Abstract
Using the whole-cell voltage clamp technique, the effect of aprindine on Na+/Ca2+ exchange current (INCX) was examined in guinea-pig single cardiac ventricular myocytes and CCL39 fibroblasts expressing a dog cardiac Na+/Ca2+ exchanger (NCX1).
INCX was recorded by ramp pulses from the holding potential of −60 mV with the external solution containing 140 mM Na+ and 1 mM Ca2+, and the pipette solution containing 20 mM Na+, 20 mM BAPTA and 13 mM Ca2+ (433 nM free Ca2+).
External application of aprindine suppressed INCX in a concentration-dependent manner. The IC50 values of outward (measured at 50 mV) and inward (measured at −100 mV) INCX components were 48.8 and 51.8 μM with Hill coefficients of 1.3 and 1, respectively.
Intracellular application of trypsin via the pipette solution did not change the blocking effect of aprindine, suggesting that aprindine does not affect the exchanger from the cytoplasmic side.
Aprindine inhibited INCX of a mutant NCX1 with a deletion of amino acids 247 – 671 in the large intracellular domain between the transmembrane segments 5 and 6 in a similar manner to that of the wild-type, suggesting that the site of aprindine inhibition is not in the large intracellular domain of NCX1.
A kinetic study indicated that aprindine was cooperatively competitive with KB-R7943, another inhibitor of NCX and that aprindine was a competitive inhibitor with respect to external Ca2+.
We conclude that aprindine may modestly inhibit INCX in a therapeutic range of concentrations (around 2.5∼6.9 μM) possibly at an external or intra-membranous site of the exchanger.
Keywords: Anti-arrhythmic drug, aprindine, cardiac myocyte, CCL39 cell, inhibitors, KB-R7943, Na+/Ca2+ exchange current, whole-cell clamp
Introduction
Aprindine hydrochloride is a class I-b anti-arrhythmic agent in Vaughan Williams classification, and is widely used to treat atrial and ventricular tachyarrhythmias (Kesteloot et al., 1973; Zipes et al., 1977; Fasola et al., 1977; Adams et al., 1984; Kodama et al., 1999). Aprindine suppresses the maximum upstroke velocity (Vmax) and duration (APD) of action potentials in Purkinje fibres and ventricular myocardium (Verdonck et al., 1974; Carmeliet & Verdonck, 1974; Steinberg & Greenspan, 1976). In electrophysiological experiments, aprindine acutely blocked the Na+ current (INa) in guinea-pig ventricular myocytes (Sato et al., 1991). In addition to Na+ channels, the drug inhibits various other ionic currents in the heart, such as the L-type Ca2+ current (ICa) (Tanaka et al., 1990; Shibasaki et al., 1991), a pacemaker current (If) (Tanaka et al., 1990, delayed rectifier K+ current (IK) (Shibasaki et al., 1991; Ohmoto-Sekine et al., 1999) and muscarinic acetylcholine receptor-operated K+ current (IKAch) (Ohmoto-Sekine et al., 1999).
Na+/Ca2+ exchange is a major mechanism of Ca2+ extrusion in the heart (Matsuda et al., 1997; Blaustein & Lederer, 1999; Kimura, 2001). Recently we found that anti-arrhythmic drugs, such as amiodarone (Watanabe & Kimura, 2000) and bepridil (Watanabe & Kimura, 2001) suppressed INCX in guinea-pig cardiac ventricular myocytes. We also found that 2,3-butanedione monoxime (BDM) inhibited INCX (Watanabe et al., 2001). The inhibitory effects of these drugs on INCX were diminished in patch-clamp studies by trypsin applied intracellularly via the pipette solution, indicating that the site of inhibitory action of those drugs may be cytoplasmic. In the present study, we examined the effect of another anti-arrhythmic drug, aprindine, on INCX in guinea-pig ventricular cells and CCL39 fibroblasts expressing NCX1.
Methods
Cell isolation
All experiments were performed in compliance with the regulations of the Animal Research Committee of the School of Medicine, Fukushima Medical University. Guinea-pigs weighing 250 – 400 g were anaesthetized by intraperitoneal injection of pentobarbital. The chest was opened under artificial ventilation, the aorta was cannulated in situ, and the heart was mounted on a Langendorff perfusion system. After washing out the blood with Tyrode solution, the perfusate was changed to nominally Ca2+-free Tyrode solution and then to one containing 0.01% w v−1 collagenase (Wako, Osaka, Japan) and 0.002% w v−1 alkaline protease (Nagase, Tokyo, Japan). After digestion for about 15 – 20 min, the enzymes were washed out by perfusing with a high K+, low Cl− solution (modified KB solution; Isenberg & Klöckner, 1982). The ventricular tissue was cut into the modified KB solution and gently shaken to isolate single ventricular cells. The cell suspension was stored in a refrigerator (4°C) for later use.
Cell cultures
CCL39 cells (American Type Culture Collection) with and without NCX1 transfection, were maintained in Dulbecco's modified Eagle's medium (Life Technologies, Inc.) supplemented with 7.5% heat-inactivated foetal calf serum, 50 units/ml−1 penicillin, and 50 μg/ml−1 streptomycin. Two types of CCL39 fibroblasts stably expressing NCX1 were used. One was transfected with wild-type NCX1 and the other with a mutant NCX1 in which amino acids 247 – 671 of TM5-6 were deleted (Pan et al., 2000). The cells were cultured for 2 or 3 days on small pieces (2×8 mm) of a cover glass and were used for the whole cell voltage clamp experiments.
