Abstract
The effects of L-NAME and zaprinast were investigated (i.v.) on reflex-evoked changes in bladder and urethral pressures in urethane-anaesthetized female rats.
L-NAME attenuated reflex-evoked urethral relaxations (65±10%), while zaprinast potentiated these responses (68±24%). L-NAME and zaprinast also increased baseline urethral pressure and urethral striated muscle (EUS-EMG) activity. These drugs had little effect on the bladder.
Following pre-treatment with α-bungarotoxin (i.v.) to block urethral striated muscle, L-NAME and zaprinast failed to increase baseline urethral pressure. Further zaprinast failed to alter the size of reflex-evoked urethral relaxations.
Intra-urethral zaprinast caused a significant increase while sodium nitroprusside (SNP) and isoprenaline caused decreases in urethral pressure (+14±3%, −25±5%, −29±7%, respectively). These changes were associated with increases in EUS-EMG activity. After chlorisondamine (i.v.), zaprinast caused a significant fall in urethral pressure, while the decrease in urethral pressure caused by SNP and isoprenaline was potentiated. No changes in EUS-EMG activity occurred.
These results indicate that a nitrergic pathway mediates reflex-evoked urethral smooth muscle relaxations. The data also indicates that there is a background release of NO, which reduces sphincter skeletal muscle activity. Further, the ability of zaprinast to potentiate nitrergic evoked urethral relaxations involves an increase in striated muscle tone. This appears to be an indirect result of smooth muscle relaxation and is mediated, at least in part, by a chlorisondamine-sensitive mechanism.
Keywords: micturition, bladder, urethra, nitric oxide, zaprinast, phosphodiesterase, urethral striated muscle, cyclic GMP
Introduction
The activities of the urinary bladder and urethra are under a complex neural control system and act synergistically in the storage and periodic elimination (micturition) of urine. During urine storage the bladder accommodates increasing volumes of urine with little change in internal pressure, whereas there is an increase in the activity of the bladder neck and urethra to close the urethral outlet and thereby maintain continence. When the volume of urine in the bladder reaches the threshold, the micturition reflex is evoked which comprises of a contraction of the bladder and relaxation of the urethral outlet (De groat et al., 1993). The urethra consists of a combination of circular and longitudinal smooth muscle layers which are surrounded by striated muscle fibres along the middle third in a number of species, including rats (Elbadawi, 1987). This area of striated muscle, in addition to the smooth muscle fibres of this region, constitutes the external urethral sphincter (EUS). Relaxation of both these types of muscle is essential for effective voiding, and similarly both muscles are thought to be important in maintaining urinary continence (Brading, 1999). In addition, in the rat, there are rapid contractions and relaxations of the striated musculature, which occur during voiding and are thought to facilitate bladder emptying (Conte et al., 1991a).
In vitro studies have demonstrated that nitric oxide (NO) is the main neurotransmitter involved in mediating relaxations of the urethral smooth muscle (Andersson & Persson, 1994). Furthermore, Ho et al. (1998; 1999) have identified the presence of nitrergic nerve fibres in the human striated urethral sphincter, suggesting that NO may also play a role in the control of the urethral striated muscle. The second messenger system mediating the effects of NO in various physiological systems is believed to be guanylate cyclase, which causes an increase in the formation of (cyclic GMP) and subsequent smooth muscle relaxation (see Zhang & Snyder, 1995). The effects of cyclic GMP are dependent not only on its rate of formation, but also on its rate of breakdown, which is fulfilled by a family of cyclic nucleotide phosphodiesterase (PDE) isoenzymes. In particular, PDE 5 has been shown to fulfil this role in a number of systems (Beavo, 1995), for instance inhibition of cyclic GMP-selective PDEs potentiates NO-mediated relaxations in gastrointestinal (Williams & Parsons, 1995), genital (Cellek & Moncada, 1998) and vascular (Liu et al., 1992) smooth muscles. Furthermore, increases in cyclic GMP levels have been associated with NO-mediated inhibitory responses of the isolated rabbit urethra (Persson & Andersson, 1994; Dokita et al., 1994), suggesting that this transduction system is also involved in the control of urethral smooth muscle. However, the role of NO/cyclic GMP signalling in urethral smooth muscle relaxation has yet to be demonstrated in vivo and, specifically, in the rat urethra. Furthermore, the potential roles of this transduction system in the control of the striated muscle of the urethra have not been investigated. Therefore, the aim of the present study was to investigate the role of this transduction system in the control of micturition. This was carried out by investigating the effects of inhibition of NO synthesis with Nω-nitro-L-arginine methyl ester (L-NAME; Moore & Handy, 1997) and inhibition of cyclic GMP-selective PDEs with zaprinast (a PDE types 1, 5, 6 and 9 inhibitor; Ballard et al., 1998; Fisher et al., 1998) on reflex-evoked changes in urethral and bladder pressures induced by bladder distension with saline infusion. Electromyographic (EMG) recordings were also made in some of the experiments so that any drug-evoked changes in urethral pressure due to effects on urethral striated muscle (EUS-EMG) could be assessed. Preliminary accounts of the findings have been published in abstract form (Wibberley et al., 1999a,1999b; 2000a,2000b).
Methods
The experiments were carried out under the Animals (Scientific Procedures) Act, 1986. After completion of experiments, animals were killed by an overdose of pentobarbitone sodium i.v.
General preparation
Experiments were performed on 104 female Sprague-Dawley rats (200 – 345 g) anaesthetized with isoflurane (4% in 100% oxygen), and maintained with urethane (1.2 g kg−1, i.v.). Depth of anaesthesia was assessed by the stability of blood pressure and heart rate, and by an absence of hind limb withdrawal in response to paw pinch (and by an absence of cardiovascular response to paw pinch in preparations where neuromuscular blockade was produced by α-bungarotoxin (0.4 mg kg−1, i.v.). Supplementary doses of urethane (0.1 g kg−1, i.v.) were given where necessary. The trachea was intubated to maintain a patent airway. The left jugular vein was cannulated for anaesthetic and drug administration, and the left common carotid artery was cannulated with a heparinised cannula (20 units ml−1 heparin in 0.9% w v−1 saline) for the measurement of arterial blood pressure and for sampling arterial blood for blood gas analysis. Blood pressure was measured using a pressure transducer (Gould Statham P23Db), and the heart rate (HR) derived electronically on-line from the blood pressure signal using AcqKnowledge version 3.5.3 software (Biopac Systems Inc, U.S.A.). Body temperature was monitored with a rectal temperature probe and maintained between 36 – 38°C using a homeothermic blanket system (Harvard). Animals were either spontaneously breathing oxygen enriched air, or artificially ventilated (rate 80 min−1, stroke volume 8 ml kg−1) with oxygen enriched room air by use of a positive pressure pump (Harvard Rodent Ventilator 683) in neuromuscular blockaded preparations. In all experiments blood gases were maintained between 90 – 130 mmHg Po2, 40 – 50 mmHg Pco2 and pH 7.3 – 7.4 with a Corning pH/blood gas analyser (Model 238). Adjustments of the supplemented oxygen levels (spontaneously breathing animals) and respiratory pump rate and volume (artificially ventilated animals) were made as necessary to maintain blood gas and pH balance. The animals were infused (6 ml kg−1 h−1, i.v.) with a solution comprising 10 ml plasma substitute (Gelofusine), 10 ml distilled water, 0.04 g glucose and 0.168 g sodium bicarbonate, to prevent the development of non-respiratory acidosis and to maintain blood volume. The rats were placed in a stereotaxic frame and the head tilted approximately 10 – 15° to allow the animal to lie in the supine position. This was to prevent the weight of the animal affecting bladder and urethral pressure recordings.
Measurement of bladder and urethral pressures
The urinary bladder was exposed by a midline abdominal incision and the ureters emerging from the base of the bladder were tied distally and cut. The proximal ends of each ureter leading from the kidneys were exposed by retroperitoneal incisions and cannulated to prevent the bladder filling with urine during experiments. A cut was made in the bladder dome and two cannulae (0.52 mm internal and 1.2 mm external diameter) were inserted into the bladder, one of which was connected to a pressure transducer (Gould Statham P23Db) to record intravesical bladder pressure, and the other connected to a syringe pump for the infusion of saline (0.9% w v−1) to evoke the micturition reflex. The rate (0.046 ml min−1) of infusion of saline into the bladder was chosen to simulate the maximal hourly diuresis rate (Klevmark, 1974). Backflow through this cannula allowed the bladder to be emptied of saline. Urethral pressure was measured based on a method developed by M. Fraser (Kakizaki et al., 1997). For detailed methods and a diagram of this urethral pressure recording see Conley et al. (2001).
EUS-EMG recordings
In preparations where the effects of test drugs were investigated on the urethral striated muscle, two fine copper wire electrodes (0.2 mm diameter) were used and inserted percutaneously approximately 0.5 cm lateral and 0.5 cm caudal on each side of the external urethral opening from which the EMG signal was measured. At the end of experiments, animals were given decamethonium (3 mg kg−1, i.v.) to confirm the electrodes were recording EMG and to determine the level of noise.
Experimental protocols
All experiments were left for 30 min after completion of surgery to stabilize. In protocol 1 (Figure 1) an ‘initial' micturition reflex was then evoked by infusion of warmed saline into the bladder. After the appearance of three consecutive reflex-evoked bladder contractions of similar amplitude, saline infusion was discontinued. The infused volume of saline was left in the bladder for a 5 min period, during which time the reflex was ongoing. The bladder was then emptied and after a further 5 min, a test drug or vehicle was administered. After 10 min, to allow stabilization of any changes in baseline variables caused by these substances, a second micturition reflex was evoked. In some of these experiments this cycle of events was continued with L-arginine being administered as the test drug. In a separate group of animals, following the initial reflex, zaprinast or vehicle was infused i.v. for 30 min and then reflexes were repeated as above.
