Abstract
The effect of docosahexaenoic acid (DHA) on nitric oxide (NO) production and inducible NO synthase (iNOS) expression induced by interleukin (IL)-1β, and whether the effect of DHA is related to its effect on mitogen-activated protein kinase (MAPK) activation were investigated in cultured rat vascular smooth muscle cells (VSMCs).
DHA and eicosapentaenoic acid (EPA), although less potent, increased the NO production induced by IL-1β (3 ng ml−1) in a concentration-dependent manner (3–30 μM) Arachidonic acid had no significant effect. The stimulatory effect of DHA (30 μM) on the NO production was more obvious at lower concentrations of IL-1β.
IL-1β induced iNOS protein and mRNA expressions, which were significantly potentiated by DHA. EPA (30 μM) had a tendency to increase the iNOS protein and mRNA expressions, but arachidonic acid had no effect.
IL-1β-induced iNOS protein expression was significantly inhibited by PD 98059 (10 μM), a selective inhibitor of p44/42 MAPK kinase, both in the absence and the presence of DHA. SB 203580 (10 μM), a selective inhibitor of p38 MAPK activity, had no significant effect, although had a tendency to inhibit slightly.
IL-1β increased the phosphorylation of p44/42 MAPK, while it did not apparently increase the phosphorylation of p38 MAPK. DHA significantly potentiated the IL-1β-induced phosphorylation of p44/42 MAPK, while it had no significant effect on the phosphorylation of p38 MAPK.
These results suggest that DHA increases NO production by potentiating iNOS expression induced by IL-1β through mechanism involving p44/42 MAPK signalling cascade in rat VSMCs. The present study may contribute to the understanding of basic mechanisms underlying the beneficial effects of DHA on various cardiovascular disorders.
Keywords: Docosahexaenoic acid, nitric oxide, inducible nitric oxide synthase, mitogen-activated protein kinase, vascular smooth muscle cells, cardiovascular protection
Introduction
There are a number of evidences showing that diets rich in fish oils attenuate the progression of several types of human and experimental cardiovascular disorders such as myocardial infarction, arrhythmia, atherosclerosis or hypertension. It is widely accepted that n-3 polyunsaturated fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) contained in fish oils are the active biological components of these protective effects (for reviews, Leaf & Weber, 1989; Horrocks & Yeo, 1999). Although the precise mechanisms are not yet fully understood, the protective effects could be partially attributed to their direct effects on vascular endothelial and smooth muscle cell functions. In fact, dietary administration of fish oils to genetically hypertensive rats has been shown to reduce exaggerated contractility of the vasculature in response to sympathetic nerve stimulation or noradrenaline (Yin et al., 1991; Mano et al., 1995; Mclennan et al., 1996). DHA has been shown to be more effective than EPA in suppressing arrhythmia induced by ischaemia in rat, inhibiting thromboxane-like vasoconstrictor responses in aorta from spontaneously hypertensive rats (SHR), and retarding hypertension development in hypertensive rat (Mclennan et al., 1996). Further, the in vitro vasorelaxant effect of DHA on isolated rat aortic rings is greater than that of EPA (Engler, 1992). These vasorelaxant effects of DHA may be explained in part by inhibitory effect on intracellular Ca2+ dynamics in vascular smooth muscle cells (VSMCs) (Asano et al., 1997; Hirafuji et al., 2001).
Nitric oxide (NO) produced by constitutive NO synthase in the endothelium, is now well recognized to have important functions as endothelium-derived relaxing factor (EDRF) in cardiovascular pathophysiology (Dusting, 1995). NO can be also produced by inducible NO synthase (iNOS) in VSMCs following stimulation with cytokines such as interleukin-1β (IL-1β) or lipopolysaccharide (Busse & Mülsch, 1990). NO regulates the platelet aggregation and adhesion, leukocyte adhesion, and VSMC proliferation and migration besides the vasodilating action (Dusting, 1995). Therefore, there is a possibility that the mechanism for protective effects of DHA on cardiovascular disorders is, at least in part, attributed to the influence on NO production by VSMCs. However, it is not known whether DHA has any effect on NO production or iNOS expression in VSMCs. The induction of iNOS expression by IL-1β is known to require activation and DNA binding of nuclear factor-κB (NF-κB), but it is not sufficient for the κB-dependent transcription (Bergmann et al., 1998). Recent studies have shown that mitogen-activated protein kinase (MAPK) cascades including p44/42 MAPK and p38 MAPK are involved in IL-1β induction of iNOS in diverse cell types including VSMCs (Guan et al., 1997; Begum et al., 1998; Jiang & Brecher, 2000; Xu & Malave, 2000). The aim of the present study was, therefore, to evaluate the effect of DHA on the NO production and iNOS expression in rat VSMCs, and to assess whether the effect of DHA is related to its effect on MAPK activation.
Methods
Animals
Wistar Kyoto rats (WKY) were kept in our laboratory at room temperature of 22±2°C and humidity of 50±10%, and were fed freely water and food. This study was conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals by the Animal Research Committee of Health Sciences University of Hokkaido.
Cell culture
VSMCs were enzymatically isolated from aortic media of 6–7-week-old WKY using collagenase and elastase as described previously (Hirafuji et al., 2001). Cells were suspended in Dulbecco's modified Eagle medium containing 10% foetal bovine serum, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin, and cultured until confluence at 37°C in 95% air plus 5% CO2 with medium changes every 2–3 days (6–8 days). Cells were seeded in either 24-well culture plates or culture dishes. Primary VSMCs were used throughout the experiments. Cells were rinsed three times with serum-free medium containing 0.1% bovine serum albumin (BSA) and then treated without or with IL-1β in the absence or the presence of test drugs for the indicated time period.
