Abstract
We aimed to characterize 5-HT receptors mediating contraction and relaxation to 5-HT in dog proximal stomach longitudinal muscle (LM) strips.
Of the tryptamine analogues tested, 5-HT was the most potent contractile agent at basal length, while 5-CT was the most potent relaxant of PGF2α-induced contraction. Neither the contractions to 5-HT, nor the relaxations to 5-CT were influenced by tetrodotoxin, illustrating that action potential propagation is not involved.
The 5-HT-induced contraction was antagonized by mesulergine (0.03 to 0.3 μM) and ketanserin (2–20 nM), but the antagonism was not of a simple competitive nature, indicating multiple receptor involvement. Ketanserin (3 to 30 nM) and mesulergine (30 nM) competitively antagonized the α-Me-5-HT-induced contraction (pKB: 8.83±0.09 and pA2: 8.25±0.06 respectively). These affinity values are in line with literature affinities of ketanserin and mesulergine at 5-HT2A receptors in various bioassays.
The 5-CT-induced inhibition of PGF2α-induced contraction was competitively antagonized by mesulergine (pKB estimate: 8.52±0.12) and by the selective 5-HT7 receptor antagonist SB-269970 (pKB estimate: 9.36±0.14). Both pKB estimates are in line with literature affinities of these compounds for 5-HT7 receptors. Mesulergine (30 nM) and SB-269970 (10 nM) shifted the relaxant curve to 5-HT parallel to the right in the presence of ketanserin (0.3 μM) (pA2 estimates of 8.08±0.10 and 8.75±0.14 respectively), indicative of 5-HT7 receptor involvement.
It is concluded that 5-HT induces dog proximal stomach (LM) contraction via smooth muscle 5-HT2A receptors and relaxation via smooth muscle 5-HT7 receptors.
Keywords: Dog, proximal stomach, fundus, 5-HT2A, 5-HT7, SB-269970
Introduction
The dog is used as a model to study gastrointestinal motility mechanisms and the way drugs interact with them. With regard to gastric motility, it was shown in conscious dogs that intravenously administered 5-HT induced contraction of a Heidenhain pouch, that was prevented by a 5-HT4 receptor antagonist (Bingham et al., 1995) and that 5-HT4 receptor agonists stimulate gastric emptying (Gullikson et al., 1993). Recent in vitro studies confirmed the presence of 5-HT4 receptors on cholinergic nerves in the canine gastric corpus (Prins et al., 2001a). In the canine antrum, also evidence for muscular inhibitory 5-HT7 receptors was obtained (Prins et al., 2001b). Careful pharmacological characterization of the receptors mediating contraction by 5-HT in the dog proximal stomach has not been done. In other species it was shown that muscular 5-HT2B receptors mediate contraction in the rat gastric fundus (Baxter et al., 1994) and muscular 5-HT2 receptors do so in the guinea-pig gastric fundus (Takemura et al., 1999).
The proximal stomach also has a great relaxant capacity to accommodate food without major pressure changes. Different clues towards 5-HT1 receptors mediating proximal gastric relaxation have been published. Kojima et al. (1992), Meulemans et al. (1993) and Takemura et al. (1999) have shown 5-HT1 receptors mediating relaxation in guinea-pig proximal stomach in vitro. In vivo, sumatriptan (a 5-HT1 receptor agonist) relaxed the proximal stomach of cat (Coulie et al., 1999) and of man (Tack et al., 2000), a process which has been suggested to involve both nitrergic and cholinergic pathways. Surprisingly, no in-depth characterization of the 5-HT receptors involved in these responses by means of isolated muscle strip studies has been reported.
Therefore the present study was set up to characterize the 5-HT receptors involved in the effects of 5-HT on longitudinal muscle strips of the canine proximal stomach. Strips were studied at basal muscle length or were precontracted. In this manner, the putative presence of contraction-mediating receptors and relaxation-mediating receptors could be identified.
Methods
Tissue preparation
Beagle dogs of both sexes, weighing between 10 and 15 kg, were sacrificed by decerebration, followed by exsanguination through the carotid artery. The entire stomach was dissected and placed in Krebs-Henseleit solution (composition in mM: glucose 10.1, CaCl2 2.51, NaHCO3 25, MgSO4 1.18, KH2PO4 1.18, KCl 4.69 and NaCl 118). The stomach was opened by cutting along the lesser curvature and the contents were rinsed out. The muscle strips were derived from the proximal ventral part of the stomach, at approximately 3 cm from the lower oesophageal sphincter. The strips were carefully cleared of mucosa, submucosa and omentum. Longitudinal muscle strips (maximum 16 per dog) of approximately 1.5 cm length and 2–3 mm width were prepared and mounted onto tissue holders. These were placed in an organ bath set-up containing Krebs-Henseleit solution at 37°C, continuously gassed with 95% O2 and 5% CO2. The mechanical activity of the preparations was recorded via isotonic transducers (2 g load) (Harvard apparatus) on a chart recorder (Model BD 112; Kipp & Sons). All strips were studied on the preparation day.
Experimental protocols
All experiments were conducted in the presence of indomethacin (1 μM) to avoid spontaneous contractions due to prostaglandins. Agonists were added on basal muscle length (protocol 1) or on contracted muscle strips (protocol 2). Both protocols began after a 30 min stabilization period.
Protocol 1
Working on basal muscle length, antagonist or solvent was added to the organ bath and left to incubate for 30 min. After this, a cumulative concentration-response curve to an agonist was established with half log-units ascending concentration increments from 1 nM onwards. Where some agonists, at lower concentrations, induced contraction, they sometimes induced, at higher concentrations, relaxation. To analyse these results, only contraction data were taken into account. (Figure 1A).
Figure 1.
