Abstract
Neuropeptide Y (NPY) is one of the most potent stimulants of food intake. It has been debated which receptor subtype mediates this response. Initially Y1 was proposed, but later Y5 was announced as a ‘feeding' receptor in rats and mice. Very little is known regarding other mammals. The present study attempts to characterize the role of NPY in feeding behaviour in the distantly related guinea-pig. When infused intracerebroventricularly, NPY dose-dependently increased food intake.
PYY, (Leu31,Pro34)NPY and NPY(2 – 36) stimulated feeding, whereas NPY(13 – 36) had no effect. These data suggest that either Y1 or Y5 receptors or both may mediate NPY induced food intake in guinea-pigs.
The Y1 receptor antagonists, BIBO 3304 and H 409/22 displayed nanomolar affinity for the Y1 receptor (Ki values 1.1±0.2 nM and 5.6±0.9 nM, respectively), but low affinity for the Y2 or Y5 receptors. When guinea-pigs were pretreated with BIBO 3304 and H 409/22, the response to NPY was inhibited.
The Y5 antagonist, CGP 71683A had high affinity for the Y5 receptor (Ki 1.3±0.05 nM) without having any significant activities at the Y1 and Y2 receptors. When CGP 71683A was infused into brain ventricles, the feeding response to NPY was attenuated.
The present study shows that NPY stimulates feeding in guinea-pigs through Y1 and Y5 receptors. As the guinea-pig is very distantly related to the rat and mouse, this suggests that both Y1 and Y5 receptors may mediate NPY-induced hyperphagia also in other orders of mammals.
Keywords: Neuropeptide Y, Y1 receptor, Y2 receptor, Y5 receptor, food intake, guinea-pig
Introduction
Neuropeptide Y (NPY), which is a member of the pancreatic polypeptide hormone family that also includes pancreatic polypeptide (PP) and peptide YY (PYY), is one of the most evolutionarily highly conserved peptides known (Larhammar, 1996). The peptide is widely distributed within the central and peripheral nervous systems in mammals. Regulation of several important physiological activities such as food intake, blood pressure and circadian rhythms has been attributed to NPY. Five distinct Y receptor subtypes that bind NPY, PYY and PP with different affinities have been cloned in mammals. The receptors belong to the rhodopsin-like G-protein-coupled receptor superfamily, and have been given designations Y1, Y2, Y4, Y5 and y6 (Larhammar et al., 2001; Michel et al., 1998). The pharmacologically defined Y3 receptor has not been cloned (Herzog et al., 1993; Jazin et al., 1993), and may not exist as a separate gene product (Michel et al., 1998).
The NPY effects that have received the greatest attention over the past decade are those on feeding and metabolism. When NPY is injected intracerebroventricularly (i.c.v.) or directly into the hypothalamus, feeding is markedly increased (Haynes et al., 1998; Stanley & Leibowitz, 1985). This stimulatory effect has been reported not only in rodents (Iyengar et al., 1999; Levine & Morley, 1984; Morley et al., 1987a, 1987b), but also in virtually all vertebrates that have been studied (Boswell et al., 1993; Kuenzel et al., 1987; Kulkosky et al., 1989; Larsen et al., 1999; Miner et al., 1989; Morris & Crews, 1990; Pau et al., 1988; Parrot et al., 1986; Richardson et al., 1995; Volkoff & Peter, 2001). The only exceptions are the dog, which did respond to PP (Inui et al., 1991), and the baboon (Sipols et al., 1996). The receptor subtype mediating the effect of NPY on food intake is not yet firmly established. Currently available pharmacological data from rats and mice (see Kalra et al., 1999, for a review ) suggest that Y1 and/or Y5 receptors mediate the stimulatory effect of NPY on feeding. However, gene ablation studies have provided conflicting results. Mice lacking the NPY gene were initially reported to show no changes in feeding and metabolism unless crossed with mice having a genetic defect in the leptin gene, but later studies did observe reduced food intake and body weight (Erickson et al., 1996). When each of the NPY receptor genes was disrupted, there was a late-onset increase in body weight, both for Y1 and Y5 as well as for Y2 (Kanatani et al., 2000b; Kushi et al., 1998; Marsh et al., 1998; Naveilhan et al., 1999; Pedrazzini et al., 1998).
It is important to study how the NPY system works in other animal species than rats and mice to get a broader perspective on this system. Evolutionary studies based on DNA data have shown that the guinea-pig (Cavia porcellus) is almost equally distantly related to rodents and humans (D'erchia et al., 1996), and thereby this animal could be a useful complementary model for NPY studies. Recently all of the guinea-pig NPY receptors have been cloned in our laboratory. The structural and pharmacological characteristics of the guinea-pig Y1 and Y2 receptors are similar to the human and rat orthologues (Berglund et al., 1999; Sharma et al., 1998). The guinea-pig Y5 receptor has highest amino acid identity to the human Y5 receptor reported for any non-primate NPY receptor orthologue and, in contrast to rat Y5, it displays a virtually identical pharmacological profile to the human Y5 (Lundell et al., 2001). Similarly, the guinea-pig Y4 receptor resembles better the human orthologue than does the rat Y4 (Eriksson et al., 1998). It has been reported that y6 is a functional gene in mice, but a pseudogene in primates and guinea-pigs, and absent in rats (Burkhoff et al., 1998; Matsumoto et al., 1996; Starbäck et al., 2000). The aim of the present study was to determine whether centrally administered NPY or the related peptides known to stimulate feeding behaviour in rodents would increase food intake in guinea-pigs. Furthermore, the receptor subtype modulating feeding behaviour was investigated by using selective Y1 and Y5 receptor antagonists.
Methods
Compounds
NPY (porcine), PYY (porcine), (Leu31,Pro34)NPY (porcine), NPY(2-36) (porcine), NPY(13-36) (porcine) were purchased from Bachem (King of Prussia, PA, U.S.A.). The peptides were dissolved in 0.9% saline. The Y1 receptor antagonist, BIBO 3304, (R)N-{[4-(aminocarbonylaminomethyl)phenyl]methyl}-N2-(diphenylacetyl)-argininamide trifluoroacetate, was a generous gift from Boehringer Ingelheim (Pharma, Germany). Another Y1 receptor antagonist, H 409/22, {(2R)-5-([amino(imino)methyl]amino)-2-[(2,2-diphenylacetyl) amino]-N-[(1R)-1-(4-hydroxyphenyl)ethylpentanamide} was provided by AstraHässle AB (Mölndal, Sweden). Both Y1 antagonists were dissolved in sterile water after brief sonication. The Y5 antagonist, CGP 71683A, [(4-{[(4-aminoquinazolin-2-yl)amino]methyl}-cyclohexyl)methyl]-(naphthylsulphonyl)amine, was synthesized by the Institute of Organic Synthesis (Riga, Latvia), and its chemical purity tests have been described elsewhere (Kask et al., 2001). It was dissolved in 30% DMSO.