Patch-clamp recording
Whole-cell membrane currents were recorded by the patch-clamp method. Single cardiac ventricular cells and CCL39 fibroblasts were placed in a recording chamber (1 ml volume) attached to an inverted microscope (Nikon, Tokyo, Japan) and were superfused with the Tyrode solution at a rate of 5 ml min−1. The temperature of the external solution was maintained at 36.0±0.5°C. The Tyrode solution contained (in mM): NaCl 140, KCl 5.4, CaCl2 1.8, MgCl2 1, NaH2PO4 0.33, glucose 5.5 and HEPES – NaOH (pH 7.4). The modified KB solution contained (in mM): KOH 70, l-glutamic acid 50, KCl 40, taurine 20, KH2PO4 20, MgCl2 3, glucose 10, EGTA 0.2 and HEPES – KOH buffer 10 (pH 7.2). Patch pipettes were forged from 1.5 mm diameter glass capillaries with a micro-electrode puller (pp-83, Narishige, Tokyo, Japan). The pipette resistance was 2∼3 MΩ when filled with the pipette solution which contained (in mM): NaCl 20, BAPTA 20, CaCl2 13 (free Ca2+ concentration 433 nM), CsCl 120, MgCl2 3, aspartic acid 50, MgATP 5 and HEPES 10 (pH 7.2 with CsOH). The extracellular solution contained (in mM): NaCl 140, CaCl2 2, MgCl2 1, ouabain 0.02, nifedipine 0.01, ryanodine 0.01 and HEPES – CsOH 5 (pH 7.2). The electrode was connected to a patch-clamp amplifier (TM-1000, Act ME, Tokyo, Japan). Recording signals were filtered at 2.5 kHz bandwidth, and the series resistance was compensated. Current signals were stored on-line and analysed by a computer (PC-9801RX, NEC, Tokyo, Japan) with non-commercial software called RAM5.
The current – voltage (I – V) relationship was obtained by ramp pulses (Kimura et al., 1987). The holding potential was set at −60 mV. The membrane was initially depolarized from −60 mV to 60 mV, then hyperpolarized from 60 to −110 mV and then depolarized back to −60 mV at a constant rate of 640 mV s−1. The descending limb (from 60 to −110 mV) was plotted in the I – V relationship without capacitance compensation. The Ca2+ current (ICa), K+ currents, Na+ – K+ pump current and Ca2+ release channels of the sarcoplasmic reticulum were blocked by nifedipine, Cs+, ouabain and ryanodine, respectively.
Drugs
Aprindine was a kind gift from Mitsui Co Ltd. (Tokyo, Japan). Ouabain, ryanodine and nifedipine were purchased from Sigma Chemical Co (St Louis, U.S.A.). KB-R7943 (2-[2-[4-1(4-nitrobenzyloxy) phenyl]ethyl] isothiourea methanesulphonate was a gift from Kanebo Co Ltd. (Osaka, Japan). Nifedipine and KB-R7943 were dissolved in dimethylsulphoxide (DMSO) and added to the extracellular solution so that the final concentration of DMSO was ⩽0.1%, which did not affect the Na+/Ca2+ exchange current. Trypsin (2.5 μg ml−1) (Difco laboratories, Detroit, MI, U.S.A.) was dissolved directly in the pipette solution. All the chemicals used were the highest grade available.
Data analysis
All data are presented as means±s.e. (number of experiments). Student's t-test and analysis of variance were used for statistical analyses. A P value of less than 0.05 was considered significant. The concentration – response data were fitted and IC50 values and Hill coefficients were obtained using Delta Graph Professional (Polaroid Computing, Tokyo, Japan) on a Macintosh computer (Apple Computer, Mariani Avenue, Cupertino, CA, U.S.A.). Per cent inhibition of the outward INCX at various concentrations of aprindine was calculated using the following logistic equation:
where [D] is the concentration of aprindine, IC50 is the half-maximum inhibitory concentration of the drug and nH is an empirical parameter describing the steepness of the fit and is equivalent to the Hill coefficient.
Results
Effect of aprindine on INCX
INCX was induced by 1 mM Ca2+ and 140 mM Na+ in the external solution and 20 mM Na+ and 433 nM free Ca2+ in the pipette solution. Under these ionic conditions, the reversal potential of the exchange current with a 3Na : 1Ca stoichiometry was calculated to be −50 mV. After establishing the whole-cell clamp mode with a holding potential, the external solution was changed from Tyrode solution to the control external solution. As shown in Figure 1A, the control external solution was then switched to one containing 300 μM aprindine. Aprindine immediately suppressed INCX. A high concentration (100 μM) of KB-R7943, a potent and selective inhibitor of INCX under these experimental conditions, was applied to completely inhibit INCX. Figure 1B illustrates the I – V relationships obtained in the control (a), in the presence of aprindine (b) and in the presence of KB-R7943. Figure 1C illustrates the I – V curves of the aprindine-sensitive component (a-b) and the KB-R7943-sensitive component (b-c) obtained by subtraction. Both I – V curves reversed at about −50 mV, indicating that the aprindine-sensitive current is INCX. Aprindine at 300 μM inhibited INCX by 95% in this cell. The inhibition was reversible. The recovery after washout of 100 μM aprindine was 86.5±7.6% (n=4) and maximum recovery was observed at 52.5±12.5 s (n=4).
