Abstract
Sumatriptan or eletriptan produced vasocontraction in common carotid artery (CCA) by stimulating 5HT1B receptors (see also Akin & Gurdal, this issue).
Naratriptan as a 5HT1B/D agonist, was unable to produce vasocontraction in this artery, but inhibited the vasocontractile response induced by sumatriptan or eletriptan.
All these agonists inhibited forskolin-stimulated cyclic AMP production with comparable potencies and maximal responses. This inhibition was mediated by 5HT1B receptors: 5HT1B antagonist SB216641 (1 μM) completeley antagonized sumatriptan-, eletriptan- or naratriptan-induced cyclic AMP inhibition, but 5HT1D antagonist BRL15572 (1 μM) did not affect this response.
Naratriptan-induced stimulation of 5-HT1B receptors resulted only in adenylate cyclase inhibition, whereas stimulation of these receptors by sumatriptan or eletriptan produced vasocontraction as well. Hence, we concluded that the 5HT1B-mediated inhibition of adenylate cyclase was not a sufficient condition to couple the receptor stimulation to vasocontraction.
We discussed agonist-induced trafficking as a plausible mechanism for the observed phenomenon.
Keywords: Sumatriptan, naratriptan, vascular contractions, cyclic AMP, adenylate cyclase inhibition, 5-HT1B receptors, 5-HT1D receptors, rabbit common carotid artery
Introduction
Antimigraine drugs sumatriptan and naratriptan are nonselective high-affinity 5HT1B/D receptor agonists, and they cause vasocontraction in several blood vessels (Hoyer et al., 1994; Wurch et al., 1997). Among the 5HT1B/D family, the 5HT1B receptor has been reported to be the predominant subtype in most of the blood vessels (Ullmer et al., 1995; Hoyer et al., 1994; Sgard et al., 1996; Nilsson et al., 1999; Geerts et al., 2000). In accordance with this observation, others and we have shown that sumatriptan-induced vasocontraction in various blood vessels is mediated by 5HT1B receptors (Hoyer et al., 1994, Nilsson et al., 1999; Geerts et al., 2000; see also Akin & Gurdal, this issue). Likewise, naratriptan has been shown to produce vasocontraction by stimulating the same subtype in several blood vessels (e.g. human and bovine cerebral artery, dog and human coronary artery, rabbit saphenous vein) (Roon et al., 1999; Maassenvandenbrink et al., 1998; Nilsson et al., 1999, Cohen & Schenk, 1999).
Stimulation of 5HT1B receptors by serotonergic agonists is negatively coupled to cyclic AMP production. Sumatriptan and naratriptan inhibits forskolin stimulated cyclic AMP accumulation in heterologous expression systems by stimulating the 5HT1B subtype (Zgombick et al., 1993; Ng et al., 1993; Pauwels & Colpaert, 1996, Wurch et al., 1997).
Here, we aimed at investigating the relationship between the abilities of some triptan agonists to inhibit adenylate cyclase and to produce vasocontraction in rabbit common carotid artery (CCA).
Methods
Contraction experiments
Common carotid arteries were obtained from New Zealand albino rabbits (1.5–2.5 kg) that were exsanguinated under thiopental (35 mg.kg−1 i.v.) anaesthesia. Surrounding tissues were removed and the vessels were placed in cold Krebs-Henseleit solution. The vessels were cut into rings (approximately 3 mm width) and the endothelium was removed by passing a cannula through the arterial lumen. Functional integrity of endothelium was tested by observing acetylcholine-mediated vasorelaxation. The rings were opened by a single cut and then fixed with stainless steel clips at both ends in organ baths of 5 ml volume containing oxygenated (5% CO2, 95% O2) and warmed (37°C) Krebs solution (pH 7.4) with the following composition (in mM): NaCl 112, KCl 5, NaHCO3 25, NaH2PO4 1, MgCl2 0.5, CaCl2 2.5, glucose 11.5. Isometric contractions were measured using force-displacement transducers (Grass FT.03) and a general purpose amplifier (MayCom, Ankara, Turkey) connected to a personal computer. All preparations were given an initial tension of 1–1.5 g and were allowed to equilibrate for 1 h by changing the bath buffer every 10 min. Following the equilibration period, the vessels were contracted by using 10 μM of 5HT and quickly washed three times. All subsequent responses were normalized with respect to this contraction. The preparations were then allowed to equilibrate for another 1 h at the above mentioned conditions. Concentration-response curves were obtained by using cumulatively increasing concentrations of agonists (in 1/2 Log steps). Prazosin (100 nM) was added to the organ baths 20 min before obtaining sumatriptan response, since our previous studies were shown that 5-HT or other serotonergic agonists may cause contraction via α1-adrenergic receptors in common carotid artery (Gurdal & Tulunay, 1992). Parameters of concentration-response curves were estimated by means of nonlinear regression of a three-parameter logistic function described by Kenakin (1984). The apparent pKb values of SB216641 or naratriptan were determined by using the method described by Kenakin (1984). These parameters were measured from the experiments in which SB216641 or naratriptan were used at 0.1 or 10 μM, respectively.
