Abstract
An experiment was conducted to examine whether a potent, orally active and highly selective neuropeptide Y Y1 receptor antagonist attenuates hyperphagia and obesity in genetically obese Zucker fatty rats.
Oral administration of the Y1 antagonist (30 and 100 mg kg−1, once daily for 2 weeks) significantly suppressed the daily food intake and body weight gain in Zucker fatty rats accompanied with a reduction of fat cell size and plasma corticosterone levels.
Despite the fact that food intake was gradually returned to near the control level, the body weight of the treated animals remained significantly less when compared to that of the controls for the duration of the treatment.
These results suggest that the Y1 receptor, at least in part, participate in pathophysiological feeding and/or fat accumulation observed in Zucker fatty rats. Y1 antagonists might be useful for the treatment of obesity.
Keywords: Neuropeptide Y, a Y1 antagonist; body weight gain; plasma corticosterone levels; Zucker fatty rats; obesity; feeding behaviour
Introduction
Neuropeptide Y (NPY) is a highly conserved 36 amino acid peptide that has been shown to have potent, centrally-mediated orexigenic effects (Tatemoto et al., 1982; Clark et al., 1984; Stanley & Leibowitz, 1984). It is a member of a peptide family which also includes peptide YY (PYY), and pancreatic polypeptide (PP) (Tatemoto & Mutt, 1980; Tatemoto et al., 1982). Chronic administration of NPY into the brain results in hyperphagia and body weight gain, reduces energy expenditure, and increases lipogenic activity in the liver and adipose tissue (Stanley et al., 1986; Zarjevski et al., 1993). It has been also reported that concentrations of NPY and its mRNA in the hypothalamus are markedly increased during food deprivation and in some genetic models of obesity in rodents (Sanacora et al., 1990; White et al., 1990; Kesterson et al., 1997; Guan et al., 1998). Moreover, it was well known that NPY-deficient ob/ob mice are less obese than ob/ob mice with reduction of food intake (Erikson et al., 1996). From these data it is inferred that NPY may be one of the major regulators of energy homeostasis.
Five distinct subtypes of NPY receptors (Y1, Y2, Y4, Y5 and Y6) have been cloned so far, and more than one receptor subtype seems to be involved in the NPY-mediated feeding (Blomqvist & Herzog, 1997). Among them, the Y1 receptor is considered to be one of the major feeding receptors, since Y1 antagonists with distinct chemical structures are reported to suppress feeding. This is also supported by a findings that mice lacking Y1 receptor showed a significantly diminished response to exogenously administered NPY (Kanatani et al., 2000b).
Genetically obese Zucker fatty rat (fa/fa) is extensively used for the study of obesity. Hyperphagia, decreased energy expenditure, hyperinsulinaemia, and hypercorticosteronaemia based on a disfunction of leptin long-form receptors are characteristics of Zucker fatty rats (Bray, 1977). As a consequence, a large dys-regulation of leptin-controlled hypothalamic neuropeptides is observed, and NPY is thought to be one responsible factor for the abnormality (Beck et al., 1990; 1993). Levels of NPY (McKibbin et al., 1991) and its mRNA (Sanacora et al., 1990), and NPY secretion (Dryden et al., 1995) are increased in the hypothalamic nuclei involved in the regulation of feeding behaviour. In addition, hyperphagia, obesity and metabolic changes such as hyperinsulinaemia, hypercorticosteroidimia and hyperlipidaemia, which were seen in Zucker fatty rats, were mimicked by repeated administration of NPY in normal rats (Stanley et al., 1986). We previously demonstrated that a potent peptidic Y1 antagonist, 1229U91 more potently inhibited the spontaneous feeding in Zucker fatty rats than lean litter mates after intracerebroventricular (ICV) administration (Ishihara et al., 1998). Taken together, we hypothesized that Y1 receptor play a key role in the generation of obesity in Zucker fatty rats.
Recently we reported that an orally-active and highly selective Y1 antagonist can suppress NPY-induced feeding in SD rats (Kanatani et al., 1999). To clarify the physiological role of the Y1 receptor in the development of obesity in Zucker fatty rats, here we further examined the chronic effect of the Y1 antagonist in this model animal.
Methods
Drugs
The NPY Y1 antagonist (6-(5-ethyl-1,3-thiazol-2-ylthiomethyl)-2-[3-methoxy-5-(2-propenyloxycarbonylamino)benzylamino]-4-morpholinopyridine) was synthesized by Banyu Pharmaceutical Co. Ltd. The structure and in vitro pharmacological profiles of the Y1 antagonist are reported previously (Kanatani et al., 1999). Other chemical substances were purchased from commercial sources.