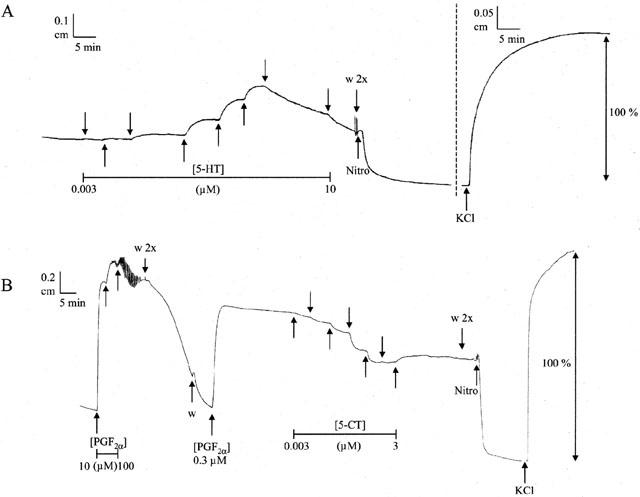
A representative recorder tracing of an example following protocol 1 (A) and protocol 2 (B) of canine proximal stomach longitudinal muscle strips. The dashed line divides areas of different amplification. Nitro=nitroglycerin (10 μM), w=wash, KCl=KCl (0.16 M). In the upper trace, 5-HT was administered with half log unit concentration increments as indicated by the arrows, in the lower trace 5-CT was administered.
Preliminary experiments were carried out to find the optimal pre-contracting agent for relaxation studies with tryptamine analogues (protocol 2). Concentration-contraction curves to KCl, prostaglandin F2 alpha (PGF2α), histamine and carbachol were constructed. After washout, the approximate EC50 concentration of each precontracting agent was added to the organ bath, after which a concentration-relaxation curve to 5-CT was established. The PGF2α-induced contraction was selected for two reasons. First, PGF2α produced a contraction that was maintained for over 2 h. Second, in comparison with the other precontracting agents, PGF2α allowed 5-CT to induce the relatively most pronounced relaxation.
Protocol 2
Prior to each relaxation curve to any tryptamine analogue, the maximum effect of PGF2α was determined and the within-strip EC50 was roughly estimated. After thorough washout, antagonist or solvent was added; after 30 min, the EC50 value of PGF2α (ranging from 0.3 to 3 μM) was added to the baths. Upon maintained contraction (usually after 5–10 min), a concentration-relaxation curve to an agonist was established in a cumulative manner. (Figure 1B).
Then, for both protocols, compounds were washed out by replacing the organ bath solution twice. Maximal relaxation to nitroglycerin (10 μM) was achieved after which maximal contraction to KCl (0.16 M) was obtained. In previous experiments 0.16 M KCl was shown to induce maximum contraction in the same tissue. All agonist-induced responses were expressed as percentage of the contraction to KCl (0.16 M) after nitroglycerin relaxation (10 μM). Only one agonist was studied per muscle strip.
Data presentation and statistical analysis
Concentration-response curves to agonists were fitted iteratively to the Hill equation, obtaining curve parameter estimates for mid-point location (pEC50), upper asymptote (α) and Hill slope (=nH). To test the influence of one concentration of a drug on the curve parameters of an agonist, one-way ANOVA was performed. A level of P<0.05 was considered to indicate significance. To test the criteria for Schild-analysis (curves must have equal slopes and upper asymptotes), one-way ANOVA was performed, followed by a post-hoc Bonferroni's test for multiple comparisons. pEC50 values in the absence and presence of different concentrations of antagonists were iteratively fitted to a modified Schild equation, as previously described by Black et al., 1985. The pKB value as true affinity estimate was calculated in case the criteria for Schild analysis were met (no alteration of slope or upper asymptote due to antagonism). If only one concentration of antagonist was tested, and the agonist curve was shifted to the right with no change in upper asymptote or slope, the antagonist affinity was expressed as an apparent dissociation constant, the pA2 value, calculated using the Schild equation.
All data were expressed as the mean±s.e.mean, where n represents the number of dogs used in one experimental protocol.
Drugs
The following drugs were used (abbreviations and respective suppliers in parentheses): (R)-3-(2-(2-(4-methyl-piperidin-1-yl)ethyl)-pyrrolidine-1-sulphonyl)-phenol (SB-269970), 5-methoxytryptamine (5-MeOT), 2-methyl-5-HT (2-Me-5-HT), granisetron HCl, mesulergine HCl, ketanserin tartrate, 1-(2-methoxyphenyl)-4-[4-(2-phthalimido)butyl]piperazine HCl (NAN-190), [1-[2-[(methylsulphonyl)amino]ethyl]-4-piperidinyl]methyl 1-methyl-1H-indole-3-carboxylate (GR113808; Janssen Research Foundation, Belgium), atropine sulphate, carbachol, histamine, NG-nitro-L-Arginine (L-NNA; Acros Chimica, Belgium), 5-hydroxytryptamine creatinine sulphate (5-HT), tetrodotoxin (TTX; Serva, Germany), α-methyl-5-HT (α-Me-5-HT), fluoxetine HCl, 5-carboxamidotryptamine (5-CT; Tocris Cookson, U.K.), potassium chloride (KCl; Sigma, Belgium), methysergide maleate (RBI, U.S.A.), Prostaglandine F2 alpha (PGF2α; diluted from Dinolytic® 5 mg ml−1; Upjohn, Animal Health, Belgium), cocaine HCl, glycerol trinitrate 1% (nitroglycerin; Merck, Germany) and pargyline HCl (Abbott, U.S.A.). All compounds were dissolved in distilled water, except for ketanserin, pargyline and 5-HT. Ketanserin was dissolved in distilled water acidified with tartaric acid in the stock solution; pargyline was dissolved in distilled water with 10% cyclodextrine in the stock solution. 5-HT was prepared with ascorbic acid in the stock solution. The solvents had no effect on the baseline tension or the curves to agonists. All stock solutions were freshly prepared on the experimental day and dilutions were prepared using distilled water.