Figure 1.
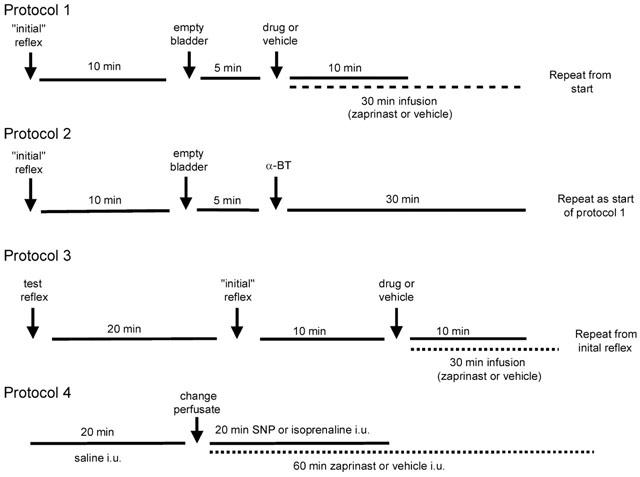
Diagram showing the experimental protocols that were used.
In experiments involving neuromuscular blockade (protocol 2; Figure 1), the initial reflex responses were carried out as above, after which the animal was artificially ventilated and α-bungarotoxin was administered. A 30 min period then followed to allow stabilization of blood gases and baseline variables, after which additional reflexes were carried out as above following further administration of test substances.
In experiments in which EUS-EMG recordings were made to assess the effects of manipulation of the NO/cyclic GMP signalling pathway on urethral striated muscle the following protocol (3; Figure 1) was used. A test reflex was carried out initially to ensure that an adequate EMG signal was being recorded. A 20 min period then followed during which any alterations in the position of the electrodes could be made. The initial micturition reflex was then evoked and further reflexes were obtained following the administration of test substances as outlined above.
In experiments examining the effects of intra-urethral perfusion of test substances (protocol 4; Figure 1), baseline urethral perfusion pressures and EUS-EMG activities were recorded with saline perfusion through the urethra (0.075 ml min−1) for 20 min. The perfusate was then changed to drug or vehicle (0.9% w v−1 saline) by changing the syringe on the syringe pump. This resulted in a transient (<30 s) loss of urethral pressure recordings. Sodium nitroprusside (SNP) and isoprenaline were infused for 20 min while zaprinast and vehicle were infused (0.075 ml min−1) for 60 min. In experiments investigating the effects of ganglionic blockade, chlorisondamine was administered i.v. 15 min before beginning protocol 4. It should be noted that baseline bladder pressures were not recorded in this set of experiments as the cannula used to fill the bladder with saline was left open to ensure that afferent activity from the bladder did not affect urethral pressure and EUS-EMG recordings
Data capture and analysis
Arterial blood pressures, bladder and urethral pressures were continuously displayed on a chart recorder (Grass Instruments) and captured (1500 samples s−1) by a MP 100 WSW interface (Biopac Systems Inc, U.S.A.) to allow data to be acquired and analysed off-line using AcqKnowledge version 3.5.3 software (Biopac Systems Inc, U.S.A.). Heart rates (HR) were derived electronically on-line from the blood pressure signal using this software (Biopac Systems Inc., U.S.A.). The amplified EMG signal was captured (1500 samples s−1) and the input integrated off-line using AcqKnowledge version 3.5.3 software (Biopac Systems Inc., U.S.A.).
Analysis of reflex-evoked bladder and urethral responses and baseline values
Saline infusion into the bladder evoked large amplitude rhythmic bladder contractions that have been assumed to represent a micturition reflex (Maggi et al., 1986). The mean amplitude (mmHg) and duration (s) of the first three bladder contractions that occurred after saline infusion was discontinued were measured (Figure 2). These three bladder contractions were analysed as they represented isovolumetric reflex bladder contractions, i.e. they occurred when the amount of saline in the bladder, and therefore resting bladder pressure, was constant. The mean amplitude (mmHg) and duration (s) of the three urethral relaxations that accompanied these bladder contractions were measured. Further, the mean amplitude (mmHg) and duration (s) of the high frequency oscillations in urethral pressure and when recorded, the mean area of the integrated EMG bursts that occurred with these three isovolumetric bladder contractions, were also measured. For each reflex, the urethral pressure between the final two reflex-evoked urethral relaxations was measured to give an indication of the overall increase in baseline urethral tone during a reflex. The micturition reflex pressure thresholds were taken as the bladder pressure (mmHg) at which the first reflex bladder contraction with concomitant reflex urethral activity (i.e. relaxation and high frequency oscillations) occurred. The volume threshold (ml) was calculated by expressing the time to evoke this first bladder contraction in volumes of saline. The frequency of the reflex was determined by measuring the number of reflex bladder contractions that occurred from the first reflex bladder contraction to the point where the bladder was emptied of saline. This was then divided by the total time over this period to express frequency as bladder contractions min−1. All baseline values were the mean values over a 3 min period and were measured 2 min before reflexes and test agent responses. The effects of bolus administration of test substances on baseline bladder and urethral pressures, MAP and HR were measured 10 min after their administration. The effects of i.v. zaprinast or zaprinast vehicle on these baseline variables were measured every 5 min until 30 min after the start of infusion. The total area of integrated baseline EUS-EMG activity was measured 2 min before the administration of test solution every 5 min over the 30 min infusion period. The area of integrated noise (measured after administration of decamethonium at the end of experiments) was subtracted from these integrated EUS-EMG values.
Figure 2.
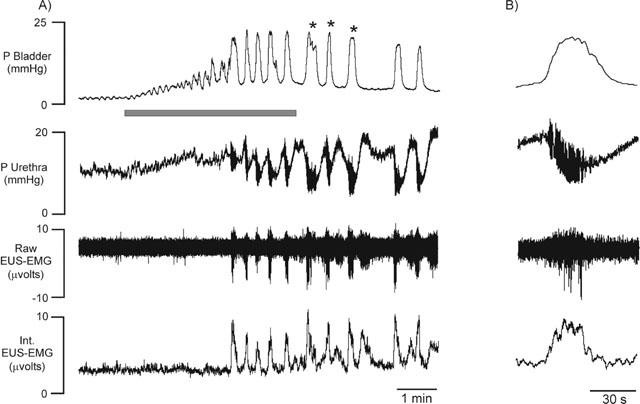
Urethane anaesthetized female rats. (A) Trace showing changes in bladder and urethral pressures, raw and integrated urethral striated muscle (EUS-EMG) activity during intravesical infusion of saline (the micturition reflex). (B) Expanded individual isovolumetric reflex-evoked bladder contraction (i.e. occurring after intravesical infusion of saline was stopped, indicated by * on trace A; see Methods) showing accompanying urethral relaxation, high frequency oscillations in urethral pressure and associated burst of urethral striated muscle activity. Shaded bar represents duration of intravesical infusion.
Analysis of urethral and EUS-EMG responses to intra-urethral perfusion of test substances
Baseline urethral pressures, integrated EUS-EMG activity (see above), MAP and HR were measured 5 min before the start of intra-urethral administration of drug or saline. The effects of test substances on these parameters were measured 5 min until 20 min after the start of intra-urethral perfusion for sodium nitroprusside and isoprenaline and for 60 min for zaprinast and saline. The effects of bolus administration of chlorisondamine on baseline bladder and urethral pressures, MAP and HR were measured 10 min after administration. Changes in baseline values were expressed as percentage changes before and after the administration of test substances.
Statistical analysis
Changes in reflex-evoked and baseline effects on the above variables measured were expressed as percentage changes before and after the administration of test substances and compared with vehicle controls by unpaired Student's t-test. Changes in baseline values caused by the infusion (i.v.) of zaprinast for 30 min were compared with vehicle controls and experiments involving pretreatment with α-Bungarotoxin by two-way analysis of variance followed by the least significance difference test (Sokal & Rohlf, 1969). Changes from baseline caused by intra-urethral infusion of test drugs were compared with vehicle controls and experiments involving pre-treatment with chlorisondamine by two-way analysis of variance followed by the least significance difference test. Values of P<0.05 were considered statistically significant. All values are expressed as mean±s.e.mean.
Drugs and solutions
Drugs and chemicals were obtained from the following sources: urethane, Nω-nitro-L-arginine methyl ester (L-NAME; hydrochloride), L-arginine (hydrochloride), α-bungarotoxin, zaprinast, decamethonium bromide, sodium nitroprusside, isoprenaline (hydrochloride) and chlorisondamine (iodide) from Sigma Aldrich Chemicals., Poole, Dorset, U.K.; pentobarbitone sodium from Rhône Mérieux Ltd, Harlow, Essex, U.K.; isoflurane from Abbott Labs, Queenborough, Kent, U.K.; gelofusine from Braun Medical Ltd, Aylesbury, Bucks, U.K.; sodium chloride, glucose, sodium bicarbonate and triethanolamine from Merck/BDH, Poole, Dorset, U.K.; heparin from CP Pharmaceuticals Ltd, Wrexham, U.K. DMPP, L-NAME, L-arginine, α-bungarotoxin, decamethonium bromide, SNP and isoprenaline were all dissolved in 0.9% w v−1 saline. Zaprinast was dissolved in 5% triethanolamine in 0.9% w v−1 saline for i.v. administration and 0.9% w v−1 saline for i.u. perfusion. All i.v. agents except zaprinast were administered in a 0.03 ml dose volume followed by a flush of 0.1 ml saline. Zaprinast or vehicle was infused i.v. at a rate of 6 ml kg−1 h−1. All drugs were given as their salts.
