Abstract
ATP and adenine dinucleotides can elicit three different types of vasomotor response in the rat mesenteric arterial bed; vasocontraction, rapid relaxation (which may be masked by contraction) and slow and prolonged vasorelaxation. Contraction is mediated by smooth muscle P2X receptors and rapid relaxation by endothelial P2Y receptors. The mechanism of prolonged relaxation is, however, controversial.
In the present study, bolus injection of doses of α,β-methylene ATP (α,β-meATP; 5 pmol – 0.5 μmol; P2X receptor agonist) in methoxamine-preconstricted rat isolated mesenteric arterial beds, mimicked the action of ATP, causing contraction (Rmax 76±9 mmHg) followed by prolonged relaxation (78±11%; t1/2 14.6±1.5 min). KCl also elicited a biphasic response (Rmax contraction 73±8 mmHg; Rmax prolonged relaxation 70±6%; t1/2 7.7±1.9 min).
P2X receptor desensitization caused by perfusion with α,β-meATP (10 μM) abolished contraction and prolonged relaxation to doses of α,β-meATP (50 nmol). Rapid relaxation (32±7%; t1/2 32±2 s) was revealed, which was abolished by removal of the endothelium using distilled water.
Sodium deoxycholate treatment blocked contractile and prolonged relaxation responses to α,β-meATP, ATP and KCl, whilst distilled water treatment had no significant effect on either phase of the biphasic responses.
These data indicate that smooth muscle P2X receptors are involved in both phases of the biphasic response (contraction followed by prolonged relaxation) to purine nucleotides in the rat isolated mesenteric arterial bed. Caution should be applied when using sodium deoxycholate to remove the endothelium because of possible damage caused by the detergent to receptors and/or the vascular smooth muscle.
Keywords: α,β-meATP; endothelium; P2 purine receptors; rat mesenteric arterial bed; vasorelaxation
Introduction
P2 receptors for extracellular purine and pyrimidine nucleotides (ATP, ADP, UTP, UDP) are expressed throughout the cardiovascular system and mediate diverse effects on cardiac and blood vessel function (Olsson & Pearson, 1990; Boarder & Hourani, 1998; Ralevic & Burnstock, 1998). They are divided into two families based on distinct molecular structures and signalling mechanisms: ionotropic P2X receptors, which are expressed principally on vascular smooth muscle (this family is composed of seven cloned subtypes), and G protein-coupled P2Y receptors (six subtypes have been cloned), which are expressed both on vascular smooth muscle and endothelial cells. ATP and ADP are agonists at subtypes of both P2X and P2Y receptors, whilst UTP and UDP are agonists at subtypes of P2Y receptors, being weak or inactive at P2X receptors (Ralevic & Burnstock, 1998).
ATP can elicit complex effects in mesenteric blood vessels; three different types of response have been described, namely vasocontraction, rapid relaxation (which may be masked by the vasocontractile response) and slow and prolonged vasorelaxation (Juul et al., 1993; Ralevic, 2001; Stanford et al., 2001). Contraction is mediated principally by smooth muscle P2X receptors and rapid relaxation by endothelial P2Y receptors (principally P2Y2-like receptors) (Ralevic & Burnstock, 1988; 1996; 1998). The mechanism of the prolonged relaxation response, which can be modulated by a variety of pharmacological agents (Ralevic, 2001; Stanford et al., 2001), is, however, controversial. Prolonged relaxation to ATP is attenuated by desensitization of smooth muscle P2X receptors with α,β-methyleneATP (α,β-meATP); a modulatory action was suggested as it is difficult to see how activation of ionotropic receptors, allowing calcium influx normally associated with vasoconstriction, could be directly involved in vasorelaxation (Ralevic, 2001). Furthermore, conflicting data show that prolonged relaxation to ATP is both unaffected by removal of the endothelium (Ralevic, 2001), and abolished by removal of the endothelium (Stanford et al., 2001). Prolonged vasorelaxation to adenine dinucleotides in rat mesenteric arteries is also unaffected by endothelial denudation (Ralevic et al., 2001). Importantly, different methods of endothelium removal were used in these studies. Ralevic et al. (2001; Ralevic, 2001) used distilled water, whilst Stanford et al. (2001) used a detergent, sodium deoxycholate.
In order to investigate the possible involvement of P2X receptors in the biphasic response (contraction and prolonged relaxation) to purine nucleotides, α,β-meATP, a selective P2X receptor agonist and desensitizing agent, was used. The principal subtype of P2X receptor in blood vessels is the P2X1 receptor, expressed on vascular smooth muscle (Ralevic & Burnstock, 1996; Lewis & Evans, 2000), so actions of α,β-meATP might seem to imply an involvement of the smooth muscle. Nonetheless, a possible involvement of the endothelium was investigated using sodium deoxycholate and distilled water treatment against responses to ATP, α,β-meATP, KCl and serotonin (5-HT). A preliminary account of some of these findings has been reported to the British Pharmacological Society (Ralevic, 2002; Ralevic & Randall, 2002).
