Abstract
Effects of the prostanoids on the growth of cultured aortic vascular smooth muscle cells (VSMCs) were examined using mice lacking prostanoid receptors.
Proliferation of VSMCs was assessed by measuring [3H]-thymidine incorporation and the cell number, and their hypertrophy by [14C]-leucine incorporation and protein content.
In VSMCs from wild-type mice, expressions of mRNAs for the EP4 and TP were most abundant, followed by those for the IP, EP3 and FP, when examined by competitive reverse transcriptase-PCR. Those for the EP1, EP2 and DP, however, could not be detected.
AE1-329, an EP4 agonist, and cicaprost, an IP agonist, inhibited platelet derived growth factor (PDGF)-induced proliferation of VSMCs from wild-type mice; these inhibitory effects disappeared completely in VSMCs from EP4−/− and IP−/− mice, respectively. In accordance with these effects, AE1-329 and cicaprost stimulated cAMP production in VSMCs from wild-type mice, which were absent in VSMCs from EP4−/− and IP−/− mice, respectively.
Effects of PGE2 on cell proliferation and adenylate cyclase were almost similar with those of AE1-329 in VSMCs from wild-type mice, which disappeared in VSMCs from EP4−/− mice.
PGD2 inhibited PDGF-induced proliferation of VSMCs from both wild-type and DP−/− mice to a similar extent. This action of PGD2 was also observed in VSMCs from EP4−/− and IP−/− mice.
In VSMCs from wild-type mice, I-BOP, a TP agonist, showed potentiation of PDGF-induced hypertrophy. I-BOP failed to show this action in VSMCs from TP−/− mice.
The specific agonists for the EP1, EP2 or EP3, and PGF2α showed little effect on the growth of VSMCs.
These results show that PGE2, PGI2 and TXA2 modulate PDGF-induced proliferation or hypertrophy of VSMCs via the EP4, IP and TP, respectively, and that the inhibitory effect of PGD2 on PDGF-induced proliferation is not mediated by the DP, EP4 or IP.
Keywords: Prostanoids, prostaglandin, thromboxane, prostacyclin, prostanoid receptor, knockout mouse, aorta, vascular smooth muscle cells, proliferation, hypertrophy
Introduction
The prostanoids, consisting of prostaglandins (PGs) and thromboxanes (TXs), exert a variety of actions in the body through the binding to their specific receptors. They include the DP, EP, FP, IP and TP, which are stimulated preferentially by PGD2, PGE2, PGF2α, PGI2 and TXA2, respectively. Furthermore, there are four subtypes of the EPs; the EP1, EP2, EP3 and EP4 (Coleman et al., 1990, 1994; Ushikubi et al., 1995; Narumiya et al., 1999). These receptors can be functionally grouped into three categories: the relaxant receptors, consisting of the IP, DP, EP2 and EP4, which mediate smooth muscle relaxation; the contractile receptors, consisting of the TP, FP and EP1, which mediate contraction of smooth muscle; and the inhibitory receptor, the EP3, which inhibits smooth muscle relaxation (Ushikubi et al., 1995).
The roles of the prostanoids in the regulation of vascular tone are well known (Coleman et al., 1990), while their roles as a modulator of proliferation or hypertrophy of vascular smooth muscle cells (VSMCs) are not so clear. PGI2 and its analogues have been reported to inhibit growth factor-induced proliferation of VSMCs (Morisaki et al., 1988; Koh et al., 1993; Wharton et al., 2000). Their inhibitory effects, however, have not been verified to be derived from their actions on the IP, because of the lack of the specific antagonists for the IP and because of the possible actions of these ligands on the prostanoid receptors other than the IP (Kiriyama et al., 1997; Okada et al., 2000). As to the effects of PGE2 on the proliferation of VSMCs, there are several reports suggesting an inhibitory effect. These reports, however, showed that PGE1 or PGI2 analogues were more potent than PGE2 as an inhibitor of the mitogenesis of VSMCs (Huttner et al., 1977; Nilsson & Olsson, 1984; Loesberg et al., 1985; Orekhov et al., 1986; Uehara et al., 1988). These results may suggest that the inhibitory effect of PGE1 on the proliferation of VSMCs was brought out by its action on the IP, because it is well known that PGE1 is a potent agonist for the IP as well as for the EPs (Coleman et al., 1990). Furthermore, PGE2 has been reported to show a stimulatory effect on the growth of VSMCs (Pasricha et al., 1992; Dorn II et al., 1992). These results indicate that the roles of PGE2 and the relevant EP subtypes in the regulation of the growth of VSMCs remain to be elucidated. In contrast, stimulatory effects of the prostanoids via the contractile receptors, such as the TP and FP, on the growth of VSMCs have been reported. It has been suggested that TP agonists stimulate the proliferation of the cultured rat VSMCs either alone (Sachinidis et al., 1995), or in the presence of insulin (Hanasaki et al., 1990), foetal bovine serum (Morinelli et al., 1994) or platelet derived growth factor (PDGF) (Sachinidis et al., 1995; Grosser et al., 1997). In addition, hypertrophic actions of a TP agonist without proliferative actions on VSMCs were reported (Dorn II et al., 1992; Craven et al., 1996), suggesting that there is a discrepancy as to the actions of TXA2 mimetics among the reported results. Hypertrophic actions of PGF2α on VSMCs have also been reported, although the proliferative action was not found (Dorn II et al., 1992; Rao et al., 1999).
To date, the effects of the prostanoids on various tissues have been examined using the prostanoids and their analogues on the assumption that they were specific to each of their own receptors. These ligands, however, have recently been revealed to be not so specific and to bind to various types of prostanoid receptors (Kiriyama et al., 1997; Abramovitz et al., 2000), and to be able to activate multiple receptors (Okada et al., 2000). Therefore, it has been difficult to evaluate the contribution of each prostanoid receptor to relevant phenomena in the tissues in which various types of the prostanoid receptors were expressed. In VSMCs, several types and subtypes of the prostanoid receptors have been reported to be expressed (Coleman et al., 1990; Narumiya et al., 1999), complicating the interpretation of the effects of the prostanoids on VSMCs when examined by pharmacological methods alone.
To systematically explore the effects of the prostanoids on the proliferation or hypertrophy of VSMCs, we used mice lacking each of the eight types and subtypes of their receptors. In addition, we utilized DI-004, AE1-259, AE-248 and AE1-329, the recently developed compounds showing higher selectivity to the EP1, EP2, EP3 and EP4, respectively, compared with those of known agonists (Suzawa et al., 2000; Okada et al., 2000).
Methods
Animals
Generation and maintenance of IP−/− (Murata et al., 1997), FP−/− (Sugimoto et al., 1997), EP1−/− and EP3−/− (Ushikubi et al., 1998), EP4−/− (Segi et al., 1998), EP2−/− (Hizaki et al., 1999) and DP−/− mice (Matsuoka et al., 2000) has been reported. Generation of TP−/− mice will be reported elsewhere. These mice and wild-type control mice have a similar genetic background to C57BL/6 mice. The EP4−/− mice have mixed genetic background of 129sv/ola and C57BL/6 mice (Segi et al., 1998). For the experiments using EP4−/− mice, F2-wild-type mice having similar mixed genetic background with EP4−/− mice were used as a control. All experiments, which were approved by the Asahikawa Medical College Committee on Animal Research, were performed using 10–12-week-old male mice.
