Abstract
Extracellular nucleotides can activate a common purinoceptor mediating various cell responses. In this study we report that stimulation of rat mesangial cells with ATP and UTP leads to a rapid activation of the protein kinase B/Akt (PKB) pathway. Time-course studies reveal a rapid and transient phosphorylation of both Ser473 and Thr308 of PKB with a maximal effect after 5 min of stimulation. The response is concentration-dependent with a maximal effect at 30 μM of ATP and UTP. Western blot analysis of mesangial cells reveals the expression of the isoenzymes PKB-α and PKB-γ, but not the PKB-β.
ATP and UTP also activate the upstream located PI 3-kinase-dependent kinase. Furthermore, the ATP- and UTP-induced PKB phosphorylation is abolished by two inhibitors of the PI 3-kinase. In addition, suramin, a putative P2Y2 receptor antagonist, and pertussis toxin, an inhibitor of Gi/Go activation, markedly block ATP- and UTP-induced PKB phosphorylation.
A series of ATP and UTP analogues were tested for their ability to stimulate PKB phosphorylation. UTP, ATP and γ-thio-ATP are the only compounds capable of activating PKB.
Stress-induced apoptosis of mesangial cells is reduced by the stable ATP analogue, γ-thio-ATP, and this inhibitory effect is reversed in the presence of LY 294002.
In summary, these results demonstrate that extracellular nucleotides are able to activate the PI 3-kinase/PDK/PKB cascade via the P2Y2-receptor and a pertussis toxin-sensitive Gi protein. Moreover, in mesangial cells this cascade may have an important role in the antiapoptotic response but not in the mitogenic or inflammatory response produced by extracellular nucleotides.
Keywords: Protein kinase B/Akt, PDK, PI 3-kinase, P2Y2 purinoceptor, ATP, UTP, apoptosis, mesangial cell
Introduction
Extracellular nucleotides, such as ATP and UTP, exert diverse effects on intact cells by activation of plasma membrane receptors, termed P2 purinoceptors. These receptors either couple through G-proteins to their intracellular effector enzymes (P2Y subtypes) or are themselves part of ligand-gated ion channels (P2X subtypes) (Ralevic & Burnstock, 1998; Boarder & Hourani, 1998).
In renal mesangial cells, ATP acts on a nucleotide receptor (P2Y2 receptor) and mediates phosphoinositide hydrolysis with generation of 1,2-diacylglycerol and inositol 1,4,5-trisphosphate (Pfeilschifter, 1990a, 1990b) leading to mobilization of intracellular calcium (Pavenstädt et al., 1993), activation of protein kinase C (Pfeilschifter & Huwiler, 1996), activation of the classical mitogen-activated protein kinase cascade (Huwiler & Pfeilschifter, 1994) and the cytosolic phospholipase A2, and stimulation of prostaglandin E2 synthesis (Pfeilschifter, 1990a; Schulze-Lohoff et al., 1992). Furthermore, in rat mesangial cells ATP can activate the stress-activated protein kinase cascade (Huwiler et al., 1997a) as well as the p38-MAPK cascade in rat mesangial cells (Huwiler et al., 2000). The serine-threonine-specific kinase protein kinase B/Akt1 (PKB) was first identified as the human homologue of a transforming oncogene (Staal, 1987; Coffer & Woodgett, 1991), and has now gained interest because of its possible function in cell survival signalling (Chan et al., 1999). Classical activators of PKB include growth factors, including platelet-derived growth factor and insulin-like growth factor, which act through tyrosine kinase receptors and involve the small G protein p21ras and phosphatidylinositol 3-kinase (PI 3-kinase). The lipid products of PI 3-kinase such as phosphatidylinositol trisphosphate (PIP3), are important cofactors for PKB activation. Additionally, PKB requires phosphorylation at two sites for full activation, namely at Thr308 in the activation loop, and at Ser473 in a hydrophobic part at the C-terminus. The phosphoinositide-dependent kinase-1 (PDK-1) was identified as the kinase responsible for Thr308 phosphorylation whereas the kinase phosphorylating Ser473, tentatively named PDK-2, has not yet been identified. A recent report suggests that Ser473 is an autophosphorylation site of PKB (Toker & Newton, 2000).
In this study in rat renal mesangial cells, we show that extracellular nucleotides, such as ATP and UTP, can activate the PKB cascade in a suramin- and pertussis toxin-sensitive manner. We also show that this pathway may play an important role in extracellular nucleotide-triggered cytoprotection against stress-induced cell death, but that it does not play an important role in the proliferative and inflammatory responses triggered by ATP and UTP.
Methods
Chemicals
All nucleotides were obtained from Fluka or Sigma Aldrich Fine Chemicals; [3H-methyl]-thymidine (specific activity: 25 Ci mmol−1), [3H]-arachidonic acid (specific activity: 240 Ci mmol−1), [32P-γ]-ATP (specific activity: >5000 Ci mmol−1), anti-rabbit and anti-mouse horseradish peroxidase-linked IgGs, protein A-sepharose 4B-CL, protein G-sepharose fast flow and Hyperfilm were purchased from Amersham Pharmacia Biotech Europe GmbH, Freiburg, Germany; donkey anti-sheep horseradish peroxidase-linked IgG was from Sigma Aldrich Chemie GmbH, Deisenhofen, Germany; the PKBα/Akt1-specific antibody and the phospho-PKB anibodies were from New England Biolabs, Frankfurt, Germany; PDK1 activity assay kit was from Upstate Biotechnology, Lake Placid, NY, U.S.A.; PKBβ/Akt-2 and PKBγ/Akt-3 antibodies, wortmannin, LY 294002 and pertussis toxin were obtained from Calbiochem-Novabiochem, Schwalbach, Germany; all cell culture nutrients were from Life Technologies, Karlsruhe, Germany.
Cell-culture
Rat renal mesangial cells were cultivated and characterized as described previously (Pfeilschifter, 1990a, 1990b). In a second step, single cells were cloned by limited dilution on 96-microwell plates. Clones with apparent mesangial cell morphology were used for further processing. In this study Passages 5–18 were used for the experiments. Glomerular endothelial cells were isolated and cultivated as previously described (Briner & Kern, 1994; Huwiler et al., 1997b). Glomerular parietal epithelial cells were cultivated in Dulbecco's modified Eagle medium containing 10% foetal bovine serum (Huwiler et al., 1993). The macrophage cell line P388D1 was cultivated in RPMI 1640 medium containing 10% foetal bovine serum.
Cell stimulation and Western blot analysis
Confluent mesangial cells in 60 mm-diameter dishes were stimulated with the indicated substances in Dulbecco's modified Eagle medium (DMEM) containing 0.1 mg ml−1 of fatty acid-free bovine serum albumin (BSA). Thereafter the medium was withdrawn and the cells washed once with ice-cold phosphate-buffered saline (PBS) solution. Cells were scraped into ice-cold lysis buffer (50 mM Tris, HCl, pH 7.4, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 2 mM EGTA, 40 mM β-glycerophosphate, 50 mM sodiumfluoride, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin, 1 μM pepstatin A, 1 mM phenylmethyl sulphonyl fluoride) and homogenized by 10 passes through a 26G-needle fitted to a 1 ml syringe. Samples were centrifuged for 10 min at 14,000×g and the supernatant taken for protein determination. Cell extracts containing 70 μg of protein were prepared in SDS-sample buffer and subjected to SDS–PAGE. Proteins were transferred onto nitrocellulose paper for 1 h at 11 V using a semi-dry blotting apparatus. The blotting buffer used was 25 mM Tris, 190 mM glycine in 20% methanol. After the transfer, immunostaining was performed as previously described in detail (Huwiler et al., 1995; 2000). Antibodies were diluted in blocking buffer as indicated in the legends of the figures. Bands were detected by the enhanced chemiluminescence (ECL) method as recommended by the manufacturer.
