Abstract
Endothelium-dependent and -independent regulation of vascular tone in small mesenteric arteries (SMA) from control (db/db +/?) and diabetic (db/db −/−) mice was compared.
Phenylephrine-induced maximum contraction, but not sensitivity, of SMA in db/db −/− compared to db/db +/? was enhanced.
Acetylcholine (ACh), but not sodium nitroprusside (SNP), -induced relaxation was reduced in SMA from db/db −/− compared to db/db +/?.
ACh-induced relaxation of SMA was inhibited by a combination of Nω-nitro-L-arginine and indomethacin in db/db +/?, but not in db/db −/−.
Acute incubation of SMA with tetrahydrobiopterin (BH4, 10 μM) and sepiapterin (100 μM) enhanced ACh-induced relaxation in SMA from db/db −/−, but not from db/db +/? 2,4-diamino-6-hydroxypyrimidine, an inhibitor of GTP cyclohydrolase I, (10 mM), impaired the sensitivity of SMA from db/db +/? to ACh, which was restored by co-incubation with BH4 (10 μM).
BH4 and superoxide dismutase (SOD, 150 u ml−1), either alone or in combination, had no effect on either ACh or SNP-induced relaxation in SMA from eNOS −/− mice.
Incubation of SMA with SOD (150 iu ml−1), catalase (200 iu ml−1) and L-arginine (1 mM) had no effect on ACh-induced relaxation of SMA. However, the combination of polyethylene glycol-SOD (200 iu ml−1) and catalase (80 u ml−1) improved the sensitivity of ACh-induced relaxation in db/db −/−, but not in db/db +/?.
These data suggest that increased production of superoxide anions and decreased availability of BH4 result in an ‘uncoupling' of nitric oxide synthase and endothelial dysfunction in SMA from db/db −/− mice.
Keywords: Endothelial dysfunction, type II diabetes, db/db mouse, endothelial nitric oxide synthase, tetrahydrobiopterin, superoxide anions, eNOS knock out mice
Introduction
The discovery of endothelium-derived relaxing factor (EDRF) and its identification as nitric oxide (NO) were key contributions to our understanding of the physiology of vasodilatation (Furchgott & Zawadzki, 1980; Palmer et al., 1987). Endothelial dysfunction is defined as an attenuated response of the blood vessels to endothelium-dependent vasodilators such as acetylcholine (ACh) and bradykinin. Endothelial dysfunction is considered as the major risk factor for the cardiovascular complications associated with type I and type II diabetes (de vriese et al., 2000).
Impaired endothelium-dependent vasodilation is primarily associated with a decreased synthesis of endothelium-derived nitric oxide (NO) and/or an increase in the production of superoxide anions (de vriese et al., 2000). Reactive oxygen species such as superoxide anions can rapidly inactivate NO, resulting in the formation of vasotoxic peroxynitrite (Gryglewski et al., 1986; Milstien & Katusic, 1999). It has been assumed that under pathophysiological conditions, such as diabetes, an imbalance between NO and superoxide anion production may contribute to impaired endothelium-dependent relaxation to ACh (Cosentino & Luscher, 1999). It has also been reported that nitric oxide synthase (NOS), under certain conditions, produces superoxide anions, in addition to NO (Pou et al., 1992). Tetrahydrobiopterin (BH4) is an essential cofactor for the synthesis of NO and, under physiological conditions, following binding of BH4 to the oxidase domain of NOS, the enzyme is activated and generates NO and L-citrulline from L-arginine and oxygen (Mayer & Werner, 1995). Reduced bio-availability/activity of BH4 has been recently reported in the fructose-fed insulin resistant rat model (Shinozaki et al., 1999) as well as in coronary arteries from insulin resistant patients (Shinozaki et al., 2001). In the presence of sub-optimal levels of BH4, NOS generates both NO and superoxide anions resulting in the formation of hydrogen peroxide and peroxynitrite (Wever et al., 1997).
Thus, restoration of BH4 to the endothelial cell should restore the activity of NOS and lead to an increased production of NO. Administration of BH4 has been demonstrated to enhance NO production in pre-hypertensive rats (Cosentino et al., 1998), restore endothelium-dependent vasodilation in coronary arteries following reperfusion injury (Tiefenbacher et al., 1996), as well as in aortae from STZ–induced diabetic rats (Pieper, 1997), coronary resistance vessels from JCR:LA corpulent rats (Brunner et al., 2000) and aortae from insulin-resistant rats (Shinozaki et al., 2000). BH4 supplementation improves endothelium-dependent relaxation in patients with hypercholesteromia (Stroes et al., 1997), endothelium-dependent relaxation in venous conduits used for coronary artery bypass graft surgery (Verma et al., 2000), patients with type II diabetes (Heitzer et al., 2000a; Verma et al., 2000), in normal epicardial coronary arteries (Setoguchi et al., 2001) and in smokers (Heitzer et al., 2000b). BH4 administration has also been demonstrated to improve functional recovery following ischaemia and reperfusion; an effect ascribed to improved coronary endothelial function and reduced oxidative stress (Verma et al., 2002). In addition, aortic tissue from the hyperphenyalaninemic (hph-1) mouse, which has a 90% deficiency in the rate limiting enzyme, GTP cyclohydrolase I, for BH4 synthesis, demonstrates reduced NO synthesis and heightened superoxide production (Cosentino et al., 2001). Furthermore, acetylcholine-mediated endothelium-dependent relaxations in aortae from hph-1, but not control, mice were inhibited by catalase and enhanced by superoxide dismutase and differences between hph-1 and control were reversed by exogenous BH4 administration (Cosentino et al., 2001).