Polymerase chain reaction (PCR) methods
Cultured VSMCs were harvested with 0.05% trypsin-0.2 mM EDTA treatment, and were washed twice with a phosphate buffered saline (PBS) (composition in mM: NaCl 136.8, KCl 2.7, Na2HPO4·12H2O 8.1 and KH2PO4 1.5). Then the cells were pelleted, and total RNA was isolated using Isogen (Nippon Gene, Toyama, Japan). Total RNA (2 μg) was reverse-transcripted using Moloney murine leukemia virus (MMLV) reverse transcriptase and oligo-dT primers. The resulting cDNA was amplified by 35 PCR cycles with an annealing temperature of 60°C using primer sets specific for each prostanoid receptor (Ma et al., 2001). To quantify expression levels of the mRNAs for the prostanoid receptors, we adopted a competitive RT–PCR method using a competitive DNA construction kit (Ma et al., 2001).
Culture of VSMCs
Culture of VSMCs was performed using the thoracic aorta of wild-type and knockout mice according to an explant method (Mcmurray et al., 1991; Zhu et al., 1998). Mice were killed by deep anaesthesia with diethyl ether, and were placed in a supine position. After thoracotomy with the incisions along the border of the sternum, the portion of descending aorta above the diaphragm was excised. The aorta was immediately immersed into PBS and was cut longitudinally. The intimal surface was gently scraped with forceps to remove endothelial cells, and incubated in PBS containing collagenase (1 mg ml−1) for 15 min at 37°C. Next, the adventitia was striped off in PBS with forceps under a stereoscope (SZ60, Olympus, Tokyo, Japan), and the remaining tissue was incubated further for 10 min at 37°C in PBS containing collagenase (1 mg ml−1) and elastase (2 U ml−1). The remaining media of the aorta was cut into 1–2 mm square, 5–6 pieces of which were set apart and put onto a 35-mm of culture dish (Primaria, Becton Dickinson, NJ, U.S.A.). To maintain the tissues close to the plate, each explant was covered with a cover glass with 5 mm sides and was cultured in 1 ml of DMEM-L medium containing 20% foetal calf serum (FCS), penicillin (50 U ml−1), streptomycin (50 μg ml−1) and amphotericin B (0.125 μg ml−1) in a humidified atmosphere of 5% CO2 and 95% air at 37°C. Culture medium was exchanged every 4 days. During 10–14 days of culture, the outgrowth of the cells was seen to form a subconfluent monolayer beyond the border of the covering glass. The cells were harvested after treatment with trypsin (0.05%)-EDTA (0.2 mM). The cells (1×105) were plated in a 35-mm dish (Iwaki, Tokyo, Japan), and cultured in 1 ml of DMEM-L medium containing 10% FCS and antibiotics for 4 days to sub-confluency. After serial subcultures, the experiments were performed using the cells of passage numbers 5–10. These cells showed a growth pattern of so called hill and valley at confluency, and consisted mostly of VSMCs (>99%), which was verified by an immunohistochemical analysis using murine monoclonal antibodies against α-smooth muscle actin (1A4: N1584, DAKO Co., Carpinteria, CA, U.S.A.), von Willebrand factor (F8/86, DAKO Co.) and prolyl-4-hydroxylase (clone 5B5, DAKO Co.) to identify the smooth muscle cells, endothelial cells and fibroblasts, respectively.
Incorporation of the [3H]-thymidine and [14C]-leucine
The proliferation and hypertrophy of the VSMCs were measured by the incorporation of [3H]-thymidine and [14C]-leucine, respectively. The VSMCs were plated in a 24-well culture plate (Iwaki, Tokyo, Japan) at 4×104 cells well−1. After 24 h of culture in 0.5 ml of DMEM-L medium containing 10% FCS and antibiotics, the medium was replaced with fresh serum-free medium supplemented with transferrin (10 μg ml−1), insulin (10 μg ml−1), sodium selenite (0.67 ng ml−1) and indomethacin (10−5 M), and the cells were cultured for 36 h to achieve a quiescent state (Libby & O'brien, 1983). Then, the prostanoids and their analogues with or without PDGF (10 ng ml−1) were added to the cell culture at the indicated concentrations. After 12 h of culture, [3H]-thymidine or [14C]-leucine were added to the concentrations of 1 μCi ml−1 and 0.1 μCi ml−1, respectively, and the cells were cultured further for 4 h. After the cells were washed twice with PBS, they were fixed with 0.5 ml of trichloroacetic acid (5%) and were washed twice with PBS. Then the cells were treated with 0.2 ml of trypsin (0.25%) at 37°C for 15 min, and they were solubilized with 0.2 ml of NaOH (0.5 M) for 10 min at 37°C. Amounts of [3H]-thymidine and [14C]-leucine incorporated into the nucleic acids and proteins, respectively, were quantified by liquid scintillation counter. We also examined the rate of [3H]-thymidine incorporation after 20 h of culture, and found that its uptakes by VSMCs stimulated by PDGF both in the presence or absence of the prostanoids were almost similar with those found after 12 h of culture (data not shown).
Measurements of cell number
The VSMCs were seeded in 6-well culture plates (2×104 cells well−1), and cultured in DMEM supplemented with 10% FCS for 24 h. They were then made quiescent by culture in serum free medium for 36 h, followed by stimulation with the agonists in the presence or absence of PDGF (10 ng ml−1) for 48 h. After the cells were harvested by trypsin-EDTA treatment and resuspended in DMEM, the numbers of the cells were counted with a haemocytometer.
Measurements of protein content
The VSMCs were seeded in 24-well culture plates (4×103 cells well−1), and cultured in DMEM supplemented with 10% FCS for 24 h. They were then made quiescent by culture in serum free medium for 12 h, followed by stimulation with the agonists in the presence or absence of PDGF (10 ng ml−1) for 24 h. The cells were washed twice with PBS, and then lysed in 0.5 ml of sodium dodecyl sulphate (0.25%). Amounts of the proteins were determined according to the method of Lowry et al. (1951).
Assessment of cell death
VSMCs were seeded in 100-mm plates at a density of 1×105 cells well−1, and cultured under the same conditions with that used for measurements of cell numbers. VSMCs were then harvested and combined with their respective supernatants, so as to include any detached cells. After the cells were washed twice with PBS, they were resuspended in 100 μl of binding buffer (mM) HEPES 10/NaOH, pH 7.4, NaCl 150, KCl 5, MgCl2 1, CaCl2 1.8, followed by sequential addition of 1 μl of 25 μg ml−1 fluorescein isothiocyanate-conjugated annexin V (FITC-annexin V) and 10 μl of propidium iodide (50 μg ml−1), according to a protocol of a kit for detection of dead cells (TA4638, R&D, MN, U.S.A.). After a 15-min incubation period at 4°C in the dark, samples (typically 1×104 cells) were analysed with a FACScan analyzer (Becton-Dickinson UK Ltd., NJ, U.S.A.) at a excitation wavelength of 488 nm and emission wavelengths of 530 and 670 nm. Acquisition and analysis were performed with the CellQuest software package (Becton-Dickinson U.K. Ltd).
Measurements of cAMP content
The VSMCs were plated in 24-well culture plates at 4×104 cell well−1 in DMEM medium containing 10% FCS. After 24 h of culture, cells were washed twice with serum-free medium, and 500 μl of DMEM containing 1 mM 3-isobutyl-1-methylxanthine (IBMX) and 0.1% bovine serum albumin was added. After preincubation for 10 min at 37°C, VSMCs were stimulated with various concentrations of PGE2, AE1-329 and cicaprost for 30 min. The incubations were terminated by adding 500 μl of 6% perchloric acid. After the cells were disrupted by sonication, the solutions were transferred to the tubes and were centrifuged for 5 min at 2000 g. The supernatants were again centrifuged for 5 min at 2000 g after the addition of 50 μl of 6 M K2CO3. The cAMP contents of the supernatants were measured using radioimmunoassay kit (Yamasa Co., Tokyo, Japan).