Polyunsaturated fatty acids were dissolved as sodium salt in physiological saline containing 0.1% BSA as carrier protein, further diluted and added to the serum-free culture medium.
Determination of nitrite/nitrate
NO production was evaluated by measuring nitrite/nitrate (designated as NOx), stable oxidation products of NO, in the culture medium. The culture medium was applied to a copperized cadmium reduction column to reduce nitrate to nitrite, and then reacted with Griess reagent, using NOx Autoanalyzer (ENO-10, Eicom, Kyoto, Japan) (Hirafuji et al., 2002). Results were expressed as NOx nmol per mg protein. Protein was determined according to the method of Lowry et al. (1951) using BSA as the standard.
Western blot analysis
Expression of iNOS protein was analysed by Western blot analysis as described previously (Hirafuji et al., 2002). Briefly, 30–50 μg of cell lysate proteins were separated on 7.5% sodium dodecyl sulphate-polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride (PVDF) transfer membrane. iNOS protein was detected with a monoclonal antibody against mouse macrophage iNOS. Phosphorylated p44/42 MAPK and p38 MAPK proteins were detected with phospho-p44/42 MAPK and phospho-p38 MAPK polyclonal antibodies, respectively. The immunoblot was incubated with appropriate horseradish peroxidase-conjugated secondary antibodies and visualized by enhanced chemiluminescence (ECL) kit.
Reverse transcription–polymerase chain reaction
Expression of iNOS mRNA was determined using semi-quantitative reverse transcription–polymerase chain reaction (RT–PCR) with total RNA, as described previously using primers specific for rat iNOS (Hirafuji et al., 2002). Total RNA was isolated from VSMCs using RNeasy total RNA Kit. RT and PCR reactions were carried out using Superscript First-Strand Synthesis System and Expand High Fidelity PCR System, respectively. Concurrent RT–PCR amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed as an internal control for variations in the efficiencies of total RNA isolation and RT. iNOS and GAPDH primers were predicted to amplify products of 250 and 306 bp, respectively. These products were separated on an ethidium bromide-stained 1.0% agarose gel and scanned in a fluorescence image analyzer (FMBIO-100, Hitachi, Japan). The quantities of each product were analysed by densitometry using NIH image and calculated relative to GAPDH.
Materials
Foetal calf serum, penicillin, streptomycin, Dulbecco's modified Eagle medium and Superscript First-Strand Synthesis System were obtained from Life Technologies (Grand Island, NY, U.S.A.). BSA was from Boehringer Mannheim (Mannheim, Germany). Collagenase, elastase, DHA, EPA, arachidonic acid and PD 98059 were from Sigma (St. Louis, MO, U.S.A.). SB 203580 was from Calbiochem (La Jolla, CA, U.S.A.). IL-1β was from Collaborative Biomedical Products (Bedford, MA, U.S.A.). Anti-iNOS monoclonal mouse antibody was from Transduction Laboratory (Lexington, KY, U.S.A.). Polyclonal rabbit antibodies to phospho-p44/42 MAPK and phospho-p38 MAPK were from Promega (Madison, WI, U.S.A.). Rabbit anti-mouse IgG and goat anti-rabbit IgG antibodies conjugated to horseradish peroxidase were from Zymed Laboratories (San Francisco, CA, U.S.A.). ECL kit was from PerkinElmer Life Sciences (Boston, MA, U.S.A.). PVDF transfer membrane was from Millipore (Bedford, MA, U.S.A.). RNeasy total RNA Kit was from Qiagen (Hilden, Germany). Expand High Fidelity PCR System was from Roche Diagnostics (Indianapolis, IN, U.S.A.). All other agents were purchased from standard supplier.
Statistical analysis
Results were expressed as mean±s.e.mean of replicate experiments. Statistical analysis of the results was performed using Student's t-test for unpaired data, and analysis of variance followed by Bonferroni/Dunn test for multiple comparisons. P values less than 0.05 were considered as significant.
Results
Effects of polyunsaturated fatty acids on NO production
When VSMCs were stimulated with IL-1β, NO was produced in a time-dependent manner, progressively increasing after 24 h up to 48 h (data not shown). VSMCs were stimulated with 3 ng ml1 IL-1β for 48 h in the absence or the presence of various concentrations (3–30 μM) of DHA, EPA and arachidonic acid to evaluate the effects of these polyunsaturated fatty acids on the NO production. As shown in Figure 1A, DHA significantly increased IL-1β-induced NO production in a concentration-dependent manner. Similarly, EPA, although less potent than DHA, significantly increased the NO production (Figure 1B). In contrast, arachidonic acid had no significant stimulatory effect (Figure 1C). DHA (30 μM) alone had no effect on the NO production (data not shown).
Figure 1.