Results
Effects of test substances on reflex-evoked responses
Initial reflex-evoked responses
Infusion of saline into the bladder in 71 female rats caused distension of the bladder, which in turn evoked the micturition reflex, characterized by the appearance of rhythmic bladder contractions of 25±1 mmHg (e.g. Figure 2), with a mean duration of 23±1 s and a frequency of 1.6±0.1 contractions min−1. The mean bladder pressure threshold to evoke the micturition reflex was 8.5±0.5 mmHg, which was reached when 0.13±0.02 ml of saline had been infused. Each rhythmic bladder contraction was accompanied by a fall in urethral pressure of 9.3±0.5 mmHg that continued for 24±1 s before returning to baseline, in some cases above baseline. During these bladder contractions urethral pressure between these contractions increased by 3±0.4 mmHg (see Figure 2 for example). In 53 out of the 71 animals, high frequency oscillations in urethral pressure (Figure 2) occurred at the peak of each bladder contraction that had a mean amplitude of 12±1.0 mmHg and continued for 11.3±1.0 s. These oscillations were abolished by pre-treatment with α-bungarotoxin, however the rise in urethral pressure during reflex-evoked bladder contractions was unaffected (compare Figure 3 with Figure 5). In the remaining 18 animals, high frequency oscillations in urethral pressure were not observed. This was probably due to variations in the position of the urethral cannula. In animals where urethral striated muscle activity was recorded (n=22), these high frequency oscillations were accompanied by bursting of the EUS-EMG (Figure 2). The mean data for the ‘initial' reflex in protocols 1 – 3 is shown in Table 1. Each reflex bladder contraction was accompanied by small increases in MAP and HR. The combined mean baseline bladder and urethral pressures, MAP and HR were 3.2±0.3, 14.5±0.6, 120±2 mmHg and 406±5 beats min−1, respectively. In experiments where EUS-EMG activity was recorded, these animals had a combined mean integrated EUS-EMG activity of 1.5±0.6 μV (n=22). The mean baseline data before the initial reflex are shown in Table 2.
Figure 3.
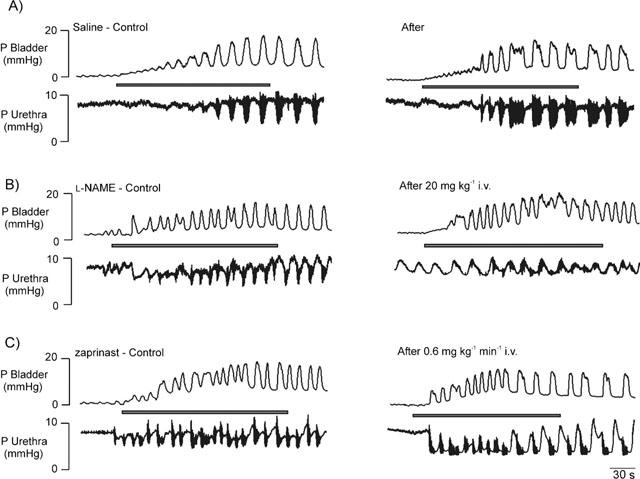
Urethane anaesthetized female rats: traces showing reflex-evoked isovolumetric (i.e. occurring when intravesical infusion of saline to evoke the reflex had been stopped; see Figure 2) changes in bladder and urethral pressures before (control) and after i.v. injections of (A) saline, (B) L-NAME, 20 mg kg−1 and an infusion of (C) zaprinast, 0.6 mg kg−1 min−1. It should be noted that the saline vehicle trace (A) is also representative of the effects of zaprinast vehicle (5% triethanolamine in saline), which has not shown for the sake of clarity. Shaded bars represent duration of intravesical infusions of saline to evoke the micturition reflex.
Figure 5.
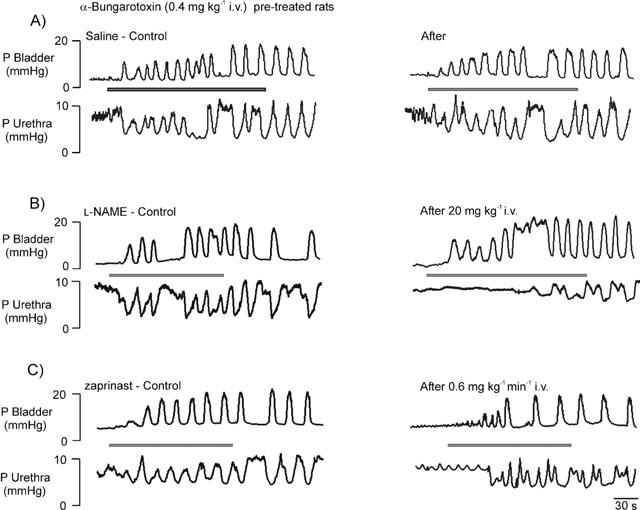
Urethane anaesthetized α-bungarotoxin (0.4 mg kg−1, i.v.) pre-treated female rats: traces showing reflex-evoked isovolumetric (i.e. occurring when intravesical infusion of saline to evoke the reflex had been stopped; see Figure 2) changes in bladder and urethral pressures before (control) and after i.v. injections of (A) saline, (B) 20 mg kg−1 of L-NAME and infusion of (C) 0.6 mg kg−1 min−1 of zaprinast. Saline vehicle trace (A) is also representative of the effects of zaprinast vehicle (5% triethanolamine in saline), which is not shown for the sake of clarity.
Table 1.
Control reflex-evoked changes caused by ‘initial' bladder distension in bladder and urethral pressures and urethral striated muscle activity (EUS-EMG) caused by intravesical infusion of saline for each experimental group in urethane anaesthetized female rats
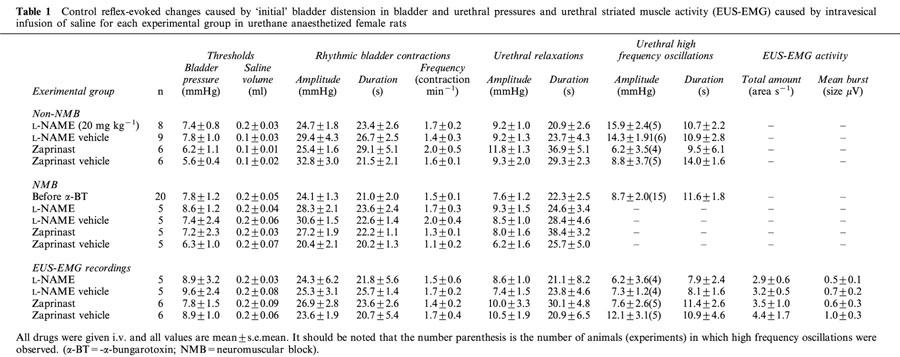
Table 2.
Baseline values before ‘initial' bladder distension-evoked reflexes for urethral and bladder pressures, urethral striated muscle activity (EUS-EMG), mean arterial blood pressure (MAP) and heart rate (HR) for all experimental groups in urethane-anaesthetized female rats
Effects (i.v.) of L-NAME and zaprinast on the micturition reflex in non- and α-bungarotoxin pre-treated rats: Reflex-evoked urethral pressure changes
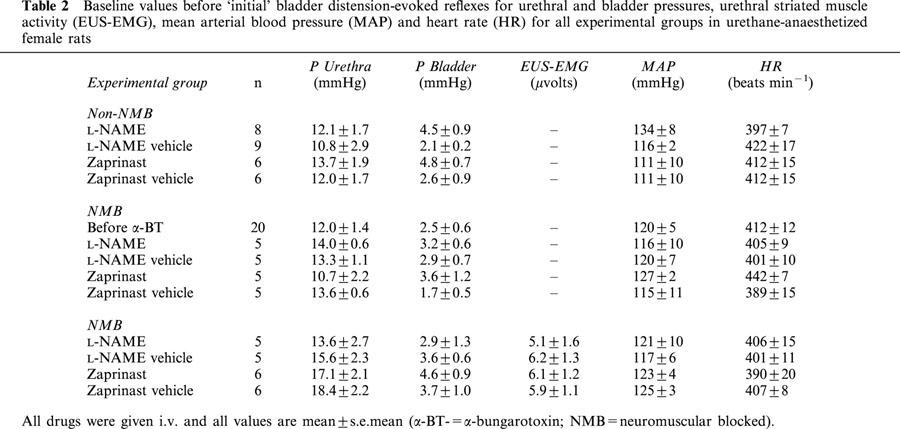
Traces showing the effects of L-NAME and zaprinast on reflex-evoked changes in urethral pressure in non- and α-bungarotoxin pre-treated rats are shown in Figures 3 and 5. L-NAME (n=13; 20 mg kg−1, i.v.) significantly attenuated the amplitude and duration of reflex-evoked urethral relaxations by 65±10% and 51±13%, respectively (Figure 4). In addition, the amplitude of reflex-evoked high frequency oscillations in urethral pressure were significantly reduced by 56±12% (n=9), but L-NAME had no effect on the duration of this high frequency bursting. In four animals, injection (i.v.) of L-arginine (150 mg kg−1) reversed the effects of L-NAME. Zaprinast (n=12; 0.6 mg kg−1 min−1, i.v., for 30 min) significantly potentiated the amplitude and duration of reflex-evoked urethral relaxations by 68±24 and 97±31%, respectively (Figure 4). Furthermore, zaprinast significantly attenuated the amplitude of reflex-evoked high frequency oscillations in urethral pressure by 30±10% (n=9), but had no effect on the duration of this high frequency bursting.
Figure 4.