Compounds and reagents
PGD2, PGE2, sulprostone, PGF2α and I-BOP were purchased from Cayman Chemical (Ann Arbor, MI, U.S.A.). Human recombinant PDGF-BB was purchased from Peprotec (Rocky Hill, NJ, U.S.A.). Specific agonists (Suzawa et al., 2000; Okada et al., 2000) for the EP1 (DI-004), EP2 (AE1-259), EP3 (AE-248) and EP4 (AE1-329) were kindly supplied by Ono Pharmaceutical (Osaka, Japan). Cicaprost was kindly donated by Schering (Berlin, Germany). Stock ethanol solutions of these compounds were stored at −20°C, and diluted with PBS for use. The concentration of the stock solutions was generally 10 mM except for 1 mM of I-BOP and 1.5 mM of cicaprost. [3H]-thymidine and [14C]-leucine were purchased from Amersham (Piscataway, NJ, U.S.A.). Collagenase, indomethacin, perchloric acid and diethyl ether were obtained from Wako Chemicals (Osaka, Japan). Elastase and IBMX were from Sigma Chemicals (St. Louis, MO, U.S.A.). DMEM-L medium, FCS, Trypsin-EDTA, oligo-dT primers, a mixture of culture reagents (insulin, sodium selenite and transferrin) and a mixture of antibiotics (penicillin, streptomycin and amphotericin B) were purchased from Gibco-BRL (Rockville, MD, U.S.A.). A competitive DNA construction kit was purchased from Takara (Tokyo, Japan). MMLV reverse transcriptase was obtained from Toyobo (Osaka, Japan).
Data analysis
All data are expressed as mean±s.e.mean. Statistical comparisons of data were made with two-way repeated-measurements ANOVA followed by Dunnett's test for multiple comparison (Dunnett, 1955) except those presented in Table 2, which were compared with Student's t-test. Differences were considered significant if P<0.05. The concentration-response curve in Figures 2, 3, 4 and 6 were constructed by non-linear regression analysis using Prism II, a computer program (GraphPad Software, SD, U.S.A.).
Table 2.
The effects of the prostanoids and their analogues on the PDGF-induced increases in [3H]-thymidine and [14C]-leucine incorporation in VSMCs from wild-type mice
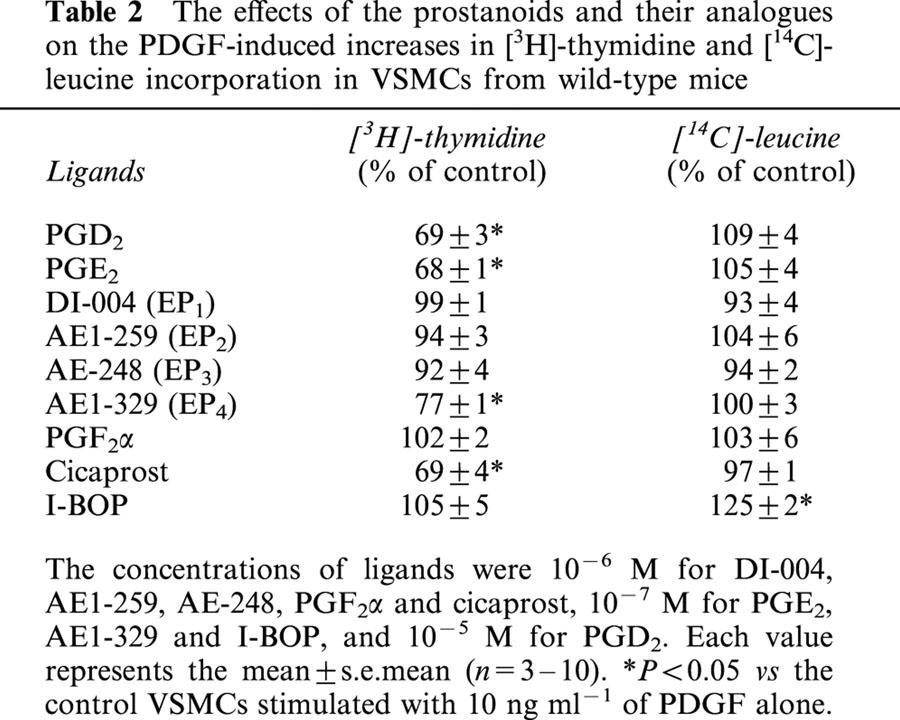
Figure 2.
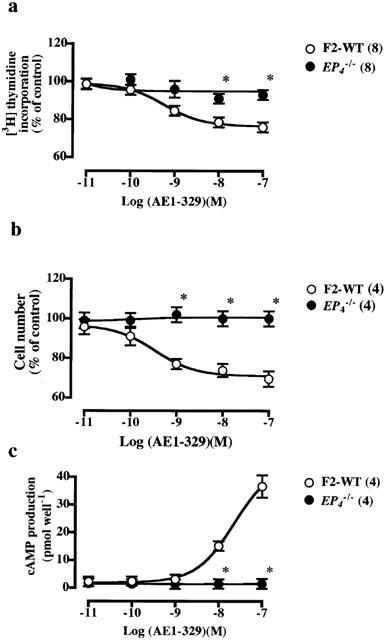
Inhibitory effects of AE1-329 on PDGF-stimulated increase in [3H]-thymidine incorporation (a), cell number (b) and cAMP production (c) in VSMCs from F2-wild-type and EP4−/− mice. The control values in the VSMCs from F2-wild-type and EP4−/− mice stimulated with PDGF (10 ng ml−1) alone were 3871±532 (n=6) and 4557±695 (n=20) c.p.m. for [3H]-thymidine incorporation, respectively, and 121±6 (n=4) and 126±8 (n=4)% increase in cell number, respectively. In a and b, the values were expressed as percentages of these control values. The inhibitory effects of AE1-329 on PDGF-induced increases in [3H]-thymidine incorporation, cell number and cAMP production were significant (P<0.05) in VSMCs from wild-type mice. Each point shows the mean value with s.e.mean shown by vertical bars. *P<0.05 vs wild-type mice. The numbers in parenthesis indicate n.
Figure 3.
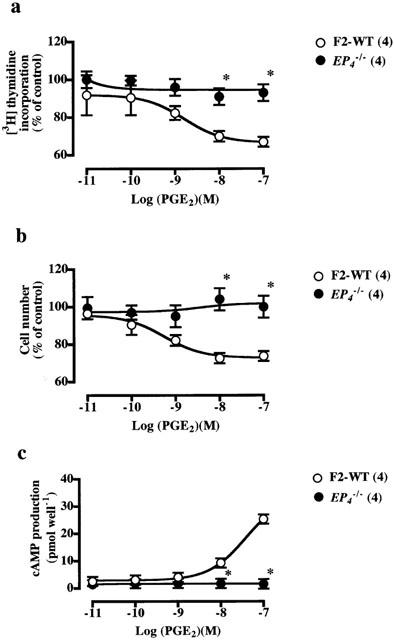
Inhibitory effects of PGE2 on PDGF-stimulated increase in [3H]-thymidine incorporation (a), cell number (b) and cAMP production (c) in VSMCs from F2-wild-type and EP4−/− mice. In a and b, the values were expressed as percentages of those of the VSMCs from F2-wild-type and EP4−/− mice stimulated with PDGF (10 ng ml−1) alone. The inhibitory effects of PGE2 on PDGF-induced increases in [3H]-thymidine incorporation, cell number and cAMP production were significant (P<0.05) in VSMCs from wild-type mice. Each point shows the mean value with s.e.mean shown by vertical bars. *P<0.05 vs wild-type mice. The numbers in parenthesis indicate n.