PDK1 Immunocomplex-kinase activity assay
The PDK1 Immunocomplex kinase activity assay was performed according to the manufacturer's (Upstate Biotechnology, Lake Placid, NY, U.S.A.) recommendations with slight modifications. In brief, mesangial cells in 100 mm-diameter dishes were incubated for 2 days in DMEM containing 0.1 mg ml−1 of fatty acid-free BSA and then stimulated at 37°C with various agents as indicated. The medium was removed and the cells were washed with ice-cold PBS to stop the reaction. Cells were then scraped directly into lysis buffer and homogenized by 10 passes through a 26-gauge needle fitted to a 1 ml syringe. The homogenate was centrifuged for 10 min at 14,000×g and the supernatant taken for immunoprecipitation. Samples containing 500 μg of protein and 5% foetal calf serum in lysis buffer, were incubated with the various antibodies overnight at 4°C. 20 μl of a 50% slurry of protein G-sepharose in PBS was then added and the mixture incubated for 1 h on a rotating wheel. After centrifugation for 3 min at 2000×g immuncomplexes were washed three times with a low salt buffer and 3× with a high salt buffer and once with 50 mM Tris, HCl pH 7.4. The beads were incubated in 30 μl of 1×PDK1 assay dilution buffer containing 500 ng of inactive serum- and glucocorticoid-regulated protein kinase (SGK) for 30 min at 30°C. Thereafter a SGK substrate peptide (RPRAATF; 66 μM final concentration) and 10 μCi [γ-32P]-ATP were added and a second kinase reaction was allowed to continue for 10 min at 30°C. 25 μl was spotted onto a P81 paper to stop the reaction, washed three times with 0.75% phosphoric acid and once with acetone and then counted in a β-counter.
Reverse transcriptase-PCR
Total RNA was isolated using guanidinium isothiocyanate solution. 1.5 μg of RNA was used for reversed transcriptase-PCR (First Strand cDNA Synthesis Kit, MBI). The following sequences were performed for PCR (Taq DNA Polymerase, recombinant, MBI): 94°C for 5 min (1 cycle), and 94°C for 30 s, 55°C (50°C for p110α) for 1.5 min, 72°C for 1 min (with variable numbers of cycles) and final extension at 72°C for 7 min. The number of cycles were: 30 for p110α and 35 for p110δ and p110γ. Sequences of the primers for analysis of mRNA: mouse p110α: forward: GAA AAT GGC TTT GAA TCT CTG G; reverse: GAT ACA TCC CAC AGG CAC G; mouse p110δ: forward: GAA AAG TGA ATG CTG ACG AGC; reverse: ACT TCG TGG CGC ATC TTC; mouse p110γ: forward: ATA TCC CTG TCC TGC CTC G; reverse: AGA GCA ATT CTT TGT CCT CTG C; GAPDH: forward: AAT GCA TCC TGC ACC ACC AA; reverse: GTC ATT GAG AGC AAT GCC AGC. PCR products (length: 779 bp for p110α, 619 bp for p110δ, 621 bp for p110γ and 470 bp for GAPDH) were run on a 1.5% agarose gel containing 0.5 μg ml−1 ethidium bromide.
Proliferation assay
Confluent mesangial cells in 24-well plates were incubated for 2 days in serum-free DMEM. Thereafter, cells were stimulated for 24 h with the agonists in the presence of 1 μCi ml−1 of [3H-methyl]-thymidine. To stop the reaction, medium was withdrawn and the cells washed twice with ice-cold PBS and incubated in 5% trichloroacetic acid for 30 min at 4°C. Thereafter, cells were washed twice with 5% trichloroacetic acid and then incubated in 0.5 M NaOH for 30 min at 37°C to solubilize the DNA. [3H]-thymidine incorporated into the DNA was then counted in a β-counter (Packard).
Determination of arachidonic acid release
Confluent mesangial cells in 16 mm-diameter wells were labelled for 24 h with [3H]-arachidonic acid (1 μCi ml−1) in DMEM, containing 0.1 mg ml−1 fatty acid-free BSA. Thereafter cells were washed three times to remove all non-incorporated [3H]-arachidonic acid. Approximately 80–90% of the added [3H]-arachidonic acid was incorporated by this method. The labelled cells were incubated in DMEM containing 1 mg ml−1 BSA as a trap for the released [3H]-arachidonic acid. The cells were then stimulated with vehicle or the indicated agonists for 30 min. Thereafter, the medium was removed and centrifuged. Cells were dissolved in 0.5 M NaOH, and radioactivity was counted in the supernatants and cell extracts in a scintillation counter. The percentage [3H]-arachidonic acid released from total incorporated radioactivity was calculated.
Apoptosis assay
Confluent mesangial cells in 30 mm-diameter dishes were incubated with the indicated stimulants in Dulbecco's modified Eagle medium (DMEM) containing 0.1 mg ml−1 fatty acid-free BSA for 24 h. Thereafter, oligonucleosomal DNA fragmentation, a characteristic biochemical feature of apoptotic cell death, was measured using a nucleosome DNA ELISA (Roche Diagnostics), which quantitatively records histone-associated DNA fragments.
Statistical analysis
Statistical analysis was performed by using one way analysis of variance (ANOVA). For multiple comparisons with the same control group, the limit of significance was divided by the number of comparisons according to Bonferroni. One-way ANOVA with Bonferroni's post test was performed using GraphPad InStat version 3.00 for Windows NT, GraphPad Software, San Diego California U.S.A.
Results
Expression of PKB isoenzymes in rat mesangial cells
To investigate the pattern of expression of PKB isoenzymes in renal mesangial cells, Western blot analysis of glomerular cell lysates was performed using polyclonal antisera selective for the three known isoforms of PKB. According to sequence information, PKB-α and -β run at a molecular size of approximately 60 kDa, whereas PKB-γ has a 25 amino acid C-terminal deletion and runs at approximately 58 kDa (Nakatani et al., 1999). As seen in Figure 1, PKB-α is expressed in all cell types present in the renal glomerulus, i.e. mesangial cells (MC), epithelial cells (EC) and endothelial cells (rGE), whereas the mouse macrophage cell line P388D1 (MΦ) does not express the α-isoform (Figure 1, upper panel). The identity of the intensive band at 45 kDa in mesangial cells is unclear but could represent a degradation product of the PKB-α. In contrast, PKB-β is not expressed in any of the glomerular cells, but shows a high expression in P388D1 macrophages (Figure 1, middle panel). PKB-γ is differentially expressed in glomerular cells, i.e. expression is found in mesangial cells but not in epithelial or endothelial cells. Again, PKB-γ shows a high expression in P388D1 macrophages (Figure 1, lower panel).
Figure 1.