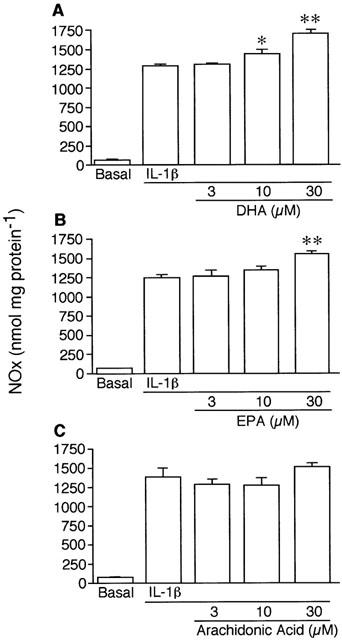
Effects of DHA (A), EPA (B) and arachidonic acid (C) on IL-1β-induced NO production by VSMCs. Cells were stimulated with 3 ng ml−1 IL-1β for 48 h in the absence or the presence of various concentrations of each fatty acid. NO was determined as nitrite/nitrate, and designated as NOx. Each result is a representative of 3–4 separate experiments with qualitatively similar results. Each column represents mean±s.e.mean of four cultures. *P<0.05, **P<0.01 versus IL-1β alone.
Figure 2 demonstrated the effect of DHA on NO production as a function of IL-1β concentration. The stimulatory effect of DHA (30 μM) on the NO production was more obvious at lower concentrations of IL-1β.
Figure 2.
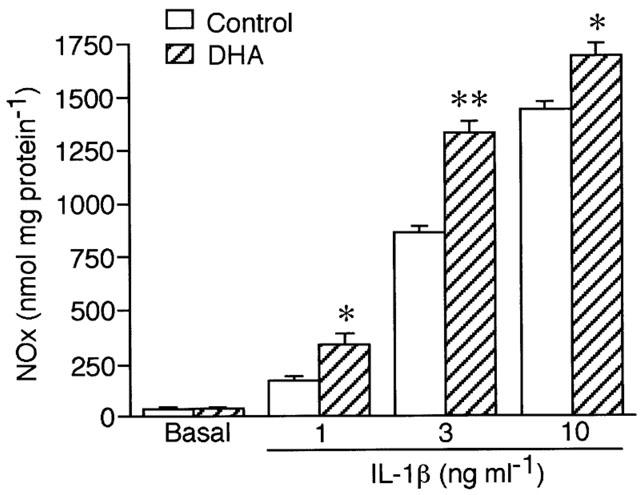
Effects of DHA on IL-1β-induced NO production by VSMCs as a function of IL-1β concentration. Cells were stimulated with the indicated concentration of IL-1β for 48 h in the absence (control) or the presence of 30 μM DHA. Result is a representative of four separate experiments with qualitatively similar results. Each column represents mean±s.e.mean of three cultures. *P<0.05, **P<0.01 versus control.
DHA (30 μM) also significantly (P<0.05) stimulated NO production induced by 3 ng ml−1 IL-1β for 24 h (161.4±23.4 versus 230.9±11.9 nmol mg protein−1, for control versus DHA, respectively, mean±s.e.mean n=6).
Effects of polyunsaturated fatty acids on iNOS protein expression
The effects of DHA, EPA and arachidonic acid on iNOS protein expression induced by IL-1β in VSMCs were investigated by Western blot analysis. Control experiment showed that iNOS expression reached the maximum almost at 24 h after IL-1β stimulation, and unchanged up to 48 h. Therefore, VSMCs were stimulated with 3 ng ml−1 IL-1β for 24 h in the absence or the presence of polyunsaturated fatty acids. As shown in Figure 3A, IL-1β stimulation induced the expression of iNOS protein, which was enhanced by DHA in a concentration-dependent manner. Figure 3B,C showed a representative Western blot image and the summary of densitometric analysis for the effects of DHA, EPA and arachidonic acid (30 μM) on IL-1β-induced iNOS expression, respectively. DHA significantly (P<0.01) increased the iNOS expression to 178.4±23.0% of control (n=10). EPA had a tendency (P<0.1) to increase the iNOS expression to 135.5±9.4% of control (n=6). However, arachidonic acid had no significant effect.
Figure 3.
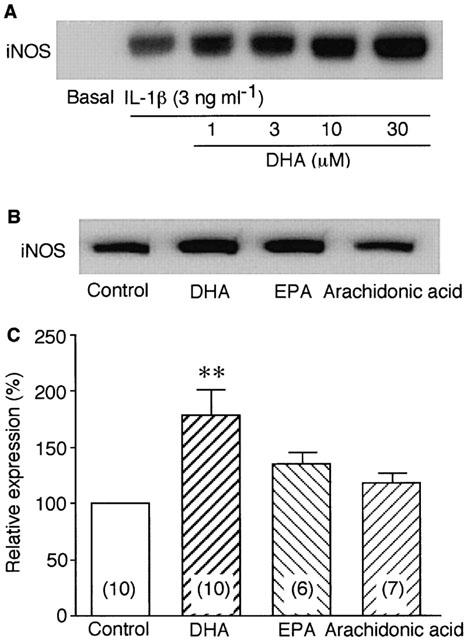
Effects of polyunsaturated fatty acids on IL-1β-induced iNOS protein expression in VSMCs. Cells were stimulated with 3 ng ml−1 IL-1β for 24 h in the absence or the presence of each fatty acid as indicated. iNOS expressions were analysed by Western blot. (A) Dose-dependent effect of DHA on iNOS expression. (B) Effects of 30 μM DHA, EPA and arachidonic acid on IL-1β-induced iNOS expression. Control means IL-1β alone. (C) Summary of densitometric analysis expressed as percentage taking the control as 100%. Each column represents mean±s.e.mean of replicate experiments indicated in parentheses. **P<0.01 versus control.