Methods
Male Wistar rats (250 – 300 g) were killed by exposure to CO2 and decapitation. Mesenteric beds were isolated and perfused, via the superior mesenteric artery, as described previously (Ralevic & Burnstock, 1996). Briefly, the abdomen was opened and the superior mesenteric artery exposed and cannulated with a hypodermic needle. The superior mesenteric vein was cut, blood flushed from the preparation with about 0.5 ml of Krebs' solution and the gut dissected carefully away from the mesenteric vasculature. The preparation was mounted in a humid chamber and perfused at a constant flow rate of 5 ml min−1 using a peristaltic pump (model 7554-30, Cole-Parmer Instrument Co., Chicago, IL, U.S.A.). The perfusate was Krebs'-Bülbring solution of the following composition (mM): NaCl 133, KCl 4.7, NaH2PO4 1.35, NaHCO3 16.3, MgSO4 0.61, CaCl2 2.52 and glucose 7.8, gassed with 95% O2 – 5% CO2 and maintained at 37°C. Preparations were allowed to equilibrate for 30 min prior to experimentation. Responses were measured as changes in perfusion pressure (mmHg) with a pressure transducer (model P23XL, Viggo-Spectramed, Oxnard, CA, U.S.A.) on a side arm of the perfusion cannula, and recorded on a polygraph (model 7D, Grass Instrument Co., Quincy, MA, U.S.A.).
After equilibration, a submaximal concentration of methoxamine (2 – 100 μM) was added in order to increase the perfusion pressure of the preparations (by about 40 – 70 mmHg above baseline). Drug injection, in a volume of 50 μl, was made into norprene rubber tubing proximal to the preparation. Injection of this volume of distilled water has a negligible effect on perfusion pressure (see Figure 1). In methoxamine-preconstricted preparations, injection of two consecutive doses of α,β-meATP (50 nmol) was followed by perfusion with α,β-meATP (10 μM; added to the perfusate). After this, two doses of α,β-meATP (50 nmol) were again injected. The preparation was then perfused with distilled water for 10 min, after which two doses of α,β-meATP (50 nmol) were injected. In separate preparations, during the equilibrium period these were injected with sodium deoxycholate (4 ml of 2 mg ml−1) or perfused for 10 min with distilled water. After recovery (about 15 min) they were preconstricted with methoxamine and responses to injections of doses of α,β-meATP (5 pmol – 0.5 μmol) and KCl (5 – 200 μmol) were investigated. In another group of preparations responses to doses of ATP (0.5 μmol) were investigated: after two consecutive doses of ATP, preparations were injected with sodium deoxycholate solution (4 ml of 2 mg ml−1). Another dose of ATP was then injected. In one out of five preparations sodium deoxycholate treatment, followed by an ATP injection, was repeated. Relaxation responses to doses of sodium nitroprusside (SNP; 0.5 pmol – 50 nmol) and serotonin (5-HT; 50 pmol – 0.5 μmol) were then investigated. In separate control preparations the same protocol (four injections of ATP; dose-response curves to SNP and 5-HT), but without injections of sodium deoxycholate, was investigated. The integrity of the endothelium was assessed with 50 nmol acetylcholine (ACh), a dose which elicits relaxation of about 80% in the rat isolated endothelium-intact mesenteric arterial bed (Windscheif et al., 1994).
Figure 1.
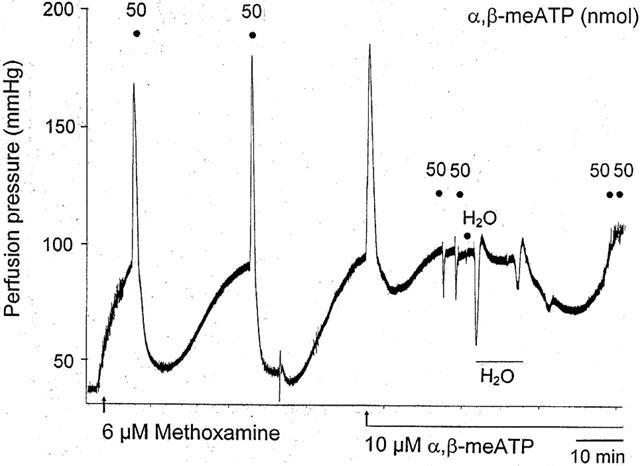
Representative trace showing effect of P2X receptor desensitization and distilled water treatment on the biphasic response to α,β-methylene ATP (α,β-meATP) in a methoxamine-preconstricted rat isolated mesenteric arterial bed. A biphasic response was elicited both by injection of α,β-meATP (two doses of 50 nmol) and by continuous perfusion with α,β-meATP (10 μM), to cause desensitization of P2X receptors. In the presence of 10 μM α,β-meATP, injection of α,β-meATP (two doses of 50 nmol) evoked only rapid relaxation. Injection of an equivalent volume of water (H2O; 50 μl bolus) had virtually no effect – note that the response is indicated by a solid circle immediately below the ‘H' of ‘H2O'. The following large drop in perfusion pressure is due to the effect of perfusion of distilled H2O through the preparation (indicated by horizontal bar). Rapid relaxation to injection of α,β-meATP was abolished after endothelium removal with distilled water.