Binding assays
Cells lines transfected with plasmids encoding the guinea-pig receptors were used for studying the selectivity and the affinities of the ligands. The agonist data shown in Table 1 have been reported previously (Berglund et al., 1999; Lundell et al., 2001; Sharma et al., 1998). The antagonists BIBO 3304, H 409/22 and CGP 71683A were tested on membrane fractions prepared using the same protocol. For binding assays, thawed aliquots of receptor membranes were resuspended in 25 mM HEPES buffer (pH 7.4) containing 2.5 mM CaCl2, 1 mM MgCl2 and 2 g l−1 Bacitracin (Sigma, St. Louis, MO, U.S.A.) and homogenized using an Ultra-Turrax homogenizer. Binding experiments were performed in a final volume of 100 μl with 2 – 10 μg protein and 125I-PYY (porcine) (Amersham Pharmacia Biotech) for 2 h at room temperature. Non-specific binding was defined as the amount of radioactivity remaining bound to the cell homogenate after incubation in the presence of 100 nM unlabelled NPY. In competition studies, various concentrations of the non-peptide compounds BIBO 3304, H 409/22 or CGP 71683A were included in the incubation mixture, along with 125I-PYY. The peptide NPY was used as a reference for each experiment. Incubations were terminated by rapid filtration through GF/C filters, which had been pre-soaked in 0.3% polyethyleneimine, using a TOMTEC (Orange, CT, U.S.A.) cell harvester. The filters were washed with 5 ml of 50 mM Tris (pH 7.4) at 4°C and dried at 60°C. The dried filters were treated with MeltiLex A (Wallac) melt-on scintillator sheets, and the radioactivity retained on the filters counted using the Wallac 1450 Betaplate counter. The results were analysed using the Prism software package (Graphpad, San Diego, CA, U.S.A.).
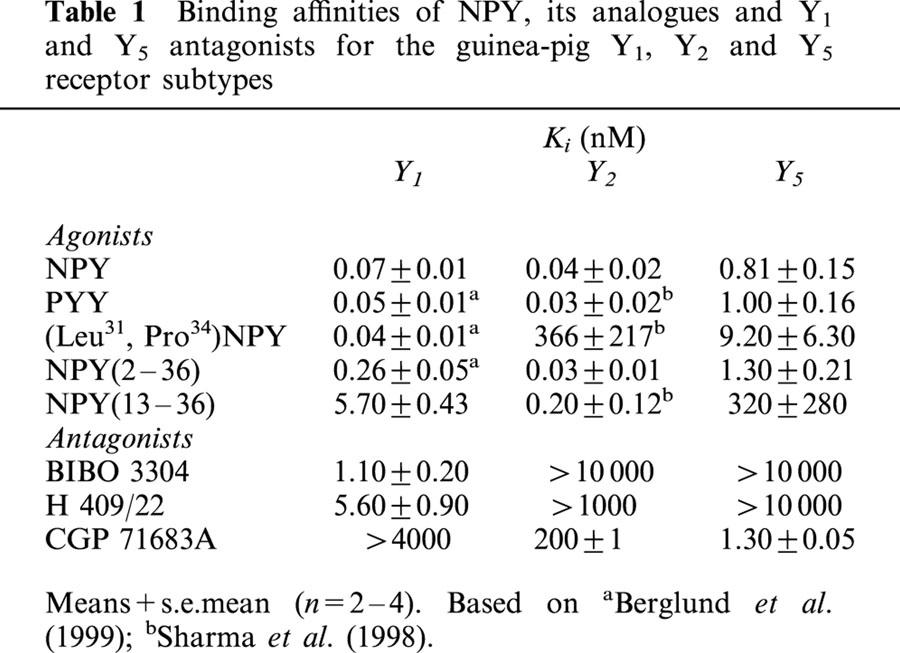
Table 1.
Binding affinities of NPY, its analogues and Y1 and Y5 antagonists for the guinea-pig Y1, Y2 and Y5 receptor subtypes
Animals and surgical procedures
The study was approved by the local ethical committee (C310/99 and C121/00). Juvenile male Dunkin – Hartley guinea-pigs (Bio Jet Service, Uppsala, Sweden) weighing 300 – 500 g were maintained in a 12-h light – dark cycle (lights on from 06.00 h to 18.00 h) in a temperature-controlled room (20 – 21°C). Two animals were housed in a polypropylene cage (60×80×25 cm) and kept separated by dividing each cage into two equal parts by a wall. Throughout the experiment, guinea-pigs were fed with powdered food (K5, Lactamin AB, Vadstena, Sweden) and hay was also freely available, except during the tests. Tap water was supplemented with approximately 0.5 mg ml−1 of L-ascorbic acid and was freely available. The feeding experiments were performed between 09.30 h and 18.00 h.
Guinea-pigs were anaesthetized by intraperitoneal (i.p.) injection of a 1 : 3 (v v−1) mixture of xylazine (Rompun vet. 20 mg ml−1, Bayer, Gothenberg, Sweden) and ketamine (Ketalar 50 mg ml−1, Parke Davis, Solna, Sweden). The animals were fixed into a stereotaxic frame (David Kopf Instruments, Tujunga, U.S.A.). The skull was exposed and a permanent stainless cannula (22 gauge) was implanted in the midline 6 mm below the bregma, according to the brain atlas of Luparello (1967). The guide cannula was fixed to the skull with screws and dental acrylic cement. The cannula was closed with a stainless steel stylet. After the surgical operation, animals were allowed to recover at least for 7 days. The animals were handled and weighed daily to habituate them to a partial restraint experience during i.c.v. infusions.
Experimental procedures
One hour before the drug administration, animals were moved into clean cages and food jars were removed. Different doses of NPY (0.9 – 10.8 nmol per animal) or saline were infused i.c.v. at a rate of 5 μl per min using a Hamilton infusion pump and syringes. The infusion volume was 10 μl. After the drug administration, the infusion cannula was left in place for an additional 1 min to avoid back diffusion along the cannula. The animals were returned to their home cage and food consumption was measured 1, 2, 3 and 4 h postinfusion. Food spillage was collected and subtracted from the intake. A video camera placed above the cage recorded the entire 4-h experiment. Afterwards, different eating parameters including the time spent on eating, number of meals and meal duration were analysed from the video tapes. Meals were divided into three categories based on duration: (1) short meals that lasted less than 1 min; (2) meals that lasted 1 – 5 min; (3) long meals that lasted over 5 min. Each animal received 2 – 4 different treatments in a randomized order with a 5 – 6 days recovery period between tests. At the end of the experiments, dye was infused and the staining of the third ventricle was examined.
Different doses of PYY (0.9 – 7.2 nmol per animal), (Leu31,Pro34)NPY (0.9 – 10.8 nmol per animal), NPY(2 – 36) (0.9 – 10.8 nmol per animal), NPY(13 – 36) (3.6 – 25 nmol per animal) were infused i.c.v. to guinea-pigs. Saline and NPY (3.6 nmol per animal) were used as reference treatments. Food consumption and different eating parameters were measured using the same protocol as above.