Animals
Male Zucker fatty (fa/fa) and age-matched lean (Fa/Fa) rats (11 – 14 weeks old, Charles River Japan) were used. They were housed in individual cages under controlled temperature (23±2°C), humidity (55±15%) and light-dark cycle (7:00 – 19:00 h). Water and pellet food (CE-2, CLEA Japan Inc.) were available ad libitum. All experimental procedures followed the Japanese Pharmacological Society Guideline for Animal Use.
Experimental procedure
Zucker fatty rats were divided into three groups to match average values of basal food intake and body weight (n=6). Each group was orally administered either vehicle or the Y1 antagonist (suspended in 0.5% methylcellulose (Wako Pure Chemical Industries, Japan) in distilled water) at doses of 30 or 100 mg kg−1 daily for 2 weeks by gavage. Daily food and water intake and body weight were measured. The administration was done during the last hour of the light period following the measurement.
After the final administration, Zucker fatty rats were fasted overnight and euthanized by collecting whole blood from the abdominal aorta under ether anaesthesia. Blood collection was done during early morning. Plasma glucose, triglyceride, free fatty acid (FFA), total cholesterol, insulin and corticosterone levels were measured. Glucose, triglyceride, FFA, total cholesterol levels were measured by enzymatic methods using commercial kits (Determiner GL-E kit, Determiner L TGII, Determiner L TCII (Kyowa Medex, Japan) and NEFA-HA testwako (II) (Wako Pure Chemical Industries, Japan)). Insulin level was measured by ELISA kit (Morinaga, Japan), and corticosterone level was measured by RIA kit (Amersham, Biosciences, Japan). Liver was taken for weighing. Epididymal adipose tissue and liver were saved for histological investigation. The adipose tissue was fixed with 4% paraformaldehyde (PFA, Chiyoda Junyaku, Japan) for overnight and imbedded in paraffin wax. Three μm thick sections were made, stained with Masson trichrome staining and cell size was measured. Liver tissues were freshly frozen and 10 μm sections were made. Slices were fixed with 4% PFA and stained with oil red O (Ohroma, Germany).
Zucker lean rats (n=6) did not undergo administration. After overnight fasting, blood and histological parameters were collected similarly to the Zucker fatty rats. These values were used as references, and not included in the statistical analysis.
Statistical analysis
ANOVA or repeated measures ANOVA followed by Bonferroni test was used for statistical analysis.
Results
Figure 1 shows the daily food (A), water intake (B) and body weight change (C) in Zucker fatty rats for 2 weeks. Orally administered Y1 antagonist at a dose of 100 mg kg−1 significantly suppressed spontaneous feeding (Figure 1A). The greatest fall in food intake was observed on the 4th day after starting drug administration. Thereafter, food intake gradually returned near to control levels, although a small reduction of food intake was still observed even after 2 weeks. The Y1 antagonist showed a tendency to suppress water intake at 100 mg kg−1, however the effect was not significant because of large variation (Figure 1B). The 2-week treatment of the higher dose of the Y1 antagonist (100 mg kg−1) significantly suppressed body weight gain (Figure 1C). Despite the return of food intake near to control level, body weight remained significantly decreased from the control until the end of the experiment period.
Figure 1.
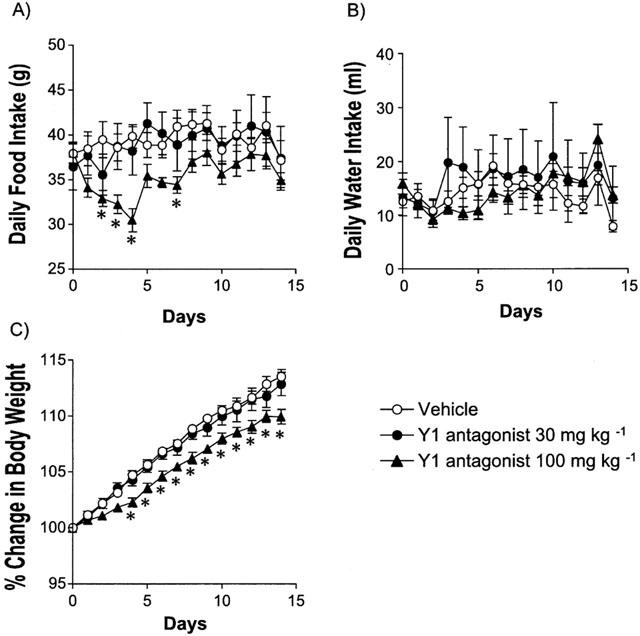
Effect of the Y1 antagonist on daily food (A) and water intake (B), and body weight change (C) in Zucker fatty rats. Values are mean±s.e.mean. *P<0.05 vs vehicle-treated group (n=6, repeated measurement ANOVA followed by Bonferroni test).
