Abstract
The new immunosuppressive agent sirolimus generally is combined in transplant patients with cyclosporine and tacrolimus which both exhibit cholestatic effects. Nothing is known about possible cholestatic effects of these combinations which might be important for biliary excretion of endogenous compounds as well as of immunosuppressants.
Rats were daily treated with sirolimus (1 mg kg−1 p.o.), cyclosporine (10 mg kg−1 i.p.), tacrolimus (1 mg kg−1 i.p.), or a combination of sirolimus with cyclosporine or tacrolimus. After 14 days a bile fistula was installed to investigate the effects of the immunosuppressants and their combinations on bile flow and on biliary excretion of bile salts, cholesterol, and immunosuppressants.
Cyclosporine as well as tacrolimus reduced bile flow (−22%; −18%), biliary excretion of bile salts (−15%;−36%) and cholesterol (−15%; −47%). Sirolimus decreased bile flow by 10%, but had no effect on cholesterol or bile salt excretion.
Combination of sirolimus/cyclosporine decreased bile flow and biliary bile salt excretion to the same extent as cyclosporine alone, but led to a 2 fold increase of biliary cholesterol excretion. Combination of sirolimus/tacrolimus reduced bile flow only by 7.5% and did not change biliary bile salt and cholesterol excretion.
Sirolimus enhanced blood concentrations of cyclosporine (+40%) and tacrolimus (+57%). Sirolimus blood concentration was increased by cyclosporine (+400%), but was not affected by tacrolimus.
We conclude that a combination of sirolimus/tacrolimus could be the better alternative to the cotreatment of sirolimus/cyclosporine in cholestatic patients and in those facing difficulties in reaching therapeutic ranges of sirolimus blood concentration.
Keywords: Cyclosporine, sirolimus, tacrolimus, cholestasis, rat
Introduction
Sirolimus (rapamycin, RAPAMUNE®) is a macrocyclic lactone isolated from Streptomyces hygroscopicus. Whereas cyclosporine (CyA) and tacrolimus (TRL), the two base therapy agents currently used in organ transplantation, achieve their effects principally by blocking calcineurin and thereby inhibiting interleukin-2 production, sirolimus (SRL) has no effect on calcineurin. Sirolimus reduces T-lymphocyte activation by inhibiting the interleukin-2-mediated signal transduction pathway (Seghal, 1995; Liu et al., 1991). As the significant nephrotoxicity, neurotoxicity, and hypertension associated with CyA and TRL can be partly attributed to calcineurin blockade (Mayer et al., 1997; Bennett et al., 1996; Andoh et al., 1997), SRL would be expected to have a different toxicity profile. In preclinical studies, SRL has been shown to be as effective as CyA in maintaining survival of renal and cardiac allografts without causing nephrotoxicity (Granger et al., 1995). When SRL was added to CyA-therapy in phase I (Murgia et al., 1996) and phase II studies (Kahan et al., 1999), no deleterious effects on renal function were observed, and the incidences of neurotoxicity and hypertension were unchanged. The most notable side-effects were hyperlipidaemia (both hypercholesterolaemia and hypertriglyceridaemia), thrombocytopenia, and leukopenia. The exact mechanisms of CyA- and SRL-induced hyperlipidaemia are not known yet. In the case of CyA, drug-induced cholestasis could be one important reason of hypercholesterolaemia, because CyA is known to cause cholestasis by inhibiting both basal and canalicular hepatocellular bile salt transporters (Stacey & Kotecka, 1988; Böhme et al., 1994). In experiments with α-naphthylisothiocyanate-treated mice, cholestasis was induced and increased levels of free cholesterol in the plasma were observed (Chisholm et al., 1999). Because experimental transplantation studies suggest a synergism between SRL and TRL (Vu et al., 1997), the combination of SRL/TRL is of increasing interest in clinical trials, too (Mcalister et al., 2000). The first results of these studies indicate that the incidence of hypercholesterolaemia is lower in SRL/TRL-treated patients than in patients under SRL/CyA therapy. In studies with rats TRL was shown to reduce bile flow, as well (Sanchez-Campos et al., 1998), but was less effective on cholestasis than CyA (Mizuta et al., 1999). In a previous study in an acute bile fistula model in rats we could show that SRL/CyA reduced bile flow and biliary excretion of bile salts and cholesterol (Deters et al., 2001). On the other hand, these parameters were not attenuated by SRL/TRL. To augment the clinical impact of these observations we investigated the effects of SRL, CyA, TRL, and the combination of either SRL/CyA or SRL/TRL on bile flow after treatment with these immunosuppressants in a subchronic rat bile fistula model over 2 weeks. Additionally, we measured the concentrations of these immunosuppressants and the main CyA metabolites in bile and blood to explore the role of biliary excretion of them for possible pharmacokinetic interactions that are of clinical importance, as well.
Methods
Animals
Male Wistar rats (300–400 g; breeder Medizinische Hochschule, Hannover Germany) were used throughout. The animal experiments were authorized by the ethical commitee of the local government Hannover, Germany. The animals were treated in compliance with the indications of the Guide to the Care and Use of Experimental Animals, routinely used at our laboratory. During the time of immunosuppressant treatment they were housed in an individually ventilated cage system. They had free access to pellet feed (Altromin® standard diet, Lage, Germany) and tap water until surgery.
Chemicals
Sirolimus (Rapamune®) was obtained from Wyeth-Pharma GmbH, Münster, Germany, as a solution (1 mg ml−1) for oral application. Thirty-two-desmethoxyrapamycin was kindly given by Wyeth-Ayerst, Princeton, U.S.A. Cyclosporine (Sandimmun®) as a solution (50 mg ml−1) for i.v. administration and tacrolimus (Prograf®) as a solution (5 mg ml−1) for i.v. administration were obtained by the local pharmacy. For intraperitoneal administration cyclosporine and tacrolimus were diluted 1/10 with 0.9% NaCl.