Inhibition of forskolin-induced cyclic AMP accumulation
Cyclic AMP accumulation was measured as described previously with some modifications (Ugur & Onaran, 1997). Briefly, the 3 mm rings were prepared as described above and placed in individual tubes containing HEPES buffer pH 7.4 at 37°C (total incubation volume is 500 μl). The rings were equilibrated with or without antagonists for 30 min. IBMX was added in the tubes (final 5 mM) and the reaction was started by adding forskolin (5 μM) and agonist and lasted for 30 min. The reaction was terminated by removing 500 μl of incubation buffer and adding 500 μl of ice-cold 15% trichloroacetic acid. Tubes were then left on ice for 60 min. Then 50 μl of the samples were used to measure cyclic AMP level. Cyclic AMP was determined using radioimmunoassay with the acetylation protocol. High affinity rabbit anti-cyclic AMP antibodies were raised in our laboratory using bovine serum albumin-conjugated cyclic AMP. Succinyl cyclic AMP tyrosinemethylester (ScAMP-TME) was iodinated using chloramine-T method. Mono and di iodo ScAMP-TME that were used as tracer ligands for RIA, were purified using gel filtration chromatography (Sefadex G25 superfine, equilibrated and eluted with Na+ acetate 1 M, pH 5).
Incubation with pertussis toxin
The 3 mm rings were transferred to 25-cm2 flasks containing 5–8 ml of Dulbecco's modified Eagle's medium with 250 U ml−1 penicillin/streptomycin and with or without 1 μg ml−1 pertussis toxin (Sigma) and placed in a 37°C incubator containing 5% CO2, 95% air and incubated for 12 h. After incubation, rings were washed with oxygenated (5% CO2, 95% O2) and warmed (37°C) Krebs solution (pH 7.4) and cyclic AMP level was measured as described above.
Statistics
Results are presented as arithmetic means with standard error of the mean from n observations. Student's unpaired t-test was used to assess the significance of differences between mean values, significance being defined by a P value less than 0.05.
Drugs
Source of compounds used were as follows: 5HT creatinin sulphate, prazosin (Sigma, Munich, Germany), eletriptan (Pfizer, Sandwich Kent, U.K.), naratriptan, sumatriptan, GR127,935 (Glaxo, Hertfordshire, U.K.). BRL15572 and SB216641 (Tocris, Bristol, U.K.).
Results
Vasocontractile responses
Sumatriptan or eletriptan, but not naratriptan, produced a vasocontractile response in CCA with pD2 values of 5.1±0.1 (Figure 1, see also Akin & Gurdal, this issue) or 4.9±0.2 (Figure 2), respectively. Apparent pKb values of 5HT1B antagonist SB216641 (Price et al., 1997) in inhibiting sumatriptan- or eletriptan-induced responses were calculated to be 8.6±0.11 or 8.9±0.13 (Figure 2; see also Akin & Gurdal, this issue), respectively. The 5HT1D receptor antagonist, BRL15572 (0.1 or 1 μM) (Price et al., 1997) did not significantly inhibit the vasocontractile response to eletriptan, (Figure 2).
Figure 1.