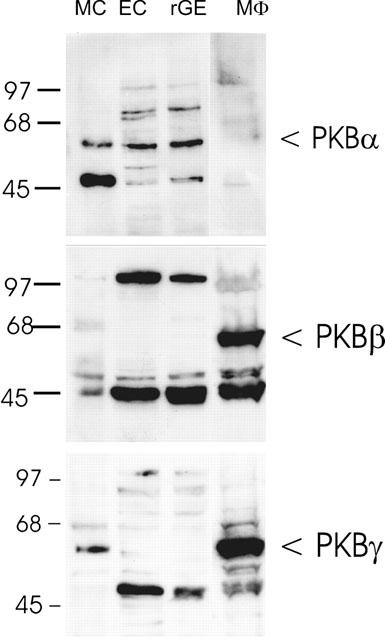
Western blot analysis of PKB isoforms in glomerular cells. Lysates of glomerular mesangial cells (MC), parietal epithelial cells (EC), endothelial cells (rGE) and the mouse macrophage cell line P388D1 (MΦ) were separated on SDS–PAGE (10% acrylamide gel), transferred to a nitrocellulose filter and Western blot analysis was performed using specific PKBα, PKBβ and PKBγ antibodies at a dilution of 1 : 1600, 1 : 1000 and 1 : 1000, respectively. Bands were detected by the ECL method according to the manufacturer's recommendation. Molecular size standards are indicated on the left side (in kDa).
ATP and UTP stimulate the PI 3-kinase/PDK/PKB cascade in mesangial cells
We investigated next the effect of extracellular nucleotides on the phosphorylation pattern of PKB in mesangial cells, because phosphorylation of PKB is considered to reflect activation of the enzyme (Andjelkovic et al., 1996).
Time-course experiments reveal a rapid phosphorylation of Ser473 within 3 min of stimulation with ATP (Figure 2A, upper panel). Maximal phosphorylation is obtained after 5 min which is followed by a rapid decrease after 10 min. A similar increase in phosphorylation is observed in the case of Thr308 (Figure 2A, middle panel), whereas the total level of PKB-α is not affected by stimulation (Figure 2A, lower panel). Stimulation with UTP causes a rapid and transient phosphorylation of Ser473 (Figure 2B upper panel) and Thr308 (middle panel) similar to that produced by ATP. Again, the total PKB level does not change on stimulation with UTP (Figure 2B, lower panel). Long-term stimulation of mesangial cells with ATP and UTP (30 min til 24 h) gives no second peak of phosphorylation (data not shown). In contrast to the transient activation of PKB by ATP and UTP, PDGF-BB causes a more sustained activation of PKB, which is still maximal after 30 min of stimulation (Figure 2C). The phosphorylation of PKB by ATP and UTP occurs in a concentration-dependent manner, with a maximal effect with 30 μM ATP (Figure 3A) or UTP (Figure 3B) after 5 min of stimulation.
Figure 2.
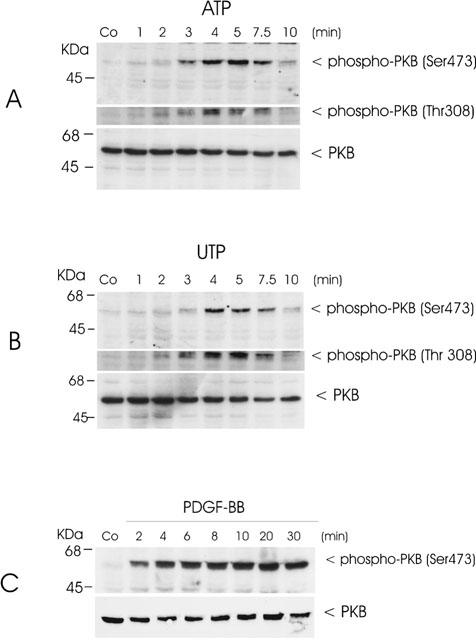
Time-course of ATP- and UTP-stimulated PKB phosphorylation in mesangial cells. Quiescent mesangial cells were treated with either vehicle (co), 100 μM of ATP (A), 100 μM of UTP (B) or 25 ng ml−1 of PDGF-BB (C) for the indicated time periods. Thereafter cells were harvested and Western blot analyses were performed using specific phospho-Ser473-PKB (upper panel), phospho-Thr308-PKB (middle panel) and total PKBα (lower panel) antibodies at a dilution of 1 : 1000, 1 : 1000 and 1 : 1600, respectively. Bands were detected by the ECL method according to the manufacturer's recommendation. Data are representative of 2 (C) or 3 (A and B) independent experiments giving similar results.
Figure 3.
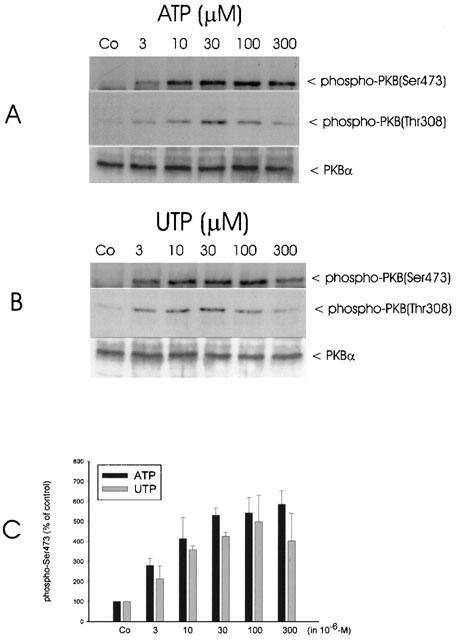
Concentration dependency of ATP- and UTP-stimulated PKB phosphorylation in mesangial cells. Quiescent mesangial cells were treated with either vehicle (co) or the indicated concentrations of ATP (A) and UTP (B) for 5 min. Thereafter cells were harvested and Western blot analysis was performed using specific phospho-Ser473-PKB (upper panel), phospho-Thr308-PKB (middle panel) and total PKBα antibodies at a dilution of 1 : 1000, 1 : 1000 and 1 : 1600, respectively. Bands were detected by the ECL method according to the manufacturer's recommendation. Data are representative of three independent experiments giving similar results. (C): The phospho-Ser473-PKB bands of the ATP and UTP stimulated samples were densitometrically evaluated. Results are expressed as per cent of vehicle-stimulated control values and are means±s.d.. (n=3).
Since mesangial cells do express PKB-α and PKB-γ, the possibility that extracellular nucleotides can activate both isoforms was investigated. For this purpose, the two isoforms were immunoprecipitated from stimulated cells and subjected to Western blot analysis using phospho-specific PKB antibodies. As seen in Figure 4, immunoprecipitated PKB-α is clearly phosphorylated on residue Thr308 (upper panel) and also on Ser473 (middle panel) after ATP, UTP and PDGF-BB stimulation. Immunoprecipitated PKB-γ, which lacks the C-terminal Ser473, still shows a marked phosphorylation on Thr308 (lower panel). These findings suggest that both isoforms are activated by extracellular nucleotides.
Figure 4.