BIBO 3304 (30 nmol per animal), H 409/22 (100 – 200 nmol per animal) or saline were infused i.c.v. in a volume of 5 – 7 μl given 15 min before NPY (3.6 nmol in 5 μl) or saline. When the effect of CGP 71683A (60 nmol per animal) was tested, 30% dimethylsulphoxide (DMSO) was used as the vehicle. CGP 71683A or DMSO were infused in a volume of 10 μl 15 min before NPY (3.6 nmol in 5 μl) or saline. Food consumption (4 h response) and eating parameters were measured as above.
Calculations and statistical analysis
The mean and standard error of the mean (s.e.mean) were calculated. The statistical differences between groups were determined with one-way analysis of variance followed by the post hoc comparisons with the Dunnett test. When the presumptions of the one-way of analysis of variance were not fulfilled, the non-parametric tests (Kruskal – Wallis or Mann – Whitney) were used.
Results
NPY dose-dependently increased food intake in guinea-pigs. The NPY doses 3.6 nmol or higher caused statistically significant changes in food consumption (Figure 1). The treatment increased also time spent on eating and number of short and intermediate meals (⩽5 min) (Table 2). The number of long meals (>5 min) remained unchanged. NPY treatment had no effect on the eating rate, but at high doses it reduced the meal size (Table 2). It generally doubled the water consumption, but due to the large variation the changes were not statistically significant (Table 2). The effects of the reference treatments (saline and NPY 3.6 nmol) on food intake were similar in all parts of the study, confirming the reliability of the test conditions (Tables 2,3,4).
Figure 1.
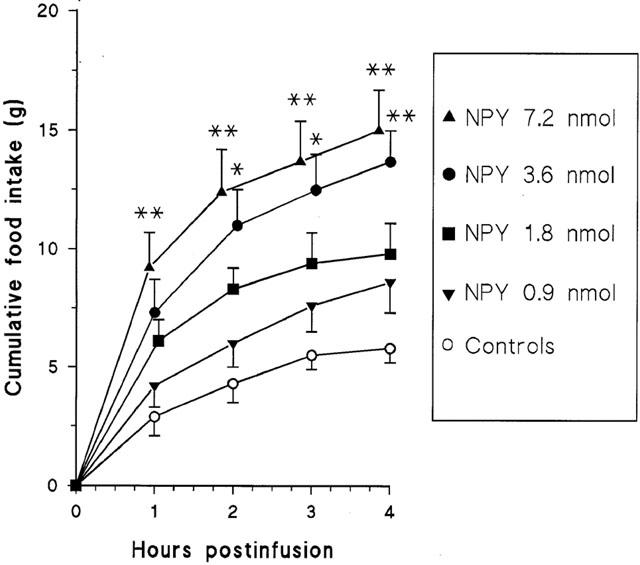
Cumulative food intake induced by different doses of NPY following i.c.v. administration to conscious guinea-pigs. Mean±s.e.mean, n=8 in each group. Asterisks indicate values which are statistically different from the control values: *P<0.05; **P<0.01.
Table 2.
Eating parameters and water consumption after i.c.v. infusion of saline or different NPY doses in conscious guinea-pigs
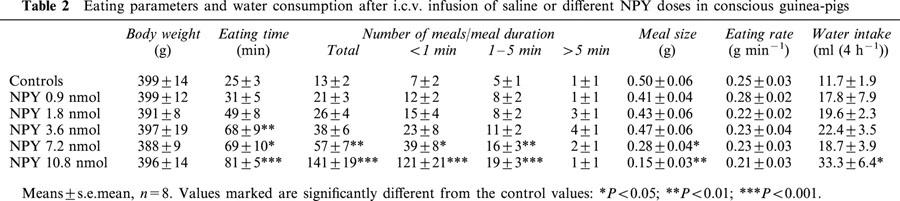
Table 3.
Eating parameters and water consumption after i.c.v. infusion of NPY analogues or saline in conscious guinea-pigs
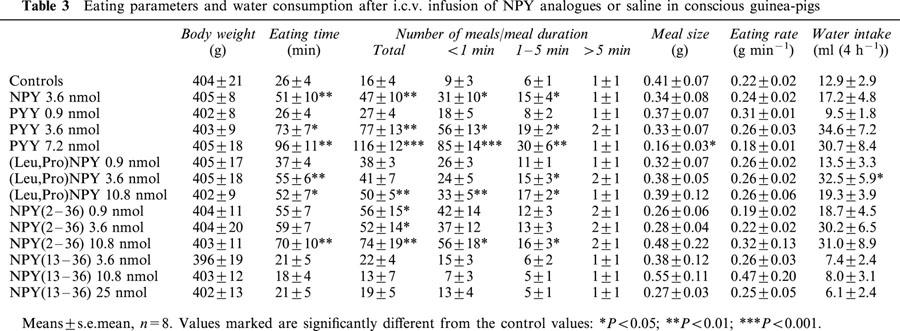
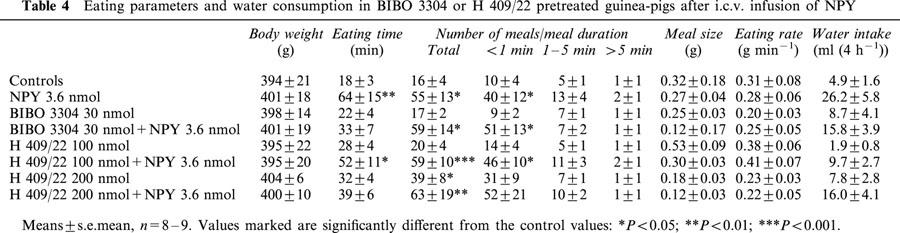
Table 4.
Eating parameters and water consumption in BIBO 3304 or H 409/22 pretreated guinea-pigs after i.c.v. infusion of NPY
Peptides PYY, (Leu31,Pro34)NPY and NPY(2 – 36) were able to increase the 4-h feeding response in guinea-pigs (Figure 2). In this respect, these three peptides were almost equipotent with NPY. PYY potently increased the time spent on eating and the meal frequency. The highest PYY dose reduced the average meal size. (Leu31,Pro34)NPY and NPY(2 – 36) also increased the time spent on eating and the meal frequency, but their dose-response curves were flatter compared to NPY and PYY (Figure 2). All peptides tended to increase the water intake (Table 3). On the other hand, NPY(13 – 36) caused no marked changes in food (Figure 2) or water consumption (Table 3).
Figure 2.
