Abstract
Nocistatin (NST) antagonizes several actions of nociceptin/orphanin FQ (N/OFQ), but acts on distinct receptors. As N/OFQ exerts anxiolytic-like actions in various tests, its behavioural actions in the elevated plus-maze (EPM) test were compared with those of bovine NST.
Five minutes after i.c.v. treatment, mice were placed on the EPM for 5 min and entries into and time spent on open and closed arms were recorded alongside other parameters.
NST (0.1 – 3 pmol) reduced percentages of entries into (control 39.6±3.1%, peak effect at 1 pmol NST 8.5±2.9%) and time spent on open arms (control 30.8±2.3%, NST 2.7±1.5%). The C-terminal hexapeptide of NST (NST-C6; 0.01 – 10 pmol) closely mimicked these actions of NST, with peak effects at 0.1 pmol.
N/OFQ (1 – 100 pmol) increased percentages of entries into (control 38.5±3.4%; peak effect at 10 pmol N/OFQ 67.9±4.9%) and time spent on open arms (control 32.0±3.8%; N/OFQ 74.9±5.8%). Closed arm entries, an index of locomotor activity, were unchanged by all peptides.
Effects of NST or NST-C6, but not N/OFQ, were still detectable 15 min after injection. Behaviour of animals co-injected with NST (1 pmol) or NST-C6 (0.1 pmol) plus N/OFQ (10 pmol) was indistinguishable from that of controls.
These results reveal potent anxiogenic-like actions of NST and NST-C6, and confirm the anxiolytic-like properties of N/OFQ. As NST and N/OFQ both derive from preproN/OF, anxiety may be modulated in opposing directions depending on how this precursor is processed.
Keywords: Anxiety, plus-maze, mice, nociceptin/orphanin FQ, nocistatin, NOP receptor, nocistatin C-terminal hexapeptide
Introduction
Prepronociceptin/orphanin FQ (ppN/OFQ) contains the sequences of at least three distinct mature peptides, nociceptin/orphanin FQ (N/OFQ), nociceptin II (or orphanin FQ2) and nocistatin (NST) (Florin et al., 1997; Okuda-Ashitaka et al., 1998; for review see Calo' et al., 2000). Though structurally similar to dynorphin A, the hepta-decapeptide N/OFQ does not bind to the classical μ-, δ- and κ-opioid receptors, but acts as a highly selective ligand for the opioid-like G protein-coupled NOP receptor (also known as ORL1 or OP4 receptor; Meunier et al., 1995; Reinscheid et al., 1995; Cox et al., 2000). To the best of our knowledge, the binding sites/receptors underlying the actions of the hepta-decapeptide nociceptin II have not yet been identified. NST, on the other hand, has been shown to associate with specific binding sites in membranes from mouse brain and spinal chord which are clearly distinct from the NOP receptors (Okuda-Ashitaka et al., 1998). The length of NST varies considerably among species (comprising 17, 30, 35 and 41 amino-acid residues in oxen, humans, rats and mice, respectively), but the last six residues of the C-terminal are fully conserved in all variants (Okuda-Ashitaka & Ito, 2000). Importantly in this regard, synthetic NST C-terminal hexapeptide (NST-C6) has been shown to retain the biological agonistic activity of full-length bovine NST (Okuda-Ashitaka et al., 1998).
Intracerebroventricular injections of N/OFQ cause various behavioural effects in rodents, including stimulation of food intake (Pomonis et al., 1996), inhibition or stimulation of spontaneous locomotion (Reinscheid et al., 1995; Florin et al., 1996), antagonism of the reinforcing properties of ethanol or morphine (Ciccocioppo et al., 1999; Murphy et al., 1999) and impairment of spatial learning (Sandin et al., 1997), among other actions. The peptide also affects nociceptive transmission/perception, inducing either hyperalgesia (Meunier et al., 1995; Reinscheid et al., 1995) or reversal of analgesia mediated or induced by opioids (Mogil et al., 1996; Grisel et al., 1996). Given intrathecally, however, N/OFQ appears to exert a dual effect comprised of hyperalgesia/allodynia at low doses and a clear antinociceptive influence at higher doses (Okuda-Ashitaka et al., 1996; Erb et al., 1997; Sakurada et al., 1999). Both of these spinal effects of N/OFQ are absent in NOP receptor null mutant mice (Inoue et al., 1999). Interestingly, although the other ppN/OFQ-derived peptide, NST, does not bind to the NOP receptor, it seems to act as a functional antagonist of N/OFQ. Indeed, several biological effects of N/OFQ are antagonized by NST, such as nociception in various experimental conditions (Martin et al., 1998; Okuda-Ashitaka et al., 1998; Yamamoto & Sakashita, 1999; Nakano et al., 2000), morphine analgesia (Zhao et al., 1999), N/OFQ impairing effects on learning/memory (Hiramatsu & Inoue, 1999), food intake (Olszewski et al., 2000) and glutamate release (Nicol et al., 1998).