Figure 4.
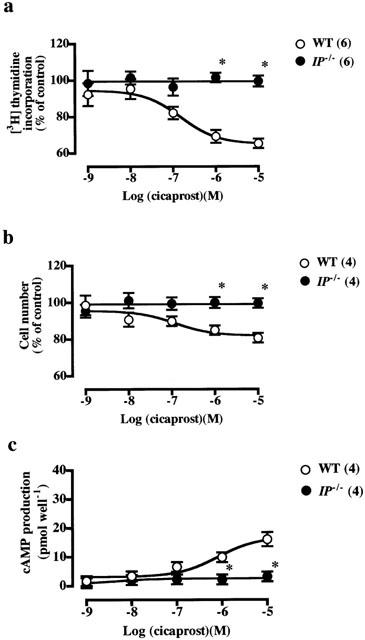
Inhibitory effects of cicaprost on PDGF-stimulated increase in [3H]-thymidine incorporation (a), cell number (b) and cAMP production (c) in VSMCs from wild-type and IP−/− mice. The control values in the VSMCs from wild-type and IP−/− mice stimulated with PDGF (10 ng ml−1) alone were 3838±120 (n=20) and 3779±438 (n=14) c.p.m. for [3H]-thymidine incorporation, respectively, and 131±6 (n=4) and 121±6 (n=4)% increase in cell number, respectively. In a and b, the values were expressed as percentages of these control values. The inhibitory effects of cicaprost on PDGF-induced increases in [3H]-thymidine incorporation, cell number and cAMP production were significant (P<0.05) in VSMCs from wild-type mice. Each point shows the mean value with s.e.mean shown by vertical bars. *P<0.05 vs wild-type mice. The numbers in parenthesis indicate n.
Figure 6.
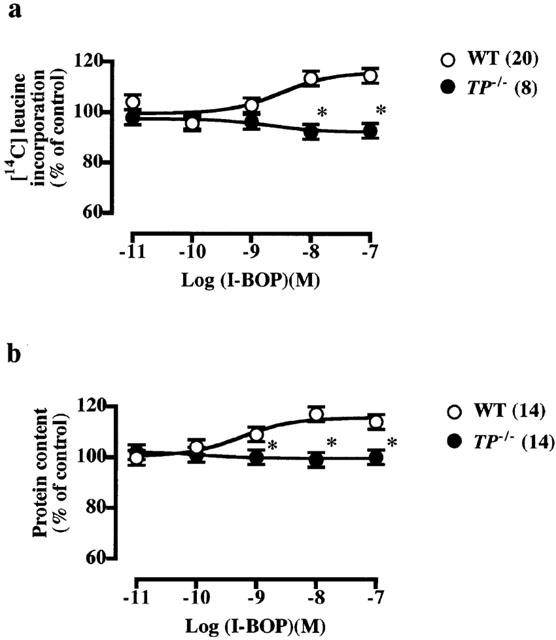
Stimulatory effects of I-BOP on [14C]-leucine incorporation (a) and protein content (b) in PDGF-stimulated VSMCs from wild-type and TP−/− mice. The control values in the VSMCs from wild-type and TP−/− mice stimulated with PDGF (10 ng ml−1) alone were 287±5 (n=12) and 245±8 (n=19) c.p.m. for [14C]-leucine incorporation, respectively, and 17±1 (n=43) and 19±5 (n=12)% increase in protein content, respectively. The values were expressed as percentages of these control values. The stimulatory effects of I-BOP on PDGF-induced increases in [14C]-leucine incorporation and protein content were significant (P<0.05) in VSMCs from wild-type mice. Each point shows the mean value with s.e.mean shown by vertical bars. *P<0.05 vs wild-type mice. The numbers in parenthesis indicate n.
Results
Expression of mRNAs for the prostanoid receptors in VSMCs
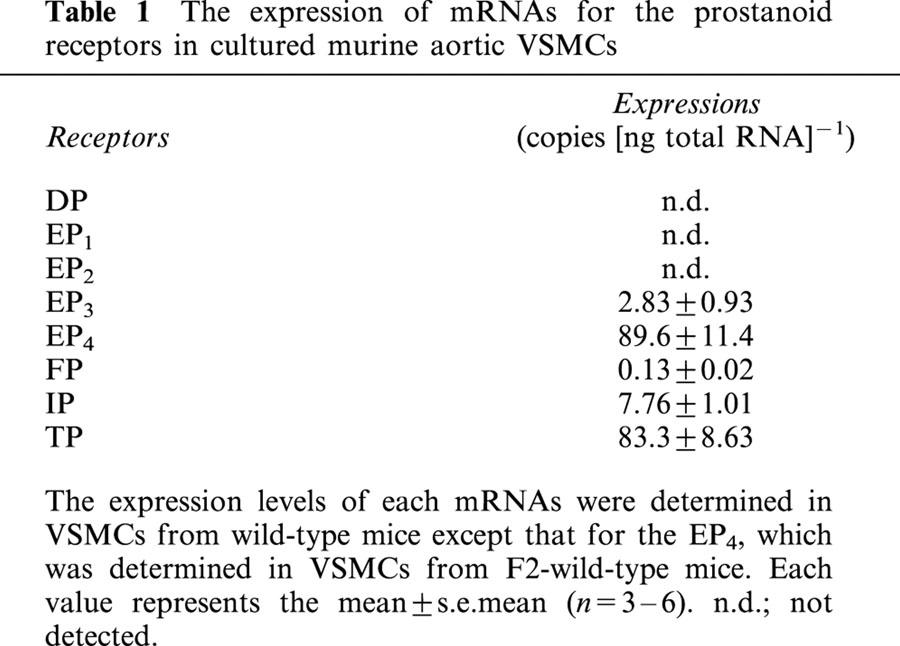
We examined which types and subtypes of the prostanoid receptors were expressed in murine VSMCs using the RT–PCR method. As shown in Figure 1, mRNAs for the EP3, EP4, TP, IP and FP were found to be expressed in VSMCs from wild-type mice. Those for the EP1, EP2 and DP, however, could not be detected. Next, we compared the expression levels of mRNAs for these receptors using a competitive RT–PCR method (Table 1). The expressions of mRNAs for the EP4 and TP were most abundant followed by those for the IP and EP3, and that for the FP was almost negligible. We could not detect the expression of mRNAs for each prostanoid receptor in VSMCs from respective knockout mice (data not shown). These results indicate that the EP4, TP, IP and EP3 may participate in the prostanoid-mediated regulation of proliferation and/or hypertrophy of VSMCs.
Figure 1.

The expression of mRNAs for the eight types and subtypes of the prostanoid receptors.
Table 1.
The expression of mRNAs for the prostanoid receptors in cultured murine aortic VSMCs
Effects of the prostanoids on the hypertrophy and proliferation of VSMCs derived from wild-type mice
We first examined the effects of the prostanoids and their analogues on both the cell proliferation and hypertrophy of the VSMCs from wild-type mice in a basic culture media containing a low concentration of insulin (10−6 M). The used ligands include PGD2, PGE2, the specific agonists for each EP subtype (DI-004, AE1-259, AE-248 and AE1-329 for the EP1, EP2, EP3 and EP4, respectively), PGF2α, cicaprost and I-BOP. All of these ligands alone, however, showed little effects, if any, on the proliferation or hypertrophy of VSMCs except I-BOP (data not shown). When I-BOP was administered alone, it showed a tendency to increase [14C]-leucine incorporation, although the effect was statistically not significant (112±3% of control values, n=4, P=0.82).