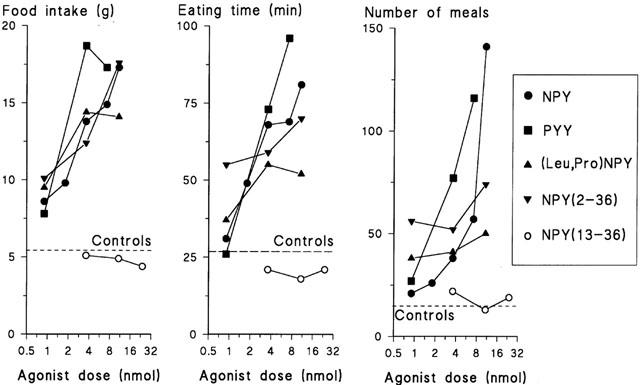
Food intake (left panel), eating time (middle panel) and number of meals (right panel) after i.c.v. administration of NPY, PYY, (Leu31,Pro34)NPY, NPY(2 – 36) and NPY(13 – 36) to conscious guinea-pigs. Means, n=8. Original data are shown in Tables 2 and 3.
The Y1 antagonists, BIBO 3304 and H 409/22, as expected had high affinity for membranes from cells transfected with the guinea-pig Y1 receptor subtype, but not for membranes with either Y2 or Y5 receptors as determined by competition with 125I-PYY (Table 1). The Y5 antagonist, CGP 71683A had high affinity for membranes from cells with the guinea-pig Y5 receptor, but was almost inactive on cell membranes with either guinea-pig Y1 or Y2 (Table 1).
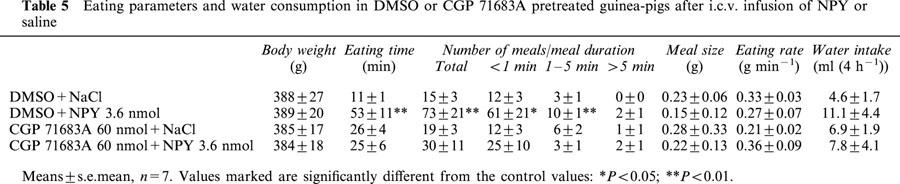
BIBO 3304 at the dose of 30 nmol and H 409/22 at the dose of 200 nmol were able to inhibit the feeding response to NPY, while the lower H 409/22 dose was ineffective (Figure 3). Both Y1 antagonists tended to reduce the time spent on eating but caused no change in the number of meals (Table 4). When H 409/22 was given alone, it had a modest stimulatory effect on feeding (Figure 3), but we did not observe any other notable changes in guinea-pigs' behaviour. As shown in Figure 4, the Y5 antagonist, CGP 71683A suppressed NPY induced food intake. The compound tended to decrease the time spent on eating and the meal frequency in NPY treated animals, but the changes were not statistically significant (Table 5).
Figure 3.

The effect of NPY (3.6 nmol i.c.v.) on 4 h food intake (upper panel) and number of meals (lower panel) in conscious guinea-pigs pretreated with the Y1 receptor antagonists, BIBO 3304 and H 409/22. Mean±s.e.mean, n=8 – 9. Asterisks indicate values which are statistically different from the controls or the group otherwise indicated: *P<0.05; **P<0.01; ***P<0.001.
Figure 4.
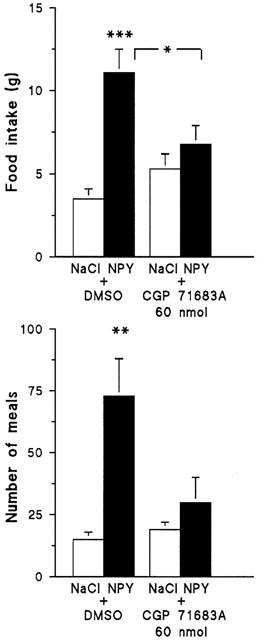
The effect of NPY (3.6 nmol i.c.v.) on 4 h food intake (upper panel) and number of meals (lower panel) in conscious guinea-pigs pretreated with the Y5 receptor antagonist, CGP 71683A. Mean±s.e.mean, n=7. Asterisks indicate values which are statistically different from the controls or the group otherwise indicated: *P<0.05; **P<0.01; ***P<0.001.
Table 5.
Eating parameters and water consumption in DMSO or CGP 71683A pretreated guinea-pigs after i.c.v. infusion of NPY or saline
Discussion
After infusion into the third brain ventricle, NPY increased food intake in guinea-pigs in a dose-related manner. This feeding-stimulating effect of NPY has been reported in most vertebrates that have been studied, including mouse (Iyengar et al., 1999), rat (Levine & Morley, 1984; Morley et al., 1987a, 1987b), golden hamster (Kulkosky et al., 1989), ground squirrel (Boswell et al., 1993), rabbit (Pau et al., 1988), pig (Parrot et al., 1986), sheep (Miner et al., 1989), rhesus monkey (Larsen et al., 1999), bird (Kuenzel et al., 1987; Richardson et al., 1995), snake (Morris & Crews, 1990) and fish (Volkoff & Peter, 2001). In the dog, feeding was stimulated by PP, not by NPY or PYY (Inui et al., 1991). The single exception is a study with male baboons that was unable to demonstrate any effect of central NPY administration on food intake (Sipols et al., 1996). Here, the effective i.c.v. dose range for guinea-pigs was comparable to that reported for rats and mice (Levine & Morley, 1984; Morley et al., 1987a).
The herbivorous guinea-pig has a stomach of small volume that necessitates a frequent intake of small meals (Hirsch, 1973). The animal does not show diurnal fluctuation in its feeding pattern, and thus it eats as much during the light as the dark period (Hirsch, 1973; Horton et al., 1975), whereas the rat as a nocturnal animal eats most of its daily intake during the dark period. In the present study, the control animals spent 11 – 26 min during the 4 h follow-up on eating. Their meal frequency was between 13 – 17 per 4 h, and the meals usually lasted less than 5 min. In agreement with this, Jilge (1985) has reported that guinea-pigs in laboratory conditions eat approximately 99 times per 24 h and the average meal duration is 67 s. Longer meals (6 – 10 min) have been observed by Hirsch (1973), but the number of meals in his study was also 4 – 5 times smaller than in our study. According to Hirsch (1973), meal frequency is a parameter independent of age, lighting conditions and water availability. As juvenile animals grow, they start to eat larger meals at a faster rate. Therefore, it was important that the average animal weights in experimental groups were adjusted to be as close to each other as possible (390±15 g).
When NPY was infused i.c.v. to guinea-pigs, not only the amount of food eaten increased but also other components of feeding behaviour such as the time spent on eating and the number of meals were elevated (Table 2). In a manner similar to NPY, food intake was stimulated by PYY, the analogue (Leu31,Pro34)NPY and the N-terminally truncated fragment, NPY(2 – 36). The peptides were all equally potent (Figure 2). The orexigenic activity of the peptide analogues did not directly correlate with affinity for one subtype of NPY receptors. As shown in Table 1, NPY and PYY bind with high affinity to the guinea-pig receptors Y1, Y2 and Y5, whereas NPY(2 – 36) is potent at Y2 and Y5. On the other hand, (Leu31,Pro34)NPY binds with high affinity to the Y1 receptor, but its affinity for the guinea-pig Y5 receptor is only moderate. Among the peptides tested, those activating both Y1 and Y5 receptors increased eating time and number of meals more potently than those stimulating either receptor subtype alone. Since food intake was not stimulated by NPY(13 – 36), a peptide which exhibits low affinity for Y1 and therefore could be expected to stimulate mainly Y2 receptors, the Y2 receptor activation seems not to elicit feeding. Nor did this Y2 agonism inhibit food intake. The combined peptide data are interpreted to mean that ligands activating either Y1 or Y5 receptors are capable of stimulating food intake in guinea-pigs.
