Abstract
Neuropeptide Y (NPY) has been shown to suppress synaptic excitation in rat hippocampus by a presynaptic action. The Y2 (Y2R) and the Y5 (Y5R) receptors have both been implicated in this action. We used the non-peptide, Y2R-selective antagonist, BIIE0246, to test the hypothesis that the Y2R mediates both the presynaptic inhibitory and anti-epileptic actions of NPY in rat hippocampus in vitro.
NPY and the Y2R-selective agonist, [ahx5-24]NPY, both inhibited the population excitatory postsynaptic potential (pEPSP) evoked in area CA1 by stratum radiatum stimulation in a concentration-dependent manner. BIIE0246 suppressed the inhibitory effects of both agonists, suppressing the maximal inhibition without causing a change in the agonist EC50, in a manner inconsistent with competitive antagonism.
BIIE0246 washed out from hippocampal slices extremely slowly. Application of agonist at high concentrations (1–3 μM) for prolonged periods did not alter the rate of washout, but did partially overcome the antagonism, inconsistent with an insurmountable antagonism by BIIE0246.
In the stimulus train-induced bursting (STIB) model of ictal activity in hippocampal slices, both NPY and [ahx5-24]NPY inhibited primary afterdischarge (1°AD) activity. BIIE0246 (100 nM) completely suppressed the actions of NPY and [ahx5-24]NPY in this model. In contrast, the potent Y5R-selective agonist, Ala31Aib32NPY, affected neither 1°AD activity in the presence of BIIE0246, nor, by itself, even the pEPSP in CA1.
BIIE0246 potently suppresses NPY actions in rat hippocampus, suggesting a dominant role for Y2R there. The apparently insurmountable antagonism observed may result from the lipophilic nature of the antagonist.
Keywords: Neuropeptide Y, Y2 receptor, Y5 receptor, BIIE0246, STIB, epilepsy, hippocampus, presynaptic inhibition
Introduction
Neuropeptide Y (NPY) is widely distributed in the central and peripheral nervous system, and acts through at least six receptor subtypes (Y1–Y6), five of which have been identified structurally. All known NPY receptors belong to the G-protein-coupled receptor superfamily (Michel et al., 1998). The effects of NPY on neuronal activity have been studied in several brain regions, including the hippocampus, hypothalamus and thalamus (Colmers & Bleakman, 1994; Ho et al., 2000; Pronchuk et al., 2002; Sun & Miller, 1999). Extensive electrophysiological and pharmacological studies have demonstrated that NPY negatively modulates excitatory synaptic transmission in the hippocampus (Colmers et al., 1985; 1991; Klapstein & Colmers, 1992; Greber et al., 1994; McQuiston & Colmers, 1996). This action results from a reduction in glutamate release from presynaptic nerve terminals (McQuiston & Colmers, 1996) mediated by the suppression of voltage-dependent Ca2+ influx (Qian et al., 1997) NPY's actions in rat hippocampal area CA1 are selective, as it does not affect GABAergic inhibition there (Colmers et al., 1988). It has been postulated that the presynaptic inhibitory effect of NPY is mediated by the activation of the neuropeptide Y Y2 (Y2R) receptor subtype (Colmers et al., 1991; Greber et al., 1994).
The pharmacological profile of the Y2R is based on its high affinity for C-terminal fragments of NPY such as NPY2-36 and NPY13-36 (Gerald et al., 1995). However, more recently, studies revealed that many of these agonists also possess a significant affinity for the NPY Y5 receptor (Y5R; Gerald et al., 1996). Thus, conclusions regarding receptor subtype identity based exclusively on the actions of such agonists must be viewed with caution. More recently, however, unequivocally selective agonists and antagonists have been developed for several of the NPY receptors, including the Y2R.
For example, a centrally truncated agonist, [ahx5-24]NPY has recently been shown to be highly selective for the Y2R (Cabrele & Beck-Sickinger, 2000). Furthermore, a selective non-peptide antagonist, BIIE0246 ((S)-N2-[[1-[2-[4-[(R,S)-5,11-dihydro-6(6h)-oxodibenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxoethyl]cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5[4H)-dioxo-1,2 -diphyenl-3H-1,2,4-triazol-4-yl]ethyl]argininamide) was reported to be the first potent and selective non-peptide NPY Y2R antagonist (Doods et al., 1999). BIIE0246 has a high affinity for the Y2R (IC50=4 nM; Weiser et al., 2000), and displayed no apparent effect on neuropeptide Y Y1, Y4 or Y5 receptor subtypes (Dumont et al., 2000). Furthermore, 1 μM BIIE0246 antagonized the inhibitory effect of 300 nM NPY on the population spike evoked in hippocampal slices (Weiser et al., 2000). By contrast, the peptide, T4-[neuropeptide Y 33-36)]4, which was also claimed to be a Y2R antagonist (Grouzmann et al., 1997), was subsequently shown to have poor potency (Pheng et al., 1999), being 100 fold less potent than BIIE0246 (Doods et al., 1999).
The aim of the present study is to test the hypothesis that the receptor involved in the NPY-mediated inhibition of excitatory transmission in rat hippocampal area CA1 and CA3 is indeed the Y2R. We studied the effect of BIIE0246 against the concentration-dependent actions of NPY and a Y2R-selective and a Y5R-selective agonist in the hippocampal slice in vitro. The evidence is consistent with the predominance of the Y2R in this preparation, but also highlights some peculiar properties of the antagonist.