Jenck et al. (1997) have described anxiolytic-like effects of i.c.v. N/OFQ administration in various rodent models of anxiety, such as light-dark place preference, elevated plus-maze (EPM), novel environment exploration and operant conflict tests. A similar profile of action was observed with a non-peptidic synthetic agonist for the NOP receptor (Jenck et al., 2000). To date, the influence of NST on anxiety-related behaviour has not yet been examined.
The present study has therefore investigated the effects of i.c.v. injections of bovine NST and NST-C6 on behaviour of mice submitted to the EPM test, comparing them with those elicited by N/OFQ over a broad range of doses. The EPM is a widely accepted paradigm of anxiety states (for review see Rodgers & Dalvi, 1997).
Methods
Animals
Experiments were conducted with male Swiss mice (30 – 40 g), raised under controlled environmental conditions (12 h light – dark cycle, with lights on from 700 h; room temperature set at 22±2°C). Food and water were available ad libitum, except during the experiments. Animals were transferred to the laboratory (at the Universidade Federal de Santa Catarina) at least 24 h before testing, and all experimental observations were carried out between 1300 and 1700 h. Animal housing conditions and all experimental procedures were previously approved by the Ethics Committee on Use of Animals in Research of the Universidade Federal de Santa Catarina and were carried out in accordance with the Guiding Principles for the Care and Use of Animals approved by the Brazilian Society of Neuroscience and Behaviour (1992).
Treatments
NST (10 fmol to 1 nmol), NST-C6 (1 fmol to 1 nmol) or N/OFQ (100 fmol to 3 nmol) were injected i.c.v. into the right brain lateral ventricle in a constant volume of 2 μl. Alternatively, in some experiments, mice were given a combined i.c.v. injection of N/OFQ (10 pmol) plus either NST (1 pmol) or NST-C6 (0.1 pmol). Other animals received i.c.v. injections of either diazepam (DZP; 7 nmol) or pentylenetetrazol (PTZ; 200 nmol), as reference anxiolytic and anxiogenic treatments, respectively (Teixeira et al., 1996). Control mice were always similarly treated with vehicle alone (phosphate-buffered saline; PBS) and tested in parallel with drug-treated animals. All i.c.v. injections were carried out using the ‘free hand' technique proposed by Haley & McCormick (1957), as modified by Laursen & Belknap (1986) and employed previously by our group (Teixeira et al., 1996; Ribeiro & De Lima, 1998). In brief, under light ether anaesthesia (i.e. just sufficient for loss of the postural reflex), a 27 gauge needle attached to a 10 μl Hamilton syringe was inserted perpendicularly 3 mm deep through the skull, at a position 2 mm lateral from the midline on the line drawn through the anterior base of the ears. Each animal only received one i.c.v. injection. Upon termination of the experiment (including testing on the rota-rod apparatus, see below), each mouse was decapitated and its brain examined a fresco. Results from mice presenting cannula misplacement or any signs of cerebral haemorrhage were discarded from the statistical analysis (less than 5% of the animals overall).
The EPM test
The putative anxiolytic or anxiogenic activity of the various drug treatments was assessed using the EPM test, as adapted for the mouse by Lister (1987). This test is based on the natural aversion of rodents for open spaces. The EPM was made of Plexiglas and consisted of two opposed open arms (30×5× 0.25 cm) and two opposed closed arms (30×5×15 cm), all facing a central platform (5×5 cm), elevated 45 cm from the floor. The apparatus was placed in a small closed room lit by a 15 W red light and could be adequately viewed by the experimenter through a glass window. Five, 15 or 30 min after the i.c.v. treatment, each mouse was placed on the central platform, facing a closed arm, and observed for a 5 min period. The frequencies of entry into either open or enclosed arms, as well as the times spent in each arm type were recorded (in s). An entry was scored as such only when the animal placed all four limbs into any given arm. The incidence of ethological parameters such as time spent on the central platform, grooming, unprotected head-dipping (i.e. an exploratory forward head/shoulder movement over the side an open arm of the maze directed down towards the floor) and protected stretch-attend postures (i.e. when animal stretches forward and retracts to original position without actually walking forward, a behaviour which occurs in or from the relative security of the closed arms or central platform of the maze) were also recorded (Cole & Rodgers, 1994).