Figure 1.
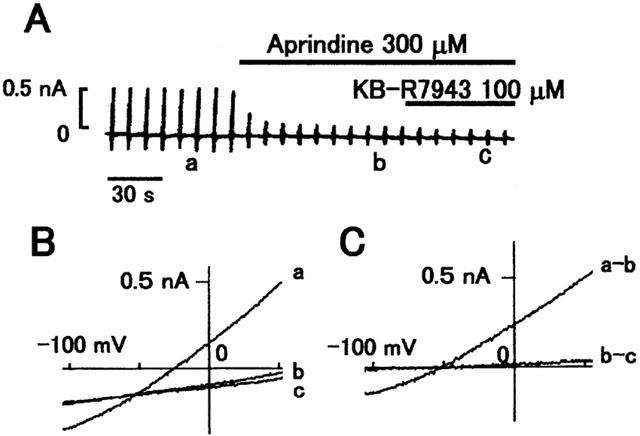
Effect of 300 μM aprindine on INCX. (A) Typical chart recording of membrane current. The bars above the current indicate when 300 μM aprindine and 100 μM KB-R7943 were applied externally. (B) I – V curves obtained at the time points corresponding to the labels in (A). (a) is the control and (b) in the presence of aprindine and (c) in the presence of KB-R7943. (C) Difference I – V curves between a and b (a-b) and between b and c (b-c) in (B).
The concentration – response relation of aprindine is shown in Figure 2, in which it can be seen that it inhibited INCX in a dose-dependent manner. The current magnitude was measured at 50 mV for the outward component of INCX and the per cent inhibition was calculated assuming that 100 μM KB-R7943 completely inhibited INCX. The fitted sigmoidal curve yielded an IC50 of 48.8 μM with a Hill coefficient of 1.3 (n=38). The IC50 of the inward component measured at −100 mV was 51.8 μM with a Hill coefficient of 1.1 (n=28) (figure not shown). These results indicate that aprindine inhibited inward and outward INCX with equal potency.
Figure 2.
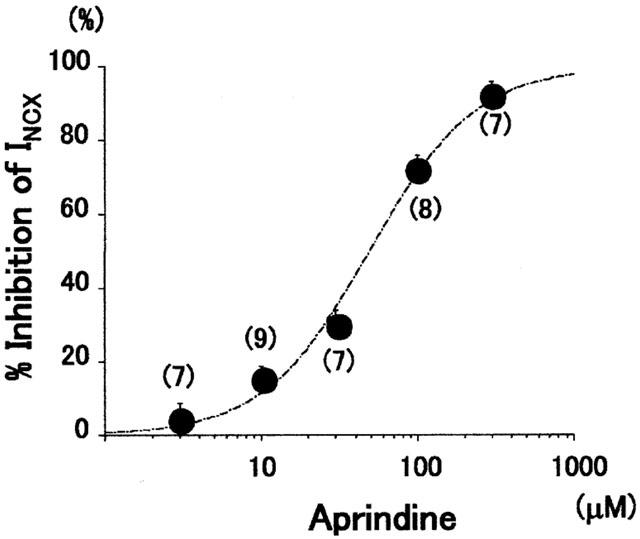
Aprindine concentration – inhibition curve. Average INCX values measured at +50 mV were fitted to a logistic equation. The IC50 of aprindine was 48.8 μM and the Hill coefficient was 1.3. Each point indicates mean±s.e. (number of cells).
Effect of trypsin on aprindine inhibition of INCX
We examined whether aprindine inhibited INCX from the cytoplasmic side of the membrane by including trypsin (2.5 μg ml−1) in the pipette solution. We previously showed that BDM (2,3-butanedione monoxime) lost its inhibitory effect on INCX in the presence of trypsin (Watanabe et al., 2001). Therefore, 10 mM BDM was applied before aprindine to verify the effectiveness of trypsin. As shown in Figure 3, BDM did not significantly inhibit INCX, but subsequent application of 300 μM aprindine dramatically inhibited INCX. KB-R7943 at 100 μM was applied after aprindine. Aprindine at 300 μM inhibited INCX by 89.7±6.9% (n=5) after trypsin treatment and by 93.5±6.3% (n=7) without trypsin (Figure 2C). Since the inhibition by aprindine was not changed by trypsin, it is probable that aprindine affects the Na+/Ca2+ exchanger from the extracellular or in the membrane and not from the cytoplasmic side.
Figure 3.