Bile duct fistula model
Rats were divided into six groups (Table 1) and dosed daily for 14 days with SRL (1 mg kg−1 p.o.), CyA (10 mg kg−1 i.p.), TRL (1 mg kg−1 i.p.), or a combination of SRL (1 mg kg−1 p.o.) with either CyA (10 mg kg−1 i.p.) or TRL (1 mg kg−1 i.p.). After starving for 24 h the rats were anaesthesized with ethylether and blood was taken from the animals by retro-orbital puncture for determination of bile salt, bilirubin, cholesterol, and triglyceride serum concentration just before the first application of the immunosuppressants. This procedure was repeated after 1 and 2 weeks of daily immunosuppressant treatment. Twelve hours after receiving the last dose of the immunosuppressants the rats were anaesthesized with urethane (6 ml kg−1 20% urethane solution ≈1.2 g kg−1 intraperitoneally). The abdomen of the animals was opened by a central incision below the sternum. The common bile duct was prepared and cannulated with a PE 10 polyethylene tube just below the bifurcation of the common duct. After fixation of the fistula the abdomen was closed and the bile was collected every 15 min in Eppendorf vials. The whole experiment lasted for 90 min and was performed on a heated surgery table keeping the body temperature of the rats at 37°C. After 90 min whole blood was taken by heart puncture for determination of immunosuppressant blood concentrations and animals were killed by exsanguination.
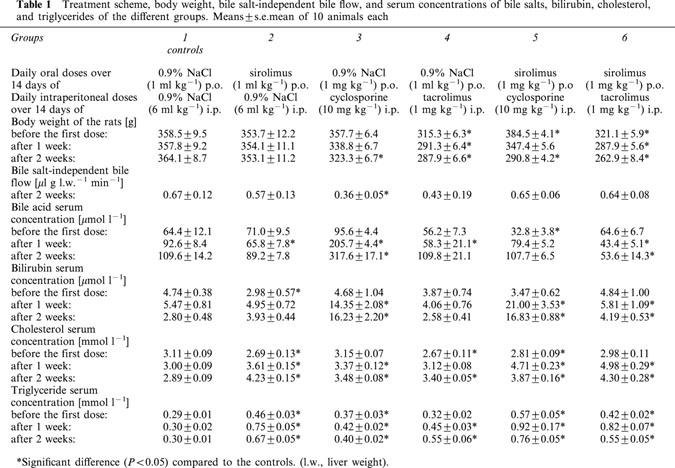
Table 1.
Treatment scheme, body weight, bile salt-independent bile flow, and serum concentrations of bile salts, bilirubin, cholesterol, and triglycerides of the different groups. Means±s.e.mean of 10 animals each
Analytical procedures
Bile flow was determined gravimetrically without correction for specific gravity and assuming a bile density of 1.0 g ml−1. The concentrations of triglycerides (Mcgowan et al., 1983) and bilirubin in serum (Walters & Gerarde, 1970) and that of cholesterol (Siedel et al., 1983) and bile salts (Mashige et al., 1981) in bile and serum were determined by enzymatic colorimetric tests. Reagents for all colorimetric measures, which were performed on a DYNATECH MR5000, were purchased from Sigma-Aldrich (Deisenhofen, Germany). Biliary excretion of bile salts, cholesterol, and bilirubin were calculated as the concentrations of these parameters multiplied by bile flow. Bile salt-independent bile flow was obtained by calculating a correlation curve between bile flow versus biliary excretion of bile salts. The intercept with the ordinate (bile flow in the face of zero bile salt secretion) is an indirect measure for bile salt-independent bile flow (Erlinger, 1982). Cyclosporine and its main metabolites AM1, AM9, AM1c, and AM4N (Table 2) in blood and bile were measured by h.p.l.c as described (Christians et al., 1988) with slight modifications. In brief, cyclosporine and its metabolites were extracted from blood by solid-liquid extraction and were quantified using cyclosporine D as internal standard. A liquid chromatograph 1090 Series II (Hewlett Packard, Waldbronn, Germany) was used as chromatographic system. One hundred μl of the extracted sample was injected into the chromatographic system. Acetonitrile and water with pH 3.0, adjusted with H2SO4, were used as eluents and the flow rate was 0.25 ml min−1. A precolumn (Hypersil C18, 5 μ, 10×2 mm) from Schambeck SFD GmbH, Deisenhofen, Germany, and a reversed phase column (Hypersil C18, 5 μ, 250×2 mm, phenomenex®, Aschaffenburg, Germany) were used and the column temperature was set to 67°C. Cyclosporine and its metabolites were eluted by a linear gradient. The proportion of acetonitrile/water was kept stable at 43/57 v v−1 during the first 20 min of analysis. Then the ratio of acetonitrile/water was slowly enhanced from 20 to 35 min to 65/35 v v−1 and from 35 to 45 min to 75/25 v v−1. This proportion of the eluents was kept stable for 7 min. Then the column was washed for 6 min with acetonitrile/water 95/5 v v−1. Afterwards the system was reequilibrated by washing the columns 4 min with 0.25 ml min−1 acetonitrile/water 43/57 v v−1. SRL and TRL were measured by liquid chromatography coupled with electrospray ionization/mass spectrometry (LC-ESI/MS) as previously described (Kirchner et al., 2001) with slight modifications. EDTA whole blood samples (300 μl) were treated with 600 μl methanol/0.2 M ZnSO4 (80/20 v v−1) plus 6 μl 32-desmethoxyrapamycin (1 ng μl−1). Then samples were vortexed (30 s) and centrifuged (20000 ×g, 5 min). Fifty μl of the supernatant was injected into the LC-ESI/MS system. A liquid chromatograph (LC) coupled with an electrospray ionization MS (LC/MSD 1100 SL Series, Agilent, Waldbronn, Germany) was used for all measurements. After injection the sample was transported by a stream of water (0.35 ml min−1 for 3 min) to an extraction column (20×2.1 mm, Waters Oasis® HLB, Eschborn, Germany). After turning the column-switching valve SRL, TRL, or CyA were eluted (flow: 0.25 ml min−1) from the extraction column in a back flash mode onto an analytical column (Hypersil C18, 5μ, 250×2 mm, phenomenex®, Aschaffenburg, Germany) by methanol/water (90/10 v v−1). After 20 min the extraction column was washed for 2 min with methanol and for 3 min with water before starting the next run. The temperature of the extraction and analytical columns was kept at 33°C. For single ion detection the mass spectrometer was focused on the [M+Na]+ of SRL (m/z 936.6), TRL (m/z 824.0), CyA (m/z 1224.6) and 32-desmethoxyrapamycin (m/z 906.6) in the positive ion mode. The accuracy of the sirolimus, tacrolimus, and cyclosporine measurement was controlled by monthly participation in the International Proficiency Testing Scheme (Analytical Services International, London, U.K.).
Table 2.