Results
Most tissue strips showed no phasic contractions. Nevertheless, about 20 per cent of the strips showed a slow and gradual decrease in muscle length shortly after the stabilization period. Since this in general led to maximal tissue contraction, strips could not contract any further. This prevented establishment of reproducible contractile responses to agonists, therefore, these strips were not used. The remaining strips had a stable muscle length on top of which clear-cut agonist-induced effects could be observed.
Response to 5-HT at basal muscle length (protocol 1)
In general, 5-HT induced contraction at lower concentrations (approximately 1 nM to 1 μM), and tended to induce relaxation at higher concentrations (approximately 1 μM to 3 mM). As we were interested in the contractile effect of 5-HT, 5-HT was not further added as soon as relaxation occurred after attainment of maximal contractile response. In about a third of all tissues, 5-HT merely induced a moderate relaxation (Figure 2A).
Figure 2.
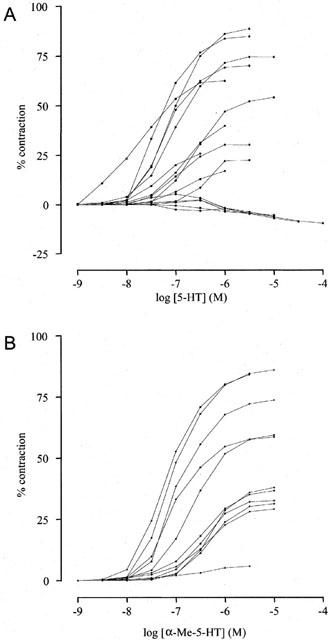
Individual concentration-contraction curves to 5-HT (A; n=16) and α-Me-5-HT (B; n=11) of canine proximal stomach longitudinal muscle preparations on basal muscle length. The responses are expressed as percentage of muscle strip contraction to KCl (0.16 M).
Inhibition of re-uptake-1 by cocaine (30 μM), of selective 5-HT re-uptake by fluoxetine (0.3 μM) and of monoamine oxidase by pargyline (0.1 mM) changed neither the potency nor the upper asymptote of the 5-HT-induced concentration contraction curve (n=4; data not shown). Tetrodotoxin (TTX; 0.3 μM; α=0.55±0.06; pEC50=6.91±0.11), NG-nitro-L-Arginine (L-NNA; 0.1 mM; α=0.37±0.07; pEC50=6.80±0.13) and atropine (1 μM; α=0.38±0.07; pEC50=6.76±0.15) did not affect the concentration-contraction curve to 5-HT (α=0.57±0.11; pEC50=6.89±0.12; n=6; data not shown).
Neither the selective 5-HT3 receptor antagonist granisetron (Sanger & Nelson, 1989; 0.3 μM), nor the selective 5-HT4 receptor antagonist GR113808 (Gale et al., 1994; 1 μM), altered the concentration-contraction curve to 5-HT (n=5 to 7; Table 1). Although the 5-HT1, 5-HT2, 5-ht5, 5-HT6 and 5-HT7 receptor antagonist methysergide (Gommeren et al., 1998; 0.1 μM) displayed a non-significant decrease of the maximal effect, it was feasible to estimate a pA2 value (pA2=7.93±0.35; n=6; Table 1).
Table 1.
Curve parameters for the concentration-contraction curves to 5-HT and α-Me-5-HT on basal muscle length in control conditions and in the presence of the antagonists indicated
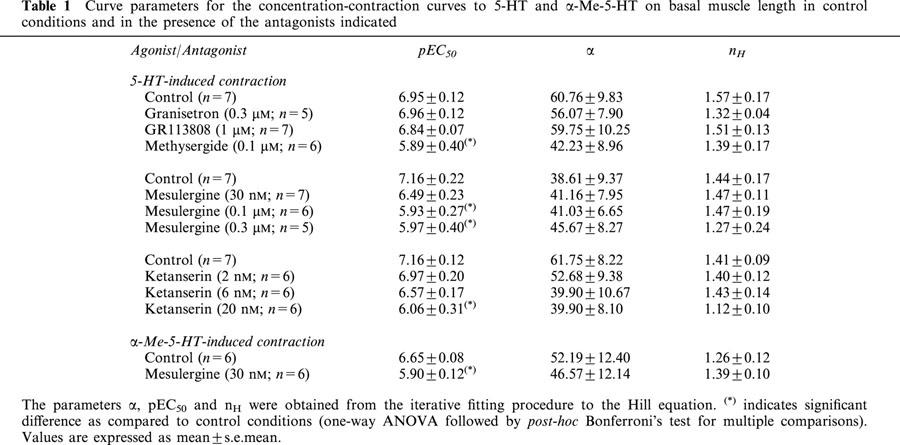
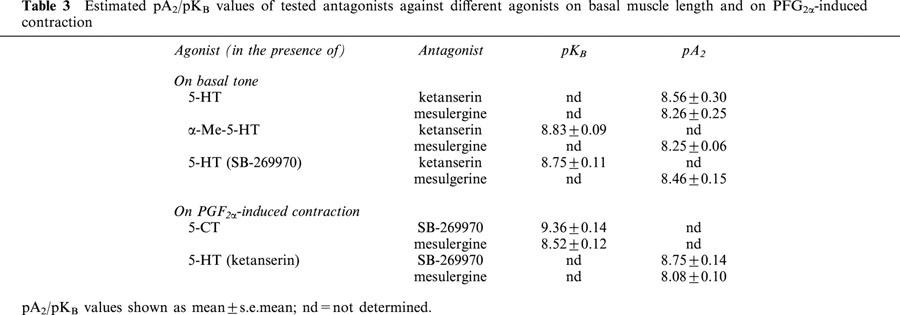
The 5-HT2 and 5-HT7 receptor antagonist mesulergine (Hoyer et al., 1994; 0.03 and 0.3 μM) produced a parallel shift to the right but this was only significant at 0.1 and 0.3 μM mesulergine (Table 1). At the highest concentration (0.3 μM), mesulergine was not able to shift the curve to 5-HT any further. The 5-HT-induced contraction curve shifted to the right in the presence of the selective 5-HT2A receptor antagonist ketanserin (Hoyer et al., 1994; 2 to 20 nM) but only at the highest concentration (20 nM) this was significant. Although a marked decrease of the upper asymptotes was observed, this is not significant. It was feasible to estimate pA2 values for both mesulergine (0.1 μM) and ketanserin (20 nM) (8.26±0.25 and 8.56±0.30 respectively; Table 3).