Drugs
α,β-meATP, ACh, ATP, deoxycholic acid (sodium salt), 5-HT, methoxamine (hydrochloride), and sodium nitroprusside were from Sigma Chemical Co. All drugs and reagents were dissolved in distilled water.
Data analysis
Vasoconstrictor responses of the mesenteric arterial beds were measured as increases in perfusion pressure in mmHg. Vasorelaxant responses were measured as changes in perfusion pressure (mmHg) and expressed as percentage relaxation of the methoxamine-induced increase in tone above baseline. Data are expressed as mean±s.e.mean and analysed by Student's t-test or by analysis of variance with Tukey's multiple comparison post-hoc test. A value of P<0.05 was taken to indicate a statistically significant difference. t1/2 is the time to half recovery of the relaxation response.
Results
Effect of α,β-meATP in preconstricted mesenteric beds
Bolus injection of α,β-meATP elicited a biphasic response of contraction followed by prolonged relaxation (Figures 1 and 2). Contractile and relaxant dose – response curves were constructed: Rmax contraction=76±9 mmHg; Rmax relaxation=77±10% (n=4) (see Figures 3a and 4a). At a dose of 50 nmol α,β-meATP (which evoked a maximal response) the t1/2 of the prolonged relaxation was 14.6±1.5 min (n=6). In many preparations two phases of contractile response to α,β-meATP (an early rapidly decaying response and a more slowly decaying secondary phase) could be identified (Figure 3a,c).
Figure 2.
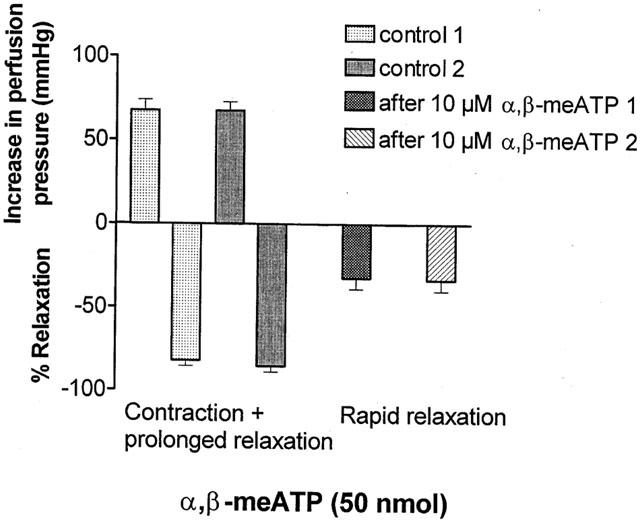
Effect of P2X receptor desensitization on the biphasic response to α,β-methylene ATP (α,β-meATP). Under control conditions two consecutive injections of α,β-meATP (50 nmol) each elicit contraction followed by prolonged relaxation in methoxamine-preconstricted rat isolated mesenteric arterial beds (n=6). In the same preparations in the presence of α,β-meATP (10 μM), to cause desensitization of P2X receptors, both contraction and prolonged relaxation are abolished, and injection of α,β-meATP (two doses of 50 nmol) elicits only rapid relaxation. Data are presented as means and vertical bars indicate s.e.mean.
Figure 3.
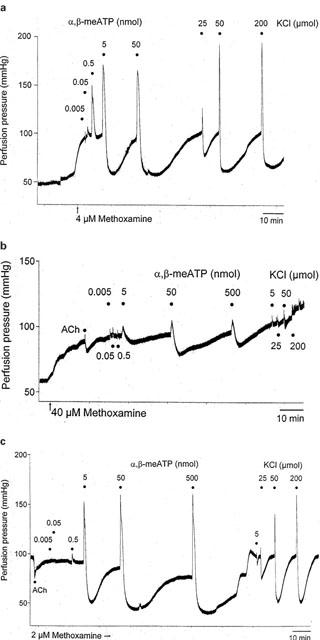
Representative traces showing biphasic responses (contraction followed by prolonged relaxation) to injection of doses of α,β-methylene ATP (0.005 – 500 nmol) and KCl (5 – 200 μmol) in methoxamine-preconstricted rat isolated mesenteric arterial beds under (a) control conditions, (b) after sodium deoxycholate treatment (4 ml of 2 mg ml−1), (c) after distilled water treatment. The response to a single dose of ACh (50 nmol) is also shown in (b) and (c).
Figure 4.
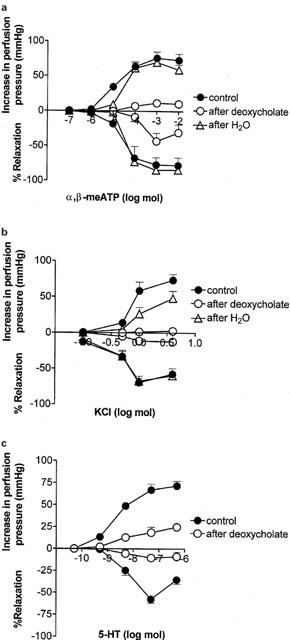
Contractile and relaxant dose-response curves for the biphasic response to: (a) α,β-methylene ATP, (b) KCl, (c) 5-HT, in methoxamine-preconstricted rat isolated mesenteric arterial beds. Contractile and relaxant response curves are shown under control conditions (n=4 – 5) and after treatment with sodium deoxycholate (n=4 – 5) or distilled water (n=5). Data are presented as means and vertical bars indicate s.e.mean.