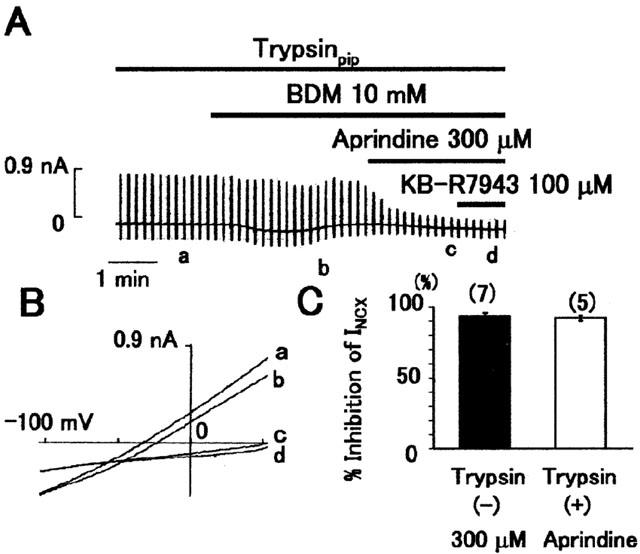
Effect of trypsin on inhibition by aprindine. (A) Typical chart recording of current. The pipette solution contained 2.5 mg ml−1 trypsin. BDM, a trypsin-sensitive inhibitor of INCX, did not inhibit INCX, indicating the presence of trypsin in the cell. (B) I – V curves obtained at the points a∼d in (A). (C) Summarized data of the inhibitory effect of 300 μM aprindine on INCX in the absence (left) and presence of trypsin (right) in the pipette solution. The values are means±s.e. *P<0.05 based on unpaired t-test.
Effects of aprindine on expressed INCX
To confirm that the site of inhibition of aprindine is not the cytoplasmic side, we tested the effects of aprindine on INCX with CCL39 cells expressing NCX1 or a mutant deleted of amino acids 247 – 671, which is a large portion of the long intracellular domain between the transmembrane segments (TM) 5 and 6, and contains the XIP region (He et al., 1997). INCX was induced by the same external and pipette solutions that were used in the experiment shown in Figure 1. As shown in Figure 4A and B, 100 μM aprindine inhibited both wild-type and mutant INCX by 70%. As summarized in Figure 4C, the inhibitory effect of 100 μM aprindine was not significantly different between the wild-type and the mutant NCX1 expressed in CCL39 cells and myocytes. The average INCX densities of the wild-type and mutant NCX1 measured at +50 mV were 3.5±1.5 pA/pF (n=5) and 2.0±1.1 pA/pF (n=6), respectively. These results indicate that the long internal loop between TM5 and 6 was not involved in the inhibition by aprindine.
Figure 4.
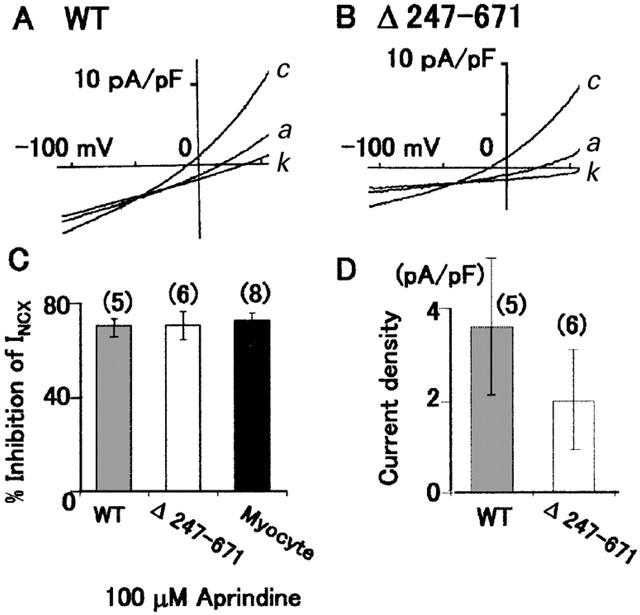
Effects of aprindine on INCX of wild-type (WT) NCX1 and a mutant with a deletion of amino acids 247 – 671 (Δ247 – 671) expressed in CCL39 cells. (A and B) I – V curves of control (c), in the presence of 100 μM aprindine (a) and 100 μM KB-R7943 (k). (C) Summary of the results of A and B. Per cent inhibition of WT, Δ247 – 671 and cardiac myocytes INCX by aprindine. (D) Comparison of INCX densities between WT and Δ247 – 671.
Mode of inhibition by aprindine with respect to KB-R7943
Watano et al. (1996) showed that KB-R7943 inhibits INCX competitively with respect to external Ca2+. We found that the inhibitory effect of 100 μM KB-R7943 on INCX was trypsin-insensitive (Watanabe & Kimura, 2000; 2001; Watanabe et al., 2001) and in this study inhibition by aprindine was also trypsin-insensitive. Therefore, aprindine and KB-R7943 may affect the same site of the exchanger. We determined if aprindine and KB-R7943 inhibit INCX in a competitive or non-competitive manner by measuring INCX in the presence of various concentrations of KB-R7943. Figure 5A is a Dixon plot of the reciprocal values of normalized INCX at 0, 10, 30 or 50 μM aprindine obtained in the presence of 0, 1 or 3 μM KB-R7943. The three fitted lines crossed at a point to the left of the Y-axis and close to the X-axis. This indicates that aprindine and KB-R7943 were co-operative pure competitive inhibitors.
Figure 5.