Systematics of the Hawk's Cay Nomenclature (Consensus Document, 1999). The name of a metabolite consists of an ‘A' for cyclosporine A, an ‘M' to indicate that is is a metabolite and a sequence of arabic numbers (x) and letters indicating position and type of the amino acid of the cyclosporine molecule modified by metabolism.
Statistics
Results are expressed as means±s.e.mean. The data were compared by analysis of variance. When the analysis indicated a significant difference between the controls and the experimental group, the means were compared using Dunnett's multiple comparisons test. A probability of <0.05 was defined as significant.
Results
Effects of SRL, CyA, TRL, SRL/CyA, and SRL/TRL on the body weight of the rats
During the daily treatment of the rats with immunosuppressants over 2 weeks none of the animals died. However, concerning the loss of body weight, big differences between the single animal groups could be observed. While in the controls and the SRL-treated rats the body weight of the rats was stable between 353 and 364 g, the body weight of the CyA- and TRL-treated rats was reduced by 9 and 10% within 2 weeks (Table 1). The loss of body weight was amplified after combining SRL with either CyA (−25%) or TRL (−18%).
Effects of SRL, CyA, TRL, SRL/CyA, and SRL/TRL on bile flow and biliary excretion of bile salts and cholesterol
The bile flow was 1.20±0.08 μl per gram liver weight per min (μl g.l.w.−1 min−1) in the controls and was reduced by SRL (−10%; statistically not significant), CyA (−22%), as well as by TRL (−18%) (Figure 1). When SRL was combined with CyA the bile flow was decreased to the same degree as by CyA given alone. After combination of SRL with TRL the bile flow was only slightly attenuated by 7.5% compared to the control values (Figure 1). The biliary excretion of bile salts (20.3±1.6 nmol g l.w. −1 min−1 in the controls) was diminished by CyA (−15%) as well as by TRL (−36%), but not affected by SRL (Figure 1). Cotreatment of SRL with CyA led to a decrease of biliary bile salt excretion by 44% of the controls. However, this parameter was not altered by the combination of SRL with TRL (Figure 1). The biliary excretion of cholesterol was 0.72±0.05 nmol g l.w.−1 min−1 and was attenuated by CyA (−16%) as well as by TRL (−47%), but not influenced by SRL (Figure 1). Surprisingly, we observed a 2 fold increase of the biliary cholesterol excretion in the SRL/CyA-treated group. On the other hand, this parameter remained unchanged compared to the controls after simultaneous administration of SRL and TRL (Figure 1).
Figure 1.
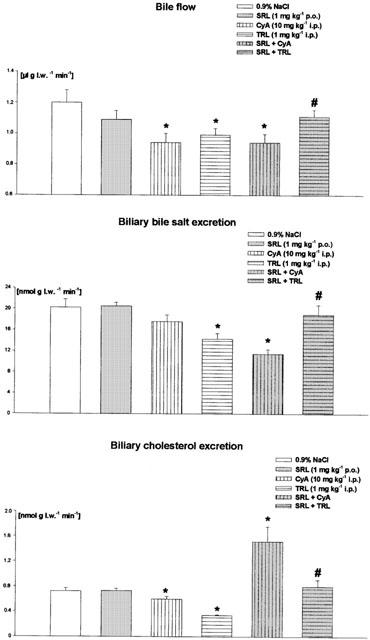
Bile flow and biliary excretion of bile salts and cholesterol after daily treatment over 2 weeks with sirolimus (SRL, 1 mg kg−1 p.o.), cyclosporine (CyA, 10 mg kg−1 i.p.), tacrolimus (TRL, 1 mg kg−1 i.p.) and of SRL (1 mg kg−1 p.o.) plus CyA (10 mg kg−1 i.p.) or TRL (1 mg kg−1 i.p.). Means±s.e.mean of 10 animals each. *Significant difference (P<0.05) compared to the controls. #Significant difference (P<0.05) between SRL+CyA- and SRL+TRL-treated groups. (l.w., liver weight).
Effects of SRL, CyA, TRL, SRL/CyA, and SRL/TRL on serum concentrations of bile salts, bilirubin, cholesterol, and triglycerides
In the controls the serum concentration of bile salts was determined as 109.6±14.2 μmol l−1. We observed a 3 fold increase of serum bile salt concentration only in those animals treated with CyA (Table 1). When rats were given either SRL, TRL, or SRL/CyA, bile salt serum concentration remained constant and after the cotreatment of SRL with TRL this parameter was even reduced to the half (Table 1). Bilirubin serum concentration (2.80±0.48 μmol l−1) was 5 fold increased by CyA. Neither SRL nor TRL led to a significant change of this parameter. Cotreatment with SRL and CyA enhanced the bilirubin serum concentration to the same degree as CyA alone. After the combination of SRL with TRL a less pronounced increase (+49%) of bilirubin serum concentration was measured (Table 1). Serum cholesterol concentration (2.89±0.09 mmol l−1) was elevated after treatment with SRL (+46%), CyA (+18%) as well as with TRL (+18%). The SRL induced increase of cholesterol serum concentration was neither exacerbated by cotreatment with CyA nor with TRL (Table 1). A similar picture was seen after determination of triglyceride serum concentration. Triglyceride serum concentration (0.30±0.01 mmol l−1) was again enhanced by SRL (+123%), CyA (+33%) as well as by TRL (+83%). When SRL was combined with CyA triglyceride serum concentration was 13% higher than in the SRL-treated animals and 17% lower after combination with TRL (Table 1).
Interactions of SRL/CyA and SRL/TRL on their blood concentration and biliary excretion
Biliary excretion and blood concentrations of the immunosuppressive drugs used were analysed by sensitive and specific chromatographic procedures. In the animal groups treated with a single immunosuppressant the mean blood level of SRL was 2.8±0.3 μg l−1 (Figure 2A), of CyA 4703±285 μg l−1 (Figure 3A), and of TRL 7.1±0.6 μg l−1 (Figure 4B), respectively. When SRL was given simultaneously with CyA or TRL, the mean blood level of SRL was significantly increased 5 fold by CyA but not changed by TRL (Figure 4A). The biliary excretion of SRL (2.2±0.3 pg g l.w.−1 min−1) was enhanced 25 fold by CyA but was not affected by TRL (Figure 4C). On the other hand, SRL increased blood concentration of CyA by 56% (Figure 3A) and of TRL by 33% (Figure 4B). The blood concentrations of the main CyA metabolites AM1 (195±41 μg l−1), AM9 (328±49 μg l−1), AM1c (26±11 μg l−1), and AM4N (not detectable in the controls) were elevated 2–5 fold (Figure 3B) by SRL, as well. Whereas the biliary excretion of CyA (22.1 ng g l.w.−1 min−1) was reduced by 20% (Figure 2A) and the excretion of the CyA metabolites was 2–4 fold increased by SRL (Figure 2B), the biliary excretion of TRL (2.7±0.8 pg g l.w.−1 min−1) was only slightly attenuated by SRL (Figure 4D).