These findings from a variety of different animal models as well as from clinical studies support the hypothesis that BH4 could be a new and an effective therapeutic approach for the treatment of endothelial dysfunction in a variety of pathophysiological conditions. The objective of the present study was to investigate endothelial function, and the role of the co-factor, BH4, and oxidative stress in small mesenteric arteries (SMA) from spontaneously diabetic (db/db −/−) mice, an animal model of type II diabetes that lacks the leptin receptor. In our investigations we used the db/db +/? mouse, that includes the homozygous +/+ as well as the heterozygous +/−, as the control animal.
Methods
Animals
Twelve to 16-week-old male C57BL/KsJ db/db mice (db/db −/−; db/db is the gene that encodes for the leptin receptor and −/− refers deficiency of leptin receptor) and non-diabetic (db/db +/?) controls, as well as eNOS −/− mice, were purchased from Jackson Laboratories (Bar Harbor, ME, U.S.A.). Plasma glucose (Sigma & Co., U.S.A.), total cholesterol (Sigma & Co., U.S.A.), triglycerides (Sigma & Co., U.S.A.) and serum non-esterified fatty acid (Wako & Co., Germany) were assayed using commercial kits.
Vascular reactivity
In accordance with a protocol approved by the University of Calgary Animal Care Committee, mice were killed by cervical dislocation and the mesenteric arcade was removed. First order branches of the mesenteric artery were dissected out into cold Krebs solution of the following composition (in mM): NaCl 120, NaHCO3 25, KCl 4.8, NaH2PO4 1.2, MgSO4 1.2, dextrose 11.0, CaCl2 1.8, aerated with 95% O2 and 5% CO2. Arteries were cut into 2 mm rings and mounted on a Mulvany-Halpern myograph as previously described (Mulvany & Halpern, 1977). The passive tension-internal circumference was determined by stretching to achieve an internal circumference equivalent to 90% of that of the vessel under a transmural pressure of 100 mmHg. All experiments were performed at 37°C.
Experimental protocols
After a 45-min equilibration period, the vascular reactivity to phenylephrine (PE) was studied in SMA from db/db +/? and db/db −/− mice. After 30 min stabilization, endothelium-dependent relaxation to ACh and endothelium-independent relaxation to SNP were recorded in preparations pre-contracted with a sub-maximal concentration of PE (EC75–80).
The first series of experiments was performed in order to study the contribution of NO to the ACh-induced relaxation of SMA derived from db/db +/? and db/db −/− mice. Tissues were pre-treated with Nω-nitro L-arginine (L-NNA, 100 μM) and indomethacin (10 μM) for 30 min and then a cumulative concentration-response curve to ACh was obtained.
The second series of experiments was performed in order to study the potential beneficial effect of exogenous BH4 on ACh- and SNP-induced relaxation of SMA from db/db +/?, db/db −/− and eNOS −/− mice. BH4 (10 μM), either alone or in combination with superoxide dismutase (SOD; 150 iu ml−1), was incubated for 30 min before constructing a cumulative concentration-response curve to ACh and SNP in SMA from db/db +/?, db/db −/− and eNOS −/− mice. Further, the effects of the acute incubation with sepiapterin (100 μM) on ACh-induced relaxation of SMA from db/db +/? and db/db −/− mice was investigated. An additional set of experiments was performed to study the effect of BH4 deficiency on endothelial function in db/db +/? mice. SMA from db/db +/? were exposed to DAHP (10 mM) for 3 h before constructing a cumulative concentration-response curve to ACh. In another group, BH4 (10 μM) was exposed for the last 30 min in the presence of DAHP before constructing a cumulative concentration-response curve to ACh.
The third series of experiments was designed to determine the effects of oxidative stress and NOS substrate on ACh-induced relaxation of SMA from db/db +/? and db/db −/− mice. SOD (150 iu ml−1), catalase (200 u ml−1), a combination of PEG-SOD (200 u ml−1) and catalase (80 u ml−1) or L-arginine (1 mM) were pre-incubated with the SMA for 30 min before constructing a cumulative concentration-response curve to ACh in SMA from db/db −/− and db/db +/?.
Drugs
All drugs were obtained from Sigma. All drugs were dissolved in distilled water except for indomethacin, which was dissolved in 95% ethanol and sepiapterin, which was dissolved in DMSO. BH4 was prepared fresh in deoxygenated distilled water and stored in the dark until use.
Data analysis
Data are expressed as pEC50 values, defined as the negative logarithm to base ten of the EC50 value, which was used in this study as a measure of sensitivity to vasoactive drugs, and maximum relaxation/contraction, as the maximum response obtained at the highest concentration tested. In all experiments, n equals the number of animals used in the protocol. Relaxation is expressed as mean percentage of PE-induced tone±standard error of mean. Statistical significance of difference between means of different groups was performed using paired or unpaired student t-test or one-way ANOVA. Multiple comparisons of the different groups were performed using Student Newman Keul test. A P value of <0.05 was considered statistically significant.
Results
Biochemical characteristics in spontaneously diabetic (db/db −/−) mice
Spontaneously diabetic mice showed significantly higher body weight compared to their littermates (47±1 vs 29±1 g respectively, n=10–11, P<0.01). Plasma glucose (475±1 vs 204±4 mg dl−1 respectively, P<0.01), triglycerides (83±10 vs 43±2 mg dl−1 respectively, P<0.01) and total cholesterol (143±25 vs 43±6 mg dl−1 respectively, P<0.01) were significantly elevated in db/db −/− compared to db/db +/?. Serum non-esterified fatty acid was significantly higher in db/db −/− compared to db/db +/? (2.45±0.3 vs 1.26±0.1 m Eq L−1 respectively, P<0.01).
Characterization of endothelial function in spontaneously diabetic (db/db −/−) mice
PE initiated a concentration-dependent contraction of SMA from db/db +/? and db/db −/− mice (Figure 1). Maximum contraction expressed as mN/mm, and sensitivity expressed as pEC50 value for db/db +/? and db/db −/− were 3.2±0.2 and 6.2±0.4 and 4.4±0.3 and 6.4±0.1 respectively. Maximum contraction to PE was significantly enhanced (P<0.01) without a change in the sensitivity in db/db −/− compared to db/db +/?.