In binding studies using cell lines stably expressing guinea-pig NPY receptors, BIBO 3304 and H 409/22, a novel Y1 antagonist structurally related to BIBP 3226, the first Y1 antagonist developed, were potent and selective ligands that could discriminate between Y1 and Y5 receptors. They displayed nanomolar affinity for the Y1 receptor, BIBO 3304 being five times more potent than H 409/22 (Table 1). Both were devoid of affinity for the Y5 receptor. The pretreatment with either 30 nmol of BIBO 3304 or by 200 nmol of H 409/22 was able to block the feeding response to exogenous NPY in guinea-pigs. The Y1 antagonists suppressed the amount of food consumed and tended to reduce eating time after NPY, but caused no change in the number of meals. When H 409/22 was given alone, it had a modest orexigenic activity. A similar kind of elicitation of feeding has been observed after administration of 1229U91, another Y1 receptor antagonist (Haynes et al., 1998). Since BIBO 3304 and H 409/22 do not block Y5 receptors, their inhibitory actions on NPY induced food intake imply that Y1 receptors are mediating this response. In agreement with the present results, it has been reported that BIBO 3304 inhibits NPY, (Leu31,Pro34)NPY and NPY(2 – 36) induced as well as fasting induced food intake in rats (Polidori et al., 2000; Wieland et al., 1998). The compound also inhibits the hyperphagia after orexin-A administration (Yamanaka et al., 2000), but not that obtained after galanin or norepinephrine (Wieland et al., 1998).
At present, no other Y5 antagonists than CGP 71683A has been publicly available, and therefore it has been used as a reference compound when studying the role of the Y5 receptor. CGP 71683A has been shown to be a competitive antagonist of the Y5 receptor in functional assays in cell lines and isolated organs (Criscione et al., 1998; Duhault et al., 2000; Dumont et al., 2000). In the present study, it had high affinity for the guinea-pig Y5 receptor without showing any significant activities at the Y1 and Y2 subtypes (Table 1). When guinea-pigs were pretreated with CGP 71683A, the feeding response to NPY was markedly attenuated, suggesting that the compound is an effective antagonist of NPY-induced feeding mediated through Y5 receptors. Previous rodent data have also shown the inhibitory effects of CGP 71683A on NPY-induced food intake in lean and obese animals (Della Zuana et al., 2001; Duhault et al., 2000; Polidori et al., 2000) and on spontaneous food intake in diabetic, 24 h fasted and free-feeding animals (Criscione et al., 1998; Kask et al., 2001).
It is interesting that the blockade of Y1 and Y5 receptors had different effects on the number of meals consumed by NPY treated animals. We had initially expected that the number of meals should correlate with the amount of food ingested, but both Y1 antagonists suppressed food intake in NPY treated animals without reducing meal frequency (Figure 3), whereas the Y5 antagonist reduced both food intake and meal frequency (Figure 4). Since NPY has a robust anxiolytic-like effect (Heilig et al., 1989), while the blockade of Y1 receptors increases anxiety (Kask et al., 2001), it is possible that the effects of ligands on the anxiety level may also modulate feeding behaviour, assuming that the compounds can reach the centres for anxiety, primarily the amygdala, during the 4 h period of the experiment. On the other hand, high doses of NPY and PYY seemed to induce hyperactivity in guinea-pigs, and one reflection of this activation is the increased amount of small meals consumed (Tables 2 and 3). Indeed, injections of NPY into frontal cortex or lateral brain ventricle in rats have been shown to increase locomotion and exploration (Levine & Morley, 1984; Morley et al., 1987b; Smialowski et al., 1992), although not in all studies (Heilig et al., 1988). Our observation that increased locomotion might be connected to Y5 receptor stimulation is in agreement with the previous finding showing that the Y5 antagonist, CGP 71683A, decreases exploratory behaviour in rats (Kask et al., 2001). The suppression of food intake after CGP 71683A administration is not, however, related to the changes in locomotor activity (Kask et al., 2001).
It has been reported that CGP 71683A binds to the serotonin (5-HT) re-uptake recognition site and cholinergic muscarinic receptors with virtually identical affinity to its binding to Y5 receptors (Della Zuana et al., 2001). Furthermore, chronic administration of CGP 71683A may produce local inflammatory changes near the site of injection (Della Zuana et al., 2001). Due to these non-specific actions and its poor solubility, CGP 71683A is not a good tool to use in vivo, and new selective Y5 antagonists are needed to characterize the physiological importance of the Y5 receptor. Recent reports on novel agents blocking Y5 receptors do not entirely support the view that Y5 receptors are crucial for NPY-induced feeding in rats (Kanatani et al., 2000a; Polidori et al., 2000). Therefore, our results with CGP 71683A in the guinea-pig should be regarded with caution until investigated with other Y5 antagonists.
The role of NPY in the modulation of food intake is well established in rats and mice (see Kalra et al., 1999 for review). Here, we have shown that NPY stimulates feeding behaviour also in guinea-pigs. Even if there are species differences in the level of expression and distribution of the NPY receptor subtypes in the brain (Dumont et al., 1998; Gehlert & Gackenheimer, 1997), and in the binding characteristics of the cloned receptors (Lundell et al., 2001; Eriksson et al., 1998; Starbäck et al., 2000), NPY and its analogues, as well as the Y1 and Y5 antagonists, seemed to behave in a similar manner in guinea-pigs as reported earlier for rats (Haynes et al., 1998; Polidori et al., 2000; Wieland et al., 1998). Our results favour the hypothesis that both Y1 and Y5 receptors mediate NPY-induced food intake. Duhault et al. (2000) have recently shown that combined administration of Y1 and Y5 receptor antagonists is a more efficient intervention to inhibit food intake than the use of either antagonist alone. Although Y1 or Y5 knockout mice do not show overt disturbances of body weight and food intake, they develop late-onset obesity through different mechanisms. Y1 deficient mice have lowered metabolic rate, while Y5 deficient mice appear to be hyperphagic (Kanatani et al., 2000b; Kushi et al., 1998; Marsch et al., 1998; Pedrazzini et al., 1998). Furthermore, feeding responses to NPY are lowered in both Y1 and Y5 deficient mice compared to wild-type animals (Kanatani et al., 2001). These observations generally support the view that both receptor subtypes are involved in food intake regulation, but through different mechanisms. This issue could be addressed by developing mutant mice lacking both the Y1 and Y5 receptors. Another approach to study the contribution of Y1 and Y5 receptors to food intake regulation is to develop highly subtype selective agonists for the NPY receptors. Such compounds have been described for the Y1 and Y5 receptors (Cabrele et al., 2000; Söll et al., 2001) and their in vivo effects in guinea-pigs are under investigation in our laboratory.