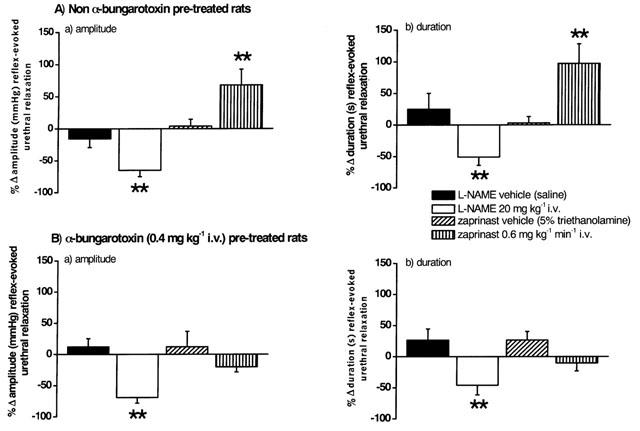
Urethane anaesthetized female rats: histograms comparing the changes (Δ) caused by i.v. injection of vehicle for L-NAME (saline) and L-NAME (20 mg kg−1), and infusions of zaprinast vehicle (5% triethanolamine in saline) and zaprinast (0.6 mg kg−1 min−1) on (a) the amplitude and (b) the duration of reflex-evoked urethral relaxations in (A) non- and (B) α-bungarotoxin (0.4 mg kg−1, i.v.) pre-treated rats. Each bar represents the mean value and vertical bars show s.e.mean. Changes caused by drugs were compared with vehicle controls using Student's unpaired t-test. *P<0.05 **P<0.01.
Following neuromuscular blockade with α-bungarotoxin, L-NAME (n=5) significantly attenuated the amplitude and duration of reflex-evoked urethral relaxations by 69±9 and 46±15%, respectively. These effects of L-NAME were not significantly different from those obtained in non-α-bungarotoxin pre-treated animals and were reversed after further administration of L-arginine (n=5). The rise in urethral pressure between reflex-evoked bladder contractions was also unaffected. However, for zaprinast (n=6) pretreated rats the rise in urethral pressure, between the rhythmic bladder contractions observed during the reflex (compare Figure 3C with Figure 5C), was now inhibited by 95±7% and the amplitude of the relaxations was not significantly different compared with vehicle control (Figures 4 and 7). No reflex-evoked high frequency oscillations were observed in α-bungarotoxin pre-treated animals.
Figure 7.
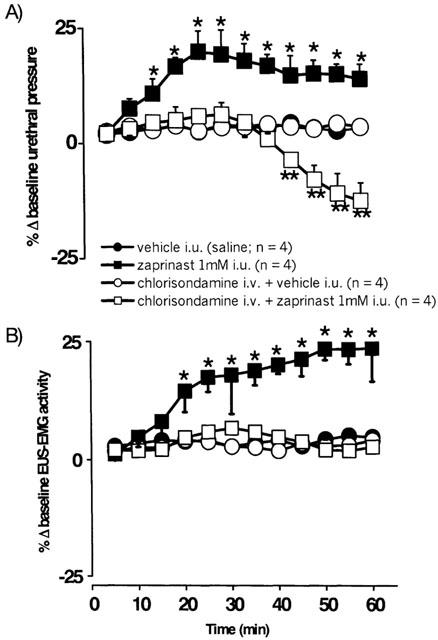
Urethane anaesthetized female rats: a comparison of the effects of i.u. perfusion (0.075 ml min−1) of vehicle (saline) and zaprinast (1 mM) for 60 min in the absence and presence of pre-treatment with chlorisondamine (10 mg kg−1, i.v.) on percentage changes (Δ) in (A) baseline urethral pressure and (B) integrated urethral striated muscle activity (EUS-EMG). Each point represents the mean value and vertical bars show s.e.mean. Changes caused by zaprinast were compared with the appropriate controls using two-way analysis of variance followed by the least significance difference test. *P<0.05 **P<0.01.
Baseline urethral pressure and EUS-EMG activity
Traces showing the effects of L-NAME and zaprinast on baseline urethral pressure and EUS-EMG activity are shown in Figure 6. L-NAME (n=13) significantly increased baseline urethral pressure (Figure 6A) by 12±10% after 5 min in non neuromuscular-blocked rats, but had no effect in α-bungarotoxin pre-treated animals. Baseline EUS-EMG activity was significantly increased by 908±201% following L-NAME. Furthermore, reflex-evoked EUS-EMG bursting was now unsynchronized with the reflex-evoked high frequency oscillations in urethral pressure, such that there was now a pattern of continuous bursting (n=4). These effects of L-NAME on urethral striated muscle activity were reversed following treatment with L-arginine. Zaprinast caused a significant increase of 20±8% in baseline urethral pressure after 10 min in non neuromuscular-blocked rats (Figure 6B). Urethral pressure remained significantly increased for a further 10 min, after which it decreased to baseline levels. After 30 min baseline urethral pressure was non-significantly increased by 14±12%. Zaprinast had no effect on baseline urethral pressure in α-bungarotoxin pre-treated rats. Baseline EUS-EMG activity (Figure 6B) was significantly increased by 120±44% following zaprinast. However, the continuous burst of EUS-EMG activity caused by L-NAME was not observed following zaprinast, which tended to cause a low level of continuous EUS-EMG activity (Figure 6). Zaprinast also significantly increased the burst size of reflex-evoked increases in EUS-EMG activity that accompanied the high frequency oscillations in urethral pressure by 178±85% (n=6).
Figure 6.
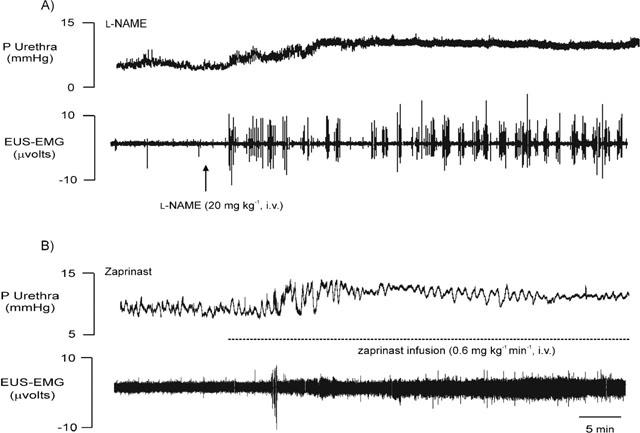
Urethane anaesthetized female rats: traces showing the effects of i.v. injections of 20 mg kg−1 of L-NAME (A) and 0.6 mg kg−1 min−1 zaprinast (B) on baseline urethral pressure (P, mmHg) and raw urethral striated muscle activity (EUS-EMG, μvolts). Dotted line indicates duration of zaprinast infusion i.v.
Bladder pressure
In non- and α-bungarotoxin pre-treated animals L-NAME and zaprinast (and further treatment with L-arginine) had no effect on the amplitude, duration, frequency, volume and pressure thresholds of reflex-evoked bladder contractions (Figures 3 and 5). L-NAME caused a significant increase in baseline bladder pressure of 82±21% (n=5) which was, surprisingly, significantly reduced (43±10%; n=5) in α-bungarotoxin pre-treated rats. This raised bladder pressure was unaffected following administration of L-arginine (n=9). Baseline bladder pressure was unaffected by zaprinast in all experimental groups.
Effects of vehicles and α-bungarotoxin (i.v.) on reflex-evoked responses and baseline values
Administration (i.v.) of vehicles for L-NAME, L-arginine (saline) and zaprinast (5% triethanolamine in saline) had no effect on reflex-evoked changes in bladder and urethral pressures or EUS-EMG activities in all experimental groups. In addition, none of these vehicles had any effect on baseline bladder and urethral pressures, MAP and HR. α-Bungarotoxin (n=15; 0.4 mg kg−1, i.v.) had no effect on reflex-evoked changes in bladder and urethral pressures (Figures 5 and 7). However, the reflex-evoked high frequency oscillations in urethral pressure that accompanied reflex bladder contractions and urethral relaxations were abolished (compare Figures 3 and 5). α-Bungarotoxin also had no effect on baseline bladder and urethral pressures, MAP and HR.
Effect of L-NAME and zaprinast on baseline MAP and HR
In non- and α-bungarotoxin pre-treated animals, L-NAME produced a significant increase in MAP of 35±3 and 32±6%, respectively. These increases were associated with a significant decrease of 11±2 and 10±3%, respectively, in baseline HR. Administration of L-arginine following L-NAME pre-treatment produced a significant decrease of 9±2% from the L-NAME-induced rise in MAP but had no effect on the raised HR (n=4). Infusion of zaprinast caused a significant decrease in baseline MAP of 21±4% (n=12) and 19±5% (n=5) after 30 min, and a significant increase in HR of 7±3% (n=12) and 8±4% (n=5) after 10 min, in non- and α-bungarotoxin pre-treated rats, respectively.
Effects of i.u. perfusion of test substances
The combined mean baseline urethral pressures, integrated EUS-EMG activities, MAP and heart rate, were 14.1±0.3 mmHg, 6.3±1.6 μV, 128±4 mmHg and 415±8 beats min−1, respectively. The mean baseline data for individual experimental groups are shown in Table 3.
Table 3.