Sumatriptan but not naratriptan produced vasocontractile response in common carotid artery. Data are presented as mean±s.e.mean (n=5–6).
Figure 2.

Effect of 5HT1B receptor antagonist SB216641 or 5HT1D receptor antagonist BRL15572 on concentration-response curve of eletriptan in common carotid artery. Maximal contraction induced by 5-HT was (mN) 19±2 in common carotid artery. Data are presented as mean±s.e.mean (n=4).
Naratriptan (10, 100 μM) inhibited vasocontractile responses to sumatriptan with a pKb value of 5.9±0.2 or eletriptan with a pKb value of 5.7±0.7 in CCA (Figure 3A,B).
Figure 3.

Effect of naratriptan on sumatriptan (A) or eletriptan (B) mediated vasocontractile response. Maximal contraction induced by 5-HT was (mN) 19±2 in common carotid artery. Data are presented as mean±s.e.mean (n=4).
Cyclic AMP accumulation
Sumatriptan inhibited forskolin-stimulated cyclic AMP accumulation. This response was sensitive to PTX treatment or GR127,935 (Figure 4).
Figure 4.

Sumatriptan (100 μM) inhibited forskolin stimulated cyclic AMP accumulation and this response was sensitive to PTX treatment or GR127935 (1 μM). Responses were expressed as a percentage of forskolin-stimulated cyclic AMP acumulation. Average forskolin stimulated-cyclic AMP response of 3–5 mm rings of common carotid artery was 2.9±0.6 pmol min−1. Data are presented as mean±s.e.mean (n=7–8) (*Indicates statistical difference, Student t-test, P<0.05).
Sumatriptan, eletriptan or naratriptan inhibited forskolin-stimulated cyclic AMP production with comparable pD2 values (7.12±0.5 for sumatriptan and 7.6±0.2 for eletriptan and 7.55±0.1 for naratriptan). 5HT, sumatriptan, naratriptan or eletriptan produced similar maximal inhibition of cyclase activity (50–60% of forskolin stimulation). 5HT1B receptor antagonist SB216641 (0.1 or 1 μM) antagonized the inhibitory response to sumatriptan, eletriptan or naratriptan in CCA. Apparent pKb values of SB21664 in inhibiting sumatriptan- or naratriptan-induced response were 8.89±0.29 and 9.12±0.14, respectively. These responses were insensitive to the 5HT1D receptor antagonist BRL15572 (1 μM) (Figures 5, 6, and 7).
Figure 5.

Effect of 5HT1B receptor antagonist SB216641 or 5HT1D receptor antagonist BRL15572 on inhibitory response of sumatriptan in forskolin-stimulated cyclic AMP accumulation in common carotid artery. Forskolin stimulated-cyclic AMP response of 3–5 mm rings of common carotid artery was 2.9±0.6 pmol min−1. Data are presented as mean±s.e.mean (n=5–6).
Figure 6.
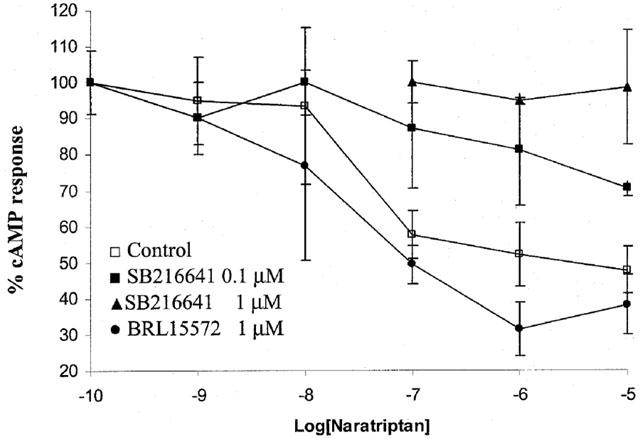
Effect of 5HT1B receptor antagonist SB216641 or 5HT1D receptor antagonist BRL15572 on inhibitory response of naratriptan in forskolin-stimulated cyclic AMP accumulation in common carotid artery. Forskolin stimulated-cyclic AMP response of 3–5 mm rings of common carotid artery was 2.9±0.6 pmol min−1. Data are presented as mean±s.e.mean (n=5–6).