Figure 2 shows the adipose cell size of epididymal fat depot. Data from Zucker lean rats are also shown as a reference. The Y1 antagonist significantly reduced the fat cell size at both doses.
Figure 2.
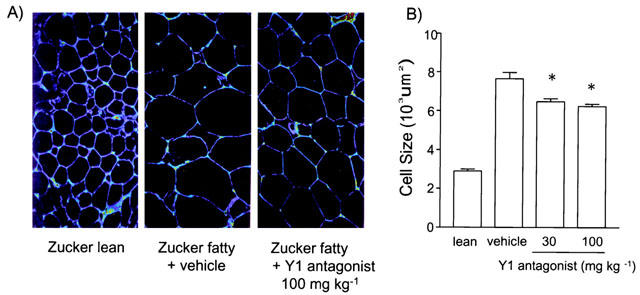
Chronic effect of Y1 antagonist on epididymal adipose cell size. (A) Microscopic observation of epididymal fat tissues from lean, vehicle- and 100 mg kg−1 of the Y1 antagonist-treated Zucker fatty rats. (B) Fat cell size of the epididymal adipose tissue from vehicle- and Y1 antagonist-treated Zucker fatty rats. Values are mean±s.e.mean. *P<0.05 vs vehicle-treated group (n=6, ANOVA followed by Bonferroni test). Values of Zucker lean rats are shown as a reference (not included in statistical analysis).
Figure 3 shows the liver weight. Liver weight of the Zucker fatty rats was twice as that of lean rats. The Y1 antagonist showed no effect on liver weight. However, histological observation showed that the Y1 antagonist tended to decrease liver lipid accumulation assessed by oil red-O staining.
Figure 3.
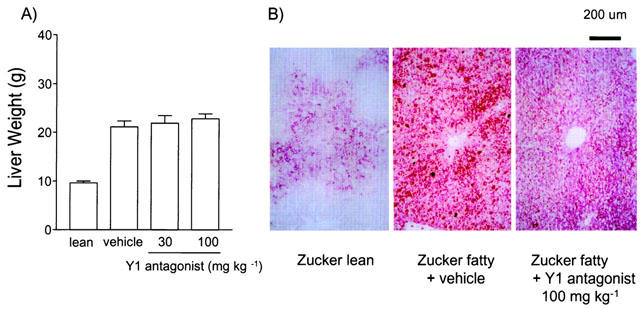
Chronic effect of Y1 antagonist on liver weight and fat accumulation. (A) Liver weight of the vehicle- or Y1 antagonist-treated Zucker fatty rats. Values are mean±s.e.mean. (n=6). Values of Zucker lean rats are shown as a reference. (B) Microscopic observation of liver from lean, vehicle- or 100 mg kg−1 of the Y1 antagonist-treated Zucker fatty rats.
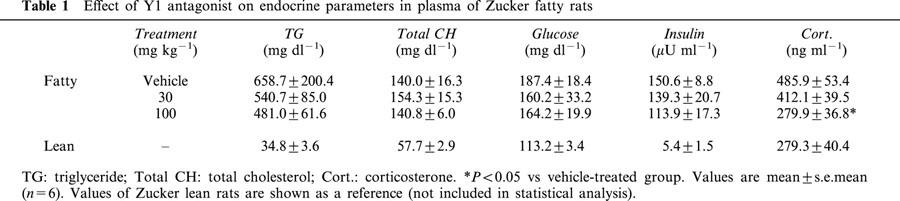
Table 1 shows the fasting plasma biochemical parameters. Data from lean rats are also shown as a reference. After 2 weeks of treatment, fasting plasma levels of glucocorticoid were reduced significantly by the treatment, while we could not detect significant change of plasma levels of glucose, triglyceride, cholesterol and insulin.
Table 1.
Effect of Y1 antagonist on endocrine parameters in plasma of Zucker fatty rats
Discussion
The present findings show that chronic treatment with a Y1-selective antagonist can suppress the body weight gain in Zucker fatty rats. Two-week treatment with the Y1 antagonist at 100 mg kg−1 induced a slight, but sustained reduction of the body weight. The body weight was 3% less than that of vehicle-treated rats at the end of the experiment. This change in body weight was accompanied by feeding suppression, reduction of adipose cell size, and plasma corticosterone levels. The present results indicate that NPY Y1 system is, at least in part, involved in the development of obesity in Zucker fatty rats.