Figure 1.
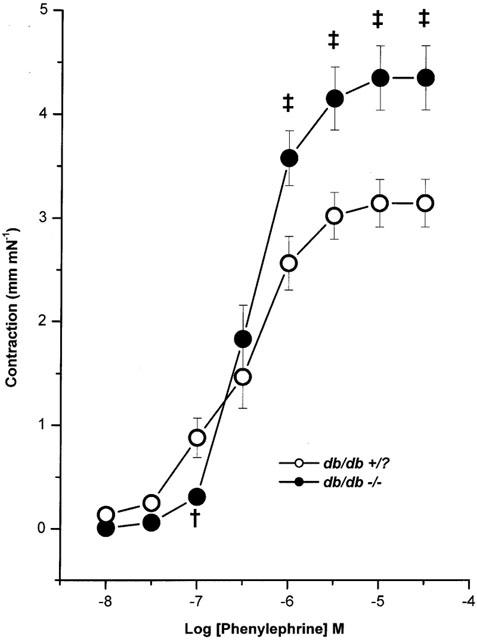
Mean concentration-response curves to phenylephrine in mouse mesenteric arteries from db/db +/? (n=10) and db/db −/− (n=12). Symbols are mean values with s.e.mean shown by vertical bars. †P<0.05 and ‡P<0.01 compared to db/db +/?.
ACh-induced maximum relaxation of SMA was significantly reduced (P<0.01) in db/db −/− compared to db/db +/?. Sensitivity and maximum relaxation to ACh for db/db +/? and db/db −/− were 6.6±0.1 and 81±4 and 6.5±0.1 and 53±6 respectively (Figure 2). Incubation of the SMA with L-NNA (100 μM) and indomethacin (10 μM) for 30 min significantly reduced the maximum relaxation induced by ACh from db/db +/? (P<0.01), but not in db/db −/− mice (n=10). Sensitivity and maximum relaxation to ACh before and after incubation with L-NNA (100 μM) and indomethacin (10 μM) for 30 min were 7.0±0.1 and 86±3 and 6.4±0.1 and 52±6 respectively in db/db +/? and 6.8±0.2 and 54±7 and 7.0±0.2 and 40±6 respectively in db/db −/−. However indomethacin alone had no effect on endothelium-dependent relaxation of SMA in both db/db +/? and db/db −/− mice (data not shown).
Figure 2.

Mean concentration-response curves to ACh in mouse mesenteric arteries from db/db +/? (n=18) and db/db −/− (n=15). Symbols are mean values with s.e.mean shown by vertical bars. ‡P<0.01 compared to db/db +/?.
SNP-mediated relaxation remained unchanged in both groups. Sensitivity and maximum relaxation to SNP for db/db +/? and db/db −/− were 6.6±0.2 and 96±1 and 6.4±0.2 and 86±5 respectively (Figure 3).
Figure 3.

Mean concentration-response curves to SNP in mouse mesenteric arteries from db/db +/? (n=16) and db/db −/− (n=15). Symbols are mean values with s.e.mean shown by vertical bars.
Effect of co-factor, tetrahydrobiopterin, on endothelium-dependent and endothelium-independent relaxation of SMA from db/db +/?, db/db −/− and eNOS −/− mice
Pre-incubation of SMA from db/db +/? mice with BH4 (10 μM) alone or in combination with SOD (150 iu ml−1) did not affect either the sensitivity (7.0±0.1, 6.9±0.2, 6.9±0.1 for the ACh control, BH4, BH4 plus SOD group respectively) or the maximum relaxation (90±2, 82±5, 86±3 for the ACh control, BH4, BH4 plus SOD group respectively) mediated by ACh (Figure 4a). However, pre-incubation with BH4 (10 μM) and the combination with SOD (150 u ml−1) significantly (P<0.01; Figure 4b) enhanced the sensitivity (6.5±0.2, 7.5±0.1, 7.5±0.1 for the ACh control, BH4, BH4 plus SOD group respectively) to ACh in SMA from db/db −/− mice without affecting the maximum relaxation (61±13, 73±6, 83±3 for the ACh control, BH4, BH4 plus SOD group respectively). Incubation with sepiapterin (100 μM), which is converted to BH4 intracellularly, significantly enhanced the sensitivity to ACh without affecting the maximum relaxation in db/db −/−, but not in db/db +/? (Table 1).
Figure 4.

Mean concentration-response curves to ACh in mouse mesenteric arteries from db/db +/? (a) and db/db −/− (b) before (control) and after incubation with either BH4 (10 μM) or combination of BH4 (10 μM) and SOD (150 iu ml−1) for 30 min (n=7). Symbols are mean values with s.e.mean shown by vertical bars. †P<0.05 and ‡P<0.01 compared to control and *P<0.05 compared to BH4 treated group.
Table 1.
Effect of the co-factor BH4 and the substrate L-arginine for eNOS and oxidative stress on endothelial-dependent relaxation to acetylcholine in spontaneously diabetic (db/db−/−) and control (db/db+/?) mice

BH4 alone, or in combination with SOD, had no effect on SNP-mediated concentration-dependent relaxation of SMA from db/db +/? and db/db −/− (Figure 5a,b), however a small apparent potentiating effect was observed in db/db −/− mice, this did not reach a statistical significance. Sensitivity and maximum relaxation were 6.7±0.2 and 97±1, 6.7±0.2 and 96±1, 7.0±0.1 and 95±1 for SNP control, BH4, BH4 plus SOD group respectively for db/db +/? (n=5–6) and 6.1±0.2 and 82±12, 7.0±0.3 and 82±11, 6.8±0.3 and 83±10 for SNP control, BH4, BH4 plus SOD group respectively for db/db −/−.