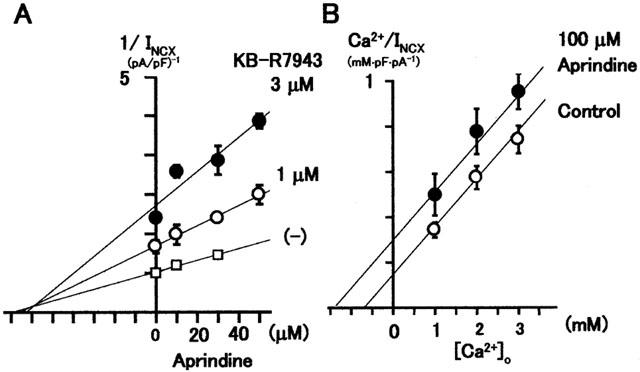
(A) Dixon plot of reciprocal or normalized INCX versus aprindine concentration under control conditions and in the presence of fixed concentrations of KB-R7943. The three fitted lines intersect at a point, indicating that aprindine and KB-R7943 are co-operative pure competitive inhibitors. (B) Hanes – Woolf plot for determining the mode of aprindine inhibition of INCX with respect to external Ca2+. Parallel fitted lines indicate that aprindine is a competitive inhibitor with respect to external Ca2+.
If aprindine and KB-R7943 were co-operative pure competitive inhibitors, aprindine should be a competitive inhibitor of INCX with respect to external Ca2+. This was tested by applying 100 μM aprindine to inhibit INCX at three different concentrations of external Ca2+. As shown in the Hanes – Wolf plot of the results in Figure 5B, the pair of fitted lines were parallel, indicating that aprindine is a competitive inhibitor with respect to external Ca2+.
Discussion
In this study, we found that acute application of aprindine suppressed INCX in a concentration-dependent manner in guinea-pig cardiac ventricular cells. The IC50 values were 48.8 and 51.8 μM and the Hill coefficients 1.3 and 1.1 at 50 and −100 mV, respectively.
We explored the site of aprindine inhibition on the exchanger by using trypsin in the pipette solution and mutant NCX1 expressed in CCL39 cells. It has been shown that trypsin or α-chymotrypsin treatment removed intracellular regulation of the Na+/Ca2+ exchange by cytoplasmic modulators such as Ca2+, Na+, ATP and protons (Hilgemann, 1990; Doering & Lederer, 1993; Espinosa-Tanguma et al., 1993). We previously showed that a calmodulin inhibitor W-7, BDM and anti-arrhythmic drugs such as amiodarone and bepridil inhibited INCX, and that the inhibition by those agents was attenuated by intracellular cymotrypsin or trypsin treatment via the pipette solution (Kimura, 1993; Watanabe et al., 2001; Watanabe & Kimura, 2000; 2001). From that we concluded that those drugs affected the exchanger from the cytoplasmic side. However, in this study, the inhibition of INCX by aprindine was trypsin-insensitive. In addition, aprindine inhibited a mutant NCX1 which had a deletion of amino acids 247 – 671 in the large internal domain between TM5 and 6. This indicates that aprindine affects the exchanger from the external side or intramembrane site and not the cytoplasmic side of NCX.
Recently, Chen et al. (2000) reported that digestion of scallop muscle membrane fractions with trypsin led to release of soluble polypeptides derived from the large cytoplasmic domain of a Na+/Ca2+ exchanger. In the presence of Ca2+, the major product was a ∼37 kDa peptide, with an N-terminus corresponding to residue 369 of NCX1 processed polypeptide sequence according to Nicoll & Philipson (1991). In the absence of Ca2+, ∼16 kDa and ∼19 kDa peptides were the major products. The 16 kDa fragment corresponded to the N-terminal part of the 37 kDa peptide. Polyclonal antibody raised against the 37 kDa peptide also bound to the ∼16 kDa and ∼19 kDa soluble tryptic peptides. Therefore, they concluded that the ∼16 kDa and ∼19 kDa peptides are the tryptic products of 37 kDa peptides.
Assuming an average residue mass of 110 Da, the ∼16 kDa and ∼37 kDa fragments were approximately 145 and 336 amino acids long and corresponded approximately to NCX1 amino acid sequences of 369 – 514 and 369 – 705, respectively. The large cytoplasmic domain of NCX1 consists of amino acids 218 – 764 (Nicoll et al., 1999; Iwamoto et al., 1999). The mutant we used was deleted of the 247 – 671 amino acid sequence. Therefore, the deleted sequence of NCX1 overlaps the domain clipped-off by trypsin. This strongly indicates that in cardiac myocytes a part of the large cytoplasmic domain of the exchanger is clipped off by trypsin. If this region is involved in the binding of an inhibitor of NCX, trypsin treatment should diminish its inhibitory effect. This was most likely the case for amiodarone and BDM, which were trypsin-sensitive NCX1 inhibitors. Consistent with this is the observation that the inhibitory effect of amiodarone was diminished in the deletion mutant NCX1. In the present study, aprindine was trypsin-insensitive and it inhibited the mutant and wild-type NCX1 equally. This suggests that the aprindine binding site is not in the cytoplasmic domain which is sensitive to trypsin.