Table 3.
Estimated pA2/pKB values of tested antagonists against different agonists on basal muscle length and on PFG2α-induced contraction
Response to 5-HT receptor agonists at basal muscle length
As described above, relaxation was occasionally seen using 5-HT. α-Me-5-HT on the other hand induced systematically well-defined concentration-contraction curves (Figure 2B). Although 5-MeOT and 2-Me-5-HT never induced relaxation, large variability for all curve parameters was observed in the contraction response. On six experiments 2-Me-5-HT induced no effect twice, 5-MeOT induced no effect once. The preferential 5-HT1 and 5-HT7 receptor agonist 5-CT induced, in all but one preparation, a small relaxation (α=−5±2%). Thus, except for α-Me-5-HT, variability in the response to the different tryptamine analogues was considerable. All concentration-contraction curves were fitted to obtain curve parameters, except for 5-CT, that in all but one preparation induced a small relaxation (see for curve parameters: Table 2). The following rank order of agonist potency was found: 5-HT>α-Me-5-HT>5-MeOT⩾2-Me-5-HT.
Table 2.
Curve parameters for the concentration-response curves to 5-HT and tryptamine analogues on basal muscle length and on PGF2α-induced contraction
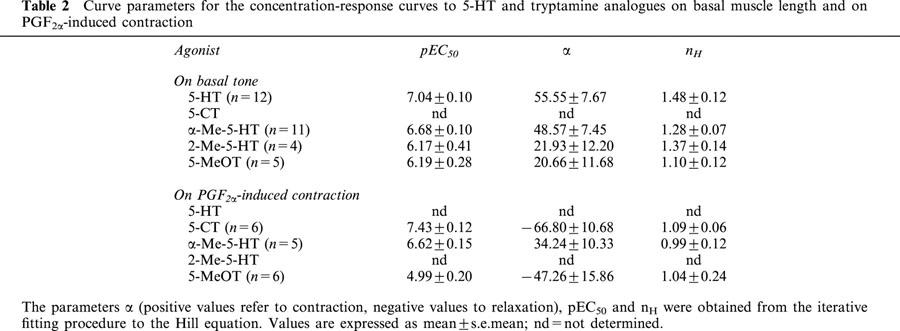
Ketanserin (3–30 nM) produced a parallel rightward displacement of the concentration-contraction curve to α-Me-5-HT (Figure 3), yielding a Schild slope (1.16±0.14) that was not significantly different from unity. After constraining the Schild slope to unity, a pKB estimate was obtained (8.83±0.09; n=11; Table 3). Mesulergine (30 nM) produced a parallel rightward displacement of the concentration-contraction curve to α-Me-5-HT, yielding a pA2 estimate (8.25±0.06; n=6; Tables 1 and 3).
Figure 3.
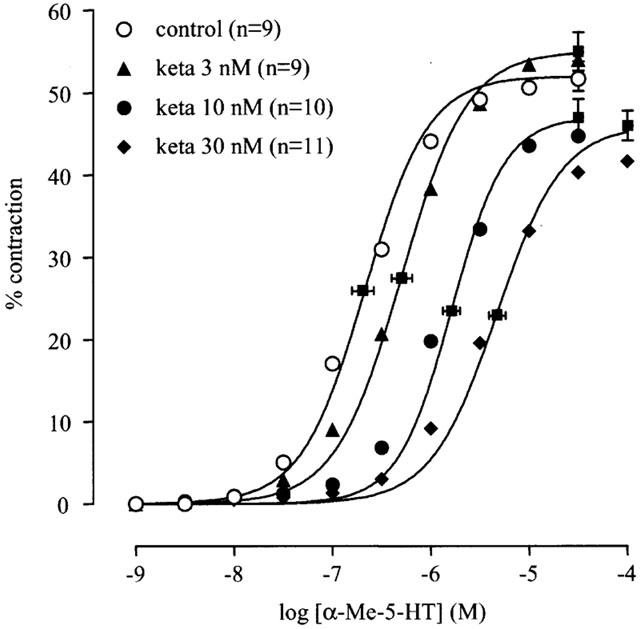
Influence of increasing concentrations of ketanserin (keta) on the α-Me-5-HT-induced contractions of canine proximal stomach longitudinal muscle preparation. Contractions are expressed as percentage of muscle strip contraction to KCl (0.16 M). The curves represent simulations using the Hill equation; the parameters for α (with vertical error bars) and EC50 (shown with horizontal error bars) were obtained from the iterative fitting procedure.
Responses to 5-HT and 5-HT receptor agonists on pre-contracted strips (protocol 2)
Since the experiments with 5-CT on basal muscle length indicated the putative presence of relaxatory 5-HT receptors, we decided to study the relaxant effect of 5-HT and 5-HT receptor agonists more in depth on strips contracted by the EC50 value (ranging from 0.3 to 3 μM) of PGF2α (53±9% of the KCl contraction (n=6)).