Figure 7.

Effect of 5HT1B receptor antagonist SB216641 (1 μM) or 5HT1D receptor antagonist BRL15572 (1 μM) on inhibitory response of eletriptan in forskolin-stimulated cyclic AMP accumulation in common carotid artery. Forskolin stimulated-cyclic AMP response of 3–5 mm rings of common carotid artery was 2.9±0.6 pmol min−1. Data are presented as mean±s.e.mean (n=5–6). (*Indicates statistical difference, Student t-test, P<0.05).
Discussion
Here we observed that stimulation of CCA by 5HT1B/D agonist sumatriptan, eletriptan or naratriptan inhibited cyclic AMP production. However, sumatriptan or eletriptan, but not naratriptan could contract the vessel. In the following few paragraphs we briefly discussed the implications and possible explanations of the observed phenomenon.
We showed that sumatriptan or eletriptan-induced contraction of CCA is mediated by 5HT1B receptors, and that the contribution of 5HT1D subtype to the response is imperceptible (see also Akin & Gurdal, this issue). Likewise, naratriptan has been known for its ability to stimulate 5HT1B (as well as 5HT1D) receptors, and the agonistic efficacy of naratriptan at 5HT1B receptors has been reported to be comparable to that of sumatriptan (Pauwels & Colpaert, 1996; Wurch et al., 1997; Nilsson et al., 1999). We also verified these observations by comparing the agonistic potencies of sumatriptan, eletriptan and naratriptan in inhibiting forskolin-induced cyclic AMP production in CCA. We found that (1) potencies of these agonists in inhibiting adenylate cyclase were indeed comparable in the present experimental system, (2) all these ligands (including 5HT) produced the same level of maximal cyclase inhibition (i.e. they all look like full agonist in this sense), (3) the inhibitory effects of the three agonists were mediated by 5HT1B receptors (as assessed by using specific antagonists for 5HT1B or 5HT1D receptors), and (4) agonist-induced inhibition of adenylate cyclase was sensitive to PTX treatment, as expected from a Gi/o-coupled receptor, like 5HT1B (Zgombick et al., 1993; Pauwels & Colpaert, 1996; Wurch et al., 1997). In summary, all of the three agonists can bind to 5HT1B receptor which leads to an inhibition of adenylate cyclase with comparable potencies, whereas only two of these bindings couple to vasocontraction (induced by sumatriptan or eletriptan which behaved as partial agonists compared to 5HT in vasocontraction). Our conclusion at this point was that inhibition of adenylate cyclase by the stimulation of 5HT1B receptors was not a sufficient condition to couple the receptor to vasocontraction, since all these ligands inhibited the adenylate cyclase down to the same level but they did not all produce vasocontraction.
Note that apparently no other receptor but 5HT1B was involved in sumatriptan or eletriptan-induced vasocontraction. In this case one may expect that naratriptan should behave as an antagonist, when the observed response is 5HT1B-stimulated vasocontraction. Experiments indeed verified this prediction: naratriptan inhibited sumatriptan- or eletriptan-induced vasocontraction. Thus, naratriptan apparently behaves as an antagonist in 5HT1B-mediated vasocontraction, but behaves as an agonist in adeylate cyclase inhibition. Such a behaviour may be expected from a weak partial agonist in weakly- and strongly-coupled systems such as vasocontraction and cyclase, respectively. However, this does not explain why the same level of adenylate cyclase inhibition (induced by all of the agonists used here) does not lead to the same level of contraction, when we assume a causal relationship between the level of intracellular cyclic AMP and contraction. Therefore the above scenario actually implies that the coupling of 5 HT1B receptor to adenylate cyclase and contraction is not sequential, in the sense that intracellular concentration of cyclic AMP does not seem to determine the level of contraction directly. Instead, the receptor-signal appears to be bifurcating at the level of Gi/o system: considering that both responses (i.e. cyclase activity and contraction) are sensitive to PTX (see also Akin & Gurdal, this issue) but cyclic AMP inhibition does not seem to be cause of vasocontraction. Hence, Gi/o can couple to these systems somehow independently and one of these couplings can be much stronger than the other, which may eventually explain the observed discrepancy between naratriptan and other ligands. However, this requires that 5HT1B receptors are strongly coupled to adenylate cyclase but weakly coupled to contraction. Nevertheless, this does not seem to be the case in the present experimental system. The measured potencies of these agonists in inhibiting adenylate cyclase are not greater than their binding affinities to 5HT1B (Pauwels & Colpaert, 1996; Wurch et al., 1997), which implies a linear (i.e. weak) coupling of 5HT1B receptors to adenylate cyclase in the present system.