Watano et al. (1996) showed that KB-R7943 inhibited INCX competitively with respect to external Ca2+. Iwamoto et al. (2001) suggested that KB-R7943 affects the exchanger at its external side, because external application but not intracellular application of KB-R7943 inhibits NCX. However, this was challenged by Elias et al. (2001) who demonstrated that cytoplasmic application of KB-R7943 inhibited INCX in the giant-patch oocyte membrane expressing NCX1.1. If aprindine and KB-R7943 affect NCX at external sites, they might interact competitively. Therefore, we determined whether aprindine and KB-R7943 are competitive inhibitors. The Dixon plot of the data (Figure 5A) indicated that the three fitted lines intersected at a point to the left of the Y-axis and close to the X-axis. Since KB-R7943 is competitive with respect to external Ca2+, this result suggests that aprindine and KB-R7943 are co-operative (or synergistic) pure competitive inhibitors, indicating that the two inhibitors may compete for different portions of the substrate binding site, or they may continue with the exchanger at specific sites in such a way as to distort the substrate binding site (Segel, 1964). This relation between aprindine and KB-R7943 was further supported by the finding that aprindine was a competitive inhibitor with respect to external Ca2+. The Hanes – Woolf plot in Figure 5B clearly shows this. Iwamoto et al. (2001) found that the most important amino acid for KB-R7943 binding to NCX1 is Gly833 in the α-2 repeat re-entrant domain between TM7 and TM8. α-2 repeat together with α-1 repeat are assumed to form the ion transport pathway, because mutations of these regions reduce the affinity of the exchanger for extracellular Ca2+ (Iwamoto et al., 2000). Since we found that aprindine is a competitive inhibitor with respect to external Ca2+, α-2 repeat may also be involved in aprindine binding.
With regard to action potentials, aprindine decreased the maximum upstroke velocity (Vmax) with an IC50 value of ∼2 μM without affecting the resting potential in atrial and ventricular cells and Purkinje fibres of guinea-pigs, cats, cows and dogs (Verdonck et al., 1974; Carmeliet & Verdonck, 1974; Steinberg & Greenspan, 1976; Kodama et al., 1999). There are reports that the following ionic currents are affected by aprindine. INa of guinea-pig ventricular myocytes was inhibited with IC50 values of 37.7 μM at the holding potential (HP) of −140 mV, and 0.74 μM at −100 mV HP (Sato et al., 1991). In rabbit AV node, the Ca2+ current was blocked by aprindine in a voltage-dependent manner with an estimated dissociation constant of 10 μM and a Hill coefficient of 0.8 (Tanaka et al., 1990). Shibasaki et al. (1991) showed that 5 μM aprindine suppressed the calcium current (ICa) by 35% in guinea-pig atrial myocytes. Aprindine affects most types of K+ channels in cardiac ventricular cells. In a double-microelectrode voltage-clamp study carried out in rabbit SA node and AV nodes, 1∼4 μM aprindine inhibited the delayed rectifier K+ current (IK) (Tanaka et al., 1990). Aprindine at 3 or 5 μM inhibited the IK with little influence on the inward rectifier K+ current or Ca2+ current (Shibasaki et al., 1991; Ohmoto-Sekine et al., 1999). In addition, it inhibited IKr but not IKs (Ohmoto-Sekine et al., 1999). The ligand-gated K+ currents were also susceptible to inhibition. Aprindine inhibited the carbachol-induced and GTPγS-induced IKAch of guinea-pig atrial cells with the IC50 values of 0.4 and 2.5 μM, respectively (Ohmoto-Sekine et al., 1999). Aprindine at 1∼4 μM also inhibited the pacemaker current (If) of rabbit SA node and AV node (Tanaka et al., 1990).
Acute and chronic clinical administration of aprindine results in plasma levels in the range of 0.9 to 2.5 μg ml−1, which corresponds to 2.5 to 6.9 μM (Zipes et al., 1977; Vlay et al., 1985). The concentrations of aprindine that inhibit INa, ICa, IKr and IKAch are in the therapeutic concentration range of the drug. As for INCX, the minimum concentration of aprindine required to block INCX by 5.4±3.2% (n=7) was 3 μM. Therefore, it is possible that aprindine might modestly inhibit INCX at therapeutic concentrations. Whether such modest inhibition of INCX is therapeutically beneficial or detrimental needs to be clarified. Further research is needed to elucidate the mechanism of inhibition of INCX by aprindine and its functional significance.
Acknowledgments
We thank Dr Isao Matsuoka for his helpful discussions, and Dr Tomoyuki Ono and Ms Sanae Sato for their technical assistance. This work was supported by grants-in-aid from the Ministry of Education, Science, Sports and Culture of Japan (09670098, 11670096, 11357020).