We next examined the effects of these ligands on the PDGF-stimulated proliferation and hypertrophy of VSMCs from wild-type mice. PDGF induced the proliferation and hypertrophy of the VSMCs concentration-dependently with both showing half-maximal responses at 1.5 ng ml−1 of PDGF, and plateauing at 50 ng ml−1 of PDGF. When stimulated with 10 ng ml−1 of PDGF, [3H]-thymidine incorporation and cell numbers as percentages of those for the controls in the absence of PDGF were 351±28 (n=112) and 138±6 (n=32)%, respectively. Those for [14C]-leucine incorporation and protein content were 157±3 (n=105) and 117±1 (n=43)%, respectively. The values for [3H]-thymidine and [14C]-leucine incorporation in VSMCs from F2-wild-type mice and mice lacking the prostanoid receptors individually were not significantly different from those in VSMCs from wild-type mice (data are presented in Legends for figures), showing that there are no difference in the sensitivity to PDGF among these VSMCs.
Among the ligands used, PGD2, PGE2, AE1-329 and cicaprost showed significant inhibitory effects on the PDGF (10 ng ml−1)-induced increases in [3H]-thymidine incorporation (Table 2). We examined if the inhibitory effects of these ligands on cell proliferation are derived from their induction of cell death. The proportion of dead cells consisting of both apoptotic and necrotic cells, when treated with each of the ligands, was below 3%, which was similar with that found in control cells, suggesting no effects of these ligands on cell death.
In contrast, I-BOP potentiated significantly the PDGF-induced increase in [14C]-leucine incorporation without affecting the PDGF-induced increases in [3H]-thymidine incorporation. DI-004, AE1-259, AE-248 and PGF2α did not show significant effects on the PDGF-induced increase in [3H]-thymidine and [14C]-leucine incorporation.
The inhibitory effects of AE1-329 and PGE2 on PDGF-induced proliferation of VSMCs
AE1-329, a specific EP4 agonist, showed concentration-dependent inhibition of the PDGF-induced increase in [3H]-thymidine incorporation (Figure 2a) and cell number (Figure 2b) in VSMCs from F2-wild-type mice without affecting the [14C]-leucine incorporation (Table 2). These inhibitory effects of AE1-329 disappeared completely in VSMCs from EP4−/− mice, suggesting that the EP4 mediates the inhibitory effects of PGE2. Indeed, the concentration-dependent inhibitory effects of PGE2 on the PDGF-induced increase in [3H]-thymidine incorporation (Figure 3a) and cell number (Figure 3b) again disappeared in VSMCs from EP4−/− mice, indicating that the EP4 is a major receptor mediating the inhibitory effect of PGE2 on PDGF-induced proliferation of VSMCs.
The inhibitory effect of cicaprost on PDGF-induced proliferation of VSMCs
Cicaprost, an IP agonist, inhibited concentration-dependently the PDGF-induced increase in [3H]-thymidine incorporation (Figure 4a) and cell number (Figure 4b) in VSMCs from wild-type mice without affecting the [14C]-leucine incorporation (Table 2). These inhibitory effects of cicaprost disappeared completely in VSMCs from IP−/− mice (Figure 4a,b), indicating that they are mediated by the IP.
The stimulatory effects of AE1-329, PGE2 and cicaprost on cAMP production in VSMCs
AE1-329, PGE2 and cicaprost stimulated cAMP production concentration-dependently (Figures 2c, 3c, 4c). The stimulatory effects of AE1-329 and PGE2 on adenylate cyclase disappeared in VSMCs from EP4−/− mice, and that of cicaprost disappeared in VSMCs from IP−/− mice, suggesting that the inhibitory effects of these ligands on cell proliferation are mediated by their stimulatory actions on cAMP production.
The inhibitory effect of PGD2 on PDGF-induced proliferation of VSMCs
PGD2 (10−5 M) inhibited the PDGF-induced increase in [3H]-thymidine incorporation in VSMCs from both wild-type and DP−/− mice to a similar extent (Figure 5a), indicating that the action of PGD2 is not mediated by the DP. This result is in accordance with the fact that the inhibitory effect of PGD2 on PDGF-induced [3H]-thymidine incorporation was apparent only at 10−5 M or higher concentrations of PGD2, because DP-mediated effects of PGD2 are usually observed at nM order concentrations. Next, we examined the effects of PGD2 on the PDGF-induced increase in [3H]-thymidine incorporation in VSMCs from EP4−/− or IP−/− mice, because only these two receptors along with the DP mediate the inhibitory effects of the prostanoids (Table 2), and because PGD2 may act on these receptors to induce its action. As shown in Figure 5b, however, this inhibitory effect of PGD2 was also observed in VSMCs from EP4−/− andIP−/− mice, showing that the effect is not derived from actions of PGD2 on the EP4 or IP.
Figure 5.
Inhibitory effects of PGD2 on PDGF-stimulated increase in [3H]-thymidine incorporation in VSMCs from wild-type and DP−/− (a), and EP4−/− and IP−/− (b) mice. The concentration of PGD2 used was 10−5 M. The control value in the VSMCs from DP−/− mice stimulated with PDGF (10 ng ml−1) alone was 4557±695 (n=20) c.p.m. The values were expressed as percentages of the respective control values in the VSMCs from wild-type, DP−/−, EP4−/− and IP−/− mice. Each column shows the mean value with s.e.mean shown by vertical bars. There was no significant difference in the degree of inhibition by PGD2 among the four groups of VSMCs. The numbers in parenthesis indicate n.
The potentiating effect of I-BOP on PDGF-induced hypertrophy of VSMCs
I-BOP, a TP agonist, showed concentration-dependent potentiation of the PDGF-induced increase in [14C]-leucine incorporation (Figure 6a) and protein content (Figure 6b) in VSMCs from wild-type mice. These potentiating effects of I-BOP disappeared completely in VSMCs from TP−/− mice (Figure 6), indicating that they are mediated by the TP. In contrast, I-BOP (10−11 to 10−7 M) did not affect PDGF-induced increase in [3H]-thymidine incorporation and cell number (data not shown).
Discussion
Among the expression levels of mRNAs for the four subtypes of the EPs, mRNA for the EP4 was most abundant, and that for the EP3 was slight (Table 1). The EP4 has been known to couple to Gs and to stimulate adenylate cyclase, leading to a rise in cytosolic cAMP level (Narumiya et al., 1999). Recently, a role of PGE2 via the EP4 in the vascular system has been reported to be in the regulation of a closure of the ductus arteriosus after birth (Nguyen et al., 1997; Segi et al., 1998). However, the action of PGE2 via the EP4 on the hypertrophy or proliferation of VSMCs was not known. The present study clearly demonstrated that the signalling via the EP4 leads to an inhibition of the PDGF-induced proliferation of VSMCs possibly through the increase in cAMP concentration as suggested in rat VSMCs (Indolfi et al., 1997). Moreover, it was demonstrated for the first time that PGE2 itself showed an inhibitory effect on the PDGF-induced proliferation of VSMCs via the EP4. On the other hand, AE-248, a specific EP3 agonist, failed to affect both the hypertrophy and proliferation of VSMCs either in the presence or absence of PDGF; this may be due to the low expression level of the EP3 in VSMCs (Table 1).