An alternative explanation, in this case, may be that agonists can possess diverging efficacies for the two systems (i.e. adenylate cyclase and contraction) which vary independently from one agonist to another. Thus, naratriptan may have relatively low efficacy for the contraction system whereas all of the agonists used in the present study possess comparable efficacies in cyclase inhibition. In other words, 5HT1B receptor couples to adenylate cyclase (through Gi/o) when stimulated by sumatriptan, eletriptan or naratriptan, but couples to an additional signalling system (through a PTX-sensitive G protein) to produce vasocontraction when stimulated by sumatriptan or eletriptan (see Akin & Gurdal, this issue). One possibility for the contraction pathway would be the activation of PLC, however we were unable to detect a sumatriptan-induced inositol phosphate accumulation in this experimental system. On the other hand, the vasocontraction was sensitive to L-type voltage dependent Ca+2- channel blockers, such as nicardipine or nitrendipine (see also Akin & Gurdal, this issue), which suggests the contribution of an inward Ca2+ current to the observed response. This scenario is schematized in Figure 8.
Figure 8.

Schematic representation of agonist-induced differential coupling of 5HT1B receptor to adenylate cyclase (AC) and Ca2+ channels.
Such an idea, which is known as agonist-induced signal trafficking, has actually been suggested for many other receptor systems (Kenakin, 1995). The underlying idea is that a receptor can adopt different conformations depending on the identity of the agonist, so that each conformation can couple to different signalling molecules with different efficiencies. Hence, the identity of the agonist determines not only the quantity of the signal (i.e. the efficacy), but also its quality by targeting the receptor to different signalling molecules. Although it is hard to prove the validity of this scenario directly for the present case, such an idea seems to provide a working hypothesis, which constitutes a plausible explanation for the unusual response pattern of these agonists in CCA.
Acknowledgments
This study has been supported by the following grants: Turkish Scientific and Technical Research Council SBAG 2288, Ankara University Research Found 98-09-00-54.
Abbreviations
- CCA
common carotid artery
- IP
inositol phosphate
- PTX
pertussis toxin
References
- COHEN M.L., SCHENK K. 5-Hydroxytryptamine (1F) receptors do not participate in vasoconstriction: lack of vasoconstriction to LY344864, a selective serotonin (1F) receptor agonist in rabbit saphenous vein. J. Pharmacol. Exp. Ther. 1999;290:935–939. [PubMed] [Google Scholar]
- GEERTS I.S., DE MEYER G.R.Y., BULT H. Collar-induced elevation of mRNA and functional activity of 5-HT1B receptor in the rabbit carotid artery. Br. J. Pharmacol. 2000;131:1723–1731. doi: 10.1038/sj.bjp.0703732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GURDAL H., TULUNAY F.C.The interaction of serotonergic agonists with α-adrenoceptors in rabbit aorta 5-Hydroxytryptamine Mechanisms in Primary Headaches 1992New York: Raven Press; 146–150.ed. Olesen, J. & Saxena, P.R. pp [Google Scholar]
- HOYER D., CLARK D.E., FOZARD J.R., HARTIG P.R., MARTIN D.R., MYLECHARLANE E.J., SAXENA P.R., HUMPHREY P.A. VIIth International Union of Pharmacology classification of receptors for 5-hydroxytryptamine. Pharmacol. Rev. 1994;46:157–204. [PubMed] [Google Scholar]