Effect of P2X receptor desensitization, followed by water treatment, on responses to α,β-meATP in preconstricted mesenteric beds
Perfusion with α,β-meATP (10 μM) elicited a biphasic response of contraction (79±6 mmHg) followed by prolonged relaxation (39±12%) (n=6), mimicking the effects of bolus application of doses of α,β-meATP (Figure 1). After desensitization of P2X receptors and recovery of tone, the biphasic response to bolus injection of a dose of α,β-meATP (50 nmol) was abolished and rapid relaxation, 32±7%; t1/2 32±2 s (n=6) was revealed. This was reproducible when application of the dose of α,β-meATP was repeated; 33±7%; t1/2 30±2.2 s (n=6) (Figures 1 and 2). The rapid relaxation response to α,β-meATP (50 nmol) was abolished following endothelium removal with distilled water (n=6) (Figure 1).
Effect of 5-HT and KCl in preconstricted mesenteric beds
KCl elicited a biphasic response in the mesenteric arterial beds: Rmax contraction=73±8 mmHg; Rmax relaxation=70±6% (n=4) (Figures 3 and 4b). At a dose of 50 μmol KCl (which evoked a maximal response) the t1/2 of the prolonged relaxation was 7.7±1.9 min (n=6). 5-HT also elicited a biphasic response: Rmax contraction=72±5 mmHg; Rmax relaxation=35±5% (n=5) (Figure 4c). At a dose of 50 nmol 5-HT (which evoked a maximal response) the t1/2 for prolonged relaxation was 9.3±0.8 min (n=5).
Effect of sodium deoxycholate treatment on responses to α,β-meATP, 5-HT and KCl
Sodium deoxycholate blocked contractile and relaxant responses to α,β-meATP: Rmax contraction=12±5 mmHg; Rmax relaxation=43±12% (n=4) (Figures 3b and 4a). Contractile and relaxant responses to KCl were also blocked: Rmax contraction=3±2 mmHg; Rmax relaxation=12±12% (n=4) (Figures 3b and 4b). After sodium deoxycholate treatment contractile and relaxant responses to 5-HT were also attenuated: Rmax contraction=25±4 mmHg; Rmax relaxation=9±5% (n=5) (Figure 4c).
Sodium deoxycholate treatment blocked the relaxation response to 50 nmol ACh (15.4±6.1%; n=6) but there was no significant effect on relaxations to SNP (see Figure 7).
Figure 7.
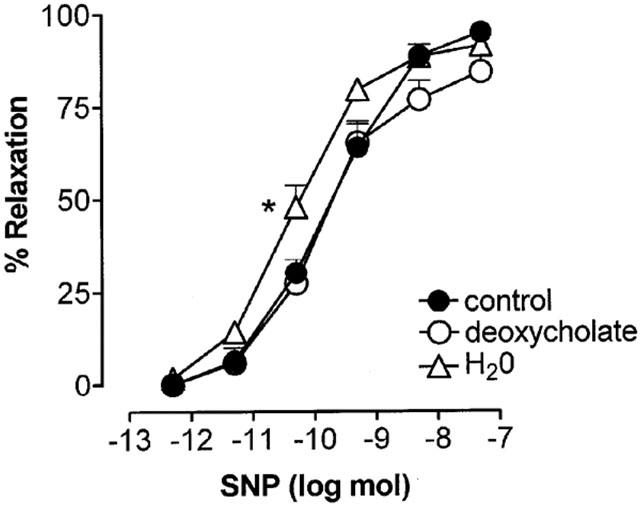
Vasorelaxant dose-response curves to sodium nitroprusside (SNP) generated under control conditions (n=6) or after treatment with sodium deoxycholate (n=6) or distilled water (n=5) in methoxamine-preconstricted rat isolated mesenteric arterial beds. Data are presented as means and vertical bars indicate s.e.mean. *P<0.05, indicates significant difference between pD2 value after water treatment compared to the other two groups.
Effect of distilled water treatment on responses toα,β-meATP and KCl
Distilled water treatment had no significant effect on the biphasic response to α,β-meATP (Rmax contraction=70±4 mmHg; Rmax relaxation=84±3%; t1/2 at 50 nmol=18.8±1.2 min; n=5) (Figures 3c and 4a). There was also no significant effect ofthis treatment on the biphasic response to KCl (Rmax contraction=48±10 mmHg; Rmax relaxation=68±7%; t1/2 50 μmol=4.7±2 min; n=5) (Figures 3c and 4b). In contrast, distilled water treatment blocked relaxation to 50 nmol ACh (28±5%, n=5). This residual relaxant activity of ACh was not significantly different to that remaining after sodium deoxycholate treatment. The sensitivity of vasorelaxation to SNP was augmented after distilled water treatment (pD2 value 10.35±0.12) compared to controls (pD2 value 9.79±0.17) (P<0.05) producing a leftward shift in the dose – response curve (see Figure 7).