Effects of polyunsaturated fatty acids on iNOS mRNA expression
The effects of DHA, EPA and arachidonic acid on iNOS mRNA expression induced by IL-1β in VSMCs were also determined by semi-quantitative RT–PCR method. Figure 4A showed a representative fluorescence image of RT–PCR products. Densitometric analysis showed that IL-1β-induced iNOS mRNA expression was potentiated by DHA (30 μM) and slightly by EPA (30 μM), but not by arachidonic acid. As shown in Figure 4B, DHA significantly (P<0.01) increased the IL-1β-induced iNOS mRNA expression by 24.8% of control (n=4).
Figure 4.
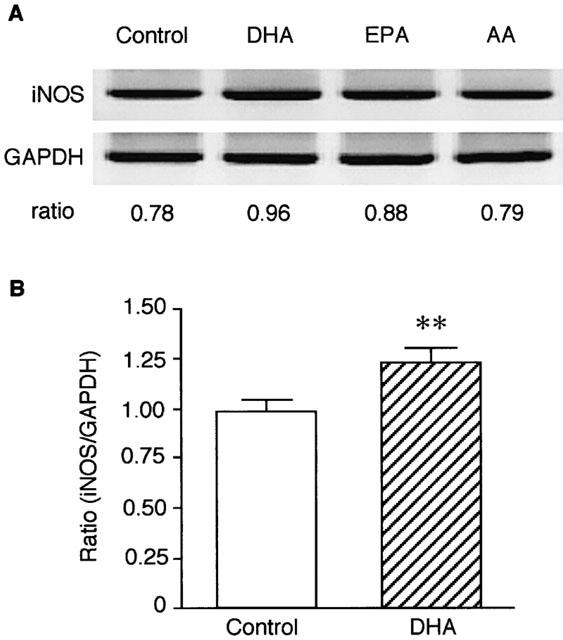
Effects of DHA, EPA and arachidonic acid (AA) on IL-1β-induced iNOS mRNA expression in VSMCs. Cells were stimulated with 3 ng ml−1 IL-1β for 24 h in the absence (control) or the presence of 30 μM each fatty acid. iNOS mRNA expression was analysed by semi-quantitative RT–PCR. (A) Fluorescence images of RT–PCR products for iNOS and GAPDH. Each ratio of iNOS/GAPDH according to densitometric analysis was demonstrated below the image. (B) Summary of densitometric analysis expressed as the ratio. Each column represents mean±s.e.mean of four separate experiments. **P<0.01 versus control.
Effects of MAPK inhibitors on iNOS protein expression
Figure 5 demonstrated the effects of PD 98059 (10 μM), a selective inhibitor of p44/42 MAPK kinase, and SB 203580 (10 μM), a selective inhibitor of p38 MAPK activity, on IL-1β-induced iNOS protein expression in the absence or the presence of DHA (30 μM). Figure 5A, B and C showed a representative Western blot image of an experiment and the summary of densitometric analysis, respectively. iNOS expression induced by IL-1β (3 ng ml−1) in VSMCs was significantly inhibited by PD 98059 both in the absence and the presence of DHA. SB 203580 had no significant effect, although had a tendency to inhibit slightly.
Figure 5.
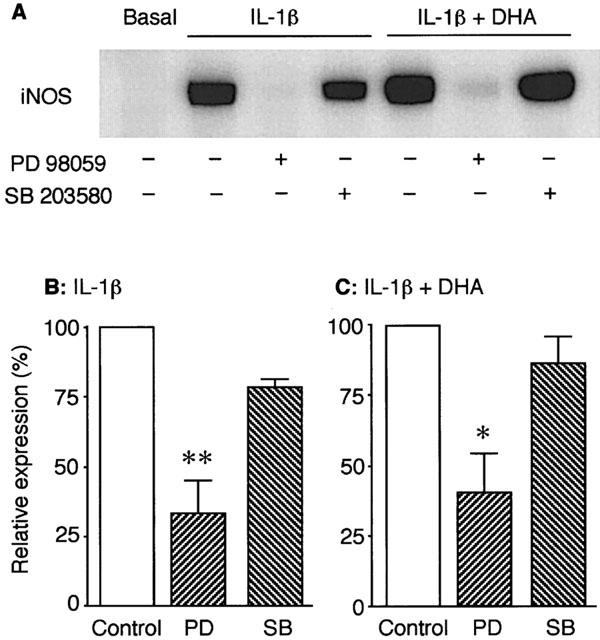
The effects of PD 98059, a selective inhibitor of p44/42 MAPK phosphorylation, and SB 203580, a selective inhibitor of p38 MAPK activity, on IL-1β-induced iNOS protein expression in VSMCs. Cells were stimulated with 3 ng ml−1 IL-1β for 24 h in the absence or the presence of DHA (30 μM) and the inhibitors (10 μM) as indicated. iNOS expression was analysed by Western blot. (A) A representative blot image of an experiment. (B and C) Summary of densitometric analysis expressed as percentage taking each control (IL-1β alone and IL-1β plus DHA, respectively) as 100%. Each column represents mean±s.e.mean of four separate experiments. PD: PD 98059; SB: SB 203580. *P<0.05, **P<0.01 versus each control.
Effects of DHA on MAPK activation
The effect of DHA on MAPK activation was determined by Western blot analysis of phosphorylated p44/42 and p38 MAPK protein expressions. Figure 6A demonstrated representative Western blot images for phosphorylated p44/42 MAPK (upper) and p38 MAPK (lower) proteins. Stimulation of VSMCs with IL-1β (3 ng ml−1) increased the phosphorylation of p44/42 MAPK, which reached the maximum within 10 min after the stimulation both in the absence and the presence of 30 μM DHA. IL-1β did not increase apparently the phosphorylation of p38 MAPK both in the absence and the presence of DHA. As summarized in Figure 6B, DHA significantly potentiated the IL-1β-induced phosphorylation of p44/42 MAPK. However, as summarized in Figure 6C, DHA had no significant effect on the phosphorylation of p38 MAPK.