PGI2 and its analogues have been reported to inhibit growth factor- or serum-induced proliferation of VSMCs (Morisaki et al., 1988; Koh et al., 1993; Wharton et al., 2000; Clapp et al., 2002). This inhibitory effect on the proliferation of VSMCs has been utilized for the treatment of patients with primary pulmonary hypertension. In this disease, however, other non-vasodilatory effects of PGI2 analogues, such as those on the hypertrophy of VSMCs and/or matrix production, may also have a role. Furthermore, PGI2 synthase, when transfected into the rat carotid artery, suppressed neointima formation induced by balloon-injury (Todaka et al., 1999), suggesting therapeutic potency of PGI2 for the vascular remodeling. It was not known, however, whether the inhibitory effect of PGI2 on the proliferation of VSMCs is mediated by the IP, because there has been no known antagonist for the IP. In the present study, it was clearly shown that the inhibitory effect of PGI2 on the proliferation of VSMCs is indeed mediated by the IP. The potency of cicaprost in inhibition of cell proliferation and in stimulation of cAMP production, however, is lower than that obtained from the examination using the cloned murine IP expressed in Chinese hamster ovary cells (Namba et al., 1994). This difference in potency may be derived from the difference of cell types examined, because some investigators have also reported low potency of IP agonists (EC50 values of over 10−6 M) in growth inhibition of VSMCs (Morisaki et al., 1988; Uehara et al., 1988). Another possibility may include the mechanism of rapid receptor desensitization as suggested in platelets (Jaschonek et al., 1988).
PGD2 showed potent inhibitory effects on PDGF-induced [3H]-thymidine incorporation. Similar effects of PGD2, however, were found on VSMCs derived from DP−/− mice, suggesting that this action was not derived from its action on the DP. Moreover, this action of PGD2 was observed in EP4−/− and IP−/− mice, excluding the possibility of the cross-action of PGD2 on the EP4 or IP. On the other hand, PGJ2 and its derivatives, which were produced in the process of the degradation of PGD2, have been reported to inhibit the growth of VSMCs through the binding to a nuclear receptor, peroxisome proliferator-activated receptor γ (Wakino et al., 2000; Narumiya et al., 1986). The present results, therefore, along with the reported data suggest that the potent inhibitory effect of PGD2 on the proliferation of VSMCs may be derived from the action of its degradation products on the peroxisome proliferator-activated receptor γ.
Previous reports have suggested that the TP agonists including I-BOP can increase [3H]-thymidine incorporation and cell proliferation in rat aorta-derived cultured VSMCs (Hanasaki et al., 1990). In some studies, TXA2 mimetics, such as U-46619, CTA2 and I-BOP, can stimulate proliferation of VSMCs, partially doing so by upregulating the synthesis and release of endogenous growth factors such as PDGF or basic fibroblast growth factor, and by a synergistic action with growth factors in intracellular signalling via the MAP kinase pathway (Craven et al., 1996; Grosser et al., 1997; Zucker et al., 1998). In murine VSMCs, we were unable to confirm these observations of a proliferative effect of I-BOP. In contrast, we found that I-BOP, like angiotensin II (Geisterfer et al., 1988), stimulates protein synthesis of VSMCs without stimulating the cell proliferation. Several investigators have also reported a similar action of a TP agonist on cultured rat VSMCs (Dorn II et al., 1992; Craven et al., 1996). Therefore, whether stimulation of the TP leads to either proliferation or hypertrophy of VSMCs may depend on the experimental conditions, though species difference may be present between rat and mouse.
PGF2α has been reported to show hypertrophic effect on cultured rat VSMCs (Dorn II et al., 1992; Rao et al., 1999); however, we were unable to find this effect of PGF2α in the present study using murine VSMCs. Hypertrophic actions of PGF2α were also reported in cultured skeletal muscle (Vandenburgh et al., 1990) and in cultured neonatal rat cardiomyocytes (Adams et al., 1996). In rat cardiomyocytes, PGF2α was suggested to induce the hypertrophy via the Gq-cJun NH2-terminal kinase pathway (Adams et al., 1998), whereas, in murine cardiomyocytes, PGF2α lacked the hypertrophic action and could not activate the Gq protein (Hilal-Dandan et al., 2000; Deng et al., 2000), suggesting species difference in the signalling of the FP in the cardiomyocytes between the rat and mouse. Our result suggest that the species difference in VSMCs may also be present between the rat and mouse. Although the precise biochemical mechanism of the difference would remain to be clarified, one explanation for the lack of the effect of PGF2α may be due to the very low expression level of FP mRNA in murine VSMCs.
In addition to the species difference of VSMCs in responses to the prostanoids, the receptors regulating pro- or anti-proliferative actions of the prostanoids are likely to differ in different blood vessels and even in different segments of the same vessel (Archer, 1996; Wharton et al., 2000). In this mean, there may need some caution to apply present results derived from cultured aortic VSMCs to other VSMCs from different vessels.
In conclusion, the inhibitory effects of PGE2 and PGI2 on proliferation via the EP4 and IP, respectively, and the potentiating effect of TXA2 on hypertrophy of VSMCs via the TP were verified in this study using VSMCs derived from knockout mice. These prostanoids may play important roles in the pathogenesis of vascular diseases such as pulmonary hypertension, atherosclerosis and vascular remodelling, in which the growth of VSMCs is a major phenotype.
Acknowledgments
We thank K. Nakaya and T. Yokoyama for help in breeding and maintenance of mice, and T. Nakamura for secretarial assistance. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan, and by the Research Grant for Cardiovascular Disease (11C-1) from the Ministry of Health and Welfare. This work was also supported by grants from Ono Pharmaceutical Co., the Uehara Memorial Foundation and the Smoking Research Foundation.
Abbreviations
- CTA2
carbocyclic thromboxane A2
- I-BOP
1S-[1α,2β(5Z),3α(1E,3S),4α] -7-[3-(hydroxy-4-(p-iodophenoxy)-1-butenyl)-7-oxabicyclo-[2.2.1] hept-2-yl]-5′-heptenoic acid
- U-46619
(15S)-hydroxy-11α, 9α(epoxymethano)prosta-5Z,13E-dienoic acids
- VSMC
vascular smooth muscle cell
References