Methods
Slice preparation
Male Sprague-Dawley rats (18–34 days) were killed by decapitation in accordance with Canadian Council of Animal Care guidelines in a protocol approved by the University of Alberta Health Sciences Laboratory Animal Committee. The brains were rapidly removed and transferred into ice-cold (2–3°C) cutting solution consisting of (in mM) NaCl 118, KCl 3, NaH2PO4 1.4, MgSO4 1.3, MgCl2 5.0, NaHCO3 26, CaCl2 1.5, glucose 10. Kynurenic acid (1 mM) was added to the cutting solution only to block glutamate receptor activation during slice preparation. The solution was bubbled continuously with 95% O2 and 5% CO2.
Transverse hippocampal slices (400 μm) were obtained as described previously (Klapstein & Colmers, 1997; Ho et al., 2000) using one of two vibrating slicers: a Vibratome (TPI, St. Louis, MO, U.S.A.) or a Slicer HR-2 (Sigmann-Elektronik, Hüffenhardt, Germany). The resulting slices were submerged immediately in a holding chamber, and allowed to equilibrate in artificial cerebrospinal fluid (ACSF, composed of, in mM, NaCl 124, KCl 3, NaH2PO4 1.4, MgSO4 1.3, NaHCO3 26, CaCl2 1.5, glucose 10), bubbled continuously with 95% O2/5% CO2, and held at 32°C for 30 min, and subsequently allowed to equilibrate to room temperature where they were held for ⩾30 min before being transferred to a submersion-type recording chamber. During the experiment, slices were continously superfused with oxygenated ACSF (32–34°C) at a rate of ≈2.5 ml min−1. For stimulus-train induced bursting (STIB) experiments, slicing procedures were identical, except that slices were cut at 600 μm thickness (Klapstein & Colmers, 1997; Ho et al., 2000). Slices from young animals provide stable, uniform epileptiform discharges for prolonged periods of time (Lewis et al., 1990), which is essential for the present experiments. The studies on the actions of NPY, [ahx5-24]NPY and BIIE0246 on the pEPSP were thus also performed on slices from animals of this age to permit direct comparisons with results from the STIB model.
Electrophysiological studies
Extracellular recordings of area CA1 were performed with borosilicate glass micropipettes (2–5 MΩ) filled with ACSF. The recording pipette was attached to the headstage of an Axoclamp 2A amplifier (Axon Instruments, Foster City, CA, U.S.A.) used in bridge-current clamp model
A bipolar, sharpened tungsten electrode placed in the stratum radiatum of CA1 was used for orthodromic stimulation. Stimuli were applied as square-wave, monophasic pulses (100–200 μs, 6–20 V, 0.1 Hz), from a stimulus isolation unit (IsoFlex, AMPI, Jerusalem). For each data point, three successive field potentials were digitally averaged and stored on-line for subsequent analysis by using pClamp 5.5 software (Axon Instruments).
The slope of the initial, linear portion of the stimulus-evoked population excitatory postsynaptic potential (pEPSP) was used to determine the effects of agonists and antagonists on evoked transmitter release (Klapstein & Colmers, 1992). At the beginning of an experiment, and if needed, during the experiment, the stimulus voltage was varied systematically to construct a stimulus–response relationship for the preparation, and a stimulus voltage chosen that elicited a response on the linear portion of the relationship, usually about 75–85% of the maximum pEPSP slope. Stimulation was continued at 0.1 Hz throughout the entire experiment.
For STIB experiments, procedures were as described previously (Klapstein & Colmers, 1997; Ho et al., 2000; see also Clark & Wilson, 1992 for review of the method). Briefly, the bipolar stimulating electrode was placed in stratum radiatum of CA2, and the recording electrode placed in the pyramidal layer of area CA3; positions of both electrodes were optimized to obtain a good quality field potential upon stimulation. Stimulation was stopped, and an ACSF with altered divalent cations applied, composed of (in mM) NaCl 120, KCl 3.3, MgSO4 0.9, CaCl2 1.6, NaH2PO4 1.23, NaHCO3 25, glucose 10), bubbled continuously with 95% O2/5% CO2, for at least 30 min prior to any further stimulation, and was used for the remainder of the experiment. To elicit afterdischarges, brief stimulus trains (4 stimuli, 30 V, 0.1 ms, 100 Hz), applied to the stimulating electrode, were repeated at 5 Hz. Initially, 15 stimulus trains were applied at 5 Hz. If this failed to elicit an afterdischarge, the number of brief trains applied was increased until an afterdischarge was elicited. Once the duration of the afterdischarge was stable, the threshold for eliciting the afterdischarge was determined by decreasing the number of trains applied until the stimulus failed to elicit an afterdischarge, then increasing the number of trains until the afterdischarge was always elicited and was of stable duration. Only preparations exhibiting such stable behaviour were used for further study.
Drug applications
Both NPY and [ahx5-24]NPY were stored at −20°C as concentrated aliquots made up in distilled water, and BIIE0246 was dissolved in 100% ethanol as a concentrate (1 mM) and diluted in ACSF immediately prior to use. Ethanol application at a final dilution of 1 : 10,000 (the maximum used here) was without effect on synaptic transmission in the hippocampal slice.
For concentration–response experiments, NPY or [ahx5-24] NPY were applied at the indicated concentrations in 25 ml of carbogenated ACSF, and superfused through the recording chamber via a switching valve. Data were acquired every 2 min from several minutes prior to application of a test substance to beyond the peak effect (>10 min after perfusion began), and the recovery of the synaptic response upon washout assessed at 5 min intervals until it had recovered from the effects of the application, or for a minimum of 20 min. BIIE0246 was applied in ACSF at each concentration tested for a minimum of 15 min prior to the application of any test agonist, which was in this case dissolved in ACSF containing the appropriate concentration of antagonist.