Figure 6.
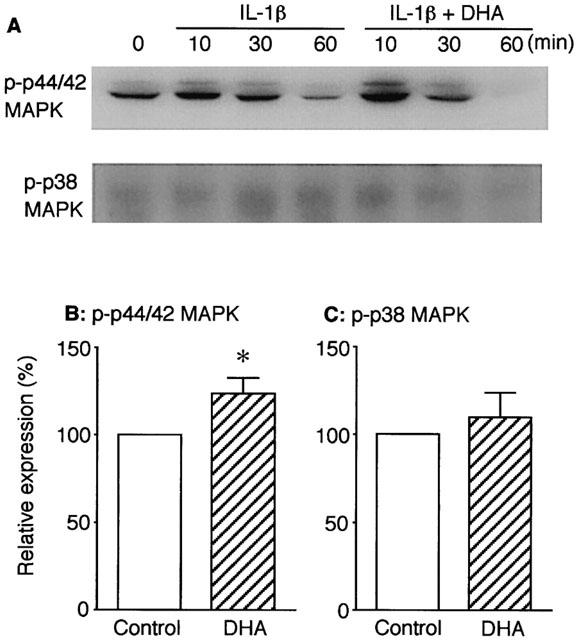
Effect of DHA on p44/42 MAPK and p38 MAPK activations induced by IL-1β in VSMCs. Expressions of phosphorylated p44/42 (p-p44/42) MAPK and p38 (p-p38) MAPK proteins were determined by Western blot analysis. (A) Representative Western blot images for p-p44/42 MAPK (upper) and p-p38 MAPK (lower). Cells were stimulated with 3 ng ml−1 IL-1β in the absence or the presence of DHA (30 μM) for the indicated time periods. (B and C) Summary of densitometric analysis for p-p44/42 MAPK and p-p38 MAPK, respectively, expressed as percentage taking each control (IL-1β alone) as 100%. Cells were stimulated with IL-1β in the absence or the presence of DHA for 10 min. Each column represents mean±s.e.mean of four separate experiments. *P<0.05 versus control.
Discussion
The present study, for the first time, demonstrated that DHA, when added to the culture medium, significantly potentiated IL-1β-induced NO production in a concentration-dependent manner in rat VSMCs. DHA alone had no stimulatory effect on the NO production. The effect of DHA was not too remarkable, but substantial especially when IL-1β concentration was low (1 ng ml−1). DHA also increased IL-1β-induced iNOS mRNA and protein expressions. These results clearly indicate that DHA enhanced the upstream signalling cascade leading to iNOS expression in IL-1β-stimulated rat VSMCs. Although less potent than DHA, EPA, but not arachidonic acid, also showed the stimulatory effect, suggesting that this effect is common to n-3 polyunsaturated fatty acids.
Recent studies have shown that MAPK cascades including p44/42 MAPK and p38 MAPK are involved in IL-1β induction of iNOS in diverse cell types including VSMCs, although there are some discrepancies in these results (Guan et al., 1997; Begum et al., 1998; Jiang & Brecher, 2000; Xu & Malave, 2000). We also demonstrated that p44/42 MAPK in VSMCs was activated by IL-1β stimulation, and that the inhibition of p44/42 MAPK phosphorylation by PD 98059, a selective inhibitor of the upstream MAPK kinase, resulted in a significant reduction of iNOS expression induced by IL-1β, suggesting the requirement for p44/42 MAPK cascade activation to induce iNOS expression. In our experiment, p38 MAPK was not apparently activated by IL-1β stimulation. Pretreatment of VSMCs with SB 203580, an effective inhibitor of p38 MAPK, at a concentration of 10 μM showed little inhibitory effect on the iNOS expression. This result indicates less importance for the p38 MAPK cascade in the IL-1β induction of iNOS in rat VSMCs, in consistency with that of Jiang & Brecher (2000).
The IL-1β-activated phosphorylation of p44/42 MAPK was further potentiated by coincubation with DHA. In contrast, the phosphorylation of p38 MAPK was not potentiated by DHA. Inhibition of p44/42 MAPK phosphorylation by the selective inhibitor PD 98059 almost completely inhibited the iNOS expression both in the absence and the presence of DHA, while the p38 MAPK inhibitor SB 203580 had little inhibitory effect. These results suggest that DHA increases NO production by potentiating iNOS expression induced by IL-1β through mechanism involving p44/42 MAPK signalling cascade in rat VSMCs. In cultured vascular cells, DHA can be easily incorporated into the membrane phospholipids (Morisaki et al., 1985) and modulates membrane properties such as membrane fluidity (Hashimoto et al., 1999). Since the MAPK cascade is initiated at the plasma membrane and modified by the membrane lipid microenvironment (Casey, 1994), the alteration in the membrane properties may directly or indirectly enhance downstream signalling pathways after IL-1β receptor stimulation. As the potentiating effect of DHA at 24 h incubation was greater in iNOS protein expression (78.4% increase) than in iNOS mRNA expression (24.8% increase), it appears that DHA may also enhance the post-transcriptional pathways. However, since iNOS protein levels are regulated by transcription, mRNA stability, translation, and protein turnover, it is hard to determine exactly how DHA upregulates iNOS protein induction. The present study did not address whether DHA had an effect on NF-κB activation pathways. Further studies are required to determine the precise mechanism.