Abbreviations
- DMSO
dimethylsulphoxide
- ICa
L-type Ca2+ current
- If
pacemaker current
- Ik
delayed rectifier K+ current
- IKAch
muscarinic acetylcholine receptor-operated K+ current
- INa
Na+ current
- INCX
Na+/Ca2+ exchange current
- I – V curve
current – voltage relation curve
- KB-R7943
2-[2-[4-(nitrobenzyloxy)phenyl]ethydil]isothiourea methanesulphonate
- NCX1
cardiac Na+/Ca2+ exchanger
References
- ADAMS P.C., CAMPBELL R.W., JULIAN D.G. The clinical pharmacology of antiarrhythmic drugs. Cardiovasc. Clin. 1984;14:153–190. [PubMed] [Google Scholar]
- BLAUSTEIN M., LEDERER J. Sodium/Calcium exchange: Its physiological implications. Physiol. Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- CARMELIET E., VERDONCK F. Effects of aprindine and lidocaine on transmembrane potentials and radioactive K efflux in different cardiac tissues. Acta. Cardiol. 1974;S18:73–90. [PubMed] [Google Scholar]
- CHEN M., ZHANG Z., TAWIAH-BOATENG M., HARDWICKE P.M.D. A Ca2+-dependent tryptic cleavage site and a protein kinase A phosphorylation site are present in the Ca2+ regulatory domain of scallop muscle Na+-Ca2+ exchanger. J. Biol. Chem. 2000;275:22961–22968. doi: 10.1074/jbc.M001743200. [DOI] [PubMed] [Google Scholar]
- DOERING A.E., LEDERER W.J. The mechanism by which cytoplasmic protons inhibit the sodium-calcium exchanger in guinea-pig heart cells. J. Physiol. 1993;466:481–499. [PMC free article] [PubMed] [Google Scholar]
- ELIAS C.L., LUKAS A., SHURRAW S., SCOTT J., OMELCHENKO A., GROSS G., HNATOWICH M., HRYSHKO L. Inhibition of Na+/Ca2+ exchange by KB-R9743: transport mode selectivity and antiarrhythmic consequences. Am. J. Physiol. 2001;281:H1334–H1345. doi: 10.1152/ajpheart.2001.281.3.H1334. [DOI] [PubMed] [Google Scholar]
- ESPINOSA-TANGUMA R., DESANTIAGO J., RASGADO-FLORES H. α-Chimotrypsin deregulation of the sodium-calcium exchanger in barnacle muscle cells. Am. J. Physiol. 1993;265:C1118–C1127. doi: 10.1152/ajpcell.1993.265.4.C1118. [DOI] [PubMed] [Google Scholar]
- FASOLA A.F., NOBLE R.J., ZIPES D.P. Treatment of recurrent ventricular tachycardia and fibrillation with aprindine. Am. J. Cardiol. 1977;39:903–909. doi: 10.1016/s0002-9149(77)80045-3. [DOI] [PubMed] [Google Scholar]
- HE Z., PETESCH N., VOGES K.-P., RÖBEN W., PHILIPSON K.D. Identification of important amino acid residues of the Na+-Ca2+ exchanger inhibitory peptide, XIP. J. Membrane Biol. 1997;156:149–156. doi: 10.1007/s002329900197. [DOI] [PubMed] [Google Scholar]
- HILGEMANN D.W. Regulation and deregulation of cardiac Na+-Ca2+ exchange in giant excised sarcolemmal membrane patches. Nature. 1990;344:242–245. doi: 10.1038/344242a0. [DOI] [PubMed] [Google Scholar]
- ISENBERG G., KLÖCKNER U. Calcium tolerant ventricular myocytes prepared by preincubation in a ‘KB' medium. Pflügers Arch. 1982;395:6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- IWAMOTO T., KITA S., UEHARA A., INOUE Y,. , TANIGUCHI Y., IMANAGA I., SHIGEKAWA M. Structural domains influencing sensitivity to isothioruea derivative inhibitor KB-R7943 in cardiac Na+/Ca2+ exchanger. Mol. Pharmacol. 2001;59:524–531. doi: 10.1124/mol.59.3.524. [DOI] [PubMed] [Google Scholar]
- IWAMOTO T., NAKAMURA T.Y., PAN Y., UEHARA A., IMANAGA I., SHIGEKAWA M. Unique topology of the internal repeats in the cardiac Na+-Ca2+ exchanger. FEBS Lett. 1999;446:264–268. doi: 10.1016/s0014-5793(99)00218-5. [DOI] [PubMed] [Google Scholar]
- IWAMOTO T., UEHARA A., IMANAGA I., SHIGEKAWA M. The Na+/Ca2+ exchange NCX1 has oppositely oriented reentrant loop domains that contain conserved aspartic acids whose mutation alters its apparent Ca2+ affinity. J. Biol. Chem. 2000;49:38571–38580. doi: 10.1074/jbc.M003788200. [DOI] [PubMed] [Google Scholar]
- KESTELOOT H., VAN MIEGHEM W., DE GEEST H. Aprindine (AC 1802), a new anti-arrhythmic drug. Acta Cardiol. (Brux). 1973;28:145–165. [PubMed] [Google Scholar]
- KIMURA J. Effects of various calmodulin antagonists on Na/Ca exchange current of single ventricular cells of guinea pig. Pflügers Arch. 1993;424:523–528. doi: 10.1007/BF00374917. [DOI] [PubMed] [Google Scholar]
- KIMURA J.Cardiac Na+-Ca2+ exchanger: Pathophysiology and pharmacology Heart Physiology and Pathophysiology 2001San Diego: Academic Press; 417–425.4th edn. ed Sperelakis, N., Kurachi, Y., Terzic, A, Cohen, M.V. pp [Google Scholar]