Baseline values for intra-urethral (i.u.) perfusion of test substances for urethral pressure, urethral striated muscle activity (EUS-EMG), mean arterial blood pressure (MAP) and heart rate (HR) for all experimental groups in urethane-anaesthetized female rats
Effects of i.u. zaprinast, SNP and isoprenaline on baseline urethral pressure and EUS-EMG activity in non- and chlorisondamine pre-treated rats
Zaprinast (n=4; 1 mM at 0.075 ml min−1, i.u.) evoked a significant increase in urethral pressure after 15 min reaching a maximum of 19±5% by 30 min and attaining an increase of 14±3% after a total of 60 min (Figure 7A). This was associated with a significant increase in EUS-EMG activity of 18±8% after 30 min, which was further increased to reach 24±7% after 60 min (Figure 7B). In animals pre-treated with chlorisondamine (10 mg kg−1, i.v.), a neuronal nicotinic receptor antagonist (Broadley, 1996), zaprinast i.u. had no effect on baseline urethral pressure (6±3%; n=4) or EUS-EMG (7±1%) activity after 30 min (Figure 7). However, after 45±2 min zaprinast caused a significant decrease in urethral pressure, reaching 18±4% after 60 min (Figure 7A), which was associated with no change in EUS-EMG activity.
Traces showing the effects of SNP on urethral pressure and EUS-EMG activity in non- and chlorisondamine pre-treated rats are shown in Figure 8. SNP (n=4; 1 mM at 0.075 ml min−1, i.u.) and isoprenaline (n=4; 1 mM at 0.075 ml min−1, i.u.) caused significant falls in baseline urethral pressure after 10±2 and 12±4 min, respectively, reaching a maximum of 25±5 and 29±7%, respectively, after 20 min (Figure 9). SNP and isoprenaline i.u. both evoked significant bursting in baseline EUS-EMG recordings, after 11±5 and 15±4 min, respectively, reaching a maximum increase of 69±12 and 76±14%, respectively, after 20 min (Figure 9). In chlorisondamine pre-treated animals, SNP (n=4; 0.075 ml min−1, i.u.) and isoprenaline (n=4; 0.075 ml min−1, i.u.) caused significant falls in baseline urethral pressure after 6±2 and 4±3 min, reaching a maximum of 36±4 and 41±10%, respectively, after 20 min (Figure 9). The fall in urethral pressure evoked by these agents was significantly greater than that produced in non-chlorisondamine pre-treated animals. Furthermore, the time point when urethral pressure began to fall was significantly earlier than the point at which this occurred in non-chlorisondamine pre-treated rats. In addition, i.u. administration of SNP and isoprenaline now had no effect on EUS-EMG activities in chlorisondamine pre-treated animals (Figure 9).
Figure 8.
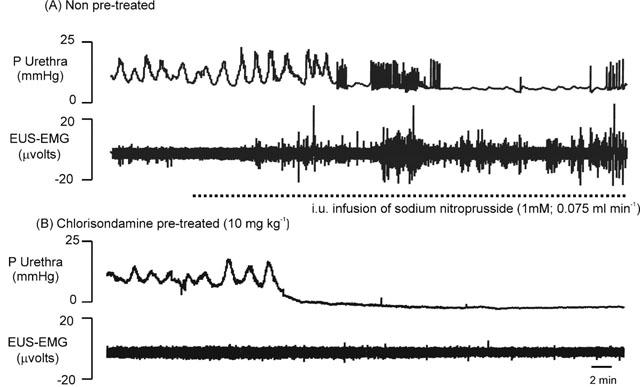
Urethane anaesthetized female rats: traces showing the effects of intraurethral (i.u.) perfusion (0.075 ml min−1) of sodium nitroprusside (SNP; 1 mM) on baseline urethral pressure (P urethra, mmHg) and raw urethral striated muscle activity (EUS-EMG, μvolts) in (A) non- and (B) chlorisondamine (10 mg kg−1, i.v.) pre-treated rats. Dotted line indicates period of sodium nitroprusside perfusion.
Figure 9.
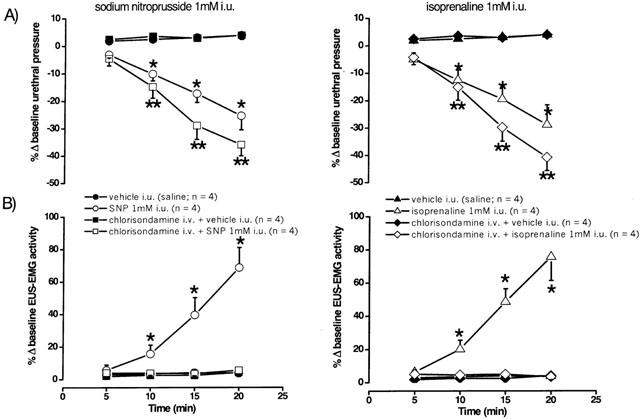
Urethane anaesthetized female rats: a comparison of the effects of i.u. perfusion (0.075 ml min−1) of saline, sodium nitroprusside (1 mM) and isoprenaline (1 mM) for 20 min in the absence and presence of pre-treatment with chlorisondamine (10 mg kg−1, i.v.) on percentage changes (Δ) in (A) baseline urethral pressure (urethra P, mmHg) and (B) integrated urethral striated muscle activity (EUS-EMG, μvolts). Each point represents the mean value and vertical bars show s.e.mean. Changes caused by drugs were compared with vehicle controls and experiments involving pre-treatment with chlorisondamine using two-way analysis of variance followed by the least significance difference test. *P<0.05 **P<0.01 in the absence of chlorisondamine pre-treatment.
Effects of i.u. zaprinast, SNP and isoprenaline on baseline MAP and HR in non- and chlorisondamine pre-treated rats
In non-chlorisondamine pre-treated rats, zaprinast i.u. failed to affect MAP and HR after 60 min. However, interestingly, following pre-treatment with chlorisondamine, zaprinast i.u. evoked a significant fall in MAP of 17±10% after 30 min, which reached a decrease of 21±2% after 60 min. This was associated with a significant increase in HR of 8±2% after 30 min, which was maintained throughout the further period of perfusion. SNP i.u. evoked a significant fall of 34±5 and 26±4% in MAP in both non- and chlorisondamine pre-treated rats, respectively, after 20 min, and caused a significant increase in HR of 6±1 and 8±3%, respectively, after 20 min. Similarly, i.u. isoprenaline evoked a significant fall of 20±7 and 22±8% in MAP in both non- and chlorisondamine-pretreated animals, respectively, after 20 min. Isoprenaline also produced a significant increase in HR of 5±2 and 6±1%, respectively, after 20 min.
Effects of vehicle and chlorisondamine on baseline values
Administration of vehicle (0.075 ml min−1) had no effect on baseline urethral pressures, EUS-EMG activities, MAP and HR in all experimental groups (n=8; Figures 7 and 9). Administration of chlorisondamine (10 mg kg−1, i.v.) had no effect on baseline urethral pressures and EUS-EMG activities (n=12), although there was an associated maintained decrease in MAP of 38±10% and a decrease in HR of 12±6%.
Discussion
Nitrergic pathway to the urethra
The present study demonstrates that reflex-evoked urethral relaxations are mediated by a nitrergic pathway in the anaesthetized female rat. However, the present data are unable to directly demonstrate that this pathway, as would be expected, is mediated by cyclic GMP transduction. Zaprinast, inhibits the breakdown of cyclic GMP (Ballard et al., 1998), and although this drug caused an expected potentiation of the effects of nitrergic stimulation to the urethra this, surprisingly, was due to an increase in urethral striated muscle tone. Whether this increase in tone is due to cyclic GMP inhibition and/or other unknown mechanism/s remains to be determined (see below). The evidence from the present experiments that a nitrergic pathway mediates urethral relaxation is based on the observation that L-NAME, given at a concentration that has previously been shown to cause changes in urodynamic parameters in the unanaesthetized female rat (Persson et al., 1992), attenuated reflex-evoked urethral relaxations. Furthermore, this nitrergic pathway seems to be only affecting smooth, and not striated muscle, as the evoked relaxations were similarly attenuated by L-NAME following neuromuscular blockade with α-bungarotoxin, although the present data suggests that there is also a tonic release of NO causing a decrease in striated (sphincter) muscle activity. These findings are consistent with in vitro studies showing that inhibitory non-adrenergic non-cholinergic (NANC) responses of isolated rat (Persson et al., 1992), rabbit (Andersson et al., 1992), sheep (Garcia-Pascual et al., 1991) and human (Klarskov et al., 1983) urethral smooth muscle are mediated by NO, and in vivo studies in conscious (Persson et al., 1992) and anaesthetized (Bennett et al., 1995) female rats.
NO and external urethral sphincter
Although the present data confirm that a nitrergic pathway controls the urethral smooth muscle, the present data also indicate that NO is involved in the basal control of the striated muscle of the urethra. This muscle exhibited a distinct type of activity during the micturition reflex with high frequency oscillations in the urethral pressure recording occurring during reflex-evoked urethral relaxations. These oscillations were α-bungarotoxin-sensitive, and were associated with bursts of EUS-EMG activity indicating that these oscillations were due to urethral striated muscle. In this respect, Conte et al. (1991a,1991b) have shown that these high frequency oscillations in urethral pressure in the female rat are abolished after sectioning of the pudendal nerves, which provide the somatic innervation to the lower urinary tract (McKenna & Nadelhaft, 1986), but are still present following dissection of the extra-urethral striated muscles of the pelvic floor, confirming that they are the direct result of urethral striated muscle activity. In the present experiments, L-NAME caused an increase in baseline urethral pressure, which was blocked in the presence of α-bungarotoxin. Further, L-NAME caused the appearance of tonic bursting in the EUS-EMG such that when the reflex was evoked it was difficult to distinguish the expected increase in EUS-EMG associated with each urethral relaxation as it had become unsynchronized. A similar change in EUS-EMG has been reported by Bennett et al. (1995) using the NOS inhibitor N-nitro-L-arginine (L-NOARG). However, these authors found that L-NOARG did not increase baseline urethral tone. This difference is probably due to ligation of the urethrovesical junction, which was not performed in the present study, and therefore the differences between these NOS inhibitors are likely experimental rather than pharmacological. The most obvious explanation that ligation of the urethral junction prevents L-NOARG from causing an increase in urethral tone is probably due to interference with the blood rather than the nerve supply to the EUS, as reflex increases in EUS-EMG activity were still observed (Bennett et al., 1995). Thus, these data suggest there is a tonic release of NO inhibiting urethral striated muscle activity and that this is most likely to be at the level of the neuromuscular junction. In this respect, NOS immunoreactivity has been identified in human urethral striated muscle (Ho et al., 1998; 1999) and NOS immunoreactivity has been identified in a number of rat skeletal muscles, including the oesphageal (Worl et al., 1994) and soleus (Kobzik et al., 1994) muscles. In addition, it has been demonstrated that in rat oesophageal striated muscle there is a close association of α-bungarotoxin binding sites and NOS immunoreactivity (Neuhuber et al., 1995). Further, NO has been demonstrated to cause inhibition of the release of acetylcholine in the rat diaphram (Mukhtarov et al., 2000). Interestingly, a physiological role for NO in the prevention of excessive increases in urethral tone during periods of increased tension has been suggested (Persson et al., 1992) and therefore these effects of NO on the urethral striated muscle may contribute to such a role.