In consistency with our results, EPA or its ethyl ester has been reported to potentiate the release of NO from cultured VSMCs (Schini et al., 1993) and endothelial cells (Boulanger et al., 1990; Okuda et al., 1997). DHA as well as EPA enhances EDRF release in isolated rat aortic rings (Lawson et al., 1991). In contrast, DHA has been demonstrated to inhibit NO production by interferon-γ plus lypopolysaccharide-stimulated mouse macrophages by inhibiting transcription of iNOS gene (Khair-El-din et al., 1996). Furthermore, DHA or EPA incubation is reported to suppress p44/42 MAPK activation in macrophages (Lo et al., 2000), a malignantly transformed colonic cell line (Collett et al., 2001) and Jurkat T cells (Denys et al., 2001). Although at present we have no clear explanation for these discrepancies, the effect of n-3 unsaturated fatty acids may depend on cell types, being different between vascular and other cell types, and/or experimental conditions such as their concentration or the stimulus used.
Besides the vasodilating effect, NO has inhibitory effects on platelet aggregation, leukocyte adhesion, VSMC proliferation and migration, and induces VMSC apoptosis, suggesting an important role of iNOS-derived NO in atherosclerosis and vascular remodelling (Dusting, 1995). The iNOS induction in VSMCs, therefore, may primarily function as a defensive and compensatory mechanism for lack of endothelial function, by preventing the development of pathological conditions. In fact, several studies have been shown that iNOS expression in aortae isolated from SHR in the established stage of hypertension is higher than that from WKY (Junquero et al., 1993; Wu et al., 1996; Briones et al., 2000), while iNOS expression in cultured VSMCs derived from SHR or stroke-prone SHR (SHRSP) is lower than that from WKY (Malinski et al., 1993; Singh et al., 1996; Dubois, 1996; Hirafuji et al., 2002). Our group has shown that diet containing DHA to young SHRSP for 14 weeks remarkably inhibits the development of hypertension (Kimura et al., 1995). The maximal concentration (30 μM) of DHA used in the present study is close to that found in the plasma of SHRSP fed diet containing 5% DHA (Minami et al., 1997). Dietary fish oils or purified DHA have been also reported to augment vascular NO release (Mcveigh et al., 1993) and plasma concentration of NO metabolites (Mohan & Das, 1997) in human. Taken together with these results, our results suggest that potentiation of NO production by DHA in VSMCs may, at least in part, contribute to its beneficial effects on various cardiovascular disorders.
In conclusion, the results of this study suggest that DHA increases NO production by potentiating iNOS expression induced by IL-1β through mechanism involving p44/42 MAPK signalling cascade in rat VSMCs. The present study may contribute to the understanding of basic mechanisms underlying the beneficial effects of DHA on various cardiovascular disorders. Further studies are still required to clarify the precise mechanism.
Acknowledgments
This work was supported in part by Grant-in-Aid for Scientific Research (No. 11672177), and by the High Technology Research Program from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations
- BSA
bovine serum albumin
- DHA
docosahexaenoic acid
- EDRF
endothelium-derived relaxing factor
- EPA
eicosapentaenoic acid
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IL-1β
interleukin-1β
- iNOS
inducible nitric oxide synthase
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- RT–PCR
reverse transcription–polymerase chain reaction
- SHR
spontaneously hypertensive rats
- SHRSP
stroke-prone spontaneously hypertensive rats
- VSMCs
vascular smooth muscle cells
- WKY
Wistar Kyoto rats
References
- ASANO M., NAKAJIMA T., IWASAWA K., HAZAMA H., OMATA M., SOMA M., YAMASHITA K., OKUDA Y. Inhibitory effects of omega-3 polyunsaturated fatty acids on receptor-mediated non-selective cation currents in rat A7r5 vascular smooth muscle cells. Br. J. Pharmacol. 1997;120:1367–1375. doi: 10.1038/sj.bjp.0701047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGMANN M., HART L., LINDSAY M., BARNES P.J., NEWTON R. IκBα Degradation and nuclear factor-κB DNA binding are insufficient for interleukin-1β and tumor necrosis factor-α-induced κB-dependent transcription. Requirement for an additional activation pathway. J. Biol. Chem. 1998;273:6607–6610. doi: 10.1074/jbc.273.12.6607. [DOI] [PubMed] [Google Scholar]