Figure 3.
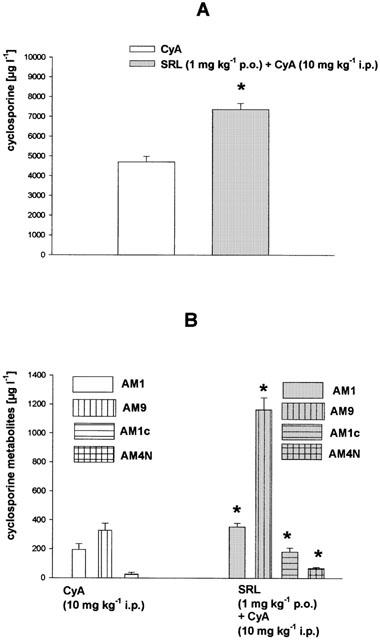
Blood concentrations of (A) cyclosporine and (B) its metabolites after daily treatment over 2 weeks with cyclosporine (CyA, 10 mg kg−1 i.p.) or sirolimus (SRL, 1 mg kg−1 p.o.) plus CyA (10 mg kg−1 i.p.). Means±s.e.mean of 10 animals each. *Significant difference (P<0.05) compared to the CyA-treated group.
Figure 4.
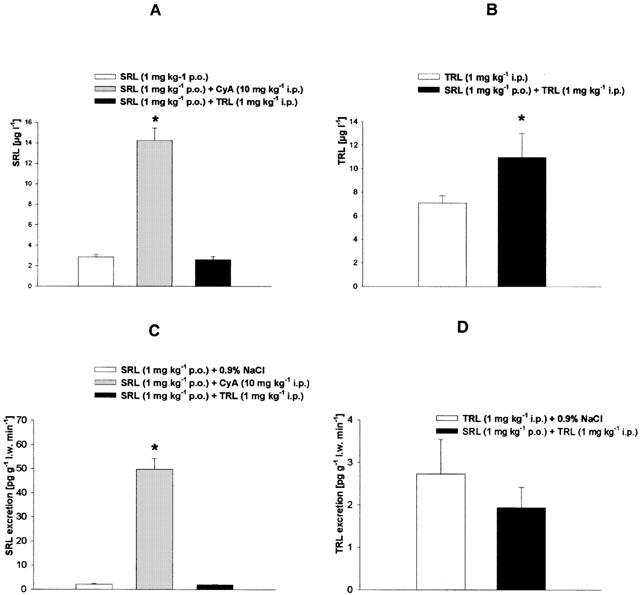
Blood concentrations and biliary excretion of (A,C) sirolimus and (B,D) tacrolimus after daily treatment over 2 weeks with sirolimus (SRL, 1 mg kg−1 p.o.), tacrolimus (TRL 1 mg kg−1 i.p.) and of SRL (1 mg kg−1 p.o.) plus CyA (10 mg kg−1 i.p.) or TRL (1 mg kg−1 i.p.). Means±s.e.mean of 10 animals each. *Significant difference (P<0.05) compared to the SRL- or TRL-treated group. (l.w., liver weight).
Figure 2.
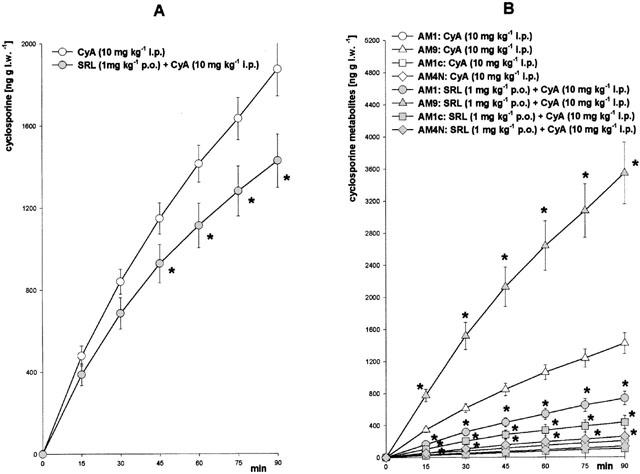
Biliary excretion of (A) cyclosporine and (B) its metabolites after daily treatment over 2 weeks with cyclosporine (CyA, 10 mg kg−1 i.p.) or with SRL (1 mg kg−1 p.o.) plus CyA (10 mg kg−1 i.p.). Means±s.e.mean of 10 animals each. *Significant difference (P<0.05) compared to the CyA-treated group. (l.w., liver weight).
Discussion
Cholestatic properties of CyA have been observed in clinical studies (Kahan, 1989) and in experiments with rats (Le Thai et al., 1988). Inhibition of the transporters involved in bile formation is a documented mechanism for the cholestatic effects of CyA (Mosley & Morrissette, 1990). Hepatic uptake of conjugated bile salts depends on Na+ and is mediated by Ntcp (Na+/taurocholate cotransporting polypeptide). The transport of unconjugated bile salts and other organic anions into hepatocytes is mediated by a transporter family called Oatps (organic anion transporting polypeptides). The biliary excretion of both conjugated and unconjugated bile salts depends on ATP. The excretion of taurine-conjugated bile salts is mediated by Bsep (bile salt export pump) and that of divalent glucuronated or sulphated bile salts by Mrp2 (multidrug resistance protein2) (Müller & Jansen, 1998). CyA was shown to decrease both the uptake of bile salts in the liver (Stacey & Kotecka, 1988) and their excretion into the bile canaliculi. The inhibitory effects of CyA on bile salt transporters of the canalicular hepatocyte membrane are described as being stronger than those on transporters of the basolateral hepatocyte membrane (Böhme et al., 1994). In experiments with vesicles isolated from Bsep-expressing Sf6 cells CyA inhibited Bsep-mediated transport of bile salts (Stieger et al., 2000). Bile salt-independent bile flow is mainly driven by the biliary excretion of bicarbonate and reduced glutathione (GSH) which is substrate of Mrp2 (Paulusma et al., 1999). In single and short-term treatment studies with rats it was observed that CyA-induced cholestasis was due not only to alterations in the hepatobiliary transport of bile salts but also to an impairment of bile formation dependent on the biliary secretion of glutathione (Moran et al., 1998). In our experiments daily treatment of the rats over 2 weeks with CyA led to a decrease of bile salt dependent (−15%) as well as of bile salt-independent bile flow (−46%) (Figure 1; Table 1). Bile salts play an important role in the biliary excretion of cholesterol (Graham et al., 1989). Therefore, the observed decrease of biliary cholesterol excretion (−16%) by CyA in our and other studies with rats (Galan et al., 1995) could be the result of impaired biliary bile salt excretion induced by CyA (Figure 1). As indirect parameters of cholestasis a 3 fold enhanced bile salt serum concentration and more than a 5 fold increase of bilirubin serum concentration compared to the controls were measured in the CyA-treated rats (Table 1).