Effect of ATP in preconstricted mesenteric beds
The rapid, endothelium-dependent, P2Y receptor-mediated relaxation to low doses of ATP (up to and including 50 nmol) is illustrated in Figure 5a). As the dose of ATP increases (50 and 500 nmol) vasoconstriction also occurs which, at 500 nmol ATP, blocks rapid relaxation. It was noted in a single preparation, that when the methoxamine-induced tone of the preparations was relatively high (greater than 80 mmHg above baseline), the observed P2X receptor-mediated contraction elicited by ATP was small, but when the preconstricted tone was lower in the same preparation (about 40 mmHg above baseline), the P2X receptor-mediated contraction was large (although absolute values of perfusion pressure achieved by the P2X receptor-mediated contractions were similar) (Figure 5b).
Figure 5.
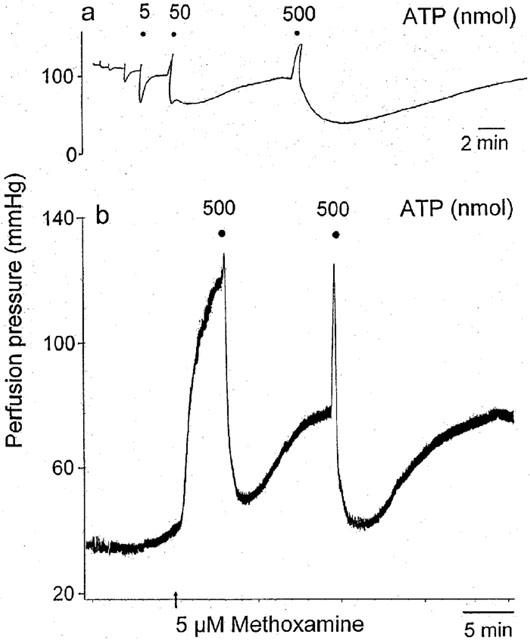
Representative traces showing responses to injection of ATP: (a) doses of 0.05, 0.5 (not labelled), 5, 50 and 500 nmol and (b) two doses of 500 nmol, in methoxamine-preconstricted rat isolated mesenteric arterial beds. (a) Doses of ATP up to and including 50 nmol elicit a rapid endothelium-dependent relaxation. Doses of ATP of 50 and 500 nmol elicit contraction (which opposes the rapid relaxation) and prolonged relaxation. (b) When tone was relatively high (>80 mmHg increase above baseline) ATP-induced contraction was small, whilst when tone was lower (about 40 mmHg increase above baseline), contraction was larger (although the absolute amplitudes of the methoxamine- plus ATP-induced contractions were similar). Note the different time scales in the two parts of the figure. (Figure 5a is reproduced, with permission, from Ralevic (2001), Br. J. Pharmacol., 132, 685 – 692).
Injection of a single dose of ATP (0.5 μmol) elicited vasoconstriction (28±7 mmHg) followed by prolonged relaxation (87±4%) (n=5), which was reproducible when repeated four times in time-control experiments (Figure 6). The t1/2 for two consecutive injections of doses of ATP (0.5 μmol) was not significantly different at 12±1.5 min and 10.4±0.9 min, respectively (n=10). Sodium deoxycholate treatment attenuated contractile and prolonged relaxation responses to ATP (Figure 6).
Figure 6.
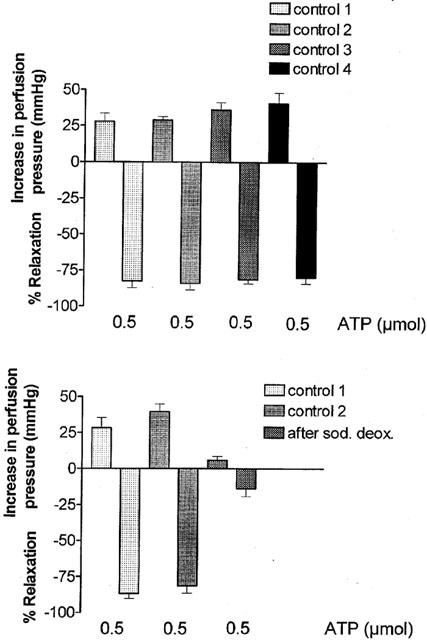
Under control conditions four repeated injections of ATP (0.5 μmol) elicit reproducible biphasic responses (contraction followed by prolonged relaxation) in methoxamine-preconstricted rat isolated mesenteric arterial beds (upper panel; n=6). In separate preparations, reproducibility is confirmed for two consecutive injections, but both components of the biphasic response are blocked following sodium deoxycholate treatment (n=6). Data are presented as means and vertical bars indicate s.e.mean.