In experiments designed to measure the washout and reversibility of the antagonist, a standard test application of 25 ml of 300 nM [ahx5-24]NPY was applied to the slice twice with a complete washout of the agonist in between, and the mean inhibition used as a control value. BIIE0246 (30 nM) was then applied to the slice for 55 min, and the response to 300 nM [ahx5-24]NPY measured twice during this time. Washout of the antagonist commenced immediately after the second response, and the rate of antagonist washout was assessed by the response to [ahx5-24]NPY, measured at 60, 110, 150 and 180 min after washout began. To determine if the washout of the antagonist was dependent on the exposure to the agonist, we assessed the effect on the pEPSP of a 25 ml application of different concentrations of [ahx5-24]NPY (without antagonist) 30 min after washout of BIIE0246 commenced.
Data analysis
Preparations served as their own controls. Data are expressed as percentage inhibition of the control pEPSP slope value. All data are from preparations that showed significant recovery from NPY agonist application effects, upon washout. Concentration–response curves were constructed by plotting the log molar concentration of agonist versus response expressed as percentage inhibition of the maximal response recorded immediately before each drug application (control).
Statistical analysis and concentration–effect curves were calculated using PRISM 3.02 (GraphPad Software, Inc. San Diego, CA, U.S.A.). Time–course data and graphs were prepared using AXUM 5.0c (MathSoft, Inc. Cambridge MA, U.S.A.). Numerical data are presented as means±s.e.m. Statistical comparisons of NPY agonist application effects were made using Student's paired t-tests, and considered significant at P⩽0.05. EC50 values were calculated by automated non-linear regression analysis using Prism.
Materials
Human NPY was purchased from Peptidec Technologies (Pierrefonds, Quebec). The centrally-truncated, Y2 receptor-selective agonist, [ahx5-24]NPY, and Ala31,Aib32NPY were synthesized by solid-state synthesis, as described previously (Rist et al., 1995; Cabrele et al., 2000). The Y2 receptor antagonist, (S)-N2-[[1-[2-[4-[(R,S)-5,11-dihydro-6(6h)-oxodibenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxoethyl]cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5[4H)-dioxo-1,2-diphyenl-3H-1,2,4-triazol-4-yl]ethyl]argininamide (BIIE0246) was a generous gift of Dr Henri Dodds, Boehringer-Ingelheim. All other chemicals were obtained from BDH Inc. (Toronto).
Results
Data were obtained from 87 preparations, with a total of 379 drug applications.
Concentration–response experiments
The pEPSP slope values varied from trial to trial in the same slice preparation and between different preparations, ranging from 0.25 to 1.45 mV ms−1. Although we attempted to perform complete concentration–response curves on each preparation under each condition, this was not always possible.
As reported earlier (Colmers et al., 1987), NPY inhibited excitatory synaptic transmission from stratum radiatum to CA1 pyramidal cells in a concentration–dependent manner. Bath application of 1 μM NPY caused a profound inhibition of the pEPSP slope (by 85.98±4.98%, n=4, P<0.01), which reversed upon washout. Under these conditions, NPY was significantly effective at concentrations tested above 10 nM (EC50=136 nM; Figure 1A), which is in agreement with earlier reports (e.g., Klapstein & Colmers, 1992). The concentration-response relationship for NPY was unexpectedly steep, with a Hill coefficient of 2.65±0.82. The inhibitory effect of NPY was mimicked by the selective Y2 agonist, the centrally-truncated analogue [ahx5-24]NPY (Rist et al., 1995; McQuiston & Colmers, 1996; Cabrele & Beck-Sickinger, 2000), consistent with the activation of a Y2 receptor (Colmers et al., 1991). Application of 1 μM [ahx5-24] NPY also reversibly inhibited the pEPSP slope by 46.15±2.59% (n=40, P<0.01). Washout of this agonist was considerably more rapid than that of NPY, as we reported earlier (McQuiston & Colmers, 1996). [ahx5-24]NPY was significantly effective at concentrations at or above 30 nM, and its EC50 was 290 nM (Figure 1B). The Hill coefficient of the concentration–resposne relationship was 1.35±0.64. By contrast with NPY and the Y2-selective agonist, application of the Y5 agonist, Ala31Aib32NPY (1 μM) had no significant effect on the pEPSP slope (n=3; not illustrated).
Figure 1.
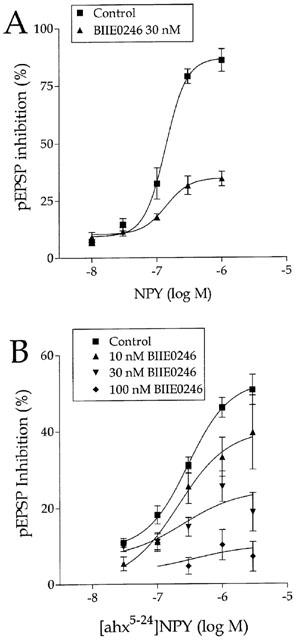
Concentration–response curves for the inhibition of the stratum radiatum-CA1 pEPSP by NPY and [ahx5-24]NPY in control and in the presence of concentrations of BIIE0246 as indicated. Data represent the mean±s.e.mean of 3–6 determinations.