Zaprinast and urethral relaxation
A role for cyclic GMP transduction in NO-mediated urethral smooth muscle relaxations has been suggested following the observation that cyclic GMP-immunoreactivity is present in urethral smooth muscle (Waldeck et al., 1998; Smet et al., 1996). Zaprinast has been previously reported to potentiate electrically-evoked relaxations (Dokita et al., 1994; Beavo, 1995) and increase cyclic GMP levels (Persson & Andersson, 1994) in the isolated rabbit urethra, most likely by inhibiting the breakdown of cyclic GMP via inhibition of PDE 5 (Ballard et al., 1998). Similarly, zaprinast has also been shown to potentiate NO-mediated relaxations in gastrointestinal (Williams & Parsons, 1995), genital (Cellek & Moncada, 1998) and vascular (Liu et al., 1992) smooth muscles. Furthermore, Persson et al. (2000) have demonstrated that urethral strips from mice lacking the gene for cyclic GMP-dependent protein kinase I, a cyclic GMP effector, exhibit an impaired relaxant response to NO. Therefore, in the present experiments, if NO/cyclic GMP signalling does play a role in reflex-evoked urethral relaxations, zaprinast would be expected to increase the availability of cyclic GMP and thus potentiate these responses. Indeed, in non-neuromuscular blocked female rats, reflex-evoked responses were increased by zaprinast, consistent with such a view. The dose of zaprinast used has previously been shown to produce decreases in MAP and significant increases in plasma and aortic cyclic GMP levels in male rats (Dundore et al., 1993). Indeed, in the present experiments, zaprinast lowered blood pressure. However, surprisingly, zaprinast increased baseline urethral pressure in similarity to the effects of L-NAME. Furthermore, this was prevented by pre-treatment with α-bungarotoxin, implying that zaprinast also increased EUS tension. α-bungarotoxin also blocked the potentiating effect of zaprinast on reflex-evoked relaxations. The failure of zaprinast to potentiate reflex-evoked urethral relaxations in the presence of α-bungarotoxin is probably related to the ability of zaprinast to decrease smooth muscle tone. This is suggested by the fact that zaprinast blocked the increase in urethral tone that occurred between reflex-evoked bladder contractions (this increase was absent in the presence of neuromuscular blockade indicating it is mediated by the urethral smooth muscle). Furthermore, zaprinast has been shown to decrease urethral tone in the isolated female rat urethral preparation (Wibberley, 2001) and in isolated genital (Cellek & Moncada, 1998) and gastrointestinal (Williams & Parsons, 1995) smooth muscles. This would be consistent with the observation that an elevation in cyclic GMP causes myosin light chain phosphorylation to be dissociated from smooth muscle contraction by an unknown mechanism (Karaki et al., 1997), thus interfering with ability of urethral smooth muscle to fully increase its tone.
Presumably the reason this is not observed when the striated muscle is not blocked is that the striated muscle compensates for the zaprinast-mediated failure of the smooth muscle to fully increase tone. In this respect, zaprinast caused a large increase in reflex-evoked striated muscle activity and, at least initially, baseline urethral tone was increased along with the increase in EUS-EMG activity. However, zaprinast did not cause a fall in baseline urethral pressure in α-bungarotoxin pretreated rats, as might be expected if zaprinast was causing a decrease in smooth muscle tone. The failure to see a decrease in pressure may be related to the sensitivity of the method being used and/or other elements such as connective and elastic tissue in the urethra, which may prevent any changes being observed. In addition, the nature of the relaxation may be different, in that zaprinast may just cause smooth muscle to loose some of its intrinsic tone causing it to become flaccid. This would be consistent with the observation that the increase in baseline urethral pressure is not maintained, although basal EMG activity was increased, in non-neuromuscular blocked rats. This could also explain why the amplitude of the high frequency oscillations, which are mediated by striated muscle activity, are decreased, although EUS-EMG recordings are increased, as a loss of tone would presumably dampen the signal. However, the precise explanation for the failure of the nitrergic-evoked urethral relaxation to be potentiated in the absence of external sphincter activity (striated muscle) remains to determined. It can only be concluded that the striated muscle of the EUS plays an essential role in the ability of zaprinast to potentiate urethral relaxation. It could even be possible that α-bungarotoxin could interfere, by some unknown mechanism, with the effects of zaprinast.
Zaprinast and external urethral sphincter
There are a number of possible explanations for the ability of zaprinast to increase urethral striated muscle activity. The observation that both L-NAME and zaprinast increased urethral striated muscle activity does not support a role for NO/cyclic GMP signalling in this musculature (see below). Indeed, if the inhibitory effects of NO on the urethral striated muscle are mediated by increases in cyclic GMP levels, then zaprinast would be expected to potentiate these effects of NO and thus further decrease urethral striated muscle activity. Therefore, the effects of zaprinast on the urethral striated muscle are unlikely to be the result of manipulation of this pathway, and the inhibitory effects of NO on the urethral striated muscle involve a mechanism independent of cyclic GMP transduction. Indeed, NO has been shown to inhibit skeletal muscle contraction by inhibiting a Ca2+-ATPase in the rabbit (Ishii et al., 1998). Alternatively, PDEs that are not inhibited by zaprinast, or alternative signalling molecules such as cyclic AMP, may mediate the effects of NO in the urethral striated muscle. Indeed, cyclic AMP levels may be increased by a direct modulation of the adenylyl cyclase-dependent protein kinase pathway by NO (Vila-Petroff et al., 1999). Finally, the effects of both L-NAME and zaprinast on the urethral striated muscle may be due to mechanisms entirely independent of the NO/cyclic GMP transduction pathway, which require further investigation.
The ability of zaprinast to increase striated muscle activity may be indirect and occur as a result of an assumed zaprinast-evoked reduction in smooth muscle tone (see above). This hypothesis was tested by comparing the effects of intra-urethal zaprinast with two other smooth muscle relaxants; SNP, a NO donor, and isoprenaline, a β-adrenoceptor agonist. Zaprinast intra-urethrally evoked a similar increase in urethral pressure as when given i.v., which was also associated with an increase in EUS-EMG, however this was smaller. Conversely, SNP and isoprenaline caused a decrease in baseline urethral pressure, which was associated with an increase in EUS-EMG. The ability of all three drugs to produce an increase in EUS-EMG was blocked by pretreatment (i.v.) with the neuronal nicotinic receptor antagonist chlorisondamine. Further, zaprinast now caused a significant fall in baseline urethral pressure, supporting the view that zaprinast does cause urethral smooth muscle relaxation, while the falls caused by sodium nitroprusside and isoprenaline were potentiated. This implies that the increase in striated muscle activity occurs in response to an evoked relaxation of the smooth muscle and may represent a mechanism by which ‘resting' urethral tone is maintained. As the pathway involves activation of neuronal nicotinic receptors, this suggests that a reflex, rather than the release of local mediators, is responsible for the maintenance of urethral tone. In this respect, urethral distension with saline at the level of the urethral striated muscle has been shown to elicit a series of hexamethonium-sensitive slow phasic urethral contractions in male rats (Conte et al., 1991a) and an increase in EUS-EMG in female rats (Jung et al., 1999). Furthermore, Conte et al. (1993) have suggested the presence of a physiological interaction between capsaicin-sensitive urethral afferents and the somatic efferent innervation to the EUS, constituting a chemonociceptive urethra-urethral neural loop, which, via pudendal nerves, leads to a supraspinally-mediated activation of the EUS. Whether the reflex reported in the present study involves the same loop remains unknown. Furthermore, the precise site/s at which chlorisondamine is acting remains to be determined, although an effect of this drug at the level of the pelvic plexus, a bilateral association of neurones and ganglia, which lies at the base of the bladder (Keast, 1999), is most likely.
A direct effect of zaprinast on the urethral striated muscle to increase its activity cannot be ruled out. For example, zaprinast may exert an inhibitory or modulatory effect on glycogen synthesis and glucose oxidation (Young et al., 1996), Ca2+ (Weber & Herz, 1968) or noradrenaline release (Berkowitz et al., 1970).