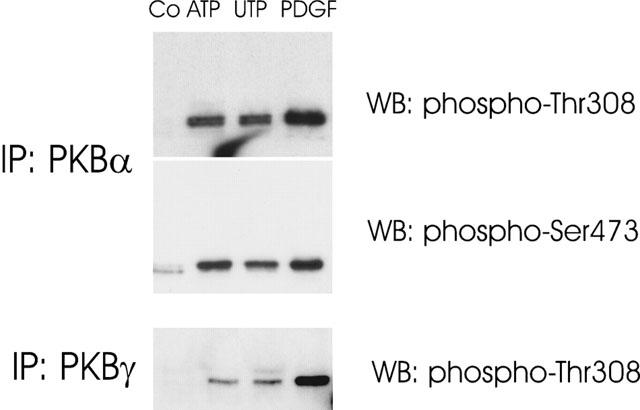
Phosphorylation of PKB isoforms α and γ by ATP, UTP and PDGF-BB in mesangial cells. Quiescent mesangial cells were stimulation for 5 min with either vehicle (Co), ATP (100 μM), UTP (100 μM) or PDGF-BB (25 ng ml−1). Thereafter, PKBα and PKBγ were immunoprecipitated from cell lysates with isoform-selective antibodies (in a dilution of 1 : 250 each) and subjected to Western blot analysis using either a phospho-Ser473-PKB- or a phospho-Thr308-PKB-specific antibody as indicated. Bands were detected by the ECL method according to the manufacturer's recommendation. Data are representative of two independent experiments giving similar results.
The possibility that ATP and UTP also activate the upstream kinase PDK-1 was examined using an immunocomplex kinase assay in an in vitro two-step kinase reaction, i.e. immunoprecipitated PDK-1 is incubated with recombinant inactive serum- and glucocorticoid-regulated protein kinase (SGK) in the first step and then with a peptide fragment of a SGK substrate in a second step. Thereafter, 32P-phosphorylated SGK substrate peptide is measured in a β-counter. Figure 5 shows that ATP within 2 min does indeed induce a rapid activation of PDK-1 in mesangial cells.
Figure 5.
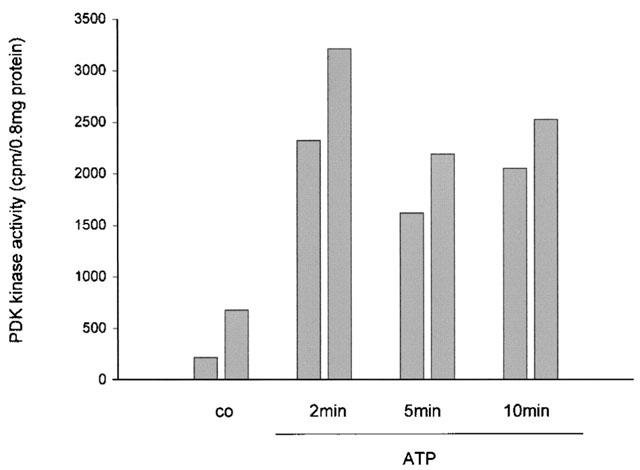
Effect of ATP on PDK-1 activity in rat mesangial cells. Quiescent mesangial cells were treated with either vehicle (co) or 100 μM of ATP for the indicated time periods. Thereafter cells were harvested and PDK-1 was immunoprecipitated and subjected to an in vitro two-step kinase reaction as described in the Methods section. Data are expressed as c.p.m. per 0.8 mg of protein and show the individual values of two independent experiments.
In order to trace the cascade one step further upstream, the PI 3-kinase was investigated since it is well accepted that PDK-1 and also PKB require the lipid products of PI 3-kinase, particularly PIP3, for full activation. By using reverse transcriptase-PCR of mouse mesangial cells, the PI 3-kinase subtypes p110α, p110γ and p110δ could be identified (Figure 6A). Evidence for the involvement of PI 3-kinase in ATP and UTP-induced PKB activation is obtained using the two inhibitors of PI 3-kinase, wortmannin (Powis et al., 1994; Okada et al., 1994) and LY 294002 (Vlahos et al., 1994), which block the ATP- and UTP-stimulated PKB activation in a dose-dependent manner (Figure 6B).
Figure 6.
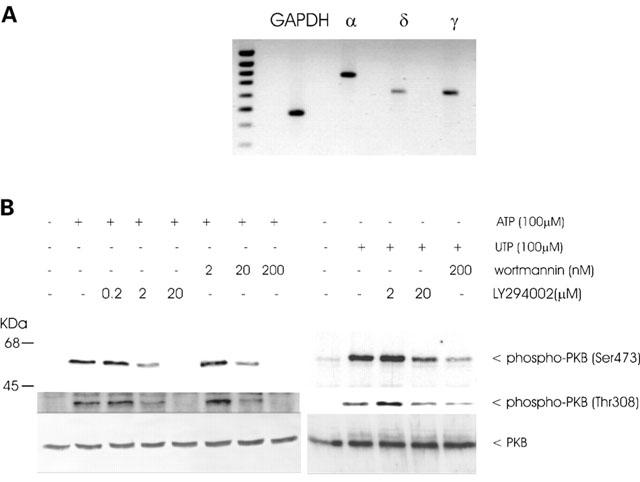
PI 3-kinase isoform expression in mesangial cells and the effect of wortmannin and LY 294002 on ATP-induced PKB phosphorylation. (A): RNA was prepared from quiescent mouse mesangial cells and reversed transcriptase-PCR of the p110α (α), p110δ (δ), p110γ (γ) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed as described in the Experimental Procedure Section. GAPDH runs at 470 bp, p110α at 779 bp, p110δ at 619 bp and p110γ at 621 bp. (B): Quiescent mesangial cells were pretreated with the indicated concentrations of wortmannin and LY 294002 for 20 min before stimulation for 5 min with 100 μM of ATP. Thereafter cell lysates, containing 70 μg of protein were subjected to SDS–PAGE (8% acrylamide gel) and Western blot analysis was performed using specific phospho-Ser473-PKB (upper panel), phospho-Thr308-PKB (middle panel) and total PKBα antibodies at a dilution of 1 : 1000, 1 : 1000 and 1 : 1600, respectively. Bands were visualized by the ECL method and evaluated on a densitometer. Data are representative of at least 3 independent experiments giving similar results.
Involvement of the P2Y2 receptor and a pertussis toxin sensitive Gi protein in PKB activation
In the next step, suramin, a putative P2Y2 purinoceptor antagonist without actions on the P2Y4 receptor (Boarder & Hourani, 1998) was tested and found to block ATP and UTP-induced PKB phosphorylation in a dosis-dependent manner as shown in Figure 7. In addition, the stimulatory action of ATP and UTP is almost completely blocked by pertussis toxin (Hewlett et al., 1983), a potent inhibitor of the Gi/Go proteins (Figure 7).
Figure 7.
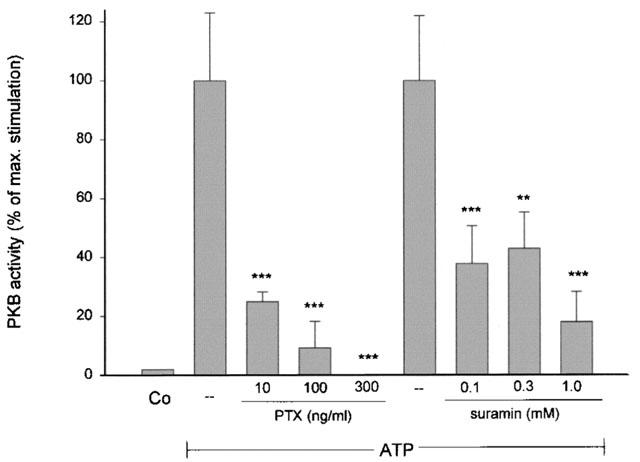
Effect of pertussis toxin and suramin on ATP- and UTP-induced PKB phosphorylation in mesangial cells. Quiescent mesangial cells were pretreated with the indicated concentrations of suramin (A) for 20 min or pertussis toxin (B) for 20 h before stimulation for 5 min with 100 μM of ATP. Thereafter cell lysates, containing 70 μg of protein were subjected to SDS–PAGE (8% acrylamide gel) and Western blot analysis was performed using specific phospho-Ser473-PKB (upper panel), phospho-Thr308-PKB (middle panel) and total PKBα antibodies at a dilution of 1 : 1000, 1 : 1000 and 1 : 1600, respectively. Bands were visualized by the ECL method and evaluated on a densitometer. Results are expressed as per cent of control value and are means±s.d. (n=3–4).