Previously, contradictory results have been reported with respect to the effect of TRL on bile flow. In a study investigating the effects of chronic tacrolimus treatment (0.8 mg kg−1) on bile secretion in rats for 6 weeks, TRL was found to induce cholestasis by inhibiting primarily the biliary excretion of GSH and, to lesser extent, of bicarbonate (Sanchez-Campos et al., 1998). In another study, rats were given TRL up to 4 mg kg−1 for 7 days. In this study TRL increased bile salt-dependent flow while bile salt-independent flow was reduced by TRL (Mizuta et al., 1999). When rats were treated with either a high dose of 10 mg kg−1 TRL only once or daily for 1 week TRL even induced choleresis through stimulation of insulin-like growth factor-I production in the liver of rats (Kawamura et al., 2001). When we administered rats TRL at a dose of 1 mg kg−1 i.p. bile flow (−18%) as well as biliary excretion of bile salts (−36%) and cholesterol (−47%) were reduced to a similar extent as in the experiments with CyA (Figure 1). TRL diminished the bile salt-independent bile flow statistically not significantly by 35% (Table 1). Surprisingly, in spite of impaired biliary excretion of bile salts no increase of serum bile salts concentration was seen in the TRL-treated animals (Table 1). From this observation we hypothesize that TRL may decrease bile salt synthesis via inhibition of the microsomal enzyme cholesterol-7α-hydroxylase, the key enzyme in bile salt synthesis. However, no effect of TRL on this enzyme has been reported in the literature so far.
Information regarding the effects of SRL on bile flow is very limited at the moment. In preliminary own experiments with rats we could show that a single dose of 6 mg kg−1 i.p SRL resulted in a decrease of bile flow mainly due to an impaired excretion of GSH (Deters et al., 2001). Coincident with these results SRL was shown to decrease mRNA expression of the transport protein Mrp2 in the liver of rats (Bramow et al., 2001). In our present study the oral treatment of SRL at a dose of 1 mg kg−1 day−1 attenuated bile flow by 10%. This effect can be contributed mainly to the statistically not significant reduction of bile salt-independent bile flow (−15%; Table 1) because SRL neither impaired biliary excretion of bile salts nor of cholesterol (Figure 1). Compatible to these results, the serum bile salt concentration was not and the serum bilirubin concentration was only slightly increased in the SRL-treated rats (Table 1).
When SRL was combined with CyA the bile flow was reduced to the same extent as in the experiments where CyA was given alone (Figure 1). The CyA-induced decrease of biliary bile salt excretion was exacerbated by cotreatment with SRL. On the other hand, the decrease of biliary bile salt excretion after SRL/CyA cotreatment did not result in elevated serum concentrations of bile salts as observed after CyA-treatment indicating reduced synthesis of bile salts out of cholesterol. Results from earlier studies indicate that CyA may alter the binding kinetics of low-density lipoproteins (LDL) to the LDL-receptor, thereby interfering with the normal feedback mechanisms that regulate cholesterol biosynthesis (Hoogeveen et al., 2001). Both mechanisms, decrease of cholesterol metabolism and increase of cholesterol synthesis, could cause a strong increase of the hepatic cholesterol concentration and consequently of the biliary cholesterol excretion as observed in our experiments (Figure 1).
These effects were not observed in the SRL- or CyA-treated rats, because in these animals the SRL or CyA blood concentrations were much lower than in the SRL/CyA-treated rats (Figures 3 and 4).
When SRL was combined with TRL bile flow was slightly attenuated and the biliary excretion of bile salts and cholesterol was not impaired in comparison to the controls (Figure 1). Bile salt serum concentration in the SRL/TRL group decreased to the half of the control values while the bilirubin serum concentration was slightly elevated (Table 1).
The first clinical studies comparing the effects of SRL/TRL and SRL/CyA on lipid serum concentration indicate lower serum concentrations of both cholesterol and triglycerides after SRL/TRL than after SRL/CyA treatment in transplant recipients (Mcalister et al., 2000; Trotter et al., 2001). However, it has been reported that TRL also caused elevated cholesterol and triglyceride serum concentrations (Ichimaru et al., 2001; Charco et al., 1999). In our experiments cholesterol serum concentration was elevated by SRL, CyA, TRL, as well as by SRL/CyA and SRL/TRL. The highest cholesterol serum concentration was observed after treatment of SRL followed by CyA and TRL which showed the same effect on cholesterol serum concentration. Neither cotreatment of SRL with CyA nor with TRL further increased SRL-induced hypercholesterolaemia (Table 1). Probably, in the case of SRL/CyA cotreatment, a further increase of the SRL-induced hypercholesterolaemia after addition of CyA could be partially compensated by the strong increase of the biliary cholesterol excretion (Figure 1).