NO/cyclic GMP signalling and the bladder
The present experiments show that pharmacological manipulation of the NO/cyclic GMP pathway had no effects on reflex-evoked changes in bladder pressure. Conversely, Persson et al., (1991; 1992) have shown that inhibition of NOS induces bladder hyperactivity and decreases bladder capacity in the unanaesthetized female rat, although these changes may reflect an effect on the urethral outlet rather than a direct effect on the bladder. Experiments involving isolated strips of detrusor muscle from various species have provided evidence for NO-mediated inhibitory neurotransmission in some reports (James et al., 1993; Chung et al., 1996), but not in others (Persson & Andersson, 1992). Zaprinast had no effect on the contractile responses of isolated guinea-pig (Longhurst et al., 1997) and porcine (Truss et al., 1996) bladder strips. The findings in the present study provide further evidence for a lack of a role for NO/cyclic GMP in reflex-evoked bladder responses. However, administration of L-NAME caused an increase in baseline bladder pressure, although these effects of L-NAME were not reversed by L-arginine, suggesting mechanisms independent of the L-arginine/NO pathway. The observation that L-NAME-evoked increases in baseline bladder pressure in both non- and α-bungarotoxin pre-treated animals provides evidence against a mechanical mechanism of action, as a result of an increase in urethral striated muscle activity. Alternatively, these effects may be the result of an L-NAME-evoked increase in MAP, as changes in blood flow to the bladder may affect smooth muscle tone.
In conclusion, the present study has demonstrated that NO mediates reflex-evoked smooth muscle relaxations. Furthermore, NO appears to exert a tonic inhibitory effect on the urethral striated muscle, which may operate as a braking mechanism in the maintenance of urethral tone. The ability of zaprinast to potentiate reflex-evoked urethral relaxations is due to an ability to increase urethral striated muscle activity by unknown mechanism/s. One possible mechanism is indirect, by activating a reflex increase in striated muscle tone in response to a loss of tone in the smooth muscle. Furthermore, the ability of zaprinast to increase urethral striated muscle tone, seemingly fairly selectively (as no gross effects on respiratory movement or skeletal muscle fasciculation were observed), indicates that it may be possible to pharmacologically target this muscle to treat various forms of incontinence.
Acknowledgments
A. Wibberley was in receipt of a MRC Industrial Scholarship with Pfizer Global Research and Development, U.K. The authors greatly appreciate the support of and useful discussions with Matthew Fraser and William C. De Groat at the University of Pittsburgh. The authors also wish to thank Steve Wilkinson and Ian Knowles for technical assistance.
Abbreviations
- EMG
electromyogram
- EUS
external urethral sphincter
- HR
heart rate
- i.u.
intra-urethral
- L-NAME
Nω-nitro-L-arginine methyl ester
- MAP
mean arterial pressure
- NO
nitric oxide
- NOS
nitric oxide synthase
- PDE
phosphodiesterase
- SNP
sodium nitroprusside
References
- ANDERSSON K.E., GARCIA-PASCUAL A., PERSSON K.E., FORMAN A., TOTTRUP A. Electrically-induced, nerve-mediated relaxation of rabbit urethra involves nitric oxide. J. Urol. 1992;147:253–259. doi: 10.1016/s0022-5347(17)37208-7. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.E., PERSSON K. Nitric oxide synthase and nitric oxide-mediated effects in lower urinary tract smooth muscles. World J. Urol. 1994;12:274–280. doi: 10.1007/BF00191207. [DOI] [PubMed] [Google Scholar]
- BALLARD S.A., GINGELL C.J., TANG K., TURNER L.A., PRICE M.E., NAYLOR A.M. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isoenzymes. J. Urol. 1998;159:2164–2171. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- BEAVO J.A. Cyclic nucleotide phosphodiesterases: Functional implications of multiple isoforms. Physiol. Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- BENNETT B.C., KRUSE M.N., ROPPOLO J.R., FLOOD H.D., FRASER M., DE GROAT W.C. Neural control of urethral activity in vivo: Role of nitric oxide. J. Urol. 1995;153:2004–2009. [PubMed] [Google Scholar]
- BERKOWITZ B.A., TARVER J.H., SPECTOR S. Release of norepinephrine in the central nervous system by theophylline and caffeine. Eur. J. Pharm. 1970;10:64–71. doi: 10.1016/0014-2999(70)90158-5. [DOI] [PubMed] [Google Scholar]
- BRADING A.F. The physiology of the mammalian urinary outflow tract. Exp. Physiol. 1999;84:215–221. doi: 10.1111/j.1469-445x.1999.tb00084.x. [DOI] [PubMed] [Google Scholar]
- BROADLEY K.J. Autonomic Pharmacology. London: Taylor & Francis; 1996. [Google Scholar]
- CELLEK S., MONCADA S. Nitrergic neurotransmission mediates the non-adrenergic non-cholinergic responses in the clitoral corpus cavernosum of the rabbit. Br. J. Pharmacol. 1998;125:1627–1629. doi: 10.1038/sj.bjp.0702278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG B.H., CHOI S.K., CHANG K.C. Effects of nitric oxide on detrusor relaxation. J. Urol. 1996;155:2090–2093. [PubMed] [Google Scholar]
- CONLEY R.K., WILLIAMS T.J., FORD A.P., RAMAGE A.G. The role of α1-adrenoceptors and 5-HT1A receptors in the control of the micturition reflex in male anaesthetized rats. Br. J. Pharmacol. 2001;133:61–72. doi: 10.1038/sj.bjp.0704043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONTE B., MAGGI C.A., GIACHETTI A., PARLANI M., LOPEZ G., MANZINI S. Intraurethral capsaicin produces reflex activation of the striated urethral sphincter in urethane-anaesthetised male rats. J. Urol. 1993;150:1271–1277. doi: 10.1016/s0022-5347(17)35759-2. [DOI] [PubMed] [Google Scholar]
- CONTE B., MAGGI C.A., PARLANI M., LOPEZ G., MANZINI S., GIACHETTI A. Simultaneous recording of vesical and urethral pressure in urethane-anaesthetized rats: Effect of neuromuscular blocking agents on the activity of the external urethral sphincter. J. Pharmacol. Meth. 1991a;26:161–171. doi: 10.1016/0160-5402(91)90041-3. [DOI] [PubMed] [Google Scholar]
- CONTE B., MAGGI C.A., PARLANI M., GIACHETTI A. Evidence for the existence of a urethro-urethral excitatory reflex in urethane anaesthetised female rats: involvement of peripheral ganglionic structures. J. Urol. 1991b;146:1627–1630. doi: 10.1016/s0022-5347(17)38201-0. [DOI] [PubMed] [Google Scholar]
- DE GROAT W.C., BOOTH A.M., YOSHIMURA N.Neurophysiology of micturition and its modification in animal models of human disease Nervous Control of the Urogenital System 1993Switzerland: Harwood Academic Publishers; 227–290.ed. Maggi, C.A. pp [Google Scholar]
- DOKITA S., SMITH S.D., NISHIMOTO T., WHEELER M.A., WEISS R.M. Involvement of nitric oxide and cyclic GMP in rabbit urethral relaxation. Eur. J. Pharmacol. 1994;269:269–275. doi: 10.1016/0922-4106(94)90136-8. [DOI] [PubMed] [Google Scholar]
- DUNDORE R.L., CLAS D.M., WHEELER L.T., HABEEB P.G., BODE D.C., ALLAN-BUCHHOLZ R., SILVER P.J., PAGANI E.D. Zaprinast increase cyclic GMP levels in plasma and in aortic tissue of rats. Eur. J. Pharmacol. 1993;249:293–297. doi: 10.1016/0014-2999(93)90525-m. [DOI] [PubMed] [Google Scholar]
- ELBADAWI A.Comparative neuromorphology in animals The Physiology of the Lower Urinary Tract 1987London: Springer-Verlag; 23–52.ed. Torrens, M. & Morrison, J.F.B. pp [Google Scholar]
- FISHER D.A., SMITH J.F., PILLAR J.S., ST-DENIS S.T., CHENG J.B. Isolation and characterization of PDE9A, a novel human cGMP-specific phosphodiesterase. J. Biol. Chem. 1998;273:15559–15564. doi: 10.1074/jbc.273.25.15559. [DOI] [PubMed] [Google Scholar]
- GARCIA-PASCUAL A., COSTA G., GARCIA-SACRISTAN A., ANDERSSON K.E. Relaxation of sheep urethral smooth muscle induced by electrical stimulation of nerves: involvement of nitric oxide. Acta Physiol. Scand. 1991;141:531–539. doi: 10.1111/j.1748-1716.1991.tb09114.x. [DOI] [PubMed] [Google Scholar]
- HO K.M.T, , MCMURRAY G., BRADING A.F., NOBLE J.G., NY L., ANDERSSON K.E. Nitric oxide synthase in the heterogeneous population of intramural striated muscle fibres of the human membranous urethral sphincter. J. Urol. 1998;159:1091–1096. [PubMed] [Google Scholar]
- HO K.M.T., NY L., MCMURRAY G., ANDERSSON K.E., BRADING A.F., NOBLE J.G. Co-localization of carbon monoxide and nitric oxide synthesizing enzymes in the human urethral sphincter. J. Urol. 1999;161:1968–1972. [PubMed] [Google Scholar]
- ISHII T., SUNAMI O., SAITOH N., NISHIO H., TAKEUCHI T., HATA F. Inhibition of skeletal muscle sarcoplasmic reticulum Ca2+-ATPase by nitric oxide. FEBS Lett. 1998;440:218–222. doi: 10.1016/s0014-5793(98)01460-4. [DOI] [PubMed] [Google Scholar]