Figure 5.

Mean concentration-response curves to SNP in mouse mesenteric arteries from db/db +/? (a) and db/db −/− (b) before (control) and after incubation with either BH4 (10 μM) or combination of BH4 (10 μM) and SOD (150 iu ml−1) for 30 min (n=6–9). Symbols are mean values with s.e.mean shown by vertical bars.
BH4 alone, or in combination with SOD, had no significant effect on ACh and SNP-mediated concentration-dependent relaxation of SMA from eNOS −/− mice (Figure 6a,b). Sensitivity and maximum relaxation of SMA to ACh were 6.1±0.2 and 76±9, 6.4±0.1 and 76±6, 6.4±0.2 and 73±9 for ACh control, BH4, BH4 plus SOD group respectively. Sensitivity and maximum relaxation of SMA to SNP were 7.2±0.2 and 99±1, 7.1±0.1 and 97±1, 7.1±0.3 and 96±1 for ACh control, BH4, BH4 plus SOD group respectively.
Figure 6.

Mean concentration-response curves to ACh (a) and SNP (b) in mouse mesenteric arteries from eNOS −/− before (control) and after incubation with either BH4 (10 μM) or combination of BH4 (10 μM) and SOD (150 iu ml−1) for 30 min (n=6). Symbols are mean values with s.e.mean shown by vertical bars.
Incubation of SMA from db/db +/? with DAHP (10 mM), a GTP cyclohydrolase I inhibitor, for 3 h significantly (P<0.05) reduced the sensitivity to ACh, without affecting the maximum relaxation. Co-incubation with BH4 (10 μM) during the last 30 min of incubation, restored the sensitivity to normal (Figure 7). pEC50 values and maximum relaxation to ACh were 7.3±0.2 and 85±11, 6.6±0.2 and 86±3, 7.2±0.1 and 90±4 for ACh control, DAHP, DAHP plus BH4 group respectively.
Figure 7.

Mean concentration-response curves to ACh in mouse mesenteric arteries from db/db +/? before (control) and after incubation with either DAHP (10 mM) for 3 h or DAHP (10 mM) for 3 h plus BH4 (10 μM) during the last 30 min (n=4–5). Symbols are mean values with s.e.mean shown by vertical bars. *P<0.05 compared to control.
Incubation of the SMA with BH4 for 30 min, however, had no effect on enhanced vascular reactivity of SMA from db/db −/− to PE. Sensitivity and maximum contraction (mN/mm) to PE were 6.5±0.1 and 4.0±0.4 and 6.5±0.1 and 4.0±0.5 for before and after incubation with BH4 respectively (n=4).
Effect of oxidative stress and substrate, L-arginine on endothelium-dependent relaxation of SMA from spontaneously diabetic mice
SOD (150 u ml−1), a superoxide anion scavenger and catalase (200 u ml−1), an enzyme that dismutases hydrogen peroxide, had no effect on sensitivity and maximum relaxation to ACh in db/db +/? and db/db −/− mice (Table 1). SOD also had no effect on SNP-induced relaxation in db/db +/? and db/db −/− mice (data not shown). The combination of PEG-SOD (200 u ml−1), membrane permeable superoxide anion scavenger and catalase (80 u ml−1) significantly (P<0.05) enhanced the sensitivity to ACh in db/db −/−, but not in db/db +/?, without affecting the maximum relaxation (Table 1). However, the combination of PEG-SOD and catalase had no effect on enhanced vascular reactivity to PE in db/db −/− mice. Sensitivity and maximum contraction (mN/mm) to PE were 6.5±0.1 and 4.0±0.4 and 6.7±0.1 and 3.9±0.5 for before and after incubation with the combination of PEG-SOD and catalase respectively (n= 4).
Pre-incubation with L-arginine (1 mM) for 30 min had no effect on sensitivity or maximum relaxation to ACh in db/db +/? and db/db −/− mice (Table 1). Similarly, pre-incubation with L-arginine had no effect on SNP-induced relaxation in db/db +/? and db/db −/− mice (data not shown).
Discussion
The present study demonstrates that SMA from spontaneously diabetic mice (db/db −/−) have enhanced vascular reactivity to PE, impaired endothelium-dependent relaxation to ACh and unaltered endothelium-independent relaxation to SNP. We have also demonstrated an improved sensitivity to ACh following acute incubation with BH4 and with a combination of PEG-SOD and catalase, but not with catalase, SOD or L-arginine pretreatment.
The spontaneously diabetic mice used in this study demonstrated severe obesity, hyperglycaemia and dyslipidaemia, which are the characteristic features of type II diabetes. Endothelium-dependent relaxation to ACh was significantly impaired in db/db −/− mice compared to non-diabetic littermates, however, endothelium-independent relaxation to SNP remained unchanged in db/db −/− compared to db/db +/?. Furthermore, vessels from db/db −/− mice demonstrated enhanced vascular reactivity to PE. Thus, impaired endothelium function exists in arteries from db/db −/− mice and these data are in agreement with earlier reports that have studied endothelial function in the thoracic aorta (Kamata & Kojima, 1997; Piercy & Taylor, 1998) and mesenteric artery (Lagaud et al., 2001) from the db/db mouse . Furthermore, ACh-induced relaxation in db/db −/−, but not db/db +/+, was resistant to a combination of L-NNA and indomethacin suggesting that changes in NOS function was a major contributor to the attenuated ACh-mediated relaxation in pathophysiological conditions.
The cellular basis for the change in NOS function may include: (a) decreased release or production of nitric oxide due to deficiency of the substrate, L-arginine or co-factor, tetrahydrobiopterin; (b) increased destruction of NO by oxygen-derived free radicals; (c) increased release of an endothelium-derived constricting factor (Harrison, 1997; Shimokawa, 1999; Hink et al., 2001); or (d) an increased abundance of caveolin-1, an endogenous inhibitor of eNOS.