In conclusion, the results presented here show that NPY through Y1 and Y5 receptors stimulates feeding behaviour in the guinea-pig as it does in the rat. As guinea-pigs and rats are very distantly related, this suggests that the dual involvement of Y1 and Y5 receptors may reflect the ancestral situation for mammals before the radiation of the present mammalian orders. Our results help provide a firmer basis for extrapolation to humans regarding the role of NPY and its receptors in the control of food intake.
Acknowledgments
The authors wish to thank Ms Suvi Salmela and Ms Ulrika Lönngren for skilful technical assistance and Dr Bengt Meyerson for advice. We are grateful to Dr E. Rusk at Boehringer Ingelheim for kindly providing the compound BIBO 3304 and Dr M. Nordlander at AstraHässle AB for H 409/22. This work was supported by grants from the Swedish Natural Science Research Council, the Wenner-Gren Foundation, the Saastamoinen Foundation and the Academy of Finland.
Abbreviations
- BIBO 3304
(R)N-{[4-(aminocarbonylaminomethyl)phenyl]methyl}-N2-(diphenylacetyl)-argininamide trifluoroacetate
- CGP 71683A
[(4-{[(4-aminoquinazolin-2-yl)amino]methyl}-cyclohexyl)methyl]-(naphthylsulphonyl)amine
- H 409/22
{(2R)-5-([amino(imino)methyl]amino)-2-(2,2-diphenylacetyl) amino]-N-[(1R)-1-(4-hydroxyphenyl)ethylpentanamide}
- NPY
neuropeptide Y
- PP
pancreatic polypeptide
- PYY
peptide YY
References
- BERGLUND M.M., HOLMBERG S.K.S., ERIKSSON H., GEDDA K., MAFFRAND J.P., SERRADEIL-LE GAL C., CHHAJLANI V., GRUNDEMAR L., LARHAMMAR D. The cloned guinea-pig neuropeptide Y receptor Y1 conforms to other mammalian Y1 receptors. Peptides. 1999;20:1043–1053. doi: 10.1016/s0196-9781(99)00098-4. [DOI] [PubMed] [Google Scholar]
- BOSWELL T., RICHARDSON R.D., SCHWARTZ M.W., D'ALESSIO D.A., WOODS S.C., SIPOLS A.J., BASKIN D.G., KENAGY G.J. NPY and galanin in a hibernator: hypothalamic gene expression and effects on feeding. Brain Res. Bull. 1993;32:379–384. doi: 10.1016/0361-9230(93)90203-n. [DOI] [PubMed] [Google Scholar]
- BURKHOFF A.M., LINEMEYER D.L., SALON J.A. Distribution of a novel hypothalamic neuropeptide receptor gene and its absence in rat. Mol. Brain. Res. 1998;53:311–316. doi: 10.1016/s0169-328x(97)00302-1. [DOI] [PubMed] [Google Scholar]
- CABRELE C., LANGER M., BADER R., WIELAND H.A., DOODS H.N., ZERBE O., BECK-SICKINGER A.G. The first selective agonist for the neuropeptide Y Y5 receptor increases food intake in rats. J. Biol. Chem. 2000;46:36043–36048. doi: 10.1074/jbc.M000626200. [DOI] [PubMed] [Google Scholar]
- CRISCIONE L., RIGOLLIER P., BATZL-HARTMANN C., RUEGER H., STRICKER-KRONGRAD A., WYSS P., BRUNNER L., WHITEBREAD S., YAMAGUCHI Y., GERALD C., HEURICH R.O., WALKER M.W., CHIESI M., SCHILLING W., HOFBAUER K.G., LEVENS N. Food intake in free-feeding and energy-deprived lean rats is mediated by the neuropeptide Y5 receptor. J. Clin. Invest. 1998;102:2136–2145. doi: 10.1172/JCI4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELLA ZUANA O., SADLO M., GERMAIN M., FELETOU M., CHAMORRO S., TISSERAND F., DE MONTRION C., BOIVIN J.F., DUHAULT J., BOUTIN J.A., LEVENS N. Reduced food intake in response to CGP 71683A may be due to mechanisms other than NPY Y5 receptor blockade. Int. J. Obesity. 2001;25:84–94. doi: 10.1038/sj.ijo.0801472. [DOI] [PubMed] [Google Scholar]
- D'ERCHIA A.M., GISSI C., PESOLE G., SACCONE C., ARNASON U. The guinea-pig is not a rodent. Nature. 1996;381:597–600. doi: 10.1038/381597a0. [DOI] [PubMed] [Google Scholar]
- DUHAULT J., BOULANGER M., CHAMORRO S., BOUTIN J.A., DELLA ZUANA O., DOUILLET E., FAUCHERE J.L., FELETOU M., GERMAIN M., HUSSON B., MONGE VEGA A., RENARD P., TISSERAND F. Food intake regulation in rodents: Y5 or Y1 NPY receptors or both. Can. J. Physiol. Pharmacol. 2000;78:173–185. [PubMed] [Google Scholar]
- DUMONT Y., CADIEUX A., DOODS H., FOURNIER A., QUIRION R. Potent and selective tools to investigate neuropeptide Y receptors in the central and peripheral systems: BIBO3304 (Y1) and CGP71683A (Y5) Can. J. Physiol. Pharmacol. 2000;78:116–125. [PubMed] [Google Scholar]
- DUMONT Y., JACQUES D., BOUCHARD P., QUIRION R. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea-pigs, and primates brains. J. Comp. Neurol. 1998;402:372–384. [PubMed] [Google Scholar]
- ERICKSON J.C., HOLLOPETER G., PALMITER R.D. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science 1996. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- ERIKSSON H., BERGLUND M.M., HOLMBERG S.K.S., KAHL U., GEHLERT D.R., LARHAMMAR D. The cloned guinea-pig pancreatic polypeptide receptor Y4 resembles more the human Y4 than does the rat Y4. Regul. Pept. 1998;75–76:29–37. doi: 10.1016/s0167-0115(98)00050-0. [DOI] [PubMed] [Google Scholar]
- GEHLERT D.R., GACKENHEIMER S.L. Differential distribution of neuropeptide Y Y1 and Y2 receptors in rat and guinea-pig brains. Neuroscience. 1997;76:215–224. doi: 10.1016/s0306-4522(96)00340-5. [DOI] [PubMed] [Google Scholar]
- HAYNES A.C., ARCH J.R.S., WILSON S., MCCLUE S., BUCKINGHAM R.E. Characterisation of the neuropeptide Y receptor that mediates feeding in the rat: a role for the Y5 receptor. Regul. Pept. 1998;75–76:355–361. doi: 10.1016/s0167-0115(98)00088-3. [DOI] [PubMed] [Google Scholar]