The effects of NPY and [ahx5-24]NPY were then tested, usually in the same slice, in the presence of BIIE0246. BIIE0246 was tested at 30 nM against both agonists, and also against [ahx5-24]NPY at concentrations of 10 and 100 nM. BIIE0246 alone had no effect on synaptic transmission at any concentration tested, indicating that it has no partial agonist activity, (Dumont et al., 2000), and also indicating that there is little if any basal activation of Y2 receptors in the rat hippocampal slice. BIIE0246 significantly antagonized the effects of NPY and of [ahx5-24]NPY. For example, in the presence of 30 nM BIIE0246, the inhibition caused by 1 μM NPY was reduced to about 40% of its control values. The Hill coefficient of the NPY concentration–response curve was not substantially altered by the antagonist (2.4±0.52). Interestingly, the antagonism of the action of NPY by BIIE0246 appeared to depend on the concentration of the agonist, and was greater at higher agonist concentrations. Similarly, the antagonism by BIIE0246 of most concentrations of [ahx5-24]NPY was more effective at greater agonist concentrations. However, concentration–response analysis indicated that there was little significant shift in the EC50 to NPY or to [ahx5-24]NPY in the presence of any concentrations of BIIE0246 tested here, despite the significant antagonism observed (Table 1). Because of this, it was not possible to estimate the affinity of the antagonist for the Y2R in this preparation.
Table 1.
Comparison of EC50 values of NPY and [ahx5–24] NPY in control and varying concentrations of BIIE0246
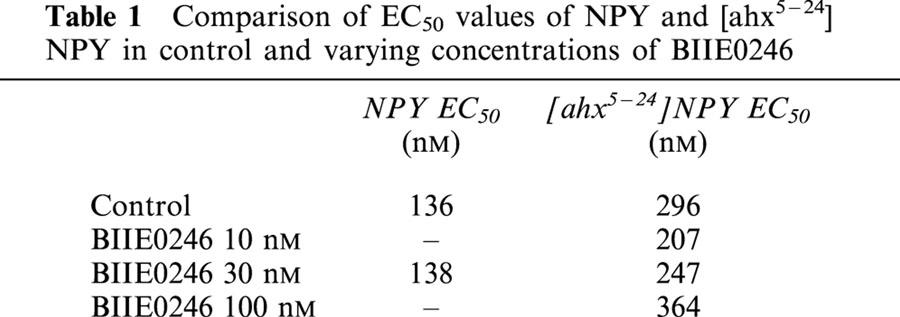
These observations, i.e. a prominent reduction by the antagonist of the maximum agonist effect, without a significant shift in the EC50, might be consistent with an insurmountable antagonism (Kenakin, 1997). This was unexpected, as previous reports had clearly indicated that BIIE0246 acts as a competitive antagonist, eliciting a parallel rightward shift in the concentration–response curve (Dumont et al., 2000; Weiser et al., 2000). Because there was no other evidence indicating that the antagonism by BIIE0246 was insurmountable, we examined the antagonism in detail.
Brain tissue has high levels of lipids in neuronal and glial membranes. Because the antagonist is highly lipophilic, we first hypothesized that the antagonist is merely difficult to wash out of brain tissue. To test this hypothesis, we measured the rate at which 30 nM BIIE0246 washed out of the slice. To do this, we assayed the Y2 receptor effect in a hippocampal slice with a 25 ml application of 300 nM [ahx5-24]NPY, which is readily and completely reversible within 20 min washout, providing us with reasonable time resolution. [ahx5-24]NPY was applied twice and washed out in control ACSF, then the antagonist was applied, and the effect of [ahx5-24]NPY measured twice at 30 min intervals, in the continued presence of the antagonist, identical conditions with those in the previous experiments. Then the antagonist was washed out with control ACSF, and the effect of [ahx5-24]NPY was measued at 30, 60, 110, 150 and 180 min after washout commenced (Figure 2). The response to [ahx5-24]NPY in antagonist and during washout was normalized to the mean control response to [ahx5-24]NPY. These data were then used to assess the rate of washout by fitting the data to a single-exponential decay function using Prism.
Figure 2.
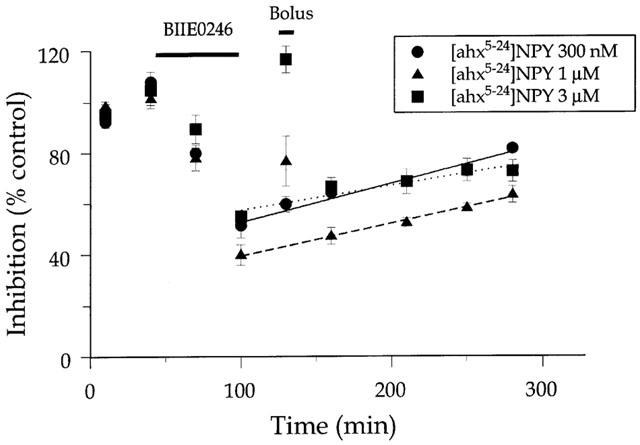
Reversal kinetics of BIIE0246 action in rat hippocampal slice. Data points represent the inhibition (expressed as per cent of the average of two initial control responses) caused by a 10 min application of 300 nM [ahx5-24]NPY of the pEPSP at the times indicated. After two control responses to agonist were measured, BIIE0246 (30 nM) was applied for 55 min (top bar) and the effect of [ahx5-24]NPY measured. Washout of the antagonist with control ACSF proceeded for 20 min, then the reversal of antagonist effect was assessed by the pEPSP inhibition caused by the application of the agonist at three different concentrations (Bolus), 300 nM, 1 μM and 3 μM. Following this, washout with control ACSF proceeded, and the response to 10 min applications of 300 nM agonist was measured at time points indicated. Lines are best-fit linear regression of the rate of recovery of the pEPSP response. Prolonged application of elevated concentrations of agonist temporarily reversed the effect of the antagonist, but failed to have a prolonged influence upon the rate at which the effect of the antagonist reversed with washout. Data represent the mean±s.e.m. of three determinations.