Among the cofactors for eNOS BH4 is critical for enzyme activity and NO production. In the event of a deficiency of BH4, constitutive NOS transfers electrons to molecular oxygen to produce superoxide instead of NO (Pou et al., 1992; 1999; Tiefenbacher, 2001). Supplementation with BH4 improves endothelium-dependent relaxation to ACh in aortic tissue from STZ-induced diabetic rats (Pieper, 1997) and in human blood vessels from type II diabetic patients (Heitzer et al., 2000a). In this study, we demonstrated that acute incubation with BH4 significantly enhanced the sensitivity of the SMA to ACh-mediated relaxation in db/db −/− mice. Auto-oxidation of BH4 can generate superoxide anion, which in turn inactivates NO with half maximal concentration of 2 μM (Mayer et al., 1995), however, the generation of superoxide anions probably occurs only at high concentrations of BH4. The generation of superoxide has been shown to have an inhibitory effect on endothelium-dependent vasorelaxation in isolated blood vessels, which can be reversed by SOD thus suggesting that these effects were mediated by superoxide (Kinoshita & Katusic, 1996). To overcome this potential problem we have also used sepiapterin, which is converted to BH4 intracellularly through a salvage pathway (Mayer & Werner, 1995). Consistent with the results from BH4, sepiapterin also improved the sensitivity to ACh in SMA from db/db −/− mice. Blockade of BH4 synthesis with DAHP, a selective GTP cyclohydrolase I inhibitor, resulted in an altered or impaired endothelial function in isolated aorta, coronary (Cosentino & Katusic, 1995; Tiefenbacher et al., 1996) and cerebral arteries (Kinoshita et al., 1997). Our results indicate that incubation with DAHP reduced the sensitivity of SMA to ACh, and that sensitivity of the tissue can be restored by incubation of SMA with BH4. Collectively these data together suggest that the dysfunction of the endothelium may be due to the lack of the co-factor BH4. However, BH4 had no effect on enhanced vascular reactivity to PE. This enhanced vascular reactivity, which is independent of NO and BH4, may thus be mediated by enhanced endothelin-1 activity (Makino et al., 2001).
It has been reported that the BH4 levels found in coronary artery endothelial cells from diabetic biobreeding (BB) rats were only 12% of that measured in normal rats and that this reduced level of BH4 correlated with a reduced production of NO (Meininger et al., 2000). This deficiency was linked to decreased activity of GTP-cyclohydrolase I; the first and the rate-limiting enzyme in the de novo biosynthesis of BH4. Moreover, the addition of sepiapterin enhanced NO production supporting the hypothesis that BH4 deficiency is the metabolic basis for impaired endothelial NO synthesis in BB rats (Meininger et al., 2000). Shinozaki et al. (1999) reported decreased levels of BH4 in aorta from the fructose-fed insulin-resistant rat model. Furthermore, in the same study, it was shown that acute incubation with BH4 restored endothelium-dependent relaxation to ACh and chronic supplementation with sepiapterin in insulin-resistant rats improved endothelium-dependent relaxation of aorta and reduced oxidative stress (Shinozaki et al., 2000).
The mechanisms involved in BH4-mediated improved endothelium function are not fully understood. It has been proposed that BH4 exerts an allosteric action to stabilize the active dimeric state of NOS and to play a redox active role in stimulating NOS (Mayer & Werner, 1995). It has also been reported that BH4 binds to NOS and facilitates the transfer of electrons and the production of NO (Hurshman et al., 1999; Schmidt et al., 2001). Other mechanisms, whereby BH4 enhances NO activity, include increasing the binding of L-arginine to NOS (Wever et al., 1998) or, because BH4 is very redox-active, scavenging reactive free radicals. In support of the later hypothesis, it has been shown that BH4 improves endothelium function in insulin-resistant rats by reducing vascular oxidative stress (Shinozaki et al., 2000). ACh-mediated relaxation is impaired in SMA from eNOS −/− but is partially compensated by an up-regulation of EDHF (Waldron et al., 1999). In the current study we observed that BH4 had no effect on ACh-mediated relaxation of SMA from eNOS −/− mice suggesting that the cellular basis for the action of BH4 to improve endothelial function in db/db −/− mice is mediated through the NOS pathway.
When the endothelial cell is presented with sub-optimal concentrations of BH4, NOS generates superoxide anions and oxygen free radicals, instead of NO (Klatt et al., 1992; Stroes et al., 1998). Reactive oxygen species readily react with NO resulting in the formation of peroxynitrite and hydroxyl ions. Peroxynitrite oxidizes BH4 to quinonoid 5,6-dihydrobiopterin, which loses its side chain to form inactive 7,8-dihydropterin. BH4 deficiency leads to eNOS dysfunction with the formation of reactive oxygen species (Milstien & Katusic, 1999). Thus, low levels of BH4 by itself can mediate its own destruction. The generation of superoxide anions might be important in endothelial dysfunction in diabetes. Superoxide may impair endothelium-dependent relaxation by rapidly inactivating NO (Gryglewski et al., 1986) and/or by oxidation of BH4 through peroxynitrite (Milstien & Katusic, 1999; Laursen et al., 2001) or by serving as a contracting factor (Katusic & Vanhoutte, 1989).