A series of ATP analogues were tested for their ability to induce PKB phosphorylation. As seen in Figure 8, ATP, UTP and γ-thio-ATP are the only substances that stimulate PKB phosphorylation. Neither ADP, AMP nor adenosine cause significant PKB phosphorylation. Furthermore, 2-methyl-thio-ATP, αβ-methylene-ATP and βγ-imido-ATP were also without effect.
Figure 8.
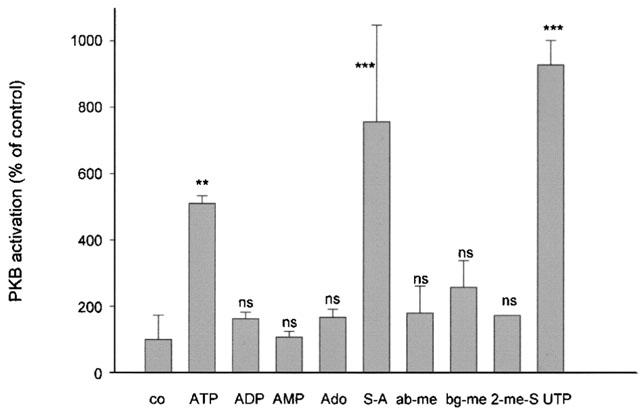
Effect of different ATP analogues on PKB phosphorylation in mesangial cells. Quiescent mesangial cells were stimulated for 5 min with 100 μM of ATP, ADP, AMP, adenosine (Ado), γ-S-ATP (S-A), αβ-methylene-ATP (ab-me), βγ-methylene-ATP (bg-me), 2-methyl-S-ATP (2-me-S) or UTP. Thereafter cell lysates, containing 70 μg of protein were subjected to SDS–PAGE (8% acrylamide gel) and Western blot analysis was performed using a specific phospho-Ser473-PKB antibody at a dilution of 1 : 1000. Bands were visualized by the ECL method and evaluated on a densitometer. Results are expressed as per cent of control value and are means±s.d. (n=2–4). **P<0.01, ***P<0.001, statistically significant difference compared to unstimulated control value.
Involvement of the PKB pathway in cell responses of mesangial cells to extracellular nucleotides
The fact that extracellular nucleotides trigger the release of arachidonic acid by a cPLA2 (Pfeilschifter, 1990a) and also stimulate cell proliferation (Huwiler & Pfeilschifter, 1994; Schulze-Lohoff et al., 1992), prompted us to investigate the contribution of the PKB pathway in nucleotide-induced arachidonic acid release and the proliferation of mesangial cells. Stimulation for 30 min with ATP and UTP causes a several fold increase in arachidonic acid release (Figure 9A) consistent with previous data (Pfeilschifter, 1990a, 1990b). However, neither wortmannin nor LY 294002 can block the extracellular nucleotide-induced arachidonic acid release, thus clearly arguing against a role of the PKB pathway in nucleotide-induced cPLA2 activation and arachidonic acid release. Similarly, extracellular nucleotide-stimulated cell proliferation is not inhibited by either wortmannin or LY 294002 when measuring [3H]-thymidine incorporation into DNA (Figure 9B). However, a maximal 50% inhibition is seen with the classical mitogen-activated protein kinase (MAPK) cascade inibitor U0126, which is not further increased in the presence of wortmannin (Figure 9B).
Figure 9.
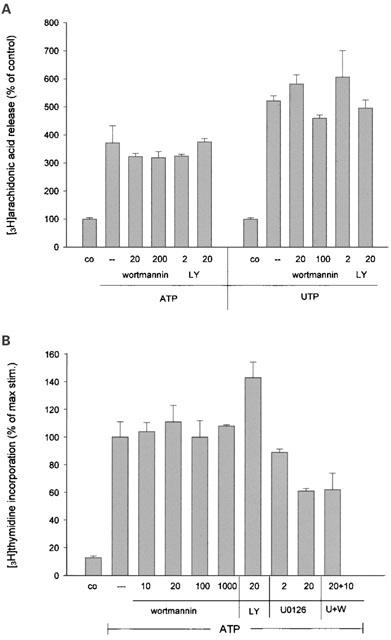
Effect of the PI 3-kinase inhibitors wortmannin and LY 294002 on ATP- and UTP-induced arachidonic acid release and cell proliferation. (A): [3H]-arachidonic acid-labelled mesangial cells were stimulated for 30 min with 100 μM of ATP or UTP in the presence of the indicated concentrations of LY 294002 (in μM) and wortmannin (in nM), which were preincubated for 30 min. Thereafter, the radioactivity released into the medium was counted as described in the Methods section. Results are expressed as per cent of control value and are means±s.d. (n=4). (B): Quiescent mesangial cells were stimulated for 24 h with 100 μM of ATP or UTP in the presence of the indicated concentrations of LY 294002 (in μM) and wortmannin (in nM), and 1 μCi ml−1 of [3H]-thymidine. Thereafter, the radioactivity incorporated into the DNA was counted as described in the Methods section. Results are expressed as per cent of control value and are means±s.d. (n=4).
In contrast to these findings, extracellular nucleotides may exert a cytoprotective effect on stress-induced apoptosis in mesangial cells. As seen in Figure 10, cotreatment of cells with tumour necrosis factor-α (TNFα) and cycloheximide (CHX) causes a marked increase in oligonucleosomal DNA fragmentation, which is a typical feature of programmed cell death (apoptosis). This increased DNA fragmentation is reduced in a dose-dependent manner in the presence of the stable ATP analogue, γ-thio-ATP, and this protective effect in turn can be reversed with the PI 3-kinase inhibitor, LY294002 (Figure 10). Stimulation of cells with LY 294002 alone, at a concentration of 20 μM, has no effect on apoptosis (Figure 10).
Figure 10.
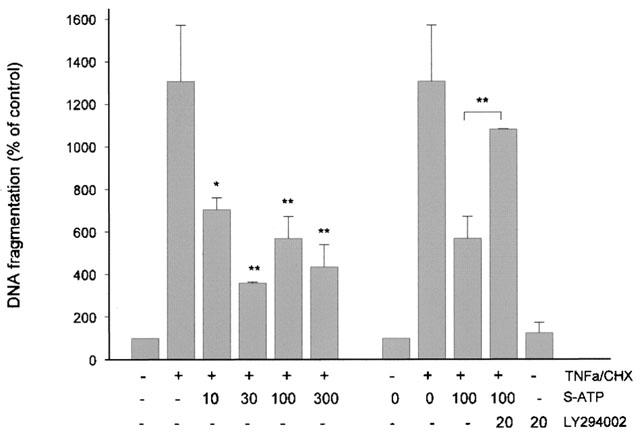
Effect of the stable ATP analog, γ-thio-ATP, on TNFα/cycloheximide-induced DNA fragmentation in meanagial cells. Confluent mesangial cells were treated for 20 h with tumour necrosis factor-α/cycloheximide (TNFa/CHX; 2 nM and 10 μM) in the presence of γ-thio-ATP (S-ATP; in μM) and where indicated in the presence of LY 294002 (20 μM). Thereafter, DNA fragmentation was measured as described in the Methods section. Results are expressed as per cent of control values and are means±s.d. (n=2–4). *P< 0.05, **P<0.01, statistically significant difference compared to TNFα/CHX-stimulated value.