- KENAKIN T.P. The classification of drug and drug receptors in isolated tissues. Pharmacol. Rev. 1984;36:165–222. [PubMed] [Google Scholar]
- KENAKIN T.P. Agonist-receptor efficacy II: agonist trafficking of receptor signals. TIPS. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- MAASSENVANDENBRINK A., REEKERS M., BAX W.A., FERRARI M.D., SAXENA P.R. Coronary side-effect potential of current and prospective antimigraine drugs. Circulation. 1998;98:25–30. doi: 10.1161/01.cir.98.1.25. [DOI] [PubMed] [Google Scholar]
- NILSSON T., LONGMORE J., SHAW D., OLESEN I.J., EDVINSON L. Contractile 5-HT1B receptors in human cerebral arteries: pharmacological characterization and localization with immunocytochemistry. Br. J. Pharmacol. 1999;128:1133–1140. doi: 10.1038/sj.bjp.0702773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NG G.Y., GEORGE S.R., CARON M., BOUIVER M., DENNIS M., O‘DOWD B.F. Human serotonin 1B receptor expression in Sf9 cells: phosphorylation, palmitoylation, and adenylyl cyclase inhibition. Biochemistry. 1993;32:11727–11733. doi: 10.1021/bi00094a032. [DOI] [PubMed] [Google Scholar]
- PAUWELS P.J., COLPAERT F.C. Selective antagonism of human 5HT1D and 5HT1B receptor-mediated responses in stably transfectd C6-glial cells by ketanserin and GR127,935. Eur. J. Pharmacol. 1996;300:141–145. doi: 10.1016/0014-2999(96)00011-8. [DOI] [PubMed] [Google Scholar]
- PRICE G.W., BURTON M.J., COLLIN L.J., DUCKWORTH M., GASTER L., GOTHERT M., JONES B.J., ROBERTS C., WATSON J.M., MIDDLEMISS D.N. SB-216641 and BRL-15572-compounds to pharmacologically discriminate h5-HT1B and h5HT1D receptors. Naunyn-Schmied. Arch. Pharmacol. 1997;356:312–316. doi: 10.1007/pl00005056. [DOI] [PubMed] [Google Scholar]
- ROON K.I., MAASSEN VAN DEN BRINK A., FERRARI M.D., SAXENA P.R. Bovine isolated middle cerebral artery contractions to antimigraine drugs. Naunyn Schmiedebergs. Arch. Pharmaco. 1999;360:591–596. doi: 10.1007/s002109900095. [DOI] [PubMed] [Google Scholar]
- SGARD F., FAURE C., GRAHAM D. Evidence for 5HT1Dβ but not 5-HT1Dα receptor subtype in canine large coronary arteries and saphenous vein. Cardiovasc. Res. 1996;31:793–799. doi: 10.1016/0008-6363(96)00014-4. [DOI] [PubMed] [Google Scholar]
- UGUR O., ONARAN H.O. Allosteric equilibrium model explains steady-state coupling of β-adrenergic receptors to adenylate cyclase in turkey erythrocyte membranes. Biochem. J. 1997;323:765–776. doi: 10.1042/bj3230765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ULLMER C., SCHMUCK K., KALKMAN H.O., LUBBERT H. Expression of serotonin receptor mRNAs in blood vessels. FEBS Lett. 1995;370:215–221. doi: 10.1016/0014-5793(95)00828-w. [DOI] [PubMed] [Google Scholar]
- WURCH T., PALMIER C., COLPAERT F.C., PAUWELS P.J. Recombinant saphenous vein 5HT1b receptors of the rabbit:comparative pharmacology with human 5-HT1B receptors. Br. J. Pharmacol. 1997;120:153–159. doi: 10.1038/sj.bjp.0700868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZGOMBICK J.M., BORDEN L.A., COCHRAN T.L., KUCHAREWICZ S.A., WEINSHANK R.L., BRANCEK T.A. Dual coupling of cloned human 5-hydroxytryptaminelda and 5-hydroxytriptamine1db receptors stably expressed in murine fibroblasts: inhibition of adenylate cyclase and elevation of intracellular calcium concentrations via pertusis toxin sensitive G protein(s) Mol. Pharm. 1993;44:575–582. [PubMed] [Google Scholar]