There is a body of evidence suggesting an increased oxidative stress in both experimental diabetic models as well as in clinical situations. It has been shown that free radical scavengers like SOD restore impaired endothelium-dependent relaxation in diabetic animals (Pieper & Siebeneich, 1998; Cosentino & Luscher, 1999; Kamata et al., 1999). In the current study, SOD and catalase had no effect on ACh-mediated relaxation. Failure of SOD and catalase to improve endothelial function may be due to the fact that their membrane permeability is poor or that their anti-oxidant action is not sufficient to scavenge overwhelming oxidative stress. Thus, in the current study, we used the membrane permeable superoxide scavenger, PEG-SOD in combination with catalase and pre-incubation of tissues with this combination improved the sensitivity of SMA from db/db −/− to ACh. Thus, superoxide anions contribute to the impaired endothelium function in the db/db mouse. The cellular sources of superoxide anions need further investigation, but might be multiple viz., auto-oxidation of glucose per se, glucose-mediated biochemical events, such as the polyol pathway, advanced glycosylation end product and receptor interactions, diacylglycerol-protein kinase C pathway or oxidized LDL, or uncoupling of NOS (de vriese et al., 2000).
A reduced availability of L-arginine seems unlikely because the concentration of L-arginine in endothelial cells is in the millimolar range and about a thousand fold higher than required for maximal activity of eNOS (Harrison, 1997). Nonetheless the beneficial effects of L-arginine supplementation have been documented in vitro in isolated tissue studies from diabetic rat as well as in intact models of type I diabetes (Pieper & Peltier, 1995; Pieper et al., 1997; Pieper & Dondlinger, 1997). However, the same groups observed that the beneficial effect is dependent on the duration of diabetes (Pieper et al., 1995). In the present study, pre-incubation with L-arginine (1 mM) did not enhance ACh-mediated relaxation of SMA, suggesting that lack of substrate was not the primary cause of endothelial dysfunction in SMA from spontaneously diabetic (db/db −/−) mice.
In conclusion, in the present study we demonstrated that SMA from spontaneously diabetic mice exhibit impaired endothelial function that can be restored by acute incubation with BH4 and a combination of PEG-SOD and catalase. These findings indicate that the cellular basis of endothelial dysfunction in the db/db−/− mouse model of type II diabetes may relate to the reduced availability of BH4 and/or increased oxidative stress resulting in the uncoupling of eNOS leading to endothelial dysfunction.
Acknowledgments
The authors wish to acknowledge the financial support of an operating grant from the Canadian Institute for Health Research (to C.R. Triggle), Canadian Diabetes Association (to T. Anderson and C.R. Triggle) and a graduate fellowship award from Pfizer/CHS/CIHR (to M. Pannirselvam). T. Anderson is a scholar of the Alberta Heritage Foundation for Medical Research. S.Verma is a CIHR Fellow.
Abbreviations
- ACh
acetylcholine
- BH4
(6R)-5,6,7,8-tetrahydrobiopterin
- DAHP
2,4-diamino-6-hydroxypyrimidine
- EDHF
endothelium-derived hyperpolarizing factor
- EDRF
endothelium-dependent relaxing factor
- eNOS
endothelial nitric oxide synthase
- L-NNA
Nω-nitro-L-arginine
- NO
nitric oxide
- PE
phenylephrine
- PEG-SOD
polyethylene glycol-superoxide dismutase
- SMA
small mesenteric artery
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- STZ
streptozotocin
References
- BRUNNER F., WOLKART G., PFEIFFER S., RUSSELL J.C., WASCHER T.C. Vascular dysfunction and myocardial contractility in the JCR:LA-corpulent rat. Cardiovasc. Res. 2000;47:150–158. doi: 10.1016/s0008-6363(00)00056-0. [DOI] [PubMed] [Google Scholar]
- COSENTINO F., BARKER J.E., BRAND M.P., HEALES S.J., WERNER E.R., TIPPINS J.R., WEST N., CHANNON K.M., VOLPE M., LUSCHER T.F. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001;21:496–502. doi: 10.1161/01.atv.21.4.496. [DOI] [PubMed] [Google Scholar]
- COSENTINO F., KATUSIC Z.S. Tetrahydrobiopterin and dysfunction of endothelial nitric oxide synthase in coronary arteries. Circulation. 1995;91:139–144. doi: 10.1161/01.cir.91.1.139. [DOI] [PubMed] [Google Scholar]
- COSENTINO F., LUSCHER T.F. Tetrahydrobiopterin and endothelial nitric oxide synthase activity. Cardiovasc. Res. 1999;43:274–278. doi: 10.1016/s0008-6363(99)00134-0. [DOI] [PubMed] [Google Scholar]