Previously we showed that ICV administration of the Y1 antagonist inhibited the NPY-induced feeding in SD rats at a dose of 200 μg, and that acute ICV (200 μg) or PO dosing (100 mg kg−1) of this compound significantly reduced the spontaneous feeding in Zucker fatty rats (Kanatani et al., 1999). The present result is very consistent with the previous one, and the extent of the feeding suppression was similar in these two studies. The brain concentration of this compound was 0.5 μM at 24 h after PO dosing at 100 mg kg−1, which is more than 100 fold higher concentration than the IC50 value in functional assay (3.2 nM, NPY-induced Ca2+ influx in cells expressing human Y1 receptors, Kanatani et al., 1999). This compound showed a high specificity for Y1 receptors (Kanatani et al., 1999). In addition, we identified that this compound hardly affected normal feeding in SD rats (Ishihara et al., unpublished observations, data not shown), which is well matched to the results from structurally diverse Y1 antagonists showing that the anti-orexigenic effects of these compounds were much smaller in lean animals than those in obese animals (Ishihara et al., 1998; Kanatani et al., 2001). Deduced from these data, it is expected that the anti-obese effect of this compound is mediated by the Y1 receptor blockade, and that the dosage level is enough to block the brain Y1 receptors for 24 h. However, further confirmation of brain Y1 receptor occupancy with this compound using ex vivo binding is remaining to be addressed.
Although both Y1 and Y5 receptors are reported to be involved in feeding regulation, it is not clear which subtype has a physiological role in development of obesity in Zucker fatty rats. In the present experiment, the reduction of body weight was accompanied by a significant feeding suppression induced by the Y1 antagonist treatment. This observation is in agreement with our previous study that the spontaneous feeding in Zucker fatty rats is remarkably suppressed by 1229U91, a peptidic NPY Y1 antagonist with weak agonistic activities for Y4 and Y5 receptors (Ishihara et al., 1998; Kanatani et al., 1998). In that study, the feeding suppression by 1229U91 was much more potent in Zucker fatty rats than that in Zucker lean rats. These findings are in agreement with the hypothesis that the NPY Y1 system is much stimulated in the brain of the Zucker fatty rats. On the other hand, an orally active and selective Y5 antagonist inhibited a Y5 agonist-induced feeding but not the NPY-induced feeding in SD rats (Kanatani et al., 2000a). In addition, we showed that structurally-distinct two Y5 antagonists did not affect the spontaneous feeding in Zucker fatty rats (Kanatani et al., 1997; 2000c). Consistent with this, it is reported that the density of Y5 or Y2, but not Y1 receptors, is significantly reduced in a number of hypothalamic and non-hypothalamic brain regions of Zucker fatty rats as compared to the lean litter mates (Widdowson, 1997). Together, the present results indicate that the NPY Y1 receptor system is involved in the hyperphagia in Zucker fatty rats.
The greatest fall in food intake was observed on the 4th day after starting drug administration. Thereafter, food intake gradually returned near to control levels, although a small reduction of food intake was still observed even after 2 weeks. This result may indicate that some other counter-regulatory mechanisms are induced by continuous blockade of the NPY Y1 system. A similar attenuation of feeding suppression is also seen in other anti-orexigenic substances such as amphetamine, fenfluramine and dexfenfluramine (Levitsky et al., 1980; Rowland & Carlton, 1986; Rowland, 1994). It is also reported that reducing body weight prior to drug administration diminished the anorectic effectiveness of amphetamine (Levitsky et al., 1980). In addition, the decrease in the effectiveness of amphetamine is limited to the anorexia; the behavioural effects of the drug such as increased motor activity and stereotypy do not show tolerance with repeated administrations (Lewander, 1971). Thus, the attenuation of feeding suppression may be common feature of drugs which affect feeding, and it may reflect a successful physiological and behavioural adjustment to an appropriate level of body weight (Levitsky et al., 1980). Precise mechanisms for these phenomena are still unclear. To clarify the mechanism, further evaluation of the change of hypothalamic levels of NPY, NPY receptors and other factors, such as leptin, a-MSH, orexin, galanin and so on, is needed.