5-MeOT systematically induced pronounced relaxations. 5-CT induced concentration-dependent relaxations but at higher concentrations (from 3 μM onwards) a small contraction occurred (9±2% of KCl contraction, n=6). α-Me-5-HT always induced contractions on top of the PGF2α-induced contraction, although sometimes, at higher concentrations (from 10 μM onwards), a relaxation of variable amplitude occurred. The relaxant responses to 5-CT and 5-MeOT and the contractile responses to α-Me-5-HT are shown in Figure 4; the curve parameters are given in Table 2. The response to 5-HT and 2-Me-5-HT was very variable. Sometimes 5-HT and 2-Me-5-HT induced relaxation only (n=3) while in other strips contraction was seen using lower concentrations (1 nM to 1 μM) of the agonist and relaxation was seen using higher agonist concentrations (from 10 μM onwards; n=3). The mean effect in all strips can be seen in Figure 4.
Figure 4.
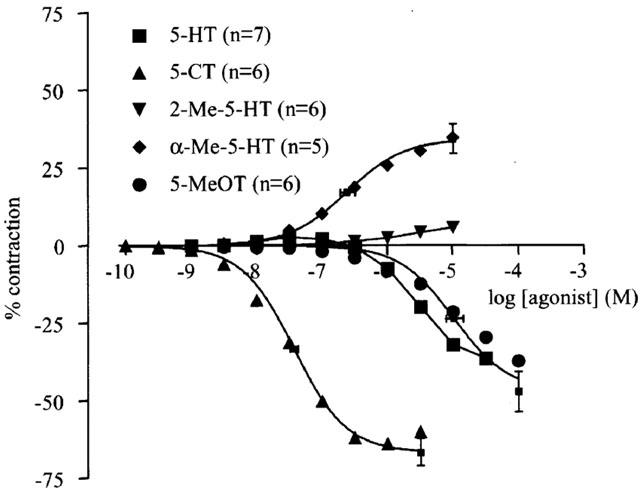
Effect of 5-HT, 5-CT, 2-Me-5-HT, 5-MeOT and α-Me-5-HT on the PGF2α-induced contraction of canine proximal stomach longitudinal muscle preparation. The curves to 5-CT, α-Me-5-HT and 5-MeOT represent simulations using the Hill equation; the parameters for α (shown in vertical error bars) and EC50 (shown with horizontal error bars) were obtained from the iterative fitting procedure. The vertically averaged data points of 5-HT and 2-Me-5-HT are connected. The responses are expressed as percentage of muscle strip contraction to KCl (0.16 M).
Influence of antagonists on the 5-CT-induced relaxation
Neither TTX (0.3 μM) nor L-NNA (0.1 mM) affected the concentration-relaxation curve to 5-CT (n=6). NAN-190 (5-HT1A receptor antagonist; Cao & Rodgers, 1997; 30 nM; pEC50=7.42±0.10; α=−39.00±7.74; nH=1.09±0.04) and ketanserin (0.1 μM; pEC50=7.60±0.14; α=−42.25±6.01; nH=0.88±0.05) did not alter the concentration-relaxation curve to 5-CT as compared with control conditions (pEC50=7.52±0.08; α=−34.62±4.74; nH=0.97±0.04) (n=5 to 8). Mesulergine (10–100 nM) produced a parallel rightward displacement of the concentration-relaxation curve to 5-CT (Figure 5A), yielding a Schild slope (0.84±0.16) that was not significantly different from unity. After constraining the Schild slope to unity, a pKB estimate was obtained (8.52±0.12; n=10; Table 3). The selective 5-HT7 receptor antagonist SB-269970 (Hagan et al., 2000; 1–10 nM) also produced a parallel rightward displacement of the concentration-relaxation curve to 5-CT (Figure 5B), yielding a Schild slope (1.15±0.17) that was not significantly different from unity. After constraining the Schild slope to unity, a pKB estimate was obtained (9.36±0.14; n=8; Table 3).
Figure 5.
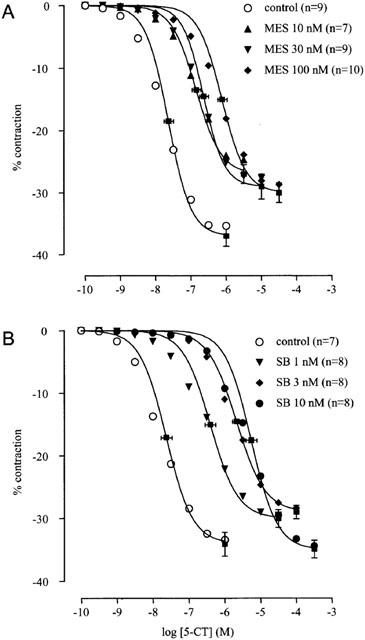
Antagonism by mesulergine (MES; A) and by SB-269970 (SB; B) of the 5-CT-induced relaxation of PGF2α-contracted longitudinal muscle preparations of dog proximal stomach. The curves represent simulations using the Hill equation and the parameters for α (shown with vertical error bars) and EC50 (shown with horizontal error bars) that were obtained from the iterative fitting procedure. The responses are expressed as percentage of muscle strip contraction to KCl (0.16 M).
5-HT-induced relaxation–pA2-estimates
In the presence of ketanserin (0.3 μM), 5-HT induced a relaxation on top of the PGF2α-induced contraction with a corresponding pEC50 of 5.96±0.17. Mesulergine (30 nM) shifted the curve to 5-HT in parallel to the right, yielding a pA2 estimate (8.08±0.10; n=6; Table 3). SB-269970 (10 nM) also produced a dextral shift of the 5-HT curve, without change in Hill slope or upper asymptote, yielding a pA2 estimate (8.75±0.14; n=5; Table 3).
5-HT-induced contraction–pA2-estimates
After blocking the 5-HT7 receptor induced relaxation with SB-269970 (0.1 μM), 5-HT induced contraction only (pEC50: 6.92±0.05). In the presence of SB-269970, ketanserin (10–100 nM) produced a parallel rightward displacement of the concentration-contraction curve to 5-HT. This yielded a Schild slope (0.94±0.13) that was not significantly different from unity. After constraining the Schild slope to unity, a pKB estimate was obtained (8.75±0.11; n=7; Table 3).