- COSENTINO F., PATTON S., D'USCIO L.V., WERNER E.R., WERNER-FELMAYER G., MOREAU P., MALINSKI T., LUSCHER T.F. Tetrahydrobiopterin alters superoxide and nitric oxide release in prehypertensive rats. J. Clin. Invest. 1998;101:1530–1537. doi: 10.1172/JCI650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE VRIESE A.S., VERBEUREN T.J., VAN d.V., LAMEIRE N.H., VANHOUTTE P.M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R.F., ZAWADZKI J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GRYGLEWSKI R.J., PALMER R.M., MONCADA S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- HARRISON D.G. Cellular and molecular mechanisms of endothelial cell dysfunction. J. Clin. Invest. 1997;100:2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEITZER T., BROCKHOFF C., MAYER B., WARNHOLTZ A., MOLLNAU H., HENNE S., MEINERTZ T., MUNZEL T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ. Res. 2000b;86:E36–E41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- HEITZER T., KROHN K., ALBERS S., MEINERTZ T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000a;43:1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- HINK U., LI H., MOLLNAU H., OELZE M., MATHEIS E., HARTMANN M., SKATCHKOV M., THAISS F., STAHL R.A., WARNHOLTZ A., MEINERTZ T., GRIENDLING K., HARRISON D.G., FORSTERMANN U., MUNZEL T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- HURSHMAN A.R., KREBS C., EDMONDSON D.E., HUYNH B.H., MARLETTA M.A. Formation of a pterin radical in the reaction of the heme domain of inducible nitric oxide synthase with oxygen. Biochemistry. 1999;38:15689–15696. doi: 10.1021/bi992026c. [DOI] [PubMed] [Google Scholar]
- KAMATA K., KOJIMA S. Characteristics of contractile responses of aorta to norepinephrine in db/db mice. Res. Commun. Mol. Pathol. Pharmacol. 1997;96:319–328. [PubMed] [Google Scholar]
- KAMATA K., NAKAJIMA M., SUGIURA M. Effects of superoxide dismutase on the acetylcholine-induced relaxation response in cholesterol-fed and streptozotocin-induced diabetic mice. J..Smooth Muscle Res. 1999;35:33–46. doi: 10.1540/jsmr.35.33. [DOI] [PubMed] [Google Scholar]
- KATUSIC Z.S., VANHOUTTE P.M. Superoxide anion is an endothelium-derived contracting factor. Am. J. Physiol. 1989;257:H33–H37. doi: 10.1152/ajpheart.1989.257.1.H33. [DOI] [PubMed] [Google Scholar]
- KINOSHITA H., KATUSIC Z.S. Exogenous tetrahydrobiopterin causes endothelium-dependent contractions in isolated canine basilar artery. Am. J. Physiol. 1996;271:H738–H743. doi: 10.1152/ajpheart.1996.271.2.H738. [DOI] [PubMed] [Google Scholar]
- KINOSHITA H., MILSTIEN S., WAMBI C., KATUSIC Z.S. Inhibition of tetrahydrobiopterin biosynthesis impairs endothelium-dependent relaxations in canine basilar artery. Am. J. Physiol. 1997;273:H718–H724. doi: 10.1152/ajpheart.1997.273.2.H718. [DOI] [PubMed] [Google Scholar]
- KLATT P., HEINZEL B., MAYER B., AMBACH E., WERNER-FELMAYER G., WACHTER H., WERNER E.R. Stimulation of human nitric oxide synthase by tetrahydrobiopterin and selective binding of the cofactor. FEBS Lett. 1992;305:160–162. doi: 10.1016/0014-5793(92)80886-l. [DOI] [PubMed] [Google Scholar]
- LAGAUD G.J.L., MASIH-KHAN E., KAI S., VAN BREEMEB C., DUBE G.P. Influence of type II diabetes on arterial tone and endothelial function in murine mesenteric resistance arteries. J. Vasc. Res. 2001;38:578–589. doi: 10.1159/000051094. [DOI] [PubMed] [Google Scholar]
- LAURSEN J.B., SOMERS M., KURZ S., MCCANN L., WARNHOLTZ A., FREEMAN B.A., TARPEY M., FUKAI T., HARRISON D.G. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- MAKINO A., ODA S., KAMATA K. Mechanisms underlying increased release of endotheliun-1 from aorta in diabetic rats. Peptides. 2001;22 4:639–645. doi: 10.1016/s0196-9781(01)00374-6. [DOI] [PubMed] [Google Scholar]
- MAYER B., KLATT P., WERNER E.R., SCHMIDT K. Kinetics and mechanism of tetrahydrobiopterin-induced oxidation of nitric oxide. J. Biol. Chem. 1995;270:655–659. doi: 10.1074/jbc.270.2.655. [DOI] [PubMed] [Google Scholar]
- MAYER B., WERNER E.R. In search of a function for tetrahydrobiopterin in the biosynthesis of nitric oxide. Naunyn Schmiedebergs Arch. Pharmacol. 1995;351:453–463. doi: 10.1007/BF00171035. [DOI] [PubMed] [Google Scholar]
- MEININGER C.J., MARINOS R.S., HATAKEYAMA K., MARTINEZ-ZAGUILAN R., ROJAS J.D., KELLY K.A., WU G. Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydrobiopterin deficiency. Biochem. J. 2000;349:353–356. doi: 10.1042/0264-6021:3490353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILSTIEN S., KATUSIC Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem. Biophys. Res. Commun. 1999;263:681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- PALMER R.M., FERRIGE A.G., MONCADA S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M. Acute amelioration of diabetic endothelial dysfunction with a derivative of the nitric oxide synthase cofactor, tetrahydrobiopterin. J. Cardiovasc. Pharmacol. 1997;29:8–15. doi: 10.1097/00005344-199701000-00002. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M., DONDLINGER L.A. Plasma and vascular tissue arginine are decreased in diabetes: acute arginine supplementation restores endothelium-dependent relaxation by augmenting cGmP production. J. Pharmacol. Exp. Ther. 1997;283:684–691. [PubMed] [Google Scholar]
- PIEPER G.M., JORDAN M., ADAMS M.B., ROZA A.M. Syngeneic pancreatic islet transplantation reverses endothelial dysfunction in experimental diabetes. Diabetes. 1995;44:1106–1113. doi: 10.2337/diab.44.9.1106. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M., PELTIER B.A. Amelioration by L-arginine of a dysfunctional arginine/nitric oxide pathway in diabetic endothelium. J. Cardiovasc. Pharmacol. 1995;25:397–403. doi: 10.1097/00005344-199503000-00008. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M., SIEBENEICH W. Oral administration of the antioxidant, N-acetylcysteine, abrogates diabetes-induced endothelial dysfunction. J. Cardiovasc. Pharmacol. 1998;32:101–105. doi: 10.1097/00005344-199807000-00016. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M., SIEBENEICH W., MOORE-HILTON G., ROZA A.M. Reversal by L-arginine of a dysfunctional arginine/nitric oxide pathway in the endothelium of the genetic diabetic BB rat. Diabetologia. 1997;40:910–915. doi: 10.1007/s001250050767. [DOI] [PubMed] [Google Scholar]
