- PACHOLCZYK T., BLAKELY R.D., AMARA S.G. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991;350:350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- PADBURY J.F., TSENG Y.-T., MCGONNIGAL B., PENADO K., STEPHAN M., RUDNICK G. Placental biogenic amine transporters: Cloning and expression. Mol. Brain Res. 1997;45:163–168. doi: 10.1016/s0169-328x(96)00309-9. [DOI] [PubMed] [Google Scholar]
- QIAN Y., GALLI A., RAMAMOORTHY S., RISSO S., DEFELICE L.J., BLAKELY R.D. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J. Neurosci. 1997;17:45–47. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAMOORTHY J.D., RAMAMOORTHY S., LEIBACH F.H., GANAPATHY V. Human placental monoamine transporters as targets for amphetamines. Am. J. Obstet. Gynecol. 1995;173:1782–1787. doi: 10.1016/0002-9378(95)90427-1. [DOI] [PubMed] [Google Scholar]
- RAMAMOORTHY S., PRASAD P.D., KULANTHAIVEL P., LEIBACH F.H., BLAKELY R.D., GANAPATHY V. Expression of a cocaine-sensitive norepinephrine transporter in the human placental syncitiotrophoblast. Biochemistry. 1993;32:1346–1353. doi: 10.1021/bi00056a021. [DOI] [PubMed] [Google Scholar]
- SEYFARTH M., RICHARDT G., MIZSNYAK A., KURZ T., SHOMIG A. Transient ischemia reduces norepinephrine release during sustained ischemia. Neural preconditioning in isolated rat heart. Circ. Res. 1996;78:573–580. doi: 10.1161/01.res.78.4.573. [DOI] [PubMed] [Google Scholar]
- SHAFQAT S., VELAZ-FAIRCLOTH M., GUADANO-FERRAZ A., FREMEAU R.T. Molecular characterization of neurotransmitter transporters. Mol. Endocrinol. 1993;7:1517–1529. doi: 10.1210/mend.7.12.7908408. [DOI] [PubMed] [Google Scholar]
- SHANNON J.R., FLATTEM N.L., JORDAN J., JACOB G., BLACK B.K., BIAGGIONI I., BLAKELY R.D., ROBERTSON D. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N. Engl. J. Med. 2000;342:541–549. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- SHEARMAN L.P., MCREYNOLDS A.M., ZHOU F.C., MEYER J.S. Relationship between [125I]RTI-55-labeled cocaine binding sites and the serotonin transporter in rat placenta. Am. J. Physiol. 1998;275:C1621–C1629. doi: 10.1152/ajpcell.1998.275.6.C1621. [DOI] [PubMed] [Google Scholar]
- SHEARMAN L.P., MEYER J.S. Norepinephrine transporters in rat placenta labeled with [3H]nisoxetine. J. Pharmacol. Exp. Ther. 1998;284:736–743. [PubMed] [Google Scholar]
- SHEARMAN L.P., MEYER J.S. Cocaine up-regulates norepinephrine transporter binding in the rat placenta. Eur. J. Pharmacol. 1999;386:1–6. doi: 10.1016/s0014-2999(99)00624-x. [DOI] [PubMed] [Google Scholar]
- SUMNERS C., RAIZADA M.K. Angiotensin II stimulates norepinephrine uptake in hypothalamus-brain stem neuronal cultures. Am. J. Physiol. 1986;250:C236–C244. doi: 10.1152/ajpcell.1986.250.2.C236. [DOI] [PubMed] [Google Scholar]
- TATSUMI M., GROSHAN K., BLAKELY R.D., RICHELSON E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur. J. Pharmacol. 1997;340:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- THOMAS S.A., MATSUMOTO A.M., PALMITER R.D. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- VATTA M.S., BIANCIOTTI L.G., FERNANDEZ B.E. Influence of atrial natriuretic factor on uptake, intracellular distribition, and release of norepinephrine in rat adrenal medulla. J. Physiol. and Pharmacol. 1993;71:195–200. doi: 10.1139/y93-030. [DOI] [PubMed] [Google Scholar]
- ZHOU Q.-Y., QUALFE C.J., PALMITER R.D. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature. 1995;374:640–643. doi: 10.1038/374640a0. [DOI] [PubMed] [Google Scholar]