The antagonist washed in with a time constant of about 38 min (n=3). Washout of the antagonist was very slow, and was never complete within the time frame of the experiment. Analysis using an exponential decay model provided unrealistic values for time constants, so we used a linear regression model to fit the washout data, which gave a better fit. The washout rate was 0.15% min−1. We then hypothesized that, if the slow antagonist washout rate as due to a very slow dissociation of the antagonist from the receptor, then the washout rate should be accelerated if the agonist were applied at greater concentrations during the washout period. To test this, a single application of agonist, at 1 μM and 3 μM was made at 30 min after washout commenced, and the rate of antagonist assessed as before (Figure 2). The rate of washout was unaffected by this manipulation. However, the response to the agonist application at 30 min washout did depend on the agonist concentration (Figure 2).
STIP experiments
NPY has been shown by a number of laboratories to have powerful inhibitory actions on epileptic discharges, both in vivo and in vitro (for review, see Vezzani et al., 1999). Because there has been considerable debate on the nature of the receptor or receptors mediating the actions of NPY on epileptiform discharges in rats (e.g., Woldbye et al., 1997; Ho et al., 2000), we examined the effects of BIIE0246 on the anticonvulsant actions of NPY and related agonists on the stimulus train-induced bursting (STIB) induced in rat hippocampal slices (Klapstein & Colmers, 1997; Ho et al., 2000).
Stable primary afterdischarges (1°AD) (Klapstein & Colmers, 1997) were elicited in three slices from three animals ranging in age from 18 to 22 days, and ranged in duration from between 20 and 38 s (Figure 3A1). As reported previously, application of NPY (300 nM) resulted in a complete suppression of the 1°AD for an average of 58±1.66 min (n=3; Figure 3A2,B), comparable to earlier results (Klapstein & Colmers, 1997; Ho et al., 2000). The effects of NPY gradually reversed upon washout. Application of BIIE0246 (100 nM) for 30 min by itself did not appear to alter the threshold or duration of the 1°AD. However in the presence of BIIE0246, a subsequent application of 300 nM NPY did not inhibit the 1°AD (Figure 3A3). Application of 1 μM [ahx5-24]NPY resulted in a suppression of the 1°AD for a maximum of 5 min, but this was completely abolished by BIIE0246 (100 nM, Figure 3C). By contrast, and consistent with the above results on the pEPSP, application of 1 μM of the Y5-specific agonist, Ala31Aib32NPY, either alone (n=2), or in the presence of BIIE0246 (n=2), had no effect on the 1°AD duration (average effect 1.7±10.8%, n=4, P>0.9), consistent with the idea that Y5 receptors have little if any effects on epileptiform discharge in the rat (Ho et al., 2000).
Figure 3.
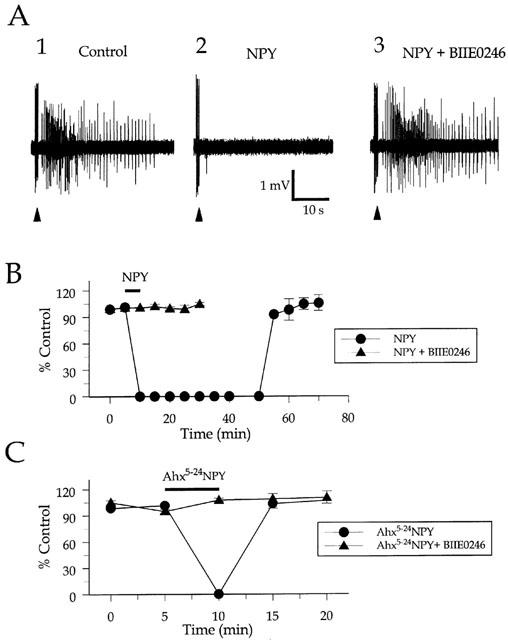
Effect of NPY receptor agonists on primary afterdischarges (1°AD) in the stimulus train-induced bursting (STIB) model. (A) extracellular recording from CA3 area, of a single preparation, exhibit tonic-clonic (control: A1) 1°AD immediately following stimulus trains (arrowheads) applied to stratum radiatum of area CA2. A2: Bath application of NPY (300 nM, 5 min) completely suppressed the 1°AD. A3: After washout of NPY and treatment with the Y2-receptor antagonist BIIE0246 (100 nM), 300 nM NPY does not inhibit the 1°AD in this preparation. (B,C) Time course of inhibition of the 1°AD by NPY (B) and [ahx5-24]NPY (1 μM) (C) in ACSF and after treatment with 100 nM BIIE0246. Stable primary afterdischarges duration was normalized to the mean duration preceding agonist application. Points represent means±s.e.mean.
Discussion
The present study confirms previous work showing that NPY can powerfully inhibit excitatory synaptic transmission in rat hippocampal area CA1 (Colmers et al., 1985; 1987; 1988; 1991; Klapstein & Colmers, 1992; Greber et al., 1994; McQuiston & Colmers, 1996). This effect was observed as a reduction in the slope of the pEPSP evoked in area CA1 from stratum radiatum. NPY inhibits glutamate release from presynaptic nerve terminals (McQuiston & Colmers, 1996), in a manner consistent with the inhibition of presynaptic voltage-dependent calcium channels in this region (Qian et al., 1997). A previous study (Weiser et al., 2000) indicated that BIIE0246 could reduce the effect of NPY on the synaptically-elicited population spike in hippocampal area CA1. The present results using this selective Y2R antagonist, confirm earlier work based entirely on agonist responses (Klapstein & Colmers, 1997; McQuiston & Colmers, 1996) that the Y2R is the dominant participant in mediating the actions of NPY on synaptic transmission in rat hippocampal area CA1, and on its effects on epileptiform discharges in the hippocampal STIB model. Nonetheless, there are some pharmacological pecularities about both the antagonist, and the natural agonist.