- BEGUM N., RAGOLIA L., RIENZIE J., MCCARTHY M., DUDDY N. Regulation of mitogen-activated protein kinase phosphatase-1 induction by insulin in vascular smooth muscle cells. Evaluation of the role of the nitric oxide signaling pathway and potential defects in hypertension. J. Biol. Chem. 1998;273:25164–25170. doi: 10.1074/jbc.273.39.25164. [DOI] [PubMed] [Google Scholar]
- BOULANGER C., SCHINI V.B., HENDRICKSON H., VANHOUTTE P.M. Chronic exposure of cultured endothelial cells to eicosapentaenoic acid potentiates the release of endothelium-derived relaxing factor(s) Br. J. Pharmacol. 1990;99:176–180. doi: 10.1111/j.1476-5381.1990.tb14673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIONES A.M., ALONSO M.J., MARÍN J., BALFAGÓN G., SALAICES M. Influence of hypertension on nitric oxide synthase expression and vascular effects of lipopolysaccharide in rat mesenteric arteries. Br. J. Pharmacol. 2000;131:185–194. doi: 10.1038/sj.bjp.0703552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSSE R., MÜLSCH A. Induction of nitric oxide synthetase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990;275:87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- CASEY P.J. Lipid modifications of G proteins. Curr. Opin. Cell Biol. 1994;6:219–225. doi: 10.1016/0955-0674(94)90139-2. [DOI] [PubMed] [Google Scholar]
- COLLETT E.D., DAVIDSON L.A., FAN Y.-Y., LUPTON J.R., CHAPKIN R.S. n-6 and n-3 polyunsaturated fatty acids differentially modulate oncogenic Ras activation in colonocytes. Am. J. Physiol. Cell Physiol. 2001;280:C1066–C1075. doi: 10.1152/ajpcell.2001.280.5.C1066. [DOI] [PubMed] [Google Scholar]
- DENYS A., HICHAMI A., KHAN N.A. Eicosapentaenoic acid and docosahexaenoic acid modulate MAP kinase (ERK1/ERK2) signaling in human T cells. J. Lipid Res. 2001;42:2015–2020. [PubMed] [Google Scholar]
- DUBOIS G. Decreased L-arginine-nitric oxide pathway in cultured myoblasts from spontaneously hypertensive versus normotensive Wistar-Kyoto rats. FEBS Lett. 1996;392:242–244. doi: 10.1016/0014-5793(96)00817-4. [DOI] [PubMed] [Google Scholar]
- DUSTING G.J. Nitric oxide in cardiovascular disorders. J. Vasc. Res. 1995;32:143–161. doi: 10.1159/000159089. [DOI] [PubMed] [Google Scholar]
- ENGLER M.B. Effect of omega-3 fatty acids, docosahexaenoic and eicosapentaenoic, on norepinephrine-induced contractions. Can. J. Physiol. Pharmacol. 1992;70:675–679. doi: 10.1139/y92-086. [DOI] [PubMed] [Google Scholar]
- GUAN Z., BAIER L.D., MORRISON A.R. p38 mitogen-activated protein kinase down-regulates nitric oxide and up-regulates prostaglandin E2 biosynthesis stimulated by interleukin-1β. J. Biol. Chem. 1997;272:8083–8089. doi: 10.1074/jbc.272.12.8083. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO M., HOSSAIN S., YAMASAKI H., YAZAWA K., MASUMURA S. Effects of eicosapentaenoic acid and docosahexaenoic acid on plasma membrane fluidity of aortic endothelial cells. Lipids. 1999;34:1297–1304. doi: 10.1007/s11745-999-0481-6. [DOI] [PubMed] [Google Scholar]
- HIRAFUJI M., EBIHARA T., KAWAHARA F., HAMAUE N., ENDO T., MINAMI M. Inhibition by docosahexaenoic acid of receptor-mediated Ca2+ influx in rat vascular smooth muscle cells stimulated with 5-hydroxytryptamine. Eur. J. Pharmacol. 2001;427:195–201. doi: 10.1016/s0014-2999(01)01274-2. [DOI] [PubMed] [Google Scholar]
- HIRAFUJI M., TSUNODA M., MACHIDA T., HAMAUE N., ENDO T., MIYAMOTO A., MINAMI M. Reduced expression of inducible nitric oxide synthase and cyclooxygenase-2 in vascular smooth muscle cells of stroke-prone spontaneously hypertensive rats. Life Sci. 2002;70:917–926. doi: 10.1016/s0024-3205(01)01464-3. [DOI] [PubMed] [Google Scholar]
- HORROCKS L.A., YEO Y.K. Health benefits of docosahexaenoic acid (DHA) Pharmacol. Res. 1999;40:211–225. doi: 10.1006/phrs.1999.0495. [DOI] [PubMed] [Google Scholar]
- JIANG B., BRECHER P. N-Acetyl-l-cysteine potentiates interleukin-1β induction of nitric oxide synthase. Role of p44/42 mitogen-activated protein kinases. Hypertension. 2000;35:914–918. doi: 10.1161/01.hyp.35.4.914. [DOI] [PubMed] [Google Scholar]
- JUNQUERO D.C., SCHINI V.B., SCOTT-BURDEN T., VANHOUTTE P.M. Enhanced production of nitric oxide in aortae from spontaneously hypertensive rats by interleukin-1β. Am. J. Hypertens. 1993;6:602–610. doi: 10.1093/ajh/6.7.602. [DOI] [PubMed] [Google Scholar]
- KHAIR-EL-DIN T., SICHER S.C., VAZQUEZ M.A., CHUNG G.W., STALLWORTH K.A., KITAMURA K., MILLER R.T., LU C.Y. Transcription of the murine iNOS gene is inhibited by docosahexaenoic acid, a major constituent of fetal and neonatal sera as well as fish oils. J. Exp. Med. 1996;183:1241–1246. doi: 10.1084/jem.183.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMURA S., MINAMI M., TOGASHI M., HAMAUE N., ENDO T., HIRAFUJI M., YAMADA M. Antihypertensive effect of dietary docosahexa-enoic acid (22: 6n-3) in stroke-prone spontaneously hypertensive rats. Biog. Amines. 1995;11:195–203. [Google Scholar]