- PIERCY V., TAYLOR S.G. A comparison of spasmogenic and relaxant responses in aortae from C57/BL/KsJ diabetic mice with those from their non-diabetic litter mates. Pharmacology. 1998;56:267–275. doi: 10.1159/000028208. [DOI] [PubMed] [Google Scholar]
- POU S., KEATON L., SURICHAMORN W., ROSEN G.M. Mechanism of superoxide generation by neuronal nitric-oxide synthase. J. Biol. Chem. 1999;274:9573–9580. doi: 10.1074/jbc.274.14.9573. [DOI] [PubMed] [Google Scholar]
- POU S., POU W.S., BREDT D.S., SNYDER S.H., ROSEN G.M. Generation of superoxide by purified brain nitric oxide synthase. J. Biol. Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- SCHMIDT P.P., LANGE R., GORREN A.C., WERNER E.R., MAYER B., ANDERSSON K.K. Formation of a protonated trihydrobiopterin radical cation in the first reaction cycle of neuronal and endothelial nitric oxide synthase detected by electron paramagnetic resonance spectroscopy. J. Biol. Inorg. Chem. 2001;6:151–158. doi: 10.1007/s007750000185. [DOI] [PubMed] [Google Scholar]
- SETOGUCHI S., MOHRI M., SHIMOKAWA H., TAKESHITA A. Tetrahydrobiopterin improves endothelial dysfunction in coronary microcirculation in patients without epicardial coronary artery disease. J. Am. Coll. Cardiol. 2001;38:493–498. doi: 10.1016/s0735-1097(01)01382-1. [DOI] [PubMed] [Google Scholar]
- SHIMOKAWA H. Primary endothelial dysfunction: atherosclerosis. J. Mol. Cell Cardiol. 1999;31:23–37. doi: 10.1006/jmcc.1998.0841. [DOI] [PubMed] [Google Scholar]
- SHINOZAKI K., HIRAYAMA A., NISHIO Y., YOSHIDA Y., OHTANI T., OKAMURA T., MASADA M., KIKKAWA R., KODAMA K., KASHIWAGI A. Coronary endothelial dysfunction in the insulin-resistant state is linked to abnormal pteridine metabolism and vascular oxidative stress. J. Am. Coll. Cardiol. 2001;38:1821–1828. doi: 10.1016/s0735-1097(01)01659-x. [DOI] [PubMed] [Google Scholar]
- SHINOZAKI K., KASHIWAGI A., NISHIO Y., OKAMURA T., YOSHIDA Y., MASADA M., TODA N., KIKKAWA R. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2- imbalance in insulin-resistant rat aorta. Diabetes. 1999;48:2437–2445. doi: 10.2337/diabetes.48.12.2437. [DOI] [PubMed] [Google Scholar]
- SHINOZAKI K., NISHIO Y., OKAMURA T., YOSHIDA Y., MAEGAWA H., KOJIMA H., MASADA M., TODA N., KIKKAWA R., KASHIWAGI A. Oral administration of tetrahydrobiopterin prevents endothelial dysfunction and vascular oxidative stress in the aortas of insulin-resistant rats. Circ. Res. 2000;87:566–573. doi: 10.1161/01.res.87.7.566. [DOI] [PubMed] [Google Scholar]
- STROES E., HIJMERING M., VAN ZANDVOORT M., WEVER R., RABELINK T.J., VAN FAASSEN E.E. Origin of superoxide production by endothelial nitric oxide synthase. FEBS Lett. 1998;438:161–164. doi: 10.1016/s0014-5793(98)01292-7. [DOI] [PubMed] [Google Scholar]
- STROES E., KASTELEIN J., COSENTINO F., ERKELENS W., WEVER R., KOOMANS H., LUSCHER T., RABELINK T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J. Clin. Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIEFENBACHER C.P. Tetrahydrobiopterin: a critical cofactor for eNOS and a strategy in the treatment of endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2484–H2488. doi: 10.1152/ajpheart.2001.280.6.H2484. [DOI] [PubMed] [Google Scholar]
- TIEFENBACHER C.P., CHILIAN W.M., MITCHELL M., DEFILY D.V. Restoration of endothelium-dependent vasodilation after reperfusion injury by tetrahydrobiopterin. Circulation. 1996;94:1423–1429. doi: 10.1161/01.cir.94.6.1423. [DOI] [PubMed] [Google Scholar]
- VERMA S., LOVREN F., DUMONT A.S., MATHER K.J., MAITLAND A., KIESER T.M., TRIGGLE C.R., ANDERSON T.J. Tetrahydrobiopterin improves endothelial function in human saphenous veins. J. Thorac. Cardiovasc. Surg. 2000;120:668–671. doi: 10.1067/mtc.2000.109000. [DOI] [PubMed] [Google Scholar]
- VERMA S., MAITLAND A., WEISEL R.D., MICKLE D.A.G., LI S.H., LI R.K., FEDAK P.W.M., RAO V.Novel cardioprotective effects of tetrahydrobiopterin following anoxia and reoxygenation: identifying cellular targets for pharmacological manipulation J. Thorac. Cardiovasc. Surg. 2002(in press) [DOI] [PubMed]
- WALDRON G.J., DING H., LOVREN F., KUBES P., TRIGGLE C.R. Acetylcholine-induced relaxation of peripheral arteries isolated from mice lacking endothelial nitric oxide synthase. Br. J. Pharmacol. 1999;128:653–658. doi: 10.1038/sj.bjp.0702858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEVER R.M., LUSCHER T.F., COSENTINO F., RABELINK T.J. Atherosclerosis and the two faces of endothelial nitric oxide synthase. Circulation. 1998;97:108–112. doi: 10.1161/01.cir.97.1.108. [DOI] [PubMed] [Google Scholar]
- WEVER R.M., VAN DAM T., VAN RIJN H.J., DE GROOT F., RABELINK T.J. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem. Biophys. Res. Commun. 1997;237:340–344. doi: 10.1006/bbrc.1997.7069. [DOI] [PubMed] [Google Scholar]