Neuropeptide Y
NPY itself powerfully inhibited synaptic excitation in hippocampal area CA1. The EC50 was 136 nM in these experiments, and at 1 μM, NPY inhibited the EPSC by about 85%. However, given the low- to sub-nanomolar affinity of NPY for all its receptors (Gerald et al., 1995), the EC50 for NPY appears rather high. The concentration–response relationship to NPY is very steep, roughly double that observed for the Y2R selective agonist, [ahx5-24]NPY. By contrast, in brain slices of similar thickness containing the hypothalamic paraventricular nucleus (PVN), the EC50 for NPY on the inhibition of the IPSC was 28 nM and the Hill coefficient was about 1.9 (Pronchuk et al., 2002). Since the recordings in hippocampal and PVN slices were done under essentially identical conditions, the explanation for this difference is probably not straightforward.
Selective Y2R agonist
The selective Y2 receptor agonist, [ahx5-24]NPY, mimicked the effect of NPY on hippocampal synaptic transmission and on STIB as reported earlier (Beck-Sickinger et al., 1992; McQuiston & Colmers, 1996; Klapstein & Colmers, 1997; Cabrele & Beck-Sickinger, 2000). We observed this analogue to be less potent than NPY itself, consistent with the lower affinity of this agonist for the Y2R, and with observations from functional studies (Beck-Sickinger et al., 1992). In addition to lower affinity, we observed earlier that [ahx5-24] NPY diffuses into and washes out of hippocampal slices far more easily than does NPY itself (McQuiston & Colmers, 1996; Klapstein & Colmers, 1997). This property made it extremely useful in the antagonist experiments here.
BIIE0246
BIIE0246 clearly and potently suppressed the actions both of NPY and of [ahx5-24]NPY, even at the low concentration of 30 nM. Indeed, the antagonist was able to block most of the effects of [ahx5-24]NPY, and even at 30 nM, it blocked over half of the effects of NPY. While BIIE0246 had powerful effects on the actions of exogenously applied agonists, there was no intrinsic effect of the antagonist on conventional synaptic transmission, suggesting that, in the hippocampal slice preparation, the ambient levels of NPY are very low. Based on published data, the IC50 of the antagonist at the human Y2 receptor is about 3.3 nM (Doods et al., 1999), and 4 nM in rat hippocampal membranes (Weiser et al., 2000). While we intended to determine the affinity of the antagonist for the Y2R in the hippocampal slice, it was not possible in this preparation because of the apparent insurmountability of the antagonism.
To address this apparent insurmountability, we first hypothesized that BIIE0246 simply was difficult to wash out of brain tissue because it is highly lipophilic, and so we measured the washout rate of the antagonist. Consistent with the hypothesis, the washout rate was extremely slow. Furthermore, the washout rate was unaffected by pre-treatment with large amounts of high concentrations of the Y2R agonist. However, the prolonged application of higher concentrations of the agonist did have significantly greater effects on the pEPSP in the presence of the antagonist than did the conventional doses. This is consistent with the competitive antagonism proposed for BIIE0246 (Weiser et al., 2000; Dumont et al., 2000). Based on these observations, we propose that in the hippocampal slice, BIIE0246 is a competitive antagonist, but because of its highly lipophilic nature, a large concentration of the antagonist builds up in the membranes near the receptors, providing a much higher actual concentration in the environment of the receptor than is applied in the bath. Although this hypothesis remains to be confirmed, it is of interest to note that in the thicker tissue of the colon preparation, the antagonism by BIIE0246 also appears to be insurmountable (Dumont et al., 2000).
STIB
Despite the peculiar nature of the antagonism, 100 nM BIIE0246 totally blocked the inhibitory actions both of [ahx5-24]NPY and of NPY itself on the epileptiform discharges in the in vitro STIB model of temporal lobe epilepsy. This is consistent with a major role for the Y2R in the control of excitability in the rat hippocampus. As in the single evoked pEPSP responses, in the STIB experiments too, BIIE0246 had little effect on the duration of the 1°AD. Previous experiments have suggested a dominant role for the Y2R in the suppression of epileptiform activity in the hippocampus (Klapstein & Colmers, 1997; Ho et al., 2000; Vezzani et al., 1999). Interestingly, the application of 1 μM of a highly-selective and potent Y5 agonist had no effect on either the pEPSP, or indeed on STIB response. While there is certainly evidence for presynaptic Y5 receptors in this preparation (Ho et al., 2000), we have little evidence in support of a significant role for Y5 receptors in the regulation of excitability, especially in comparison with Y2R.
Finally, while [ahx5-24]NPY is less potent and efficacious than NPY on the pEPSP, in the STIB model it is apparently equally efficacious as NPY as it completely blocks the 1°AD. However, we consider this to be only apparent. In the STIB experiments, we used roughly equipotent concentrations of NPY and [ahx5-24]NPY, but [ahx5-24]NPY suppressed the 1°AD for about 5 min, while NPY suppressed it for nearly 1 h. This difference is unlikely to arise from the relatively small difference in mean effect of the agonists at the concentrations used. Because we wanted to observe an effect of the antagonist, agonist concentrations that were well above threshold for the suppression of STIB were chosen. NPY itself washes out of the hippocampal slice very slowly in comparison with [ahx5-24]NPY (e.g., McQuiston & Colmers, 1996), suggesting that the concentration of NPY in the slice remains above the threshold for suppressing STIB for a much longer time than that of [ahx5-24]NPY. In any case, BIIE0246 completely blocked the effect of both these agonists.