In the presence of SB-269970 (100 nM), mesulergine (30 nM) produced a parallel rightward displacement of the concentration-contraction curve to 5-HT. A pA2 estimate was obtained (8.46±0.15; n=6; Table 3).
Discussion
This study aimed to characterize the 5-HT-receptors involved in the 5-HT-induced length changes of dog proximal stomach longitudinal muscle. The experiments with pargyline, fluoxetine and cocaine imply that neither breakdown nor re-uptake of 5-HT impacts the interaction of 5-HT with its receptors. Furthermore, the inability of TTX to affect the 5-HT-induced contractions and the 5-CT-induced relaxations indicates that the receptors involved are located on smooth muscle.
Tryptamine analogues
The rank order of tryptamine analogues potency can be used to roughly identify the 5-HT receptor subtype involved in a particular response (Baxter et al., 1994). Except for α-Me-5-HT, that always induced contraction, tryptamine analogues induced contraction as well as relaxation, expressing varying efficacy at both phenomena. Since α-Me-5-HT preferentially stimulates 5-HT2 receptors, the presence of a 5-HT2 receptor population mediating contraction is likely, especially in view of the observation that 5-HT and α-Me-5-HT are nearly equipotent. The 5-HT1 and 5-HT7 receptor agonist 5-CT and the 5-HT1, 5-HT4 and 5-HT7 receptor agonist 5-MeOT induced relaxation of the PGF2α-induced contraction, raising the possibility of 5-HT1, 5-HT4 and 5-HT7 receptors mediating relaxation. As 5-CT, that is not active at 5-HT4 receptors was the most potent relaxant agonist, the involvement of 5-HT4 receptors is unlikely. Involvement of 5-HT7 receptors is more likely than involvement of 5-HT1 receptors, due to the inability of TTX to affect the relaxation to 5-HT. 5-HT1 receptors located on smooth muscle would be expected to mediate contraction rather than relaxation, since they are negatively coupled to adenylate cyclase. In contrast, 5-HT7 receptors are positively coupled to this enzyme (Bard et al., 1993), thus when located on smooth muscle, are likely candidates to mediate relaxation. Thus, the experiments with agonists point to the presence of contractile 5-HT2 receptors and relaxatory 5-HT7 receptors.
The contractile 5-HT receptor
The inability of GR113808 and granisetron to alter the 5-HT-induced concentration-contraction curves suggests that 5-HT4 and 5-HT3 receptors are not involved (Table 1). Used at higher concentrations, the antagonism produced by mesulergine and ketanserin to 5-HT was not competitive, thus the real dissociation constant, the pKB value, could not be estimated. Nevertheless it was possible to estimate the apparent affinity, the pA2 value using the lowest effective concentration of the antagonists (Table 3). The true affinity estimate pKB can be used as a valuable tool to characterize receptors. Although pA2 estimates in general do not provide conclusive evidence, it can be used as suggestive for the presence of a given receptor subtype. As the pharmacological characterization of the relaxant 5-HT receptor (see below) suggested that the relaxation to 5-HT is due to an interaction with 5-HT7 receptors, mesulergine and ketanserin were also tested versus 5-HT in the presence of the selective 5-HT7 receptor antagonist SB-269970. In the presence of SB-269970 (100 nM), ketanserin and mesulergine competitively antagonized the 5-HT-induced contraction curve, yielding a pKB estimate for ketanserin and a pA2 estimate for mesulergine. Ketanserin also competitively antagonized the contraction to α-Me-5-HT (Table 3).
Ketanserin has an agonist-independent affinity for the contractile 5-HT receptor, as both pKB estimates (to 5-HT and to α-Me-5-HT) are not significantly different. The ketanserin affinity for the 5-HT receptor involved agrees well with literature affinities for the 5-HT2A receptor (dog colon: pKB of 8.4; Prins et al. (1997), rat caudal artery: pA2 of 8.4; Blackburn et al. (1988)). This confirms the presence of contractile 5-HT2A receptors in dog proximal stomach muscle.
Also mesulergine shows agonist-independent affinity for the contractile 5-HT receptor as the pA2 estimates to 5-HT (with (8.46±0.15) and without (8.26±0.25) SB-269970) and α-Me-5-HT (8.25±0.06) are not significantly different. For mesulergine, literature affinity estimates for 5-HT2A receptors vary from 7.4 (Jerman et al., 2001) over 8.2 (Prins et al., 1997) to 9.1 (Briejer et al., 1997). This might well be due to differences in affinity values between animal species, as described for mesulergine by Johnson et al. (1993). So, our findings for mesulergine (pA2 estimate 8.25–8.46) do not contradict 5-HT2A receptor involvement.
The relaxatory 5-HT receptor
Of all the tryptamine analogues tested on PGF2α-induced contraction, 5-CT was the most potent and most efficacious relaxant agonist. This pointed to the involvement of 5-HT1 or 5-HT7 receptors. 5-HT1 receptor involvement is highly unlikely for reasons indicated above. This is further stressed by the experiments with NAN-190 (30 nM), that did not antagonize the 5-CT-induced relaxations indicating that 5-HT1A receptors are not involved. Both mesulergine and the selective 5-HT7 receptor antagonist SB-269970 competitively antagonized the 5-CT-induced relaxation (Table 3). SB-269970 has been described as a selective and potent 5-HT7 receptor antagonist (pKi of 8.9; Lovell et al., 2000). It can be used as a selective radioligand for 5-HT7 receptors (Thomas et al., 2000) but few functional models have been tested with SB-269970. SB-269970 antagonized the 5-CT-induced stimulation of adenylyl cyclase activity in human 5-HT7/HEK293 membranes (pA2 of 8.5) and in guinea-pig hippocampal membranes (pKB of 8.3; Hagan et al., 2000). It is conceivable that the dog 5-HT7 receptor expresses higher affinity for SB-269970 than the human or guinea-pig 5-HT7 receptor. Overall, the obtained affinities strongly point to 5-HT7 receptor involvement.