- KIMURA J., MIYANMAE S., NOMA A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J. Physiol. 1987;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KODAMA I., OGAWA S., INOUE H., KASANUKI H., KATO T., MITAMURA H., HIRAOKA M., SUGIMOTO T. Profile of aprindine, cibenzoline, pilsicanide and pirmenol in the framework of the Sicilian Gambit. Jpn. Circ. J. 1999;63:1–12. doi: 10.1253/jcj.63.1. [DOI] [PubMed] [Google Scholar]
- MATSUDA T., TAKUMA K., BABA A. Na+-Ca2+ Exchanger: Physiology and Pharmacology. Jpn. J. Pharmacol. 1997;74:1–20. doi: 10.1254/jjp.74.1. [DOI] [PubMed] [Google Scholar]
- NICOLL D.A., OTTOLIA M., LU L., LU Y., PHILIPSON K.D. A new topological model of the cardiac sarcolemmal Na+-Ca2+ exchanger. J. Biol. Chem. 1999;274:910–917. doi: 10.1074/jbc.274.2.910. [DOI] [PubMed] [Google Scholar]
- NICOLL D.A., PHILIPSON K.D. Molecular studies of the cardiac sarcolemmal sodium-calcium exchanger. Ann. N.Y. Acad. Sci. 1991;639:181–188. doi: 10.1111/j.1749-6632.1991.tb17305.x. [DOI] [PubMed] [Google Scholar]
- OHMOTO-SEKINE Y., UEMURA H., TAMAGAWA M., NAKAYA H. Inhibitory effects of aprindine on the delayed rectifier K+ current and the muscarinic acetylcholine receptor-operated K+ current in guinea-pig atrial cells. Br. J. Pharmacol. 1999;126:751–761. doi: 10.1038/sj.bjp.0702334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAN Y., IWAMOTO T., UEHARA A., NAKAMURA T.Y., IMANAGA I., SHIGEKAWA M. Physiological functions of the regulatory domains of the cardiac Na+/Ca2+ exchanger NCX1. Am. J. Physiol. 2000;279:C393–C402. doi: 10.1152/ajpcell.2000.279.2.C393. [DOI] [PubMed] [Google Scholar]
- SATO R., HISATOME I., TANAKA Y., SASAKI N., KOTAKE H., MASHIBA H., KATORI R. Aprindine blocks the sodium current in guinea-pig ventricular myocytes. Naunyn Schmiedebergs Arch. Pharmacol. 1991;344:331–336. doi: 10.1007/BF00183008. [DOI] [PubMed] [Google Scholar]
- SEGEL I.H. New York: John Wiley & Sons, Inc; 1964. Enzyme Kinetics: Behavior and analysis of rapid equilibrium and steady-state enzyme systems; pp. 465–504. [Google Scholar]
- SHIBASAKI T., KOJIMA S., ITO H., HIRAKAWA S. Effects of anti-arrhythmic drugs on action potential repolarization of guinea-pig atrial myocytes. (in Japanese) Heart. 1991;23:327–331. [Google Scholar]
- STEINBERG M.I., GREENSPAN K. Intracellular electrophysiological alterations in canine cardiac conducting tissue induced by aprindine and ligocaine. Cardiovasc. Res. 1976;10:236–244. doi: 10.1093/cvr/10.2.236. [DOI] [PubMed] [Google Scholar]
- TANAKA H., NISHIMURA M., HOMMA N., HABUCHI Y., WATANABE Y. Electrogenic actions of aprindine in rabbit atrioventricular node. Naunyn Schmiedebergs Arch. Pharmacol. 1990;341:347–356. doi: 10.1007/BF00180661. [DOI] [PubMed] [Google Scholar]
- VLAY S.C., KALLMAN C.H., REID P.R. The utility of aprindine blood levels in the management of ventricular arrhythmias. J. Am. Coll. Cardiol. 1985;5:738–743. doi: 10.1016/s0735-1097(85)80403-4. [DOI] [PubMed] [Google Scholar]
- VERDONCK F., VEREECKE J., VLEUGELS A. Electrophysiological effects of aprindine on isolated heart preparations. Eur. J. Pharmacol. 1974;26:338–347. doi: 10.1016/0014-2999(74)90245-3. [DOI] [PubMed] [Google Scholar]
- WATANABE Y., IWAMOTO T., MATSUOKA I., OHKUBO S., ONO T., WATANO T., SHIGEKAWA M., KIMURA J. Inhibitory effect of 2,3-butanedione monoxime (BDM) on Na+/Ca2+ exchange current in guinea-pig cardiac ventricular myocytes. Br. J. Pharmacol. 2001;132:1317–1325. doi: 10.1038/sj.bjp.0703926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE Y., KIMURA J. Inhibitory effect of amiodarone on Na+/Ca2+ exchange current in guinea-pig cardiac myocytes. Br. J. Pharmacol. 2000;131:80–84. doi: 10.1038/sj.bjp.0703527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE Y., KIMURA J. Blocking effect of bepridil on Na+/Ca2+ exchange current in guinea pig cardiac ventricular myocytes. Jpn. J. Pharmacol. 2001;85:370–375. doi: 10.1254/jjp.85.370. [DOI] [PubMed] [Google Scholar]
- WATANO T., KIMURA J., MORITA T., NAKANISHI H. A novel antagonist, No. 7943, of the Na+/Ca2+ exchange current in guinea-pig cardiac ventricular cells. Br. J. Pharmacol. 1996;119:555–563. doi: 10.1111/j.1476-5381.1996.tb15708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIPES D.P., GAUM W.E., FOSTER P.R., ROSEN K.M., WU D., AMAT-Y-LEON F., NOBLE R.J. Aprindine for treatment of supraventricular tachycardias. With particular application to Wolff-Parkinson-White syndrome. Am. J. Cardiol. 1977;40:586–596. doi: 10.1016/0002-9149(77)90075-3. [DOI] [PubMed] [Google Scholar]