- HEILIG M., SÖDERPALM P., ENGEL J.A., WIDERLÖV E. Cenrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal models. Psychopharmacology. 1989;98:524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- HEILIG M., WAHLESTEDT C., WIDERLÖV E. Neuropeptide Y (NPY)-induced suppression of activity in the rat: evidence for NPY receptor heterogenity and for interaction with α-adrenoceptors. Eur. J. Pharmacol. 1988;157:205–213. doi: 10.1016/0014-2999(88)90384-6. [DOI] [PubMed] [Google Scholar]
- HERZOG H., HORT Y.J., SHINE J., SELBIE L.A. Molecular cloning, characterization and localization of the human homolog to the reported bovine NPY Y3 receptor: lack of NPY binding and activation. DNA Cell. Biol. 1993;12:465–471. doi: 10.1089/dna.1993.12.465. [DOI] [PubMed] [Google Scholar]
- HIRSCH E. Some determinants of intake and patterns of feeding in the guinea-pig. Physiol. Behav. 1973;11:687–704. doi: 10.1016/0031-9384(73)90255-2. [DOI] [PubMed] [Google Scholar]
- HORTON B.J., WEST C.E., TURLEY S.D. Diurnal variation in the feeding pattern of guinea-pigs. Nutr. Metabol. 1975;18:294–301. doi: 10.1159/000175607. [DOI] [PubMed] [Google Scholar]
- INUI A., OKITA M., NAKAJIMA M., INOUE T., SAKATANI N., OYA M., MORIOKA H., OKIMURA Y., CHIHARA K., BABA S. Neuropeptide regulation of feeding in dogs. Am. J. Physiol. 1991;261:R588–R594. doi: 10.1152/ajpregu.1991.261.3.R588. [DOI] [PubMed] [Google Scholar]
- IYENGAR S., LI D.L., SIMMONS R.M.A. Characterization of neuropeptide Y-induced feeding in mice: do Y1-Y6 receptor subtypes mediated feeding. J. Pharmacol. Exp. Ther. 1999;289:1031–1040. [PubMed] [Google Scholar]
- JAZIN E.E., YOO H., BLOMQVIST A.G., WENG G., WALKER M.W., SALON J., LARHAMMAR D., WAHLESTEDT C. A proposed bovine neuropeptide Y (NPY) receptor, or its human homologue, confers neither NPY binding sites nor NPY responsiveness on transfected cells. Regul. Pept. 1993;47:247–258. doi: 10.1016/0167-0115(93)90392-l. [DOI] [PubMed] [Google Scholar]
- JILGE B. The rhythm of food and water ingestion, faeces excretion and locomotor activity in the guinea-pig. Z. Versuchstierk. 1985;27:215–225. [PubMed] [Google Scholar]
- KALRA S.P., DUBE M.G., PU S., XU B., HORVATH T.L., KALRA P.S. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocrine Reviews. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- KANATANI A., HATA M., MASHIKO S., ISHIHARA A., OKAMOTO O., HAGA Y., OHE T., KANNO T., MURAI N., ISHII Y., FUKURODA T., FUKAMI T., IHARA M. A typical Y1 receptor regulates feeding behaviors: Effects of a potent and selective Y1 antagonist, J-115814. Mol. Pharmacol. 2001;59:501–505. doi: 10.1124/mol.59.3.501. [DOI] [PubMed] [Google Scholar]
- KANATANI A., HATA M., MASHIKO S., ISUGIMOTO N., ITO J., FUKURODA T., FUKAMI T., MORIN N., MACNEIL D.J., VAN DER PLOEG L.H.T., SAGA Y., NISHIMURA S., IHARA M. Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology. 2000b;141:1011–1016. doi: 10.1210/endo.141.3.7387. [DOI] [PubMed] [Google Scholar]
- KANATANI A., ISHIHARA A., IWAASA H., NAKAMURA K., OKAMOTO O., HIDAKA M., ITO J., FUKURODA T., MACNEIL D.J., VAN DER PLOEG L.H.T., ISHII Y., OKABE T., FUKAMI T., IHARA M. L-152,804: Orally active and selective neuropeptide Y Y5 receptor antagonist. Biochem. Biophys. Res. Commun. 2000a;272:169–173. doi: 10.1006/bbrc.2000.2696. [DOI] [PubMed] [Google Scholar]
- KASK A., VASAR E., HEIDMETS L.T., ALLIKMETS L., WIKBERG J.E.S. Neuropeptide Y Y5 receptor antagonist CGP71683A: the effects on food intake and anxiety-related behavior in the rat. Eur. J. Pharmacol. 2001;414:215–224. doi: 10.1016/s0014-2999(01)00768-3. [DOI] [PubMed] [Google Scholar]
- KUENZEL W.J., DOUGLASS L.W., DAVISON B.A. Robust feeding following central administration of neuropeptide Y or peptide YY in chicks, Gallus domesticus. Peptides. 1987;8:823–828. doi: 10.1016/0196-9781(87)90066-0. [DOI] [PubMed] [Google Scholar]
- KULKOSKY P.J., GLAZNER G.W., MOORE H.D., LOW C.A., WOODS S.C. Neuropeptide Y: Behavioral effects in the golden hamster. Peptides. 1989;9:1389–1393. doi: 10.1016/0196-9781(88)90207-0. [DOI] [PubMed] [Google Scholar]
- KUSHI A., SASAI H., KOIZUMI H., TAKEDA N., YOKOYAMA M., NAKAMURA M. Obesity and mild hyperinsulinemia found in neuropeptide Y-Y1 receptor-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15659–15664. doi: 10.1073/pnas.95.26.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARHAMMAR D. Evolution of neuropeptide Y, peptide YY, and pancreatic polypeptide. Regul. Pept. 1996;62:1–11. doi: 10.1016/0167-0115(95)00169-7. [DOI] [PubMed] [Google Scholar]
- LARHAMMAR D., WRAITH A., BERGLUND M.M., HOLMBERG S.K.S., LUNDELL I. Origins of the many NPY-family receptors in mammals. Peptides. 2001;22:295–307. doi: 10.1016/s0196-9781(01)00331-x. [DOI] [PubMed] [Google Scholar]
- LARSEN P.J., TANG-CHRISTENSEN M., STIDSEN C.E., MADSEN K., SMITH M.S., CAMERON J.L. Activation of central neuropeptide Y Y1 receptors potently stimulates food intake in male rhesus monkeys. J. Clin. Endocrinol. Metab. 1999;84:3781–3791. doi: 10.1210/jcem.84.10.5897. [DOI] [PubMed] [Google Scholar]
- LEVINE A.S., MORLEY J.E. Neuropeptide Y: a potent inducer of consummatory behavior in rats. Peptides. 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- LUNDELL I., ERIKSSON H., MARKLUND U., LARHAMMAR D. Cloning and characterization of the guinea-pig neuropeptide Y receptor Y5. Peptides. 2001;22:357–363. doi: 10.1016/s0196-9781(01)00338-2. [DOI] [PubMed] [Google Scholar]
- LUPARELLO T.J. Stereotaxic atlas of the forebrain of the guinea-pig. Basel: S. Karger AG; 1967. p. 79. [Google Scholar]