- ABRAMOVITZ M., ADAM M., BOIE Y., CARRIERE M., DENIS D., GODBOUT C., LAMONTAGNE S., ROCHETTE C., SAWYER N., TREMBLAY N.M., BELLEY M., GALLANT M., DUFRESNE C., GAREAU Y., RUEL R., JUTEAU H., LABELLE M., OUIMET N., METTERS K.M. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim. Biophys. Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- ADAMS J.W., MIGITA D.S., YU M.K., YOUNG R., HELLICKSON M.S., CASTRO- VARGAS F.E., DOMINGO J.D., LEE P.H., BUI J.S., HENDERSON S.A. Prostaglandin F2α stimulates hypertrophic growth of cultured neonatal rat ventricular myocytes. J. Biol. Chem. 1996;271:1179–1186. doi: 10.1074/jbc.271.2.1179. [DOI] [PubMed] [Google Scholar]
- ADAMS J.W., SAH V.P., HENDERSON S.A., BROWN J.H. Tyrosine kinase and c-Jun NH2-terminal kinase mediate hypertrophic responses to prostaglandin F2α in cultured neonatal rat ventricular myocytes. Circ. Res. 1998;83:167–178. doi: 10.1161/01.res.83.2.167. [DOI] [PubMed] [Google Scholar]
- ARCHER S.L. Diversity of phenotype and function of vascular smooth muscle cells. J. Lab. Clin. Med. 1996;127:524–529. doi: 10.1016/s0022-2143(96)90142-0. [DOI] [PubMed] [Google Scholar]
- CLAPP L.H., FINNEY P., TURCATO S., TRAN S., RUBIN L.J., TINKER A. Differential effects of stable prostacyclin analogs on smooth muscle proliferation and cyclic AMP generation in human pulmonary artery. Am. J. Resp. Cell Mol. Biol. 2002;26:194–201. doi: 10.1165/ajrcmb.26.2.4695. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., GRIX S.P., HEAD S.A., LOUTTIT J.B., MALLETT A., SHELDRICK R.L.G. A novel inhibitory prostanoid receptor in piglet saphenous vein. Prostaglandins. 1994;47:151–168. doi: 10.1016/0090-6980(94)90084-1. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., KENNEDY I., HUMPHREY P.P.A., BUNCE K., LUMLEY P.Prostanoids and their receptors Comprehensive Medicinal Chemistry Vol. 3: Membranes & Receptors 1990Oxford: Pergamon Press; 643–714.ed. Emmett, J.C [Google Scholar]
- CRAVEN P.A., STUDER R.K., DERUBERTIS F.R. Thromboxane/prostaglandin endoperoxide-induced hypertrophy of rat vascular smooth muscle cells is signaled by protein kinase C-dependent increases in transforming growth factor-beta. Hypertension. 1996;28:169–176. doi: 10.1161/01.hyp.28.2.169. [DOI] [PubMed] [Google Scholar]
- DENG X.-F., ROKOSH D.G., SIMPSON P.C. Autonomous and growth factor-induced hypertrophy in cultured neonatal mouse cardiac myocytes. Circ. Res. 2000;87:781–788. doi: 10.1161/01.res.87.9.781. [DOI] [PubMed] [Google Scholar]
- DORN G.W., II, BECKER M.W., DAVIS M.G. Dissociation of the contractile and hypertrophic effects of vasoconstrictor prostanoids in vascular smooth muscle. J. Biol. Chem. 1992;267:24897–24905. [PubMed] [Google Scholar]
- DUNNETT C.W. A multiple comparison procedure for comparing several treatments with a control. Am. J. Stat. Assn. 1955;50:1096–1121. [Google Scholar]
- GEISTERFER A.A.T., PEACH M.J., OWENS G.K. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ. Res. 1988;62:749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- GROSSER T., ZUCKER T.-P., WEBER A.-A., SCHULTE K., SACHINIDIS A., VETTER H., SCHRÖR K. Thromboxane A2 induces cell signaling but requires platelet-derived growth factor to act as a mitogen. Eur. J. Pharmacol. 1997;319:327–332. doi: 10.1016/s0014-2999(96)00860-6. [DOI] [PubMed] [Google Scholar]
- HANASAKI K., NAKANO T., ARITA H. Receptor-mediated mitogenic effect of thromboxane A2 in vascular smooth muscle cells. Biochem. Pharmacol. 1990;40:2535–2542. doi: 10.1016/0006-2952(90)90096-4. [DOI] [PubMed] [Google Scholar]
- HILAL-DANDAN R., KANTER J.R., BRUNTON L.L. Characterization of G- protein signaling in ventricular myocytes from the adult mouse heart: differences from the rat. J. Mol. Cell Cardiol. 2000;32:1211–1221. doi: 10.1006/jmcc.2000.1156. [DOI] [PubMed] [Google Scholar]
- HIZAKI H., SEGI E., SUGIMOTO Y., HIROSE M., SAJI T., USHIKUBI F., MATSUOKA T., NODA Y., TANAKA T., YOSHIDA N., NARUMIYA S., ICHIKAWA A. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP2. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10501–10506. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTNER J.J., GWEBU E.T., PANGANAMALA R.V., MILO G.E., CORNWELL D.G., SHARMA H.M., GEER J.C. Fatty acids and their prostaglandin derivatives: inhibitors of proliferation in aortic smooth muscle cells. Science. 1977;197:289–291. doi: 10.1126/science.877555. [DOI] [PubMed] [Google Scholar]
- INDOLFI C., AVVEDIMENTO E.V., DI LORENZO E., ESPOSITO G., RAPACCIUOLO A., GIULIANO P., GRIECO D., CAVUTO L., STINGONE A.M., CIULLO I., CONDORELLI G., CHIARIELLO M. Activation of cAMP-PKA signaling in vivo inhibits smooth muscle cell proliferation induced by vascular injury. Nat. Med. 1997;3:775–779. doi: 10.1038/nm0797-775. [DOI] [PubMed] [Google Scholar]
- JASCHONEK K., FAUL C., SCHMIDT H., RENN W. Desensitization of platelets to iloprost: Loss of specific binding sites and heterologous desensitization of adenylate cyclase. Eur. J. Pharmacol. 1988;147:187–196. doi: 10.1016/0014-2999(88)90777-7. [DOI] [PubMed] [Google Scholar]
- KIRIYAMA M., USHIKUBI F., KOBAYASHI T., HIRATA M., SUGIMOTO Y., NARUMIYA S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOH E., MORIMOTO S., JIANG B., INOUE T., NABATA T., KITANO S., YASUDA O., FUKUO K., OGIHARA T. Effects of beraprost sodium, a stable analogue of prostacyclin, on hyperplasia, hypertrophy and glycosaminoglycan synthesis of rat aortic smooth muscle cells. Artery. 1993;20:242–252. [PubMed] [Google Scholar]
- LIBBY P., O'BRIEN K.V. Culture of quiescent arterial smooth muscle cells in a defined serum-free medium. J. Cell Physiol. 1983;115:217–223. doi: 10.1002/jcp.1041150217. [DOI] [PubMed] [Google Scholar]
- LOESBERG C., VAN WIJK R., ZANDBERGEN J., VAN AKEN W.G., VAN MOURIK J.A., DE GROOT P.G. Cell cycle-dependent inhibition of human vascular smooth muscle cell proliferation by prostaglandin E1. Exp. Cell Res. 1985;160:117–125. doi: 10.1016/0014-4827(85)90241-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSENBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MA H., HARA A., XIAO C.-Y., OKADA Y., TAKAHATA O., NAKAYA K., SUGIMOTO Y., ICHIKAWA A., NARUMIYA S., USHIKUBI F. Increased bleeding tendency and decreased susceptibility to thromboembolism in mice lacking the prostaglandin E receptor subtype EP3. Circulation. 2001;104:1176–1180. doi: 10.1161/hc3601.094003. [DOI] [PubMed] [Google Scholar]
- MATSUOKA T., HIRATA M., TANAKA H., TAKAHASHI Y., MURATA T., KABASHIMA K., SUGIMOTO Y., KOBAYASHI T., USHIKUBI F., AZE Y., EGUCHI N., URADE Y., YOSHIDA N., KIMURA K., MIZOGUCHI A., HONDA Y., NAGAI H., NARUMIYA S. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013–2017. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- MCMURRAY H.F., PARROTT D.P., BOWYER D.E. A standardised method of culturing aortic explants, suitable for the study of factors affecting the phenotypic modulation, migration and proliferation of aortic smooth muscle cells. Atherosclerosis. 1991;86:227–237. doi: 10.1016/0021-9150(91)90219-s. [DOI] [PubMed] [Google Scholar]