In conclusion, the Y2R antagonist BIIE0246 potently suppresses the inhibition of the stratum radiatum-CA1 pEPSP by a selective Y2R agonist and by NPY itself. The antagonism appears to be insurmountable, potentially because of the highly lipophilic nature of the antagonist. However, BIIE0246 had a potent effect on the NPY-mediated suppression of epileptiform bursting in the STIB model in hippocampal slices. The data suggest that the Y2 receptor plays a critical role in the regulation of excitability in the rat hippocampus.
Acknowledgments
We would like to thank Dr Henri Doods, Boehringer-Ingelheim, Biberach, Germany for the generous gift of BIIE0246. This study was supported by the Canadian Institutes for Health Research (MT10520) and Human Frontiers Science Program RG0045-2000-B.
Abbreviations
- 1°AD
primary afterdischarge
- ACSF
artificial cerebrospinal fluid
- ahx
6-aminohexanoic acid
- BIIE0246
(S)-N2-[[1-[2-[4-[(R,S)-5,11-dihydro-6(6h)-oxodibenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxoethyl]cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5[4H)-dioxo-1,2-diphyenl-3H-1,2,4-triazol-4-yl]ethyl]argininamide
- CA1
(Cornu Ammonis (Ammon's Horn=hippocampus) area 1
- NPY
Neuropeptide Y
- pEPSP
population excitatory postsynaptic potential
- STIB
stimulus train-induced bursting
- Y2R
NPY Y2 receptor
- Y5R
NPY Y5 receptor
References
- BECK-SICKINGER A.G., GROUZMANN E., HOFFMANN E., GAIDA W., VAN MEIR E.G., WAEBER B., JUNG G. A novel cyclic analog of neuropeptide Y specific for the Y2 receptor. Eur. J. Biochem. 1992;206:957–964. doi: 10.1111/j.1432-1033.1992.tb17006.x. [DOI] [PubMed] [Google Scholar]
- CABRELE C., BECK-SICKINGER A.G. Molecular characterization of the ligand-receptor interaction of the neuropeptide Y family. J. Pept. Sci. 2000;6:97–122. doi: 10.1002/(SICI)1099-1387(200003)6:3<97::AID-PSC236>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- CABRELE C., LANGER M., BADER R., WIELAND H.A., DOODS H.N., ZERBE O., BECK-SICKINGER A.G. The first selective agonist for the neuropeptide YY5 receptor increases food intake in rats. J. Biol. Chem. 2000;275:36043–36048. doi: 10.1074/jbc.M000626200. [DOI] [PubMed] [Google Scholar]
- CLARK S., WILSON W.A.Brain slice model of epilepsy: neuronal networks and actions of antiepileptic drugs Drugs for Control of Epilepsy: Actions on Neuronal Networks Involved in Seizure Disorders 1992Boca Raton: CRC Press; 89–123.ed. Faingold, C.L. & Fromm, G.H. pp [Google Scholar]
- COLMERS W.F., BLEAKMAN D. Effects of neuropeptide Y on the electrical properties of neurons. Trends Neurosci. 1994;17:373–379. doi: 10.1016/0166-2236(94)90046-9. [DOI] [PubMed] [Google Scholar]
- COLMERS W.F., KLAPSTEIN G.J., FOURNIER A., STPIERRE S., TREHERNE K.A. Presynaptic inhibition by neuropeptide Y in rat hippocampal slice in vitro is mediated by a Y2 receptor. Br. J. Pharmacol. 1991;102:41–44. doi: 10.1111/j.1476-5381.1991.tb12129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLMERS W.F., LUKOWIAK K., PITTMAN Q.J. Neuropeptide Y reduces orthodromically evoked population spike in rat hippocampal CA1 by a possibly presynaptic mechanism. Brain Res. 1985;346:404–408. doi: 10.1016/0006-8993(85)90880-7. [DOI] [PubMed] [Google Scholar]
- COLMERS W.F., LUKOWIAK K., PITTMAN Q.J. Presynaptic action of neuropeptide Y in area CA1 of the rat hippocampal slice. J. Physiol. 1987;383:285–299. doi: 10.1113/jphysiol.1987.sp016409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLMERS W.F., LUKOWIAK K., PITTMAN Q.J. Neuropeptide Y action in the rat hippocampal slice: site and mechanism of presynaptic inhibition. J. Neurosci. 1988;8:3827–3837. doi: 10.1523/JNEUROSCI.08-10-03827.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOODS H., GAIDA W., WIELAND H.A., DOLLINGER H., SCHNORRENBERG G., ESSER F., ENGEL W., EBERLEIN W., RUDOLF K. BIIE0246: a selective and high affinity neuropeptide Y Y(2) receptor antagonist. Eur. J. Pharmacol. 1999;384:R3–R5. doi: 10.1016/s0014-2999(99)00650-0. [DOI] [PubMed] [Google Scholar]
- DUMONT Y., CADIEUX A., DOODS H., PHENG L.H., ABOUNADER R., HAMEL E., JACQUES D., REGOLI D., QUIRION R. BIIE0246, a potent and highly selective non-peptide neuropeptide Y Y(2) receptor antagonist. Br. J. Pharmacol. 2000;129:1075–1088. doi: 10.1038/sj.bjp.0703162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERALD C., WALKER M.W., CRISCIONE L., GUSTAFSON E.L., BATZL-HARTMANN C., SMITH K.E., VAYSSE P., DURKIN M.M., LAZ T.M., LINEMEYER D.L., SCHAFFHAUSER A.O., WHITEBREAD S., HOFBAUER K.G., TABER R.I., BRANCHEK T.A., WEINSHANK R.L. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature. 1996;382:168–171. doi: 10.1038/382168a0. [DOI] [PubMed] [Google Scholar]