The pKB for mesulergine against 5-CT agrees well with the affinity estimates obtained in 5-HT7 receptor bioassays of the guinea-pig ileum (7.8; Carter et al., 1995), rat jejunum (8.1; McLean & Coupar, 1996) and human colon (8.3; Prins et al., 1999). Thus, the antagonism by mesulergine emphasizes that 5-HT7 receptors mediate relaxation in this bioassay of dog stomach.
In the presence of ketanserin (0.3 μM), the 5-HT-induced relaxation could be competitively antagonized by SB-269970 and mesulergine. The estimates obtained are in good agreement with literature affinities at 5-HT7 receptors and thus strongly point to 5-HT7 receptors mediating relaxation in this bioassay of canine proximal stomach. This finding is not surprising since we recently reported smooth muscle 5-HT7 receptors mediating inhibition of electrically induced contraction in the canine distal stomach (antropyloric tissue) (Prins et al., 2001b). This firmly establishes the pharmacological impact of 5-HT7 receptor stimulation in the dog stomach in vitro. It remains to be determined as to whether this is translated into a significant role of 5-HT7 receptor stimulation as a trigger for dog gastric relaxation in vivo.
Conclusion
5-HT elicits both contraction and relaxation in canine proximal stomach longitudinal muscle strips via 5-HT receptors located on the smooth muscle cells. The receptor mediating contraction has the characteristics of a 5-HT2A receptor, while the receptor mediating relaxation is a 5-HT7 receptor.
Acknowledgments
The authors wish to thank Marcel Sysmans for his technical assistance.
Abbreviations
- α-Me-5-HT
α-methyl-5-HT
- 5-CT
5-carboxamidotryptamine
- GR113808
[1-[2-[(methylsulphonyl)amino]ethyl]-4-piperidinyl]methyl 1-methyl-1H-indole-3-carboxylate
- 5-HT
5-hydroxytryptamine
- KCl
potassium chloride
- L-NNA
NG-nitro-L-Arginine
- 2-Me-5-HT
2-methyl-5-HT
- 5-MeOT
5-methoxytryptamine
- NAN-190
1-(2-methoxyphenyl)-4-[4-(2-phthalimido)butyl]piperazine HCl
- nitroglycerin
glycerol trinitrate 1%
- PGF2α
prostaglandine F2 alpha
- SB-269970
(R)-3-(2-(2-(4-methyl-piperidin-1-yl)ethyl)-pyrrolidine-1-sulphonyl)-phenol
- TTX
tetrodotoxin
References
- BARD J.A., ZGOMBICK J., ADHAM N., VAYSSE P., BRANCHEK T.A., WEINSHANK R.L. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- BAXTER G.S., MURPHY O.E., BLACKBURN T.P. Further characterization of 5-hydroxytryptamine receptors (putative 5-HT2B) in rat stomach fundus longitudinal muscle. Br. J. Pharmacol. 1994;112:323–331. doi: 10.1111/j.1476-5381.1994.tb13072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BINGHAM S., KING B.F., RUSHANT B., SMITH M.I., GASTER L., SANGER G.J. Antagonism by SB 204070 of 5-HT-evoked contractions in the dog stomach: an in-vivo model of 5-HT4 receptor function. J. Pharm. Pharmacol. 1995;47:219–222. doi: 10.1111/j.2042-7158.1995.tb05782.x. [DOI] [PubMed] [Google Scholar]
- BLACK J.W., LEFF P., SHANKLEY N.P. Further analysis of anomalous pKB values for histamine H2-receptor antagonists on the mouse isolated stomach assay. Br. J. Pharmacol. 1985;86:581–587. doi: 10.1111/j.1476-5381.1985.tb08934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKBURN T.P., THORNBER C.W., PEARCE R.J., COX B. In vitro studies with ICI 169,369, a chemically novel 5-HT antagonist. Eur. J. Pharmacol. 1988;150:247–256. doi: 10.1016/0014-2999(88)90005-2. [DOI] [PubMed] [Google Scholar]
- BRIEJER M.R., MATHIS C., SCHUURKES J.A. 5-HT receptor types in the rat ileum longitudinal muscle: focus on 5-HT2 receptors mediating contraction. Neurogastroenterol Motil. 1997;9:231–237. doi: 10.1046/j.1365-2982.1997.d01-62.x. [DOI] [PubMed] [Google Scholar]
- CAO B.J., RODGERS R.J. Influence of 5-HT1A receptor antagonism on plus-maze behaviour in mice. II. WAY 100635, SDZ 216-525 and NAN-190. Pharmacol. Biochem. Behav. 1997;58:593–603. doi: 10.1016/s0091-3057(97)00279-7. [DOI] [PubMed] [Google Scholar]
- CARTER D., CHAMPNEY M., HWANG B., EGLEN R.M. Characterization of a postjunctional 5-HT receptor mediating relaxation of guinea-pig isolated ileum. Eur. J. Pharmacol. 1995;280:243–250. doi: 10.1016/0014-2999(95)00195-q. [DOI] [PubMed] [Google Scholar]
- COULIE B., TACK J., SIFRIM D., ANDRIOLI A., JANSSENS J. Role of nitric oxide in fasting gastric fundus tone and in 5-HT1 receptor-mediated relaxation of gastric fundus. Am. J. Physiol. 1999;276:G373–G377. doi: 10.1152/ajpgi.1999.276.2.G373. [DOI] [PubMed] [Google Scholar]
- GALE J.D., GROSSMAN C.J., WHITEHEAD J.W., OXFORD A.W., BUNCE K.T., HUMPHREY P.P. GR113808: a novel, selective antagonist with high affinity at the 5-HT4 receptor. Br. J. Pharmacol. 1994;111:332–338. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMMEREN W., RENDERS J., VAN GOMPEL P., LESAGE A., LEYSEN J.E., JURZAK M. Extensive pharmacological study of the G-protein coupled fraction of human 5-HT receptors using agonist radioligand binding. Naunyn Schmiedebergs Arch. Pharmacol. 1998;358:8.42. [Google Scholar]