Discussion
It has recently become apparent that ATP not only functions as an intracellular energy source, but also acts as an extracellular signalling molecule mediating cell–cell communication via activation of plasma membrane receptors, termed P2 purinoceptors. These receptors have been classified into two main classes, the G protein-coupled receptors (P2Y) and the ligand-gated ion channels (P2X) (Ralevic & Burnstock; 1998; Boarder & Hourani, 1998; Abbracchio & Burnstock, 1994).
In renal mesangial cells, ATP acts on a nucleotide receptor (P2Y2 receptor), that can equally well be activated by UTP, and which mediates phosphoinositide hydrolysis and synthesis of prostaglandin E2 (Pfeilschifter, 1990a, 1990b). In addition, it was observed that extracellular nucleotides stimulate growth of mesangial cells (Huwiler and Pfeilschifter, 1994; Schulze-Lohoff et al., 1992), a hallmark of chronic progressive glomerular diseases. Moreover, ATP and UTP were shown to trigger the classical MAPK cascade (Huwiler and Pfeilschifter, 1994), as well as the stress-activated protein kinase (SAPK) cascade (Huwiler et al., 1997a) and the p38-MAPK cascade (Huwiler et al., 2000). However, the detailed molecular mechansims by which ATP and UTP trigger the above mentioned cell responses is still unknown.
In this study we show that ATP and UTP are able to activate the PKB/Akt pathway, which is considered to play a central role in cell growth and survival (Chan et al., 1999). Classical activators of this pathway include various growth factors, such as platelet-derived growth factor and (PDGF) and insulin-like growth factor (IGF), that act through receptor tyrosine kinases and involve p21ras and the PI 3-kinase. In early studies, neither lysophosphatidic acid, which acts through Gi protein-coupled receptors, nor phorbol ester, which directly activates protein kinase C, were found to activate the PKB pathway in fibroblasts (Burgering & Coffer, 1995; Franke et al., 1995).
However, with the identification of the γ-isoform of PI 3-kinase, which is primarily activated by Gβγ subunits (Stoyanov et al., 1995; Hirsch et al., 2000), a link between G protein-coupled agonists and PKB activation was evident. Subsequently, several studies reported that G protein-coupled receptors can indeed activate the PKB pathway (Murga et al., 2000; Bommakanti et al., 2000). Murga et al. (2000) showed that PI3K-β or -γ subtypes are essential for signaling between G protein-coupled receptors and PKB. In cells lacking these subtypes, e.g. NIH 3T3 mouse fibroblasts, no signal propagation from G protein-coupled receptors to PKB could be detected without PI3K-β or -γ being transfected into the cells.
In this study we show that rat mesangial cells do express the α, γ and δ isoforms of PI 3-kinase as detected by reverse transcriptase-PCR. The β-isoform could not be analysed because rat and mouse sequences are not available. The involvement of PI 3-kinase in nucleotide-induced PKB activation is further corroborated by finding that the PI 3-kinase inhibitors, LY 294002 and wortmannin, completely block nucleotide-induced PKB activation.
Our data further indicate that a pertussis toxin-sensitive Gi protein is involved in ATP- and UTP-signalling to the PKB pathway. This is in line with earlier reports showing the involvement of a pertussis toxin-sensitive Gi protein in nucleotide-induced inositol trisphosphate production in mesangial cells (Pfeilschifter, 1990a). The G protein-coupled agonist N-formyl-Met-Leu-Phe consistently triggered pertussis toxin-sensitive PIP3 accumulation in neutrophils (Traynor-Kaplan et al., 1989).
Molecular cloning and functional characterization have established that extracellular nucleotides mediate their effects via several subtypes of P2 receptors (Ralevic & Burnstock; 1998; Boarder & Hourani, 1998; Communi & Boeynaems, 1997; King et al., 1998). Thus the identification of the P2Y receptor subtype responsible for signaling to the PKB cascade is of importance. It was suggested that ATP and UTP use a common nucleotide or P2u receptor for transducing their signals in mesangial cells (Pfeilschifter, 1990b). The P2u receptor has been cloned (Lustig et al., 1993; Parr et al., 1994) and renamed P2Y2 receptor by the NC-IUPHAR subcommittee on P2Y receptors (Fredholm et al., 1994). The signal transduction pathways and functional cell responses triggered by ATP and UTP in mesangial cells fit to a P2Y2 receptor subtype. This includes nucleotide-stimulated inositol trisphosphate and 1,2-diacylglycerol formation (Pfeilschifter, 1990a, 1990b), and the subsequent Ca2+ mobilization (Pavenstädt et al., 1993; Gutierrez et al., 1999), protein kinase C activation (Pfeilschifter & Huwiler, 1996) and prostaglandin E2 synthesis (Pfeilschifter, 1990a), the stimulation of phospholipase D (Pfeilschifter & Merriweather, 1993), and the activation of the classical MAPK pathway and subsequent cell proliferation (Huwiler & Pfeilschifter, 1994). Indeed, Mohaupt et al. (1998) have reported that rat mesangial cells express P2Y2 receptor mRNA, although other receptors, such as the P2Y4 (Harada et al., 2000) and P2Y6 (A. Huwiler, unpublished results) have also been identified in these cells.
Our finding that suramin, which antagonizes P2Y2 receptor activation, but not P2Y4 receptor activation (Ralevic & Burnstock; 1998; Boarder & Hourani, 1998; King et al., 1998; Bogdanov et al., 1998), is able to block nucleotide-induced PKB activation, strongly suggests the involvement of the P2Y2 receptor and not the P2Y4 receptor, in the triggering of PKB activation. The involvement of the P2Y6 receptor, which is also expressed in rat mesangial cells according to reverse transcriptase-PCR (data not shown), can be excluded here because of the lack of effect of UDP and ADP, the strongest agonists on this receptor subtype (Ralevic & Burnstock; 1998; Boarder & Hourani, 1998). Furthermore, the P2Y1 receptor as well as P2X1-7 receptors can also be excluded because αβ-methylene-ATP, a potent agonist at these receptors (Boarder & Hourani, 1998) is unable to stimulate the PKB pathway.
The additional finding that adenosine is unable to stimulate the PKB pathway, gives further evidence that ATP acts via a purinoceptor and not via degradation to adenosine, binding to adenosine receptors (A-receptors) and signalling through the adenylate cyclase pathway (Scholz-Pedretti et al., 2001). In contrast, Gao et al. (2001) reported that adenosine selectively triggers PKB activation in mast cells by A3 adenosine receptor activation, an effect which also involves a pertussis toxin sensitive Gi protein.