- GERALD C., WALKER M.W., VAYSSE P.J., HE C., BRANCHEK T.A., WEINSHANK R.L. Expression cloning and pharmacological characterization of a human hippocampal neuropeptide Y/peptide YY Y2 receptor subtype. J. Biol. Chem. 1995;270:26758–26761. doi: 10.1074/jbc.270.45.26758. [DOI] [PubMed] [Google Scholar]
- GREBER S., SCHWARZER C., SPERK G. Neuropeptide Y inhibits potassium-stimulated glutamate release through Y2 receptors in rat hippocampal slices in vitro. Br. J. Pharmacol. 1994;113:737–740. doi: 10.1111/j.1476-5381.1994.tb17055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROUZMANN E., BUCLIN T., MARTIRE M., CANNIZZARO C., DORNER B., RAZANAME A., MUTTER M. Characterization of a selective antagonist of neuropeptide Y at the Y2 receptor. Synthesis and pharmacological evaluation of a Y2 antagonist. J. Biol. Chem. 1997;272:7699–7706. doi: 10.1074/jbc.272.12.7699. [DOI] [PubMed] [Google Scholar]
- HO M.W.Y., BECK-SICKINGER A.G., COLMERS W.F. Neuropeptide Y5 Receptors mediate inhibition of synaptic excitation in proximal subiculum, but do not suppress epileptiform activity in rat hippocampal slices. J. Neurophysiol. 2000;83:723–734. doi: 10.1152/jn.2000.83.2.723. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.Allotopic, noncompetitive, and irreversible antagonism Pharmacologic Analysis of Drug-Receptor Interaction 1997New York: Lippincott-Raven; 374–394.3rd edn. pp [Google Scholar]
- KLAPSTEIN G.J., COLMERS W.F. 4-Aminopyridine and low Ca2+ differentiate presynaptic inhibition mediated by neuropeptide Y, baclofen and 2-chloroadenosine in rat hippocampal CA1 in vitro. Br. J. Pharmacol. 1992;105:470–474. doi: 10.1111/j.1476-5381.1992.tb14277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLAPSTEIN G.J., COLMERS W.F. Neuropeptide Y suppresses epileptiform activity in rat hippocampus in vitro. J. Neurophysiol. 1997;78:1651–1661. doi: 10.1152/jn.1997.78.3.1651. [DOI] [PubMed] [Google Scholar]
- LEWIS D.V., JONES L.S., MOTT D.D. Hippocampal epileptiform activity induced by magnesium-free medium: differences between area CA1 and CA2/3. Epilepsy Res. 1990;6:95–101. doi: 10.1016/0920-1211(90)90083-8. [DOI] [PubMed] [Google Scholar]
- MCQUISTON A.R., COLMERS W.F. Neuropeptide Y2 receptors inhibit the frequency of spontaneous but not miniature EPSCs in CA3 pyramidal cells of rat hippocampus. J. Neurophysiol. 1996;76:3159–3168. doi: 10.1152/jn.1996.76.5.3159. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., BECK-SICKINGER A.G., COX H., DOODS H.N., HERZOG H., LARHAMMAR D., QUIRION R., SCHWARTZ T., WESTFALL T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- PHENG L.H., PERRON A., QUIRION R., CADIEUX A., FAUCHERE J.L., DUMONT Y., REGOLI D. Neuropeptide Y-induced contraction is mediated by neuropeptide Y Y2 and Y4 receptors in the rat colon. Eur. J. Pharmacol. 1999;374:85–91. doi: 10.1016/s0014-2999(99)00296-4. [DOI] [PubMed] [Google Scholar]
- PRONCHUK N., BECK-SICKINGER A.G., COLMERS W.F. Multiple NPY receptors inhibit GABA(A) synaptic responses of rat medial parvocellular effector neurons in the hypothalamic paraventricular nucleus. Endocrinology. 2002;143:535–543. doi: 10.1210/endo.143.2.8655. [DOI] [PubMed] [Google Scholar]
- QIAN J., COLMERS W.F., SAGGAU P. Inhibition of synaptic transmission by neuropeptide Y in rat hippocampal area CA1: modulation of presynaptic Ca2+ entry. J. Neurosci. 1997;17:8169–8177. doi: 10.1523/JNEUROSCI.17-21-08169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIST B., WIELAND H.A., WILLIM K.D., BECK-SICKINGER A.G. A rational approach for the development of reduced-size analogues of neuropeptide Y with high affinity to the Y1 receptor. J. Pept. Sci. 1995;1:341–348. doi: 10.1002/psc.310010509. [DOI] [PubMed] [Google Scholar]
- SUN L., MILLER R.J. Multiple neuropeptide Y receptors regulate K+ and Ca2+ channels in acutely isolated neurons from the rat arcuate nucleus. J. Neurophysiol. 1999;3:1391–1403. doi: 10.1152/jn.1999.81.3.1391. [DOI] [PubMed] [Google Scholar]
- VEZZANI A., SPERK G., COLMERS W.F. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- WEISER T., WIELAND H.A., DOODS H.N. Effects of the neuropeptide Y Y(2) receptor antagonist BIIE0246 on presynaptic inhibition by neuropeptide Y in rat hippocampal slices. Eur. J. Pharmacol. 2000;404:133–136. doi: 10.1016/s0014-2999(00)00478-7. [DOI] [PubMed] [Google Scholar]
- WOLDBYE D.P., LARSEN P.J., MIKKELSEN J.D., KLEMP K., MADSEN T.M., BOLWIG T.G. Powerful inhibition of kainic acid seizures by neuropeptide Y via Y5-like receptors. Nature Med. 1997;3:761–764. doi: 10.1038/nm0797-761. [DOI] [PubMed] [Google Scholar]