Although the reduction of food intake diminished at the end of the experiment, the Y1 antagonist at 100 mg kg−1 day−1 suppressed body weight gain significantly. The result suggests that some mechanism(s) other than feeding suppression is also concerned with the inhibition of body weight gain. One possible mechanism is suppression of corticosterone; the Y1 antagonist significantly reduced the plasma corticosterone levels to near that of lean litter mates. Elevated circulating levels of corticosterone is thought to play a role in the development and maintenance of obesity; Obesity is often accompanied by hypercorticism, over-responsiveness of the hypothalamic-pituitary-adrenal (HPA) axis or increased 24-h urinary corticosterone output (Cunningham et al., 1986; Guillaume-Gentil et al., 1990). Treatment with corticosterone can promote the development of obesity with a regional fat accumulation (Tataranni et al., 1996). On the other hand, adrenalectomy (ADX) prevents genetic as well as dietary obesity in animals, and the effect are reversed by corticosterone replacement (Castonguay et al., 1986; Freedman et al., 1986; Bray et al., 1992). In addition, ADX is reported to prevents NPY-induced obesity (Sainsbury et al., 1997). It is known that NPY increases corticosterone levels through the action of HPA axis (Zarjevski et al., 1993), and that chronic NPY infusion is reported to induce a sustained increase in corticosterone (Raposinho et al., 2001). From these findings, the present results suggest that the stimulation of NPY system produced hypercorticosteronism in Zucker fatty rats and that the Y1 antagonist may have ameliorated the obesity via suppression of plasma corticosterone levels.
Cell size of epididymal adipose tissue was significantly reduced by the Y1 antagonist even at a dose of 30 mg kg−1, at which dose body weight was not changed. Similar results was reported that ADX decreases the size of the gonadal and retroperitoneal fat by reducing cell size, and in short-term studies, these effects were observed before significant changes in total carcass fat content or food intake (Castonguay et al., 1986). The Y1 antagonist may have specifically affected the adipose tissue cell size, although more precise examination of total/regional body fat content is necessary by body composition analysis or a densitometry.
NPY is also reported to be involved in many other physiological functions. For example, NPY suppresses thermogenesis in brown adipose tissue via suppression of sympathetic nervous activities (Egawa et al., 1991; Szreder et al., 1994). Inhibitory regulation of lipolysis in white adipocytes is also reported (Castan et al., 1994). It is known that chronic ICV administration of NPY mimics hormonal and metabolic changes of obesity in rats even when they are pair-fed with the control animals, implying that NPY has diverse effects that produce obesity via NPY receptors (Zarjevski et al., 1993). The Y1 antagonist may have elicited an anti-obese effect in Zucker fatty rats by inhibiting these effects of NPY as well as altering feeding regulation. We need a precise examination of the anti-obesity mechanism(s) of this compound such as measurement of oxygen consumption, hormones and enzyme activities concerning lipid/glucose metabolism.
In this study, the Y1 antagonist tended to suppress water intake at a dose of 100 mg kg−1, although the difference was not significant because of large variation. NPY is reported to induce water intake as well as food intake (Stanley et al., 1986). Food and water intake could change co-relatively, so the effect of the Y1 antagonist on water intake may be the secondary effect of feeding suppression. In agreement with this, the tendency of reduction in water intake is observed on the days 4 – 7 at 100 mg kg−1, which is parallel to the change of food intake. Any abnormal change in gross behaviour or motor activity was not observed after administration of the Y1 antagonist. These findings suggest that the suppression of food and water intake is not due to abnormal changes in behaviour. Likewise, it seems not likely that the anti-orexigenic effects of Y1 antagonists are due to conditioned taste aversions or changes of taste sensation, since this compound hardly affected the feeding of lean SD rats (Ishihara et al., data not shown). However, currently we have no data which can exclude the possibility that the Y1 antagonist produced a non-specific effect on taste sensation, and this is remaining to be addressed.
In conclusion, an orally active and selective Y1 antagonist significantly suppressed daily food intake and body weight gain, as well as hypercorticism in Zucker fatty rats. These results suggest that the Y1 receptor, at least in part, participates in the development of obesity in Zucker fatty rats. The Y1 receptor may be a promising target to regulate energy balance, and a Y1 antagonist is a potential agent for treatment of obesity.