- GULLIKSON G.W., VIRINA M.A., LOEFFLER R.F., YANG D.C., GOLDSTIN, WANG S.X., MOUMMI C., FLYNN D.L., ZABROWSKI D.L. SC-49518 enhances gastric emptying of solid and liquid meals and stimulates gastrointestinal motility in dogs by a 5-hydroxytryptamine4 receptor mechanism. J. Pharmacol. Exp. Ther. 1993;264:240–248. [PubMed] [Google Scholar]
- HAGAN J.J., PRICE G.W., JEFFREY P., DEEKS N.J., STEAN T., PIPER D., SMITH M.I., UPTON N., MEDHURST A.D., MIDDLEMISS D.N., RILEY G.J., LOVELL P.J., BROMIDGE S.M., THOMAS D.R. Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br. J. Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- JERMAN J.C., BROUGH S.J., GAGER T., WOOD M., COLDWELL M.C., SMART D., MIDDLEMISS D.N. Pharmacological characterisation of human 5-HT2 receptor subtypes. Eur. J. Pharmacol. 2001;414:23–30. doi: 10.1016/s0014-2999(01)00775-0. [DOI] [PubMed] [Google Scholar]
- JOHNSON M.P., AUDIA J.E., NISSEN J.S., NELSON D.L. N(1)-substituted ergolines and tryptamines show species differences for the agonist-labeled 5-HT2 receptor. Eur. J. Pharmacol. 1993;239:111–118. doi: 10.1016/0014-2999(93)90983-o. [DOI] [PubMed] [Google Scholar]
- KOJIMA S., ISHIZAKI R., SHIMO Y. Investigation into the 5-hydroxytryptamine-induced relaxation of the circular smooth muscle of guinea-pig stomach fundus. Eur. J. Pharmacol. 1992;224:45–49. doi: 10.1016/0014-2999(92)94816-e. [DOI] [PubMed] [Google Scholar]
- LOVELL P.J., BROMIDGE S.M., DABBS S., DUCKWORTH D.M., FORBES I.T., JENNINGS A.J., MIDDLEMISS D.N., RAHMAN S.K., SAUNDERS D.V., COLLIN L.L., HAGAN J.J., THOMAS D.R. A novel, potent, and selective 5-HT(7) antagonist: (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl) phenol (SB-269970) J. Med. Chem. 2000;43:342–345. doi: 10.1021/jm991151j. [DOI] [PubMed] [Google Scholar]
- MCLEAN P.G., COUPAR I.M. Characterisation of a postjunctional 5-ht7-like and a prejunctional 5-HT3 receptor mediating contraction of rat isolated jejunum. Eur. J. Pharmacol. 1996;312:215–225. doi: 10.1016/0014-2999(96)00456-6. [DOI] [PubMed] [Google Scholar]
- MEULEMANS A.L., HELSEN L.F., SCHUURKES J.A. The role of nitric oxide (NO) in 5-HT-induced relaxations of the guinea-pig stomach. Naunyn Schmiedebergs Arch. Pharmacol. 1993;348:424–430. doi: 10.1007/BF00171343. [DOI] [PubMed] [Google Scholar]
- PRINS N.H., BRIEJER M.R., SCHUURKES J.A. Characterization of the contraction to 5-HT in the canine colon longitudinal muscle. Br. J. Pharmacol. 1997;120:714–720. doi: 10.1038/sj.bjp.0700954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINS N.H., BRIEJER M.R., VAN BERGEN P.J., AKKERMANS L.M., SCHUURKES J.A. Evidence for 5-HT7 receptors mediating relaxation of human colonic circular smooth muscle. Br. J. Pharmacol. 1999;128:849–852. doi: 10.1038/sj.bjp.0702762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINS N.H., VAN DER GRIJN A., LEFEBVRE R.A., AKKERMANS L.M., SCHUURKES J.A. 5-HT(4) receptors mediating enhancement of contractility in canine stomach; an in vitro and in vivo study. Br. J. Pharmacol. 2001a;132:1941–1947. doi: 10.1038/sj.bjp.0703985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINS N.H., AKKERMANS L.M.A., LEFEBVRE R.A., SCHUURKES J.A.J. Characterization of the receptors involved in the 5-HT-induced excitation of canine antral longitudinal muscle. Br. J. Pharmacol. 2001b;134:1351–1359. doi: 10.1038/sj.bjp.0704376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER G.J., NELSON D.R. Selective and functional 5-hydroxytryptamine3 receptor antagonism by BRL 43694 (granisetron) Eur. J. Pharmacol. 1989;159:113–124. doi: 10.1016/0014-2999(89)90695-x. [DOI] [PubMed] [Google Scholar]
- TACK J., COULIE B., WILMER A., ANDRIOLI A., JANSSENS J. Influence of sumatriptan on gastric fundus tone and on the perception of gastric distension in man. Gut. 2000;46:468–473. doi: 10.1136/gut.46.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEMURA K., TAKADA K., MAMEYA S., KAIBARA M., TANIYAMA K. Regional and functional differences of 5-hydroxytryptamine-receptor subtypes in guinea pig stomach. Jpn. J. Pharmacol. 1999;79:41–49. doi: 10.1254/jjp.79.41. [DOI] [PubMed] [Google Scholar]
- THOMAS D.R., ATKINSON P.J., HO M., BROMIDGE S.M., LOVELL P.J., VILLANI A.J., HAGAN J.J., MIDDLEMISS D.N., PRICE G.W. [H-3]-SB-269970–A selective antagonist radioligand for 5-HT7 receptors. Br. J. Pharmacol. 2000;130:409–417. doi: 10.1038/sj.bjp.0703318. [DOI] [PMC free article] [PubMed] [Google Scholar]