Our data further indicate that the PKB pathway, which is rapidly activated by ATP and UTP, is not involved in either nucleotide-triggered cell proliferation or in arachidonic acid release and subsequent prostaglandin synthesis, two cell responses that are potently activated by nucleotides in mesangial cells (Pfeilschifter, 1990a; Huwiler & Pfeilschifter, 1994). However, the PKB pathway seems to play an important role in the anti-apoptotic cell response of mesangial cells, since TNFα/CHX-induced cell death is reduced in a dose-dependent manner in the presence of the stable ATP analogue γ-thio-ATP (Figure 10). This anti-apoptotic effect of γ-thio-ATP is most probably mediated by PKB, but not by the classical MAPK, because the PI 3-kinase inhibitor LY 294002 (Vlahos et al., 1994), and not the highly selective MAPK kinase (MEK) inhibitor U0126 (Davies et al., 2000) (data not shown), can reverse the inhibitory effect of γ-thio-ATP on stress-induced apoptosis. Furthermore, in addition to the biochemical indication of apoptosis we have analysed for morphological changes indicative for programmed cell death, by staining with the Hö-33342 dye, which showed a similar protective effect of γ-thio-ATP (data not shown). Of course these are only first steps in elucidating the mechanism of protection by extracellular nucleotides. One mechansim by which PKB promotes cell survival involves the phosphorylation of Ser136 of the proapoptotic Bcl-2 family member Bad, which subsequently leads to a dissociation from Bcl-2/Bcl-XL and protection of mitochondria from undergoing functional impairment, i.e. prevention of cytochrome c release and facilitation of mitochondrial ATP/ADP exchange (Datta et al., 1997). We did indeed find that ATP stimulation of mesangial cells leads to a phosphorylation of Bad at Ser136 in mesangial cells (data not shown).
In contrast to this cytoprotective effect of nucleotides involving the P2Y2 receptor, Schulze-Lohoff et al. (1998) reported that mesangial cells exposed to high concentrations (in the millimolar range) of ATP, but not UTP, undergoes apoptosis and necrosis. This effect is due to activation of the P2X7 receptor since the effect can be mimicked by the P2X7 receptor agonist, 3-O-(4-benzoylbenzoyl)-ATP.
Obviously, the concentration of ATP is a critical determinant of which purinoceptor signalling pathway is initiated and therefore of the cell response: low physiological concentrations of ATP trigger the P2Y2 receptor signalling cascade leading to proliferation via MAPK activation and an anti-apoptotic effect via PKB activation, whereas high and toxic concentrations trigger the P2X7 receptor signalling cascade leading directly to apoptosis and/or necrosis.
In summary, we have shown that extracellular nucleotides in mesangial cells can activate the PKB/Akt pathway via the P2Y2 receptor coupling to a Gi protein, and potentially mediate cytoprotection against stress-induced cell death. Future work will have to delineate whether P2Y2 receptor agonists and antagonists will be efficient in treatment of glomerular disease which is characterized by mesangial cell proliferation and apoptosis.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (HU 842/2-2, PF 361/1-1 and SFB 553), the August-Scheidel Stiftung and the VERUM Stiftung für Umwelt und Verhalten. We thank Dr B. Woodcock for critically reading the manuscript.
Abbreviations
- BSA
bovine serum albumin
- CHX
cycloheximide
- DMEM
Dulbecco's modified Eagle medium
- DTT
dithiothreitol
- ECL
enhanced chemiluminescence
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IGF
insulin-like growth factor
- MAPK
mitogen-activated protein kinase
- PBS
phosphate-buffered saline
- PDGF–BB
platelet-derived growth factor–BB
- PDK
PI 3-kinase dependent kinase
- PIP3
phosphatidylinositol trisphosphate
- PKB
protein kinase B
- SAPK
stress-activated protein kinase
- SGK
serum- and glucocorticoid-regulated protein kinase
- TNFα
tumour-necrosis factor-α
References
- ABBRACCHIO M.P., BURNSTOCK G. Purinoceptors: are there families of P2X and P2Y purinoceptors. Pharmacol. Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- ANDJELKOVIC M., JAKUBOWICZ T., CRON P., MING X.F., HAN J.W., HEMMINGS B.A. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOARDER M.R., HOURANI S.M. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol. Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- BOGDANOV Y.D., WILDMAN S.S., CLEMENTS M.P., KING B.F., BURNSTOCK G. Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Br. J. Pharmacol. 1998;124:428–430. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOMMAKANTI R.K., VINAYAK S., SIMONDS W.F. Dual regulation of Akt/protein kinase B by heterotrimeric G protein subunits. J. Biol. Chem. 2000;275:38870–38876. doi: 10.1074/jbc.M007403200. [DOI] [PubMed] [Google Scholar]
- BRINER V.A., KERN F. ATP stimulates Ca2+ mobilization by a nucleotide receptor in glomerular endothelial cells. Am. J. Physiol. 1994;266:F210–F217. doi: 10.1152/ajprenal.1994.266.2.F210. [DOI] [PubMed] [Google Scholar]
- BURGERING B.M., COFFER P.J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- CHAN T.O., RITTENHOUSE S.E., TSICHLIS P.N. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- COFFER P.J., WOODGETT J.R. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur. J. Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., BOEYNAEMS J.M. Receptors responsive to extracellular pyrimidine nucleotides. Trends Pharmacol. Sci. 1997;18:83–86. doi: 10.1016/s0165-6147(96)01035-8. [DOI] [PubMed] [Google Scholar]
- DATTA S.R., DUDEK H., TAO X., MASTERS S., FU H., GOTOH Y., GREENBERG M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- DAVIES S.P., REDDY H., CAIVANO M., COHEN P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKE T.F., YANG S.I., CHAN T.O., DATTA K., KAZLAUSKAS A., MORRISON D.K., KAPLAN D.R., TSICHLIS P.N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DALY J.W., HARDEN T.K., JACOBSON K.A., LEFF P., WILLIAMS M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- GAO Z., LI B.S., DAY Y.J., LINDEN J. A3 adenosine receptor activation triggers phosphorylation of protein kinase B and protects rat basophilic leukemia 2H3 mast cells from apoptosis. Mol. Pharmacol. 2001;59:76–82. doi: 10.1124/mol.59.1.76. [DOI] [PubMed] [Google Scholar]
- GUTIERREZ A.M., LOU X., ERIK A., PERSSON G., RING A. Ca2+ response of rat mesangial cells to ATP analogues. Eur. J. Pharmacol. 1999;369:107–112. doi: 10.1016/s0014-2999(99)00032-1. [DOI] [PubMed] [Google Scholar]
- HARADA H., CHAN C.M., LOESCH A., UNWIN R., BURNSTOCK G. Induction of proliferation and apoptotic cell death via P2Y and P2X receptors, respectively, in rat glomerular mesangial cells. Kidney Int. 2000;57:949–958. doi: 10.1046/j.1523-1755.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- HEWLETT E.L., SAUER K.T., MYERS G.A., COWELL J.L., GUERRANT R.L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect. Immun. 1983;40:1198–1203. doi: 10.1128/iai.40.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH E., WYMANN M.P., PATRUCCO E., TOLOSANO E., BULGARELLI-LEVA G., MARENGO S., ROCCHI M., ALTRUDA F. Analysis of the murine phosphoinositide 3-kinase γ gene. Gene. 2000;256:69–81. doi: 10.1016/s0378-1119(00)00328-0. [DOI] [PubMed] [Google Scholar]