Abbreviations
- ADX
adrenalectomy
- ANOVA
analysis of valiance
- Cort.
corticosterone
- ELISA
enzyme-linked immunosorbent assay
- FFA
free fatty acid
- HPA
hypothalamic-pituitary-adrenal
- ICV
intracerebroventricular
- α-MSH
α-melanocyte-stimulating hormone
- NPY
neuropeptide Y
- PFA
paraformaldehyde
- PP
pancreatic polypeptide
- PYY
peptide YY
- RIA
radioimmunoassay
- TG
triglyceride
- total CH
total cholesterol
References
- BECK B., BURLET A., NICOLAS J.P., BURLET C. Hyperphagia in obesity is associated with a central peptidergic dysregulation in rats. J. Nutr. 1990;120:806–811. doi: 10.1093/jn/120.7.806. [DOI] [PubMed] [Google Scholar]
- BECK B., BURLET A., NICOLAS J.P., BURLET C. Galanin in the hypothalamus of fed and fasted lean and obese Zucker rats. Brain Res. 1993;623:124–130. doi: 10.1016/0006-8993(93)90019-j. [DOI] [PubMed] [Google Scholar]
- BLOMQVIST A.G., HERZOG H. Y-receptor subtypes-how many more. Trends Neurol. Sci. 1997;20:294–298. doi: 10.1016/s0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- BRAY G.A. The Zucker-Fatty rat: a review. Fed. Proc. 1977;36:148–153. [PubMed] [Google Scholar]
- BRAY G.A., STERN J.S., CASTONGUAY T.W. Effect of adrenalectomy and high fat diet on the fatty Zucker rat. Am. J. Physiol. 1992;262:E32–E39. doi: 10.1152/ajpendo.1992.262.1.E32. [DOI] [PubMed] [Google Scholar]
- CASTAN I., VALET P., QUIDEAU N., VOISIN T., AMBID L., LABURTHE M., LAFONTAN M., CARPENE C. Antilipolytic effects of α2-adrenergic agonists, neuropeptide Y, adenosine, and PGE1 in mammal adipocytes. Am. J. Physiol. 1994;266:R1141–R1147. doi: 10.1152/ajpregu.1994.266.4.R1141. [DOI] [PubMed] [Google Scholar]
- CASTONGUAY T.W., DALLMAN M.F., STERN J.S. Some metabolic and behavioral effects of adrenalectomy on obese Zucer rat. Am. J. Physiol. 1986;251:R923–R933. doi: 10.1152/ajpregu.1986.251.5.R923. [DOI] [PubMed] [Google Scholar]
- CLARK J.T., KALRA P.S., CROWLEY W.R., KALRA S.P. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM J.J., CALLES-ESCADON J., GARRIDO F., CARR D.B., BODE H.H. Hypercorticosteronuria and diminished pituitary responsiveness to corticotropin-releasing factor in obese Zucker rats. Endocrinology. 1986;118:98–101. doi: 10.1210/endo-118-1-98. [DOI] [PubMed] [Google Scholar]
- DRYDEN S., PICKAVANCE I., FRANKISH H.M., WILLIAMS G. Increased neuropeptide Y secretion in the hypothalamic paraventricular nucleus of obese (fa/fa) Zucker rats. Brain Res. 1995;690:185–188. doi: 10.1016/0006-8993(95)00628-4. [DOI] [PubMed] [Google Scholar]
- EGAWA M., YOSHIMATSU H., BRAY G.A. Neuropeptide Y suppresses sympathetic activity to interscapular brown adipose tissue in rats. Am. J. Physiol. 1991;260:R328–R334. doi: 10.1152/ajpregu.1991.260.2.R328. [DOI] [PubMed] [Google Scholar]
- ERIKSON J.C., HOLLOPETER G., PALMITER R.D. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- FREEDMAN M.R., HORWITZ B.A., STERN J.S. Effect of adrenalectomy and glucocorticoid replacement on development of obesity. Am. J. Physiol. 1986;250:R595–R607. doi: 10.1152/ajpregu.1986.250.4.R595. [DOI] [PubMed] [Google Scholar]
- GUAN X.M., YU H., VAN DER PLOEG L.H.T. Evidence of altered hypothalamic proopiomelanocortin/neuropeptide Y mRNA expression in tubby mice. Mol. Brain Res. 1998;59:273–279. doi: 10.1016/s0169-328x(98)00150-8. [DOI] [PubMed] [Google Scholar]
- GUILLAUME-GENTIL C., ROHNER-JEANRENAUD F., ABRAMO F., BESTETTI G.E., ROSSI G.L., JEANRENAUD B. Abnormal regulation of the hypothalamo-pituitary-adrenal axis in the genetically obese fa/fa rat. Endocrinology. 1990;126:1873–1879. doi: 10.1210/endo-126-4-1873. [DOI] [PubMed] [Google Scholar]