The ratios ‘time spent in the open arms/time spent in all (i.e. open and closed) arms' and ‘frequency of entries into open arms/total entries into all arms' were calculated and multiplied by 100, to yield the percentages of time spent in and of frequency of entries into open arms, respectively. Both parameters are considered to reflect fear-induced inhibition from entering the open arms and can be related to the ‘anxiety' level experienced by the animal (Rodgers & Dalvi, 1997). Drugs with anxiolytic-like activity usually increase the time spent in and/or frequency of entries into open arms, whereas the reverse holds true for anxiogenic-like drugs. Furthermore, the number of entries into closed arms was used and an index of general activity. All sessions were also videotaped using a an infra-red video camera (Philco, model PVC-4H10, Manaus, Brazil), to enable playback when necessary.
Motor co-ordination
Immediately after completion of the elevated plus-maze test session, mice treated with NST, NST-C6, N/OFQ or vehicle were placed on the revolving bar (diameter 2.5 cm, 12 r.p.m.) of a rota-rod apparatus for 1 min, and both the latency to fall and number of falls from the revolving bar were recorded and used as indices of motor coordination.
Data analysis
Results were analysed by Graphpad INSTAT® version 2.05 software. All data presented are expressed as the mean±s.e.m., and each value reflects the mean of six to eight animals per group. In all cases, the means were compared by a one-way analysis of variance (ANOVA), followed by Bonferroni's test. In the co-injection experiments, additional statistical evaluation was carried out using the two-tailed Student t-test for unpaired samples, in which the means of each co-injected group were compared to those of the corresponding vehicle-treated control groups. Differences were considered significant when P<0.05.
Drugs
The following drugs were used: bovine NST (from Tocris Cookson Ltd., Bristol, U.K.), NST-C6 (from American Peptide Company, Sunnyvale, CA, U.S.A.), N/OFQ (synthesized by Dr R. Guerrini, Department of Pharmaceutical Sciences, University of Ferrara), DZP and PTZ hydrochloride (from Sigma Chemical Co., St. Louis, MO, U.S.A.). All drugs were dissolved in PBS, and stock solutions of NST-C6 and N/OFQ were stored at −20°C and diluted to the desired concentrations in PBS just prior to use. NST was dissolved in PBS plus 5% NaHCO3 (50:1) as stock solution.
Results
Effects of NST and NST-C6 on behaviour in the EPM test
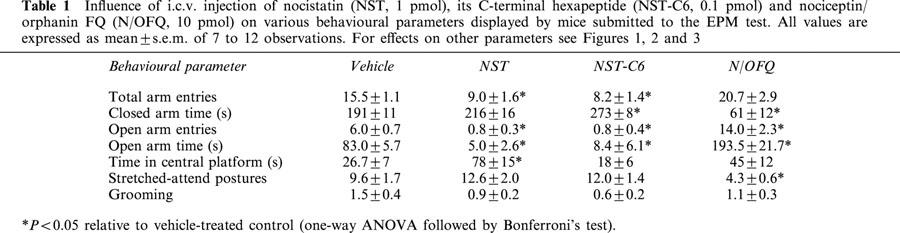
The i.c.v. administration of NST (0.1 to 3 pmol) induced significant decreases in the percentages of time spent in open arms and of entries into open arms, in addition to reductions in the number of head-dippings (F(7,62)=6.20, P<0.0001; F(7,62)=7.09, P<0.0001; F(7,62)=1.77, P>0.001, respectively). As shown in Figure 1, the dose-response curves for each of these effects of NST were clearly U-shaped, with peak reductions at 1 pmol and higher doses (up to 100 pmol) evoking progressively lesser effects. In contrast, no significant effects on locomotor activity, as assessed by the number of closed arm entries, were detected over the full range of NST doses tested (F(7,62)=1.04, P>0.05). As shown in Figure 2, qualitatively similar results were obtained with NST-C6, which also decreased the percentages of time spent in and of entries into the open arms, as well as reduced the number of head-dipping, at doses ranging from 0.01 to 1 pmol (F(8,63)=9.40, P<0.0001; F(8,63)=16.57, P<0.0001; F(8,63)=3.70, P<0.001, respectively). The dose-response curves for these NST-C6-induced effects were U-shaped, as seen with NST, but peak reductions were obtained at a lower dose (0.1 pmol). The reference anxiogenic drug, PTZ (200 nmol, i.c.v.), also decreased the percentages of time spent in and of entries into open arms (Figure 1). NST and NST-C6, over the full range of doses tested, failed to influence either locomotor activity, as assessed by the number of closed arm entries (F(7,62)=1.04, P>0.05; F(8,62)=1.42, respectively; P>0.05; Figures 1 and 2). In addition, NST (but not NST-C6) increased the time spent on the central platform, NST-C6 (but not NST) augmented the time animals spent in the closed arms, and both peptides reduced the number of total arm entries into and the time spent in open arms (Table 1 shows only results for 1 and 0.1 pmol of NST and NST-C6, respectively).