- LAWSON D.L., MEHTA J.L., SALDEEN K., MEHTA P., SALDEEN T.G.P. Omega-3 polyunsaturated fatty acids augment endothelium-dependent vasorelaxation by enhanced release of EDRF and vasodilator prostaglandins. Eicosanoids. 1991;4:217–223. [PubMed] [Google Scholar]
- LEAF A., WEBER P.C. Cardiovascular effects of n-3 fatty acids. N. Engl. J. Med. 1989;318:549–557. doi: 10.1056/NEJM198803033180905. [DOI] [PubMed] [Google Scholar]
- LO C.-J., CHIU K.C., FU M., CHU A., HELTON S. Fish oil modulates macrophage P44/P42 mitogen-activated protein kinase activity induced by lipopolysaccharide. J. Parent. Enter. Nutr. 2000;24:159–163. doi: 10.1177/0148607100024003159. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MALINSKI T., KAPTURCZAK M., DAYHARSH J., BOHR D. Nitric oxide synthase activity in genetic hypertension. Biochem. Biophys. Res. Commun. 1993;194:654–658. doi: 10.1006/bbrc.1993.1871. [DOI] [PubMed] [Google Scholar]
- MANO M.T., BEXIS S., ABEYWARDENA M.Y., MCMURCHIE E.J., KING R.A., SMITH R.M., HEAD R.J. Fish oils modulate blood pressure and vascular contractility in the rat and vascular contractility in the primate. Blood Pressure. 1995;4:177–186. doi: 10.3109/08037059509077591. [DOI] [PubMed] [Google Scholar]
- MCLENNAN P., HOWE P., ABEYWARDENA M., MUGGI R., RAEDERSTORFF D., MANO M., RAYNER T., HEAD R. The cardiovascular protective role of docosahexaenoic acid. Eur. J. Pharmacol. 1996;300:83–89. doi: 10.1016/0014-2999(95)00861-6. [DOI] [PubMed] [Google Scholar]
- MCVEIGH G.E., BRENNAN G.M., JOHNSTON G.D., MCDERMOTT B.J., MCGRATH L.T., HENRY W.R., ANDREWS J.W., HAYES J.R. Dietary fish oil augments nitric oxide production or release in patients with Type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1993;36:33–38. doi: 10.1007/BF00399090. [DOI] [PubMed] [Google Scholar]
- MINAMI M., KIMURA S., ENDO T., HAMAUE N., HIRAFUJI M., TOGASHI H., MATSUMOTO M., YOSHIOKA M., SAITO H., WATANABE S., KOBAYASHI T., OKUYAMA H. Dietary docosahexaenoic acid increases cerebral acetylcholine levels and improves passive avoidance performance in stroke-prone spontaneously hypertensive rats. Pharmacol. Biochem. Behav. 1997;58:1123–1129. doi: 10.1016/s0091-3057(97)00300-6. [DOI] [PubMed] [Google Scholar]
- MOHAN I.K., DAS U.N. Oxidant stress, anti-oxidants and essential fatty acids in systemic lupus erythematosus. Prostaglandins Leukotr. Essent. Fatty Acids. 1997;56:193–198. doi: 10.1016/s0952-3278(97)90533-0. [DOI] [PubMed] [Google Scholar]
- MORISAKI N., KANZAKI T., FUJIYAMA Y., OSAWA I., SHIRAI K., MATSUOKA N., SAITO Y., YOSHIDA S. Metabolism of n-3 polyunsaturated fatty acids and modification of phospholipids in cultured rabbit aortic smooth muscle cells. J. Lipid Res. 1985;26:930–939. [PubMed] [Google Scholar]
- OKUDA Y., KAWASHIMA K., SAWADA T., TSURUMARU K., ASANO M., SUZUKI S., SOMA M., NAKAJIMA T., YAMASHITA K. Eicosapentaenoic acid enhances nitric oxide production by cultured human endothelial cells. Biochem. Biophys. Res. Commun. 1997;232:487–491. doi: 10.1006/bbrc.1997.6328. [DOI] [PubMed] [Google Scholar]
- SCHINI V.B., DURANTE W., CATOVSKY S., VANHOUTTE P.M. Eicosapentaenoic acid potentiates the production of nitric oxide evoked by interleukin-1β in cultured vascular smooth muscle cells. J. Vasc. Res. 1993;30:209–217. doi: 10.1159/000158996. [DOI] [PubMed] [Google Scholar]
- SINGH A., SVENTEK P., LARIVIÈRE R., THIBAULT G., SCHIFFRIN E.L. Inducible nitric oxide synthase in vascular smooth muscle cells from prehypertensive spontaneously hypertensive rats. Am. J. Hypertens. 1996;9:867–877. doi: 10.1016/s0895-7061(96)00104-5. [DOI] [PubMed] [Google Scholar]
- WU C.-C., HONG H.-J., CHOU T.-C., DING Y.-A., YEN M.-H. Evidence for inducible nitric oxide synthase in spontaneously hypertensive rats. Biochem. Biophys. Res. Commun. 1996;228:459–466. doi: 10.1006/bbrc.1996.1682. [DOI] [PubMed] [Google Scholar]
- XU X., MALAVE A. p38 MAPK, but not p42/44 MAPK mediated inducible nitric oxide synthase expression in C6 glioma cells. Life Sci. 2000;67:3221–3230. doi: 10.1016/s0024-3205(00)00902-4. [DOI] [PubMed] [Google Scholar]
- YIN K., CHU Z.M., BEILIN L.J. Blood pressure and vascular reactivity changes in spontaneously hypertensive rats fed fish oil. Br. J. Pharmacol. 1991;102:991–997. doi: 10.1111/j.1476-5381.1991.tb12289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