The effects of the immunosuppressants on serum triglyceride concentrations were similiar to those on cholesterol concentrations. In our rat model all immunosuppressants induced hypertriglyceridaemia. The highest triglyceride serum concentration was measured after SRL treatment followed by TRL and CyA (Table 1). After coadministration of SRL with CyA the trigylceride serum concentration was a little bit higher than in the rats treated with SRL alone. On the other hand, in SRL/TRL-treated animals a somewhat lower triglyceride serum concentration than after SRL or SRL/CyA treatment was determined (Table 1). CyA was described to reduce LDL-receptor activity in a hepatocellular tumour line (al Rayes et al., 1997). In experiments with normolipidaemic New Zealand White rabbits tacrolimus decreased the clearance of radiolabelled human LDL and very low-density lipoproteins (VLDL) being injected into the animals (Baier et al., 2000). A similar mechanism has been postulated for the hyperlipidaemic effects of sirolimus. In clinical studies in renal transplant patients SRL could induce both hypercholesterol- and hypertriglyceridaemia. The authors concluded from their results that these hyperlipidaemic effects of SRL were not caused by an enhanced hepatic VLDL production, but rather by a decrease in the clearance of ApoB100-containing lipoproteins (Hoogeveen et al., 2001). These differences in the mechanisms of immunosuppressant-induced hypertriglyceridaemia (reduction of LDL-receptor activity in the case of CyA-induced and reduction of LDL clearance in the case of SRL- and TRL-induced hypertriglyceridaemia) may explain the slightly elevated triglycerid serum concentrations in the SRL/CyA-treated animals in comparison to the SRL- and SRL/TRL-treated rats (Table 1). Inducing effects of SRL on key enzymes of the synthesis of fatty acid and cholesterol could serve as further explanation of the observed hyperlipidaemic effects of SRL, but have not been proven in in vitro experiments, so far.
In our experiments SRL increased blood concentrations of CyA (Figure 3A) as well as of CyA metabolites (Figure 3B). This finding cannot solely be explained by the inhibition of CyA metabolism by SRL according to CYP3A4 pathway, because in this case one should observe elevated CyA blood concentrations with simultaneously decreased blood concentrations of its metabolites. An explanation for this phenomen could be that the biliary excretion of CyA was decreased by 20% by SRL (Figure 2A) implicating the involvement of a transport system for CyA into the bile (for example P-glycoprotein). Additionally, the reabsorption of CyA in the gut may also be increased in the presence of SRL by inhibition of P-glycoprotein or CYP3A4 in the mucosa cells (Lampen et al., 1998). Both mechanisms would lead to higher amounts of CyA in the body, finally resulting in higher blood concentrations of CyA metabolites, too. Interactions on biliary excretion of SRL with other drugs that are eliminated mainly via bile such as statines, protease inhibitors, or some sulfonylurea antidiabetica could be of importance, as well, in clinical situations. The biliary excretion of the CyA metabolites, however, was not impaired by SRL (Figure 2B) indicating that their transport into the bile is not specific or, alternatively, mediated by a transporter not involved in CyA elimination.
The TRL blood concentration was increased by SRL, as well (Figure 4B). Because the biliary excretion of TRL was only slightly reduced by SRL (Figure 4D), we conclude that inhibition of TRL metabolism plays a more important role for the elevated TRL blood concentrations than its decreased biliary elimination. CyA increased SRL blood concentration as well as biliary excretion of SRL, which can be explained by reduction of SRL metabolism by CyA. On the other side, neither SRL blood concentrations nor biliary SRL excretion were affected by TRL although both substances bind with the same Ks values (1–2 μmol l−1) to purified reconstituted human CYP3A4 with similiar velocities of main metabolites formation (Sattler et al., 1992).
In summary, we showed in the subchronic bile fistula model in the rat that SRL, CyA, as well as TRL have cholestatic properties and were able to induce both hypercholesterol- and hypertriglyceridaemia after daily treatment with these immunosuppressants over 2 weeks. SRL/CyA increased serum cholesterol concentrations to the same extent as SRL/TRL did. Serum triglyceride concentrations were a little bit more increased by SRL/CyA than SRL/TRL, whereas bile flow was significantly less decreased by SRL/TRL than by SRL/CyA. SRL led to an increase of both CyA and TRL blood concentrations. However, SRL blood concentration was only increased by CyA but not by TRL. Therefore, SRL/TRL could be the better pharmacological regimen than SRL/CyA in cholestatic patients and in those facing difficulties to reach the narrow therapeutic range of SRL.
Acknowledgments
We thank Annette Garbe, Ingelore Hackbarth, and Annette Linck for excellent technical assistance. Thanks to Annette Stanke for expert preparation of manuscript. We thank Wyeth-Pharma GmbH, Münster, Germany, for providing Rapamune oral solution and Wyeth-Ayerst, Princeton, U.S.A. for providing 32-desmethoxyrapamycin. This study was supported by the Deutsche Forschungsgemeinschaft grant SFB265 A7.
Abbreviations
- Bsep
bile salt export pump
- CyA
cyclosporine
- CYP3A4
cytochrome P450 3A4
- GSH
reduced glutathione
- i.p.
intraperitoneally
- i.v.
intravenously
- LC
liquid chromatography
- LC-ESI/MS
liquid chromatography-electrospray ionization/mass spectrometry
- LDL
low-density lipoproteins
- l.w.
liver weight
- Mrp2
multidrug resistance protein 2
- Ntcp
Na+/taurocholate cotransporting polypeptide
- Oatp
organic anion transporting polypeptide
- p.o.