- MARSH D.J., HOLLOPETER G., KAFER K.E., PALMITER R.D. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat. Med. 1998;4:718–721. doi: 10.1038/nm0698-718. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO M., NOMURA T., MOMOSE K., IKEDA Y., KONDOU Y., AKIHO H., TOGAMI J., KIMURA Y., OKADA M., YAMAGUCHI T. Inactivation of a novel neuropeptide Y/peptide YY receptor gene in primate species. J. Biol. Chem. 1996;271:27217–27220. doi: 10.1074/jbc.271.44.27217. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., BECK-SICKINGER A., COX H., DOODS H.N., HERZOG H., LARHAMMAR D., QUIRION R., WESTFALL T. XVI International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- MINER J.L., DELLA-FERA A., PATERSON J.A., BAILE C.A. Lateral cerebroventricular injection of neuropeptide Y stimulates feeding in sheep. Am. J. Physiol. 1989;257:R383–R387. doi: 10.1152/ajpregu.1989.257.2.R383. [DOI] [PubMed] [Google Scholar]
- MORLEY J.E., HERNANDEZ E.N., FLOOD J.F. Neuropeptide Y increases food intake in mice. Am. J. Physiol. 1987a;253:R516–R522. doi: 10.1152/ajpregu.1987.253.3.R516. [DOI] [PubMed] [Google Scholar]
- MORLEY J.E., LEVINE A.S., GOSNELL B.A., KNEIP J., GRACE M. Effect of neuropeptide Y on ingestive behaviors in the rat. Am. J. Physiol. 1987b;252:R599–R609. doi: 10.1152/ajpregu.1987.252.3.R599. [DOI] [PubMed] [Google Scholar]
- MORRIS Y.A., CREWS D. The effects of exogenous neuropeptide Y on feeding and sexual behavior in the red-sided garter snake (Thamnophis sirtalis parietalis) Brain Res. 1990;530:339–341. doi: 10.1016/0006-8993(90)91307-3. [DOI] [PubMed] [Google Scholar]
- NAVEILHAN P., HASSANI H., CANALS J.M., EKSTRAND A.J., LAREFALK Å., CHHAJLANI V., ARENAS E., GEDDA K., SVENSSON L., THOREN P., ERNFORS P. Normal feeding behavior, body weight and leptin response require the neuropeptide Y Y2 receptor. Nature Medicine. 1999;5:1188–1193. doi: 10.1038/13514. [DOI] [PubMed] [Google Scholar]
- PARROT R.F., HEAVENS R.P., BALDWIN B.A. Stimulation of feeding in the satiated pig by intracerebroventricular injection of neuropeptide. Y. Physiol. Behav. 1986;36:523–525. doi: 10.1016/0031-9384(86)90325-2. [DOI] [PubMed] [Google Scholar]
- PAU M.Y.C., PAU K.Y.F., SPIES H.G. Characterization of central actions of neuropeptide Y on food and water intake in rabbits. Physiol. Behav. 1988;44:797–802. doi: 10.1016/0031-9384(88)90065-0. [DOI] [PubMed] [Google Scholar]
- PEDRAZZINI T., SEYDOUX J., KUNSTNER P., AUBERT J.F., GROUZMANN E., BEERMANN F., BRUNNER H.R. Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nature Medicine. 1998;4:722–726. doi: 10.1038/nm0698-722. [DOI] [PubMed] [Google Scholar]
- POLIDORI C., CICCOCIOPPO R., REGOLI D., MASSI M. Neuropeptide Y receptor(s) mediating feeding in the rat: characterization with antagonists. Peptides. 2000;21:29–35. doi: 10.1016/s0196-9781(99)00170-9. [DOI] [PubMed] [Google Scholar]
- RICHARDSON R.D., BOSWELL T., RAFFETY B.D., SEELEY R.J., WINGFIELD J.C., WOODS S.C. NPY increases food intake in white-crowned sparrows: effect of short and long photoperiods. Am. J. Physiol. 1995;268:R1418–R1422. doi: 10.1152/ajpregu.1995.268.6.R1418. [DOI] [PubMed] [Google Scholar]
- SHARMA P., HOLMBERG S.K.S., ERIKSSON H., BECK-SICKINGER A.G., GRUNDEMAR L., LARHAMMAR D. Cloning and functional expression of the guinea-pig neuropeptide Y Y2 receptor. Regul. Pept. 1998;75–76:23–28. doi: 10.1016/s0167-0115(98)00049-4. [DOI] [PubMed] [Google Scholar]
- SIPOLS A.J., FIGLEWICZ D.P., SEELEY R.J., CHAVEZ M., WOODS S.C., PORTE D.J. Intraventricular neuropeptide Y does not stimulate food intake in the baboon. Physiol. Behav. 1996;60:717–719. doi: 10.1016/0031-9384(96)00105-9. [DOI] [PubMed] [Google Scholar]
- SMIALOWSKI A., LEWINSKA-GASTOL L., SMIALOWSKA M. Behavioural effects of neuropeptide Y (NPY) injection into the rat brain frontal cortex. Neuropeptides. 1992;21:153–156. doi: 10.1016/0143-4179(92)90038-x. [DOI] [PubMed] [Google Scholar]
- STANLEY B.G., LEIBOWITZS S.F. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc. Natl. Acad. Sci. U.S.A. 1985;82:3940–3943. doi: 10.1073/pnas.82.11.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARBÄCK P., WRAITH A., ERIKSSON H., LARHAMMAR D. Neuropeptide Y receptor gene y6: multiple deaths or resurrection. Biochem. Biophys. Res. Commun. 2000;277:264–269. doi: 10.1006/bbrc.2000.3656. [DOI] [PubMed] [Google Scholar]
- SÖLL R.M., DINGER M.C., LUNDELL I., LARHAMMAR D., BECK-SICKINGER A.G. Novel analogues of neuropeptide Y with a preference for the Y1-receptor. Eur. J. Biochem. 2001;268:2828–2837. doi: 10.1046/j.1432-1327.2001.02161.x. [DOI] [PubMed] [Google Scholar]
- VOLKOFF H., PETER R.E. Interactions between orexin A, NPY and galanin in the control of food intake of the goldfish, Carassius auratus. Regul. Pept. 2001;101:59–72. doi: 10.1016/s0167-0115(01)00261-0. [DOI] [PubMed] [Google Scholar]
- WIELAND H.A., ENGEL W., EBERLEIN W, , RUDOLF K., DOODS H.N. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br. J. Pharmacol. 1998;125:549–555. doi: 10.1038/sj.bjp.0702084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMANAKA A., KUNII K., NAMBU T, , TSUJINO N., SAKAI A., MATSUZAKI I., MIWA Y., GOTO K., SAKURAI T. Orexin-induced food intake involves neuropeptide Y pathway. Brain Res. 2000;859:404–409. doi: 10.1016/s0006-8993(00)02043-6. [DOI] [PubMed] [Google Scholar]