- HUWILER A., BRINER V.A., FABBRO D., PFEILSCHIFTER J. Feedback regulation of extracellular ATP-stimulated phosphoinositide hydrolysis by protein kinase C-α in bovine glomerular endothelial cells. Kidney Int. 1997b;52:329–337. doi: 10.1038/ki.1997.338. [DOI] [PubMed] [Google Scholar]
- HUWILER A., PFEILSCHIFTER J. Stimulation by extracellular ATP and UTP of the mitogen-activated protein kinase cascade and proliferation of rat renal mesangial cells. Br. J. Pharmacol. 1994;113:1455–1463. doi: 10.1111/j.1476-5381.1994.tb17160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUWILER A., SCHULZE-LOHOFF E., FABBRO D., PFEILSCHIFTER J. Immunocharacterization of protein kinase C isoenzymes in rat kidney glomeruli, and cultured glomerular epithelial and mesangial cells. Exp. Nephrol. 1993;1:19–25. [PubMed] [Google Scholar]
- HUWILER A., STABEL S., FABBRO D., PFEILSCHIFTER J. Platelet-derived growth factor and angiotensin II stimulate the mitogen-activated protein kinase cascade in rat renal mesangial cells. Biochem. J. 1995;305:777–784. doi: 10.1042/bj3050777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUWILER A., VAN ROSSUM G., WARTMANN M., PFEILSCHIFTER J. Stimulation by extracellular ATP and UTP of the stress-activated protein kinase cascade in rat renal mesangial cells. Br. J. Pharmacol. 1997a;120:807–812. doi: 10.1038/sj.bjp.0700979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUWILER A., WARTMANN M., VAN DEN BOSCH H., PFEILSCHIFTER J. Extracellular nucleotides activate the p38-stress-activated protein kinase cascade in glomerular mesangial cells. Br. J. Pharmacol. 2000;129:612–618. doi: 10.1038/sj.bjp.0703077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING B.F., TOWNSEND-NICHOLSON A., BURNSTOCK G. Metabotropic receptors for ATP and UTP: exploring the correspondence between native and recombinant nucleotide receptors. Trends Pharmacol. Sci. 1998;19:506–514. doi: 10.1016/s0165-6147(98)01271-1. [DOI] [PubMed] [Google Scholar]
- LUSTIG K.D., SHIAU A.K., BRAKE A.J., JULIUS D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOHAUPT M.G., FISCHER T., SCHWOBEL J., STERZEL R.B., SCHULZE-LOHOFF E. Activation of purinergic P2Y2 receptors inhibits inducible NO synthase in cultured rat mesangial cells. Am. J. Physiol. 1998;275:F103–F110. doi: 10.1152/ajprenal.1998.275.1.F103. [DOI] [PubMed] [Google Scholar]
- MURGA C., FUKUHARA S., GUTKIND J.S. A novel role for phosphatidylinositol 3-kinase beta in signaling from G protein-coupled receptors to Akt. J. Biol. Chem. 2000;275:12069–12073. doi: 10.1074/jbc.275.16.12069. [DOI] [PubMed] [Google Scholar]
- NAKATANI K., SAKAUE H., THOMPSON D.A., WEIGEL R.J., ROTH R.A. Identification of human Akt3 (protein kinase Bγ) which contains the regulatory serine phosphorylation site. Biochem. Biophys. Res. Commun. 1999;257:906–910. doi: 10.1006/bbrc.1999.0559. [DOI] [PubMed] [Google Scholar]
- OKADA T., KAWANO Y., SAKAKIBARA T., HAZEKI O., UI M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J. Biol. Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- PARR C.E., SULLIVAN D.M., PARADISO A.M., LAZAROWSKI E.R., BURCH L.H., OLSEN J.C., ERB L., WEISMAN G.A., BOUCHER R.C., TURNER J.T. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc. Natl. Acad. Sci. U.S.A. 1994;91:13067. doi: 10.1073/pnas.91.26.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAVENSTÄDT H., GLOY J., LEIPZIGER J., KLAER B., PFEILSCHIFTER J., SCHOLLMEYER P., GREGER R. Effect of extracellular ATP on contraction, cytosolic calcium activity, membrane voltage and ion currents of rat mesangial cells in primary culture. Br. J. Pharmacol. 1993;109:953–959. doi: 10.1111/j.1476-5381.1993.tb13713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEILSCHIFTER J. Extracellular ATP stimulates polyphosphoinositide hydrolysis and prostaglandin synthesis in rat renal mesangial cells. Involvement of a pertussis toxin-sensitive guanine nucleotide binding protein and feedback inhibition by protein kinase C. Cell. Signal. 1990a;2:129–138. doi: 10.1016/0898-6568(90)90016-4. [DOI] [PubMed] [Google Scholar]
- PFEILSCHIFTER J. Comparison of extracellular ATP and UTP signalling in rat renal mesangial cells. No indications for the involvement of separate purino- and pyrimidino-ceptors. Biochem. J. 1990b;272:469–472. doi: 10.1042/bj2720469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEILSCHIFTER J., HUWILER A. Regulatory functions of protein kinase C isoenzymes in purinoceptor signalling in mesangial cells. J. Auton. Pharmacol. 1996;16:315–318. doi: 10.1111/j.1474-8673.1996.tb00043.x. [DOI] [PubMed] [Google Scholar]
- PFEILSCHIFTER J., MERRIWEATHER C. Extracellular ATP and UTP activation of phospholipase D is mediated by protein kinase C-ε in rat renal mesangial cells. Br. J. Pharmacol. 1993;110:847–853. doi: 10.1111/j.1476-5381.1993.tb13890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWIS G., BONJOUKLIAN R., BERGGREN M.M., GALLEGOS A., ABRAHAM R., ASHENDEL C., ZALKOW L., MATTER W.F., DODGE J., GRINDEY G. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- SCHOLZ-PEDRETTI K., PFEILSCHIFTER J., KASZKIN M. Potentiation of cytokine induction of group IIA phospholipase A(2) in rat mesangial cells by ATP and adenosine via the A2A adenosine receptor. Br. J. Pharmacol. 2001;132:37–46. doi: 10.1038/sj.bjp.0703774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULZE-LOHOFF E., HUGO C., ROST S., ARNOLD S., GRUBER A., BRÜNE B., STERZEL R.B. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am. J. Physiol. 1998;275:F962–F971. doi: 10.1152/ajprenal.1998.275.6.F962. [DOI] [PubMed] [Google Scholar]
- SCHULZE-LOHOFF E., ZANNER S., OGILVIE A., STERZEL R.B. Extracellular ATP stimulates proliferation of cultured mesangial cells via P2-purinergic receptors. Am. J. Physiol. 1992;263:F374–F383. doi: 10.1152/ajprenal.1992.263.3.F374. [DOI] [PubMed] [Google Scholar]
- STAAL S.P. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc. Natl. Acad. Sci. U.S.A. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOYANOV B., VOLINIA S., HANCK T., RUBIO I., LOUBTCHENKOV M., MALEK D., STOYANOVA S., VANHAESEBROECK B., DHAND R., NÜRNBERG B., GIERSCHIK P., SEEDORF K., HSUAN J.J., WATERFIELD M.D., WETZKER R. Cloning and characterization of a G protein-activated human hosphoinositide-3 kinase. Science. 1995;269:690–693. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- TOKER A., NEWTON A.C. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J. Biol. Chem. 2000;275:8271–8274. doi: 10.1074/jbc.275.12.8271. [DOI] [PubMed] [Google Scholar]
- TRAYNOR-KAPLAN A.E., THOMPSON B.L., HARRIS A.L., TAYLOR P., OMANN G.M., SKLAR L.A. Transient increase in phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol trisphosphate during activation of human neutrophils. J. Biol. Chem. 1989;264:15668–15673. [PubMed] [Google Scholar]
- VLAHOS C.J., MATTER W.F., HUI K.Y., BROWN R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J. Biol. Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]