peroral
- SRL
sirolimus
- TRL
tacrolimus
- VLDL
very low-density lipoproteins
References
- AL RAYYES O., WALLMARK A., FLOREN C.H. Reversal of cyclosporine-inhibited low-density lipoprotein receptor activity in HepG2 cells by 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Hepatology. 1997;25:991–994. doi: 10.1002/hep.510250433. [DOI] [PubMed] [Google Scholar]
- ANDOH T.F., BURDMAN E.A., BENNETT W.M. Nephrotoxicity of immunosuppressive drugs: experimental and clinical observations. Semin. Nephrol. 1997;17:34–45. [PubMed] [Google Scholar]
- BAIER P., BUETTNER C.A., GENSCHEL J., PROEPSTING M.J., MANNS M.P., LOCHS H., SCHMIDT H.HJ. Verlangsamter Metabolismus von LDL und VLDL unter Immunsuppression im Kaninchenmodell. Transplantationsmedizin. 2000;53 Suppl. [Google Scholar]
- BÖHME M., MÜLLER M., LEIER I., JEDLITSCHKY G., KEPPLER D. Cholestasis caused by inhibition of the adenosine triphosphat-dependent bile salt transport in rat liver. Gastroenterology. 1994;107:255–265. doi: 10.1016/0016-5085(94)90084-1. [DOI] [PubMed] [Google Scholar]
- BENNETT W.M., DEMATTOS A., MEYER M.M., ANDOH T., BARRY J.M. Chronic cyclosporine nephropathy: the Achilles' heel of immunosuppressive therapy. Kidney Int. 1996;50:1089–1100. doi: 10.1038/ki.1996.415. [DOI] [PubMed] [Google Scholar]
- BRAMOW S., OTT P., NIELSEN F:T., BANGERT K., TYGSTRUP N., DALHOFF K. Cholestasis and regulation of genes related to drug metabolism and biliary transport in rat liver following treatment with cyclosporine A and sirolimus (Rapamycin) Pharmacol. Toxicol. 2001;89:133–139. doi: 10.1034/j.1600-0773.2001.d01-147.x. [DOI] [PubMed] [Google Scholar]
- CHARCO R., CANTARELL C., VARGAS V., CAPDEVILA L., LAZARO J.L., HIDALGO MURIO E., MARGARIT C. Serum cholesterol changes in long-term survivors of liver transplantation: a comparison between cyclosporine and tacrolimus therapy. Liver Transpl. Surg. 1999;5:204–208. doi: 10.1002/lt.500050303. [DOI] [PubMed] [Google Scholar]
- CHISHOLM J.W., NATION P., DOLPHIN P.J., AGELLON L.B. High plasma cholesterol in drug-induced cholestasis is associated with enhanced hepatic cholesterol synthesis. Am. J. Physiol. 1999;276:G1165–G1173. doi: 10.1152/ajpgi.1999.276.5.G1165. [DOI] [PubMed] [Google Scholar]
- CONSENSUS DOCUMENT Hawk's Cay meeting on the therapeutic drug monitoring of cyclosporine. Transplant. Proc. 1999;22:1357–1361. [PubMed] [Google Scholar]
- CHRISTIANS U., ZIMMER K.O, , WONIGEIT K., MAURER G., SEWING K.F. Liquid chromatographic measurement of cyclosporine A and its metabolites in blood, bile and urine. Clin. Chem. 1988;34:34–39. [PubMed] [Google Scholar]
- DETERS M., NOLTE K., KIRCHNER G., RESCH K., KAEVER V. Comparative study analyzing effects of sirolimus-cyclosporin and sirolimus-tacrolimus combinations on bile flow in the rat. Dig. Dis. Sci. 2001;46:2120–2126. doi: 10.1023/a:1011942310737. [DOI] [PubMed] [Google Scholar]
- ERLINGER S.Bile flow The Liver: Biology and Pathobiology 1982New York: Raven; 407–427.ed. Arias, I., Popper, H., Schacter, D. Schafritz, D. pp [Google Scholar]
- GALAN A.I., FERNANDEZ E., MORAN D., MUNOZ M.E., JIMENEZ R. Cyclosporine A hepatotoxicity: effect of prolonged treatment with cyclosporine on biliary lipid secretion in the rat. Clin. Exp. Pharmacol. Physiol. 1995;22:260–265. doi: 10.1111/j.1440-1681.1995.tb01991.x. [DOI] [PubMed] [Google Scholar]
- GRAHAM J., AHMED H., NORTHFIELD T.C.Unidirectional pathway of vesicular cholesterol transport Trends in Bile Salt Research 1989New York: Kluwer; 177–187.ed. Paumgartner, G., Dordrecht. pp [Google Scholar]
- GRANGER D.K., CROMWELL J.W., CHEN S.C., GOSWITZ J.J, , MORROW D.T., BEIERLE F.A., SEGHAL S.N., CANAFAX D.M., MATAS A.J. Prolongation of renal allograft in a large animal model by oral rapamycin monotherapy. Transplantation. 1995;59:183–186. [PubMed] [Google Scholar]
- HOOGEVEEN R.C., BALLANTYNE C.M., POWNALL H.J., OPEKUN A.R., HACHEY D.L., JAFFE J.S., OPPERMANN S., KAHAN B.D., MORRISETT J.D. Effect of sirolimus on the metabolism of ApoB100-containing lipoproteins in renal transplant patients. Transplantation. 2001;72:1244–1250. doi: 10.1097/00007890-200110150-00011. [DOI] [PubMed] [Google Scholar]
- ICHIMARU N., TAKHARA S., KOKADO Y., WANG J., HATORI M., KAMEOKA INOUE T., OKUYAMA A. Changes in lipid metabolism and effect on simvastatin in renal transplant recipients induced by cyclosporine or tacrolimus. Atherosclerosis. 2001;158:417–423. doi: 10.1016/s0021-9150(01)00438-5. [DOI] [PubMed] [Google Scholar]
- KAHAN B.D. Cyclosporine. New Engl. J. Med. 1989;321:1725–1738. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- KAHAN B.D., JULIAN B.A., PESCOVITZ M.D., VANRENTERGHEM Y., NEYLAN J. Sirolimus reduces the incidence of acute rejection episodes despite lower cyclosporine doses in caucasian recipients of mismatched primary renal allografts: a phase II trial. Rapamune Study Group. Transplantation. 1999;68:1526–1532. doi: 10.1097/00007890-199911270-00016. [DOI] [PubMed] [Google Scholar]
- KAWAMURA I., TAKESHITA S., FUSHIMI M., MABUCHI M., SEKI J., GOTO T. Induction of choleresis by immunosuppressant FK506 through stimulation of insulin-like growth factor-I production in the liver of rats. Eur. J. Pharmacol. 2001;419:99–105. doi: 10.1016/s0014-2999(01)00961-x. [DOI] [PubMed] [Google Scholar]
- KIRCHNER G.I., JACOBSEN W., DETERS M., CHRISTIANS U., NASHAN B., WINKLER M., VIDAL C., KAEVER V., SEWING K., MANNS M.P. Fast quantification method for sirolimus and its major metabolites. Transplant. Proc. 2001;33:1091–1092. doi: 10.1016/s0041-1345(00)02430-1. [DOI] [PubMed] [Google Scholar]