- MORINELLI T.A., ZHANG L.-M., NEWMAN W.H., MEIER K.E. Thromboxane A2/prostaglandin H2-stimulated mitogenesis of coronary artery smooth muscle cells involves activation of mitogen-activated protein kinase and S6 kinase. J. Biol. Chem. 1994;269:5693–5698. [PubMed] [Google Scholar]
- MORISAKI N., KANZAKI T., MOTOYAMA N., SAITO Y., YOSHIDA S. Cell cycle-dependent inhibition of DNA synthesis by prostaglandin I2 in cultured rabbit aortic smooth muscle cells. Atherosclerosis. 1988;71:165–171. doi: 10.1016/0021-9150(88)90140-2. [DOI] [PubMed] [Google Scholar]
- MURATA T., USHIKUBI F., MATSUOKA T., HIRATA M., YAMASAKI A., SUGIMOTO Y., ICHIKAWA A., AZE Y., TANAKA T., YOSHIDA N., UENO A., OH-ISHI S., NARUMIYA S. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- NAMBA T., OIDA H., SUGIMOTO Y., KAKIZUKA A., NEGISHI M., ICHIKAWA A., NARUMIYA S. cDNA cloning of a mouse prostacyclin receptor; multiple signalling pathways and expression in thymic medulla. J. Biol. Chem. 1994;269:9986–9992. [PubMed] [Google Scholar]
- NARUMIYA S., OHNO K., FUJIWARA M., FUKUSHIMA M. Site and mechanism of growth inhibition by prostaglandins. II. Temperature-dependent transfer of a cyclopentenone prostaglandin to nuclei. J. Pharmacol. Exp. Ther. 1986;239:506–511. [PubMed] [Google Scholar]
- NARUMIYA S., SUGIMOTO Y., USHIKUBI F. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- NGUYEN M., CAMENISCH T., SNOUWAERT J.N., HICKS E., COFFMAN T.M., ANDERSON P.A., MALOUF N.N., KOLLER B.H. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature. 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- NILSSON J., OLSSON A.G. Prostaglandin E1 inhibits DNA synthesis in arterial smooth muscle cells stimulated with platelet-derived growth factor. Atherosclerosis. 1984;53:77–82. doi: 10.1016/0021-9150(84)90107-2. [DOI] [PubMed] [Google Scholar]
- OKADA Y., HARA A., MA H., XIAO C.-Y., TAKAHATA O., KOHGO Y., NARUMIYA S., USHIKUBI F. Characterization of prostanoid receptors mediating contraction of the gastric fundus and ileum: studies using mice deficient in prostanoid receptors. Br. J. Pharmacol. 2000;131:745–755. doi: 10.1038/sj.bjp.0703627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OREKHOV A.N., TERTOV V.V., KUDRYASHOV S.A., KHASHIMOV K.A., SMIRNOV V.N. Primary culture of human aortic intima cells as a model for testing antiatherosclerotic drugs. Effects of cyclic AMP, prostaglandins, calcium antagonists, antioxidants, and lipid-lowering agents. Atherosclerosis. 1986;60:101–110. doi: 10.1016/0021-9150(86)90002-x. [DOI] [PubMed] [Google Scholar]
- PASRICHA P.J., HASSOUN P.M., TEUFEL E., LANDMAN M.J., FANBURG B.L. Prostaglandins E1 and E2 stimulate the proliferation of pulmonary artery smooth muscle cells. Prostaglandins. 1992;43:5–19. doi: 10.1016/0090-6980(92)90060-7. [DOI] [PubMed] [Google Scholar]
- RAO G.N., MADAMANCHI N.R., LELE M., GADIPARTHI L., GINGRAS A.-C., ELING T.E., SONENBERG N. A potential role for extracellular signal- regulated kinases in PGF2α-induced protein synthesis in smooth muscle cells. J. Biol. Chem. 1999;274:12925–12932. doi: 10.1074/jbc.274.18.12925. [DOI] [PubMed] [Google Scholar]
- SACHINIDIS A., FLESCH M., KO Y., SCHRÖR K., BÖHM M., DÜSING R., VETTER H. Thromboxane A2 and vascular smooth muscle cell proliferation. Hypertension. 1995;26:771–780. doi: 10.1161/01.hyp.26.5.771. [DOI] [PubMed] [Google Scholar]
- SEGI E., SUGIMOTO Y., YAMASAKI A., AZE Y., OIDA H., NISHIMURA T., MURATA T., MATSUOKA T., USHIKUBI F., HIROSE M., TANAKA T., YOSHIDA N., NARUMIYA S., ICHIKAWA A. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem. Biophys. Res. Commun. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- SUGIMOTO Y., YAMASAKI A., SEGI E., TSUBOI K., AZE Y., NISHIMURA T., OIDA H., YOSHIDA N., TANAKA T., KATSUYAMA M., HASUMOTO K., MURATA T., HIRATA M., USHIKUBI F., NEGISHI M., ICHIKAWA A., NARUMIYA S. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277:681–683. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- SUZAWA T., MIYAURA C., INADA M., MARUYAMA T., SUGIMOTO Y., USHIKUBI F., ICHIKAWA A., NARUMIYA S., SUDA T. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology. 2000;141:1554–1559. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]
- TODAKA T., YOKOYAMA C., YANAMOTO H., HASHIMOTO N., NAGATA I., TSUKAHARA T., HARA S., HATAE T., MORISHITA R., AOKI M., OGIHARA T., KANEDA Y., TANABE T. Gene transfer of human prostacyclin synthase prevents neointimal formation after carotid balloon injury in rats. Stroke. 1999;30:419–426. doi: 10.1161/01.str.30.2.419. [DOI] [PubMed] [Google Scholar]
- UEHARA Y., ISHIMITSU T., KIMURA K., ISHII M., IKEDA T., SUGIMOTO T. Regulatory effects of eicosanoids on thymidine uptake by vascular smooth muscle cells of rats. Prostaglandins. 1988;36:847–857. doi: 10.1016/0090-6980(88)90061-5. [DOI] [PubMed] [Google Scholar]
- USHIKUBI F., HIRATA M., NARUMIYA S. Molecular biology of prostanoid receptors; an overview. J. Lipid Mediat. Cell Signal. 1995;12:343–359. doi: 10.1016/0929-7855(95)00022-i. [DOI] [PubMed] [Google Scholar]
- USHIKUBI F., SEGI E., SUGIMOTO Y., MURATA T., MATSUOKA T., KOBAYASHI T., HIZAKI H., TUBOI K., KATSUYAMA M., ICHIKAWA A., TANAKA T., YOSHIDA N., NARUMIYA S. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- VANDENBURGH H.H., HATFALUDY S., SOHAR I., SHANSKY J. Stretch-induced prostaglandins and protein turnover in cultured skeletal muscle. Am. J. Physiol. 1990;259:C232–C240. doi: 10.1152/ajpcell.1990.259.2.C232. [DOI] [PubMed] [Google Scholar]
- WAKINO S., KINTSCHER U., KIM S., YIN F., HSUEH W.A., LAW R.E. Peroxisome proliferator-activated receptor gamma ligands inhibit retinoblastoma phosphorylation and G1→S transition in vascular smooth muscle cells. J. Biol. Chem. 2000;275:22435–22441. doi: 10.1074/jbc.M910452199. [DOI] [PubMed] [Google Scholar]
- WHARTON J., DAVIE N., UPTON P.D., YACOUB M.H., POLAK J.M., MORRELL N.W. Prostacyclin analogues differentially inhibit growth of distal and proximal human pulmonary artery smooth muscle cells. Circulation. 2000;102:3130–3136. doi: 10.1161/01.cir.102.25.3130. [DOI] [PubMed] [Google Scholar]
- ZHU Z., ZHANG S.H., WAGNER C., KURTZ A., MAEDA N., COFFMAN T., ARENDSHORST W.J. Angiotensin AT1B receptor mediates calcium signaling in vascular smooth muscle cells of AT1A receptor-deficient mice. Hypertension. 1998;31:1171–1177. doi: 10.1161/01.hyp.31.5.1171. [DOI] [PubMed] [Google Scholar]
- ZUCKER T.-Ph., BÖNISCH D., MUCK S., WEBER A.-A., BRETSCHNEIDER E., GLUSA E., SCHRÖR K. Thrombin-induced mitogenesis in coronary artery smooth muscle cells is potentiated by thromboxane A2 and involves upregulation of thromboxane receptor mRNA. Circulation. 1998;97:589–595. doi: 10.1161/01.cir.97.6.589. [DOI] [PubMed] [Google Scholar]