- ISHIHARA A., TANAKA T., KANATANI A., FUKAMI T., IHARA M., FUKURODA T. A potent neuropeptide Y (NPY) antagonist 1229U91 suppressed spontaneous food intake in Zucker fatty rats. Am. J. Physiol. 1998;43:R1500–R1504. doi: 10.1152/ajpregu.1998.274.5.R1500. [DOI] [PubMed] [Google Scholar]
- KANATANI A., FUKAMI T., FUKURODA T., IWAASA H., MACNEIL D., VAN DER PLOEG L.H.T., IHARA M. Y5 receptors are not involved in physiologically relevant feeding in rodents. Regul. Pept. 1997;71:212. doi: 10.1016/s0167-0115(98)00096-2. [DOI] [PubMed] [Google Scholar]
- KANATANI A., HATA M., MASHIKO S., ISHIHARA A., OKAMOTO O., HAGA Y., OHE T., KANNO T., MURAI N., ISHII Y., FUKURODA T., FUKAMI T., IHARA M. A typical Y1 receptor regulates feeding behaviors: effects of a potent and selective Y1 antagonist, J-115814. Mol. Pharmacol. 2001;59:501–505. doi: 10.1124/mol.59.3.501. [DOI] [PubMed] [Google Scholar]
- KANATANI A., ISHIHARA A., IWAASA H., NAKAMURA K., OKAMOTO O., HIDAKA M., ITO J., FUKURODA T., MACNEIL D.J., VAN DER PLOEG L.H.T., ISHII Y., OKABE T., FUKAMI T., IHARA M. L-152,804: Orally active and selective neuropeptide Y Y5 receptor antagonist. Biochem. Biophys. Res. Commun. 2000a;272:169–173. doi: 10.1006/bbrc.2000.2696. [DOI] [PubMed] [Google Scholar]
- KANATANI A., ITO J., ISHIHARA A., IWAAWA H., FUKURODA T., FUKAMI T., MACNEIL D.J., VAN DER PLOEG L.H.T., IHARA M. NPY-induced feeding involves the action of a Y1-like receptor in rodents. Regul. Pept. 1998;75–76:409–415. doi: 10.1016/s0167-0115(98)00096-2. [DOI] [PubMed] [Google Scholar]
- KANATANI A., KANNO T., ISHIHARA A., HATA M., SAKURABA A., TANAKA T., TSUCHIYA Y., MASE T., FUKURODA T., FUKAMI T., IHARA M. The novel nuropeptide Y Y1 receptor antagonist J-104870: a potent feeding suppressant with oral bioavailability. Biochem. Biophys. Res. Commun. 1999;266:88–91. doi: 10.1006/bbrc.1999.1750. [DOI] [PubMed] [Google Scholar]
- KANATANI A., MASHIKO S., MURAI N., SUGIMOTO N., ITO J., FUKURODA T., FUKAMI T., MORIN N., MACNEIL D.J., VAN DER PLOEG L.H.T., SAGA Y., NISHIMURA S., IHARA M. Role of Y1 receptor in the regulation of NPY-mediated feeding: comparison of wild-type, Y1 receptor-deficient and Y5 receptor-deficient mice. Endocrinology. 2000b;141:1011–1016. doi: 10.1210/endo.141.3.7387. [DOI] [PubMed] [Google Scholar]
- KANATANI A., SATO N., ISHIHARA A., IWAASA H., FUKURODA T., MACNEIL D.J., VAN DER PLOEG L.H.T., FUKAMI T., IHARA M. Role of NPY Y5 receptor in food intake elucidated by structurally diverse Y5 antagonists, J-115,856 and L-152,804. Regul. Pept. 2000c;89:67. [Google Scholar]
- KESTERSON R.A., HUSZAR D., LYNCH A.A., SIMERLY R.B., CONE R.D. Induction of neuropeptide Y gene expression in the dorsal medial hypothalamic nucleus in two models of the agouti obesity syndrome. Mol. Endocrinol. 1997;11:630–637. doi: 10.1210/mend.11.5.9921. [DOI] [PubMed] [Google Scholar]
- LEVITSKY D.A., STRUPP B.J., LUPOLI J. Tolerance to anorectic drugs: pharmacological or artifactual. Pharmac. Biochem. Behav. 1980;14:661–667. doi: 10.1016/0091-3057(81)90128-3. [DOI] [PubMed] [Google Scholar]
- LEWANDER T.A. A mechanism for the development of tolerance to amphetamine in rats. Psychopharmacologia. 1971;21:17–31. doi: 10.1007/BF00403992. [DOI] [PubMed] [Google Scholar]