- LAMPEN A., ZHANG Y., HACHBARTH I., BENET L.Z. , SEWING K.F., CHRISTIANS U. Metabolism and transport of the macrolide immunosuppressant sirolimus in the small intestine. J. Pharmacol. Exp. Ther. 1998;285:1104–1112. [PubMed] [Google Scholar]
- LE THAI B., DUMONT M., MICHEL A., ERLINGER S., HOUSSIN D. Cholestatic effect of cyclosporine in the rat. Transplantation. 1988;46:510–512. doi: 10.1097/00007890-198810000-00008. [DOI] [PubMed] [Google Scholar]
- LIU J., FARMER J.D., JR, LANE C., FRIEDMAN J., WEISSMAN I., SCHREIBER S. Calcineurin is a common target of cyclophilin-cyclosporine A and FKBP-FK 506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- MASHIGE F., TANAKA N., MAKI A., KAMEI S., YAMANAKA M. Direct spectrophotometry of total bile acids in serum. Clin. Chem. 1981;27:13452–13456. [PubMed] [Google Scholar]
- MAYER A.D., DMITREWSKI J., SQUIFFLET J.P., BESSE T., GRABENSEE B., KLEIN B., EIGLER F.W., HEEMANN U., PICHLMAYR R., BEHREND M., VANRENTERGHEM Y., DONCK J., VAN HOOFF J., CHRISTIAANS M., MORALES J.M., ANDRES A., JOHNSON R.W., SHORT C., BUCHHOLZ B., REHMERT N., LAND W., SCHLEIBNER S., FORSYTHE J.L., TALBOT D., NEUMAYR H.H., HAUSER I., ERICZON B.G., BRATTSTRÖM C., CLAESSON K., MÜHLBACHER F., POHANKA E. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection. Transplantation. 1997;64:436–443. doi: 10.1097/00007890-199708150-00012. [DOI] [PubMed] [Google Scholar]
- MCALISTER V.C., GAO Z., PELTIKIAN K., DOMINGUES J., MAHALATI K., MACDONALD A.S. Sirolimus-tacrolimus combination immunosuppression. Lancet. 2000;355:376–377. doi: 10.1016/S0140-6736(99)03882-9. [DOI] [PubMed] [Google Scholar]
- MCGOWAN M.W., ARTISS J.D., STRANDBERGH D.R., ZAK B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin. Chem. 1983;29:538–542. [PubMed] [Google Scholar]
- MIZUTA K., KOBAYASHI E., UCHIDA H., FUFIMURA A., KAWARASAKI H., HASHIZUME K. Influence of tacrolimus on bile acid and lipid composition in continuously drained bile using a rat model. Comparative Study with cyclosporine. Transpl. Int. 1999;12:316–322. doi: 10.1007/s001470050234. [DOI] [PubMed] [Google Scholar]
- MORAN D., DE BUITRAGO J.M., FERNANDEZ E., GALAN A.I., MUNOZ M.E., JIMENEZ R. Inhibition of biliary glutathione secretion by cyclosporine A in the rat: possible mechanisms and role in the cholestasis induced by the drug. J. Hepatol. 1998;29:68–77. doi: 10.1016/s0168-8278(98)80180-3. [DOI] [PubMed] [Google Scholar]
- MOSLEY R.H., JOHNSON T.R., MORRISSETTE J.M. Inhibition of bile salt transport by cyclosporine A in rat liver plasma membrane vesicles. J. Pharmacol. Exp. Ther. 1990;253:974–980. [PubMed] [Google Scholar]
- MÜLLER M., JANSEN P.L. The secretory function of the liver: new aspects of hepatobiliary transport. J. Hepatol. 1998;28:344–354. doi: 10.1016/0168-8278(88)80024-2. [DOI] [PubMed] [Google Scholar]
- MURGIA M.G., JORDAN S., KAHAN B.D. The side effect profile of sirolimus: a phase I study in quiescent cyclosporine-prednisolon-treated renal transplant patients. Kidney Int. 1996;49:209–216. doi: 10.1038/ki.1996.28. [DOI] [PubMed] [Google Scholar]
- PAULUSMA C.C., VAN GEER M.A., EVERS R., HEIJN M., OTTENHOFF R., BORST P., OUDE ELFERINK R.P. Canalicular multispecific organic anion transporter/multidrug resistance protein 2 mediates low-affinity transport of reduced glutathione. Biochem. J. 1999;338:393–401. [PMC free article] [PubMed] [Google Scholar]
- SANCHEZ-CAMPOS S., LOPEZ-ACEBO R., GONZALEZ P., CULEBRAS J.M., TUNON M.J., GONZALEZ-GALLEGO J. Cholestasis and alterations of glutathione metabolism induced by tacrolimus (FK506) in the rat. Transplantation. 1998;66:84–88. doi: 10.1097/00007890-199807150-00013. [DOI] [PubMed] [Google Scholar]
- SATTLER M., GUENGERICH F.P., YUN C.H., CHRISTIANS U., SEWING K.F. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab. Dispos. 1992;20:753–761. [PubMed] [Google Scholar]
- SEGHAL S.N. Rapamune (sirolimus, rapamycin): an overview and mechanism of action. Ther. Drug Monitor. 1995;17:660–665. doi: 10.1097/00007691-199512000-00019. [DOI] [PubMed] [Google Scholar]
- SIEDEL J., HAGELE E.O., ZIEGENHORN J., WAHLEFELD A.W. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin. Chem. 1983;29:1075–1080. [PubMed] [Google Scholar]
- STACEY N.H., KOTECKA B. Inhibition of taurocholate and ouabain transport in isolated rat hepatocytes by cyclosporin A. Gastroenterology. 1988;95:780–786. doi: 10.1016/s0016-5085(88)80028-3. [DOI] [PubMed] [Google Scholar]
- STIEGER B., FATTINGER K., MADON J., KULLAK-UBLICK G.A., MEIER P.J. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology. 2000;118:422–430. doi: 10.1016/s0016-5085(00)70224-1. [DOI] [PubMed] [Google Scholar]
- TROTTER J.F., WACHS M.E., TROUILLOT T.E., BAK T., KUGELMAS M., KAM I., EVERSON G. Dyslipidemia during sirolimus therapy in liver transplant recipients occurs with concomitant cyclosporine but not tacrolimus. Liver Transpl. 2001;7:401–408. doi: 10.1053/jlts.2001.23916. [DOI] [PubMed] [Google Scholar]
- VU M.D., QI S., XU D., WU J., FRITZSIMMONS W.E., SEHGAL S.N., DUMONT L., BUSQUE S., DALOZE P., CHEN H. Tacrolimus (FK506) and sirolimus (rapamycin) in combination are not antagonist but produce extended graft survival in cardiac transplantation in the rat. Transplantation. 1997;64:1853–1856. doi: 10.1097/00007890-199712270-00039. [DOI] [PubMed] [Google Scholar]
- WALTERS M.I., GERARDE H.W. An ultramicromethod for the determination of conjugated and total bilirubin in serum or plasma. Microchem. J. 1970;15:231. [Google Scholar]