- JAMES M.J., BIRMINGHAM A.T., HILL S.J. Partial mediation by nitric oxide of the relaxation of human isolated detrusor strips in response to electrical field stimulation. Br. J. Clin. Pharmacol. 1993;35:366–372. doi: 10.1111/j.1365-2125.1993.tb04152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNG S.Y., FRASER M.O., OZAWA H., YOKOYAMA O., YOSHIYAMA M., DE GROAT W.C., CHANCELLOR M.B. Urethral afferent nerve activity affects the micturition reflex: implications for the relationship between stress incontinence and detrusor instability. J. Urol. 1999;162:204–212. doi: 10.1097/00005392-199907000-00069. [DOI] [PubMed] [Google Scholar]
- KARAKI H., OZAKI H., HORI M., MITSUI-SAITO M., AMANO K., HARADA K., MIYAMOTO S., NAKAZAWA H., WON K.J., SATO K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- KAKIZAKI H., FRASER M.O., DE GROAT W.C. Reflex pathways controlling urethral striated and smooth muscle function in the male rat. Am. J. Physiol. 1997;272:R1647–R1656. doi: 10.1152/ajpregu.1997.272.5.R1647. [DOI] [PubMed] [Google Scholar]
- KEAST J.R. Unusual autonomic ganglia: Connections, chemistry and plasticity of pelvic ganglia. Int. Rev. Cyt. 1999;193:1–69. doi: 10.1016/s0074-7696(08)61778-7. [DOI] [PubMed] [Google Scholar]
- KLARSKOV P., GERSTENBERG T., RAMIREZ D., HALD T. Non-cholinergic, non-adrenergic nerve mediated relaxation of trigone, bladder neck and urethral smooth muscle in vitro. J. Urol. 1983;129:848. doi: 10.1016/s0022-5347(17)52397-6. [DOI] [PubMed] [Google Scholar]
- KLEVMARK B. Motility of the urinary bladder in cats during filling at physiological rates. I. Intravesical pressure patterns studied by a new method of cystometry. Acta Physiol. Scand. 1974;90:565–577. doi: 10.1111/j.1748-1716.1974.tb05621.x. [DOI] [PubMed] [Google Scholar]
- KOBZIK L., REID M.B., BREDT D.S., STAMLER J.S. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- LIU S.F., CRAWLEY D.E., ROHDE J.A.L., EVANS T.W., BARNES P.J. Role of nitric oxide and guanosine 3′,5′-cyclic monophosphate in mediating nonadrenergic, noncholinergic relaxation in guinea-pig pulmonary arteries. Br. J. Pharmacol. 1992;107:861–866. doi: 10.1111/j.1476-5381.1992.tb14538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONGHURST P.A., BRISCOE J.A., ROSENBERG D.J., LEGGET R.E. The role of cyclic nucleotides in guinea-pig bladder contractility. Br. J. Pharmacol. 1997;121:1665–1672. doi: 10.1038/sj.bjp.0701328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGI C.A., SANTICIOLI P., MELI A. The nonstop transvesical cystometrogram in urethane-anesthetized rats: a simple procedure for quantitative studies on the various phases of urinary bladder voiding cycle. J. Pharmacol. Meth. 1986;15:157–167. doi: 10.1016/0160-5402(86)90064-1. [DOI] [PubMed] [Google Scholar]
- MCKENNA K.E., NADELHAFT I. The organisation of the pudendal nerve in the male and female rat. J. Comp. Neurol. 1986;248:532–537. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- MOORE P.K., HANDY R.L.C. Selective inhibitors of neuronal nitric oxide synthase-is no NOS really good NOS for the nervous system. TiPS. 1997;18:204–211. doi: 10.1016/s0165-6147(97)01064-x. [DOI] [PubMed] [Google Scholar]
- MUKHTAROV M.R., URAZAEV A.K., NIKOLSKY E.E., VYSKOCIL F. Effect of nitric oxide and NO synthase inhibition on nonquantal acetylcholine release in the rat diaphragm. Eur. J. Neurol. 2000;12:980–986. doi: 10.1046/j.1460-9568.2000.00992.x. [DOI] [PubMed] [Google Scholar]
- NEUHUBER W.L., WORL J., BERTHOUD H.B., CONTE B. NADPH-diaphorase positive nerve fibres associated with motor endplates in the rat oesphagus: new evidence for co-innervation of striated muscle by enteric neurons. Cell Tiss. Res. 1995;276:23–30. doi: 10.1007/BF00354780. [DOI] [PubMed] [Google Scholar]
- PERSSON K., ANDERSSON K.E. Nitric oxide and relaxation of pig lower urinary tract. Brit. J. Pharm. 1992;106:416–422. doi: 10.1111/j.1476-5381.1992.tb14349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERSSON K., ANDERSSON K.E. Non-adrenergic, non-cholinergic relaxation and levels of cyclic nucleotides in rabbit lower urinary tract. Eur. J. Pharmacol. 1994;268:159–167. doi: 10.1016/0922-4106(94)90185-6. [DOI] [PubMed] [Google Scholar]
- PERSSON K., IGAWA Y., MATTIASON A., ANDERSSON K.E. Inhibition of L-arginine/nitric oxide pathway causes bladder hyperactivity in the rat. Acta. Physiol. Scand. 1991;144:107–108. doi: 10.1111/j.1748-1716.1992.tb09273.x. [DOI] [PubMed] [Google Scholar]
- PERSSON K., IGAWA Y., MATTIASON A., ANDERSSON K.E. Effects of inhibition of the L-arginine/nitric oxide pathway in the rat lower urinary tract in vivo and in vitro. Br. J. Pharmacol. 1992;107:178–184. doi: 10.1111/j.1476-5381.1992.tb14483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERSSON K., PANDITA R.K., ASZODI A., AHMAD M., PFEIFER A., FASSLER R., ANDERSSON K.E. Functional Characteristics of urinary tract smooth muscle in mice lacking cGMP protein kinase type I. Am. J. Physiol. 2000;279:R1112–R1120. doi: 10.1152/ajpregu.2000.279.3.R1112. [DOI] [PubMed] [Google Scholar]
- SMET P.J., JONAVICIUS J., MARSHALL V.R., DE VENTE J. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71:337–348. doi: 10.1016/0306-4522(95)00453-x. [DOI] [PubMed] [Google Scholar]
- SOKAL R.R., ROHLF F.J. Biometry: The Principles and Practice of Statistics in Biological Research. San Francisco: Freeman; 1969. [Google Scholar]
- TRUSS M.C., UCKERT S., STEIF C.G., FORSSMANN W.G., JONAS U. Cyclic nucleotide phosphodisterase (PDE) isoenzymes in human detrusor smooth muscle. II. Effect of various PDE inhibitors on smooth muscle tone cyclic nucleotide levels in vitro. Urol. Res. 1996;24:129–134. doi: 10.1007/BF00304075. [DOI] [PubMed] [Google Scholar]
- VILA-PETROFF M.G., YOUNG A., EGAN J., LAKATTA E.G., SOLLOTT S.J. Activation of distinct camp-dependant and cGMP-dependant pathways by nitric oxide in cardiac myocytes. Cir. Res. 1999;84:1020–1031. doi: 10.1161/01.res.84.9.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALDECK K., NY L., PERSSON K.E., ANDERSSON K.E. Mediators and mechanisms of relaxation in rabbit urethral smooth muscle. Br. J. Pharmacol. 1998;123:617–624. doi: 10.1038/sj.bjp.0701645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER A., HERZ R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J. Gen. Physiol. 1968;52:750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIBBERLEY A.Pharmacology of the autonomic control of the female rat urethra: relevance to micturition 2001PhD Thesis, Univ. London
- WIBBERLEY A., NUNN P.A., NAYLOR A.M., RAMAGE A.G. The role of NO and cGMP in reflex- and DMPP-evoked changes in bladder and urethral pressures in the anaesthetised rat. Br. J. Pharmacol. 1999a;128:225P. [Google Scholar]
- WIBBERLEY A., NUNN P.A., NAYLOR A.M., RAMAGE A.G. An investigation of the pathways involved in changes in bladder and urethral pressures evoked by DMPP, tyramine and bladder distension in anaesthetised rats. Br. J. Pharmacol. 1999b;146:31P. [Google Scholar]
- WIBBERLEY A., NUNN P.A., NAYLOR A.M., RAMAGE A.G. Inhibition of cGMP selective phosphodiesterase potentiates reflex-evoked relaxations of the urethra in anaesthetised rats: role of smooth and striated muscle. FASEB J. 2000a;14:304.8. [Google Scholar]
- WIBBERLEY A., NUNN P.A., NAYLOR A.M., RAMAGE A.G. Evidence that decreases in urethral smooth muscle tone produce reflex increases in urethral striated muscle activity in the anaesthetised female rat. J. Physiol. 2000b;526:173P. [Google Scholar]
- WILLIAMS S.J., PARSONS M.E. Nitric oxide, an enteric nonadrenergic-noncholinergic relaxant transmitter: evidence using phosphodiesterase V and nitric oxide synthase inhibition. Br. J. Pharmacol. 1995;116:1789–1796. doi: 10.1111/j.1476-5381.1995.tb16664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORL J., MAYER B., NEUHUBER W.L. Nitrergic innervation of the rat oesphagus: focus on motor endplates. J. Auton. Nerv. Syst. 1994;49:227–233. doi: 10.1016/0165-1838(94)90169-4. [DOI] [PubMed] [Google Scholar]
- YOUNG M.E., RADDA B., LEIGHTON B. Nitric oxide stimulates glucose transport and metabolism in rat skeletal muscle in vitro. Biochem. J. 1996;322:223–228. doi: 10.1042/bj3220223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG J., SNYDER S.H. Nitric oxide in the nervous system. Ann. Rev. Pharmacol. Toxicol. 1995;35:213–233. doi: 10.1146/annurev.pa.35.040195.001241. [DOI] [PubMed] [Google Scholar]