- MCKIBBIN P.E., COTTON S.J., MCMILLAN S., HOLLOWAY B., MAYERS R., MCCARTHY H.D., WILLIAMS G. Altered neuropeptide Y concentrations in specific hypothalamic regions of obese (fa/fa) Zucker rats: possible relationship to obesity and neuroendocrine disturbances. Diabetes. 1991;40:1423–1429. doi: 10.2337/diab.40.11.1423. [DOI] [PubMed] [Google Scholar]
- RAPOSINHO P.D., PIERROZ D.D., BROQUA P., WHITE R.B., PEDRAZZINI T., AUBERT M.L. Chronic administration of neuropeptide Y into the lateral ventricle of C57BL/6J male mice produces an obesity syndrome including hyperphagia, hyperleptinemia, insulin resistance, and hypogonadism. Mol. Cell. Endocrinol. 2001;185:195–204. doi: 10.1016/s0303-7207(01)00620-7. [DOI] [PubMed] [Google Scholar]
- ROWLAND N.E. Tolerance to the anorectic effect of dexfenfluramine in rats: role of serotonin, cholecystokinin, and neuropeptide Y. Physiol. Behav. 1994;55:201–207. doi: 10.1016/0031-9384(94)90124-4. [DOI] [PubMed] [Google Scholar]
- ROWLAND N.E., CARLTON J. Tolerance to fenfluramine anorexia: fact or fiction. Appetite. 1986;7 suppl:71–83. doi: 10.1016/s0195-6663(86)80053-8. [DOI] [PubMed] [Google Scholar]
- SAINSBURY A., CUSIN I., ROHNER-JEANRENAUD F., JEANRENAUD B. Adrenalectomy prevents the obesity syndrome produced by chronic central neuropeptide Y infusion in normal rats. Diabetes. 1997;46:209–214. doi: 10.2337/diab.46.2.209. [DOI] [PubMed] [Google Scholar]
- SANACORA G., KERSHAW M., FINKELSTEIN J.A., WHITE D.J. Increased hypothalamic content of preproneuropeptide Y messenger ribonucleic acid in genetically obese Zucker rats and its regulation by food deprivation. Endocrinology. 1990;127:730–737. doi: 10.1210/endo-127-2-730. [DOI] [PubMed] [Google Scholar]
- STANLEY B.G., KYRKOULI S.E., LAMPERT S., LEIBOWITZ S.F. Neuropeptide Y chronically injected into the hypothalamus: A powerful neurochemical inducer of hyperphagia and obesity. Peptides. 1986;7:1189–1192. doi: 10.1016/0196-9781(86)90149-x. [DOI] [PubMed] [Google Scholar]
- STANLEY B.G., LEIBOWITZ S.F. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- SZREDER Z., HORI T., KAIZUKA Y. Thermoregulatory effect of intracerebral injections of neuropeptide Y in rats at different environmental temperatures. Gen. Pharmac. 1994;25:85–91. doi: 10.1016/0306-3623(94)90014-0. [DOI] [PubMed] [Google Scholar]
- TATARANNI P.A., LARSON D.E., SNITKER S., YOUNG J.B., FLATT J.P., RAVUSSIN E. Effects of glucocortioids on energy metabolism and food intake in humans. Am. J. Physiol. 1996;271:E317–E325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- TATEMOTO K., CARLQUIST M., MUTT V. Neuropeptide Y-a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- TATEMOTO K., MUTT V. Isolation of two novel candidate hormones using a chemical method for finding naturally occurring polypeptides. Nature. 1980;285:417–418. doi: 10.1038/285417a0. [DOI] [PubMed] [Google Scholar]
- WHITE J.D., OLCHOVSKY D., KERSHAW M., BERELOWITZ M. Increased hypothalamic content of preproneuropeptide Y messenger ribonucleic acid in streptozotocin-diabetic rats. Endocrinology. 1990;126:765–772. doi: 10.1210/endo-126-2-765. [DOI] [PubMed] [Google Scholar]
- WIDDOWSON P.S. Regionally-selective down-regulation of NPY receptor subtypes in the obese Zucker rat. Relationship to the Y5 ‘feeding' receptor. Brain Res. 1997;758:17–28. doi: 10.1016/s0006-8993(97)00160-1. [DOI] [PubMed] [Google Scholar]
- ZARJEVSKI N., CUSIN I., VETTOR R., ROHNER-JEANRENAUD F., JEANRENAUD B. Chronic intracerebroventricular neuropeptide Y administration to normal rats mimics hormonal and metabolic changes of obesity. Endocrinology. 1993;133:1753–1758. doi: 10.1210/endo.133.4.8404618. [DOI] [PubMed] [Google Scholar]