Discussion
The present study has shown that vascular smooth muscle P2X receptors are involved in the prolonged vasorelaxation response to purine nucleotides in the rat isolated mesenteric arterial bed. Evidence for this comes from the observation that α,β-meATP, a selective P2X receptor agonist, evoked contraction followed by prolonged relaxation, thus mimicking responses to ATP in the rat mesenteric arterial bed, and both types of response were blocked by desensitization of P2X receptors. P2X receptors are known to be expressed on the smooth muscle and, accordingly, endothelium removal with distilled water (which attenuated relaxations to ACh) had no effect on the biphasic response to α,β-meATP.
α,β-meATP is a highly selective P2X receptor agonist, having little or no activity at P2Y receptors (Ralevic & Burnstock, 1988). Thus, the fact that α,β-meATP mimicked the biphasic response to ATP, and to adenine dinucleotides, in the rat mesenteric arterial bed (Ralevic, 2001; Ralevic et al., 2001; Stanford et al., 2001; present study), strongly indicates an involvement of P2X receptors. A biphasic response to α,β-meATP has also been described in rat isolated mesenteric resistance arteries, by Juul et al. (1993). Unequivocal evidence for an involvement of P2X receptors was, however, provided with the demonstration that P2X receptor desensitization abolished both contraction and prolonged relaxation to α,β-meATP. This is consistent with a recent report that P2X receptor desensitization blocks similar responses to ATP in the rat isolated mesenteric arterial bed (Ralevic, 2001).
Activation of inotropic smooth muscle P2X receptors causes an initial influx of Ca2+, and the depolarization that follows allows an influx of additional Ca2+. Summation of these events leads to smooth muscle constriction. These two events may account for the rapidly decaying and more slowly decaying phases of contraction, respectively, observed for α,β-meATP in the present study. The role of smooth muscle P2X receptors in the prolonged relaxation response is, however, less clear. A biphasic response was also observed for both 5-HT and KCl, indicating that the response is not specific to ionotropic receptors and, importantly, that it can be evoked by receptor-independent smooth muscle depolarization. An involvement of an after-hyperpolarizing event and the opening of Ca2+-activated K+ channels in the prolonged relaxation that follows contraction is possible. Indeed, modulation of the prolonged relaxation by ouabain, glibenclamide and high extracellular potassium indicates an involvement of membrane hyperpolarization (Ralevic, 2001; Stanford et al., 2001). Although an additional involvement of P2Y receptors in prolonged relaxation to ATP can not be excluded, as this response was not abolished by P2X receptor desensitization (Ralevic, 2001), it is possible that the residual response in that study was overestimated because of overlap with the inactivating tail of the initial rapid endothelium-dependent relaxation.
In blood vessels, P2X receptors are expressed principally on the vascular smooth muscle, and in rat mesenteric arteries the main subtype is P2X1 (Ralevic & Burnstock, 1996; Lewis & Evans, 2000). An involvement of P2X receptors, therefore, implies that the endothelium is not crucial for prolonged relaxation to purines, consistent with earlier reports (Steinmetz et al., 2000; Ralevic, 2001; Ralevic et al., 2001). This was confirmed in the present study with the demonstration that endothelium removal with distilled water had no significant effect on prolonged relaxation to α,β-meATP. In contrast, sodium deoxycholate treatment blocked both contraction and prolonged relaxation to α,β-meATP, ATP, 5-HT and KCl. The sodium deoxycholate treatment used was more aggressive than used by us previously to remove the endothelium (2 ml of 2 mg ml−1; Ralevic & Burnstock, 1988), and is more aggressive than the water treatment, as indicated by the trend for a smaller residual relaxation to ACh after deoxycholate treatment. However, the fact that a small ACh-mediated vasorelaxation remained after sodium deoxycholate treatment, suggests that the dose used in the present study may be more appropriate for endothelium removal than that used previously, and does not alter the conclusion regarding the endothelium independent nature of prolonged relaxation to purine nucleotides. The two treatments were not perfectly matched for endothelium removal, so it is conceivable that longer periods of perfusion with water could have effects on the smooth muscle. Conversely, a lower dose of sodium deoxycholate would likely have less pronounced inhibitory effects on smooth muscle responses. Nonetheless, this also does not alter the main conclusions drawn as there was no significant effect of water treatment on the biphasic response to α,β-meATP when the endothelium was clearly damaged.
These findings likely explain a current controversy regarding the endothelial dependency of prolonged relaxation to ATP in the rat mesenteric arterial bed (Ralevic, 2001; Ralevic et al., 2001; Stanford et al., 2001) by showing that damage caused by sodium deoxycholate, to receptors and/or the vascular smooth muscle, can account for attenuation of the response. Other investigators have also reported an impairment of smooth muscle vasocontractile responses following treatment with detergent to remove the endothelium (Chiba & Tsukada, 1984; Samata et al., 1986). Relaxation responses to SNP, a nitric oxide (NO) donor, were unaffected by sodium deoxycholate treatment, despite attenuation of responses to α,β-meATP, ATP, 5-HT and KCl, indicating that caution should be applied in using this agent as a test of vascular smooth muscle function after this treatment. Distilled water treatment caused an augmentation of responses to SNP, as reported previously using inhibitors of NO synthesis, and suggested to be due to an increase in the available pool of guanyl cyclase/cyclic GMP for activation following relief from activation by tonically produced NO (Busse et al., 1989; Lüscher et al., 1989; Ralevic et al., 1991). Augmentation was not observed following sodium deoxycholate treatment, consistent with the suggestion that this had caused damage to the vascular smooth muscle.
There is recent immunohistochemical evidence for the expression of P2X receptors on endothelium (Hansen et al., 1999; Loesch & Burnstock, 2000; Glass & Burnstock, 2001). Northern blot analysis has shown expression of P2X4 mRNA in cultured endothelial cells (Yamamoto et al., 2000b; Korenaga et al., 2001). Moreover, there is evidence that shear stress causes Ca2+ influx via activation of P2X4 receptors on human endothelial cells (Yamamoto et al., 2000a). The present study is to my knowledge, however, the first report of a vasorelaxant action of α,β-meATP mediated via the endothelium. Paradoxically, the rapid endothelial response elicited by α,β-meATP was resistant to desensitization of P2X receptors by prolonged exposure to α,β-meATP, which does not fit with the pharmacological profile of known homomeric P2X receptors (Ralevic & Burnstock, 1998). As α,β-meATP is inactive at P2Y receptors one explanation is an action at slowly-desensitizing heteromeric P2X receptors. A recent report similarly showed that desensitization of P2X receptors and block of prolonged relaxation, uncovers rapid endothelium-dependent relaxations to adenine dinucleotides (Ralevic et al., 2001). In that study, experiments to characterize the response, using the P2 receptor antagonists suramin and pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), were inconclusive, partly because suramin seemed to prevent full desensitization of the P2X response (Ralevic et al., 2001). If the endothelial response is mediated by a P2X4 receptor, this subtype is insensitive to suramin and PPADS (Ralevic & Burnstock, 1998). Thus, there are currently no selective antagonists with which to characterize the α,β-meATP-sensitive endothelium-dependent relaxation. A less exciting possibility is that the response is mediated by nucleotide contaminants of the α,β-meATP solution acting at endothelial P2Y1 and P2Y2 receptors.
The broader implications of the present results are that whilst activation of P2X receptors in blood vessels can elicit pronounced contraction, an autoregulatory mechanism (prolonged relaxation) exists to limit the extent of this response. As the prolonged relaxation response is also mediated via the smooth muscle it will operate even when there is damage to the endothelium. Two main sources of ATP in blood vessels are perivascular sympathetic nerves (from which ATP is released as a cotransmitter) and activated platelets (Ralevic & Burnstock, 1998). In preliminary studies designed to identify a physiological correlate for the present findings, there was no prolonged relaxation following contraction due to stimulation of sympathetic nerves in preconstricted mesenteric arterial beds (unpublished observations), suggesting that the prolonged relaxation response may be more significant for modulation of vasospasm evoked by high levels of purines released from activated platelets.
In conclusion, the present study has shown that activation of P2X receptors expressed on the vascular smooth muscle evokes a biphasic response consisting of contraction and prolonged relaxation in the rat isolated mesenteric arterial bed. Thus, P2X receptors are likely involved in the prolonged relaxation response previously observed to ATP and purine dinucleotides in this vascular preparation. Caution should be applied when using sodium deoxycholate to remove the endothelium as the detergent can impair vascular smooth muscle function, even when relaxation to sodium nitroprusside (the archetypal test of smooth muscle function following this treatment) is unimpaired.
Acknowledgments
The support of the Royal Society is gratefully acknowledged.
Abbreviations
- α,β-meATP
α,β-methylene ATP
- 5-HT
serotonin
References
- BOARDER M.R., HOURANI S.M.O. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol. Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- BUSSE R., POHL U., MÜLSCH A., BASSENGE E. Modulation of the vasodilator action of SIN-1 by the endothelium. J. Cardiovasc. Pharmacol. 1989;14 Suppl. 11:S81–S85. doi: 10.1097/00005344-198906152-00015. [DOI] [PubMed] [Google Scholar]
- CHIBA S., TSUKADA M. Vasoconstrictor responses induced by α-adrenoceptor agonists before and after removal of the endothelial cells of dog mesenteric arteries. J. Auton. Pharmacol. 1984;4:257–260. doi: 10.1111/j.1474-8673.1984.tb00103.x. [DOI] [PubMed] [Google Scholar]
- GLASS R., BURNSTOCK G. Immunohistochemical identification of cells expressing ATP-gated cation channels (P2X receptors) in the adult rat thyroid. J. Anat. 2001;198:569–579. doi: 10.1046/j.1469-7580.2001.19850569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSEN M.A., DUTTON J.L., BALCAR V.J., BENNETT M.R. P2X (purinergic) receptor distributions in rat blood vessels. J. Auton. Nerv. Syst. 1999;75:147–155. doi: 10.1016/s0165-1838(98)00189-1. [DOI] [PubMed] [Google Scholar]
- JUUL B., PLESNER L., AALKJAER C. Effects of ATP and related nucleotides on the tone of isolated rat mesenteric resistance arteries. J. Pharmacol. Exp. Ther. 1993;264:1234–1240. [PubMed] [Google Scholar]
- KORENAGA R., YAMAMOTO K., OHURA N., SOKABE T., KAMIYA A., ANDO J. Sp1-mediated downregulation of P2X4 receptor gene transcription in endothelial cells exposed to shear stress. Am. J. Physiol. 2001;280:H2214–H2221. doi: 10.1152/ajpheart.2001.280.5.H2214. [DOI] [PubMed] [Google Scholar]
- LEWIS C.J., EVANS R.J. Lack of run-down of smooth muscle P2X receptor currents recorded with the amphotericin permeabilized patch technique, physiological and pharmacological characterization of the properties of mesenteric artery P2X receptor ion channels. Br. J. Pharmacol. 2000;131:1659–1666. doi: 10.1038/sj.bjp.0703744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOESCH A., BURNSTOCK G. Ultrastructural localisation of ATP-gated P2X2 receptor immunoreactivity in vascular endothelial cells in rat brain. Endothelium. 2000;7:93–98. doi: 10.3109/10623320009072204. [DOI] [PubMed] [Google Scholar]
- LÜSCHER T.F., RICHARD V., YANG Z. Interaction between endothelium-derived nitric oxide and SIN-1 in human and porcine blood vessels. J. Cardiovasc. Pharmacol. 1989;14 Suppl. 11:S76–S80. [PubMed] [Google Scholar]
- OLSSON R.A., PEARSON J.D. Cardiovascular purinoceptors. Physiol. Rev. 1990;70:761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- RALEVIC V. Mechanism of prolonged vasorelaxation to ATP in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 2001;132:685–692. doi: 10.1038/sj.bjp.0703868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V. Activation of P2X receptors by α,β-methylene ATP evokes a biphasic response (contraction and prolonged relaxation) in the rat isolated mesenteric arterial bed Br. J. Pharmacol. 2002135286P [Google Scholar]
- RALEVIC V., BURNSTOCK G. Actions mediated by P2-purinoceptors in the isolated perfused mesenteric bed of the rat. Br. J. Pharmacol. 1988;95:637–645. doi: 10.1111/j.1476-5381.1988.tb11686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Discrimination by PPADS between endothelial P2Y- and P2U-purinoceptors in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 1996;118:428–434. doi: 10.1111/j.1476-5381.1996.tb15420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- RALEVIC V., JANKOWSKI J., SCHLÜTER H. Structure-activity relationships of diadenosine polyphosphates (ApnAs), adenosine polyphospho guanosines (ApnGs) and guanosine polyphospho guanosines (GpnGs) at P2 receptors in the rat mesenteric arterial bed. Br. J. Pharmacol. 2001;134:1073–1083. doi: 10.1038/sj.bjp.0704341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., MATHIE R.T., ALEXANDER B., BURNSTOCK G. NG-Nitro-L-arginine methyl ester attenuates vasodilator responses to acetylcholine but enhances those to sodium nitroprusside. J. Pharm. Pharmacol. 1991;43:871–874. doi: 10.1111/j.2042-7158.1991.tb03199.x. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., RANDALL M.D. Effects of sodium deoxycholate and distilled water treatment on the biphasic response to α,β-methylene ATP in the rat isolated mesenteric arterial bed Br. J. Pharmacol. 2002135287P [Google Scholar]
- SAMATA K., KIMURA T., SATOH S., WATANABE H. Chemical removal of the endothelium by saponin in the isolated dog femoral artery. Eur. J. Pharmacol. 1986;128:85–91. doi: 10.1016/0014-2999(86)90561-3. [DOI] [PubMed] [Google Scholar]
- STANFORD S.J., GITLIN J.M., MITCHELL J.A. Identification of two distinct vasodilator pathways activated by ATP in the mesenteric bed of the rat. Br. J. Pharmacol. 2001;133:825–832. doi: 10.1038/sj.bjp.0704139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINMETZ M., SCHLATTER E., BOUDIER H.A.J.S., RAHN K.H., DE MEY J.G.R. Diadenosine polyphosphates cause contraction and relaxation in isolated rat resistance arteries. J. Pharmacol. Exp. Ther. 2000;294:1175–1181. [PubMed] [Google Scholar]
- WINDSCHEIF U., RALEVIC V., BÄUMERT H.G., MUTSCHLER E., LAMBRECHT G., BURNSTOCK G. Vasoconstrictor and vasodilator responses to various agonists in the rat perfused mesenteric arterial bed: selective inhibition by PPADS of contractions mediated via P2X-purinoceptors. Br. J. Pharmacol. 1994;113:1015–1021. doi: 10.1111/j.1476-5381.1994.tb17094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO K., KORENAGA R., KAMIYA A., ANDO J. Fluid shear stress activates Ca2+ influx into human endothelial cells via P2X4 purinoceptors. Circ. Res. 2000a;87:385–391. doi: 10.1161/01.res.87.5.385. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO K., KORENAGA R., KAMIYA A., QI Z. P2X4 receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am. J. Physiol. 2000b;279:H285–H292. doi: 10.1152/ajpheart.2000.279.1.H285. [DOI] [PubMed] [Google Scholar]