Figure 1.
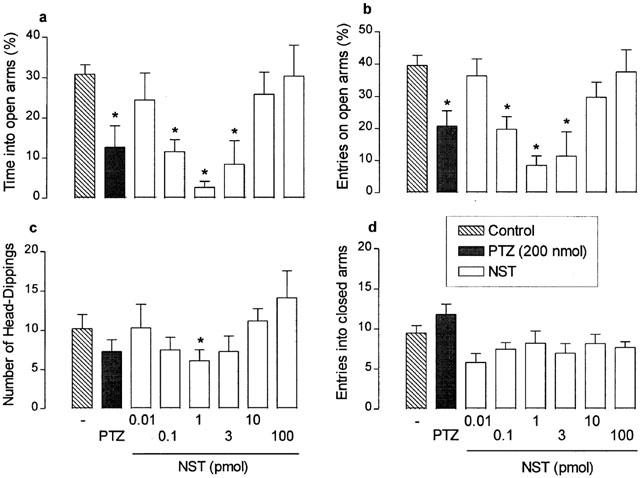
Effects of nocistatin (NST) on the percentages of time spent in (a) and entries into (b) open arms, and on the number of head-dippings (c) and entries into enclosed arms (d) in mice placed, for 5 min, in the elevated plus-maze 5 min after i.c.v. injection. Control mice were treated with vehicle only (PBS). Results obtained following similar injection of pentylenetetrazol (PTZ; 200 nmol) are also shown for comparison. Each value represents the mean±s.e.m. of 6 – 8 animals. *P<0.05 as compared to the PBS-treated control group (one-way ANOVA followed by Bonferroni's test).
Figure 2.
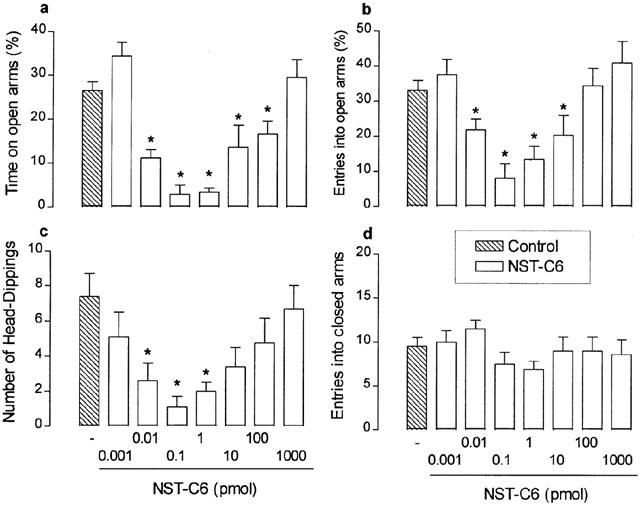
Effects of nocistatin C-terminal hexapeptide (NST-C6) on the percentages of time spent in (a) and entries into (b) open arms, and on the number of head-dippings (c) and entries into enclosed arms (d) in mice placed, for 5 min, in the elevated plus-maze 5 min after i.c.v. injection. Control mice were treated with vehicle only (PBS). Each value represents the mean±s.e.m. of 6 – 8 animals. *P<0.05 as compared to the PBS-treated control group (one-way ANOVA followed by Bonferroni's test).
Table 1.
Influence of i.c.v. injection of nocistatin (NST, 1 pmol), its C-terminal hexapeptide (NST-C6, 0.1 pmol) and nociceptin/orphanin FQ (N/OFQ, 10 pmol) on various behavioural parameters displayed by mice submitted to the EPM test. All values are expressed as mean±s.e.m. of 7 to 12 observations. For effects on other parameters see Figures 1, 2 and 3
Effects of N/OFQ on behaviour in the EPM test
As shown in Figure 3, i.c.v. administration of N/OFQ, at either 10 or 100 pmol, induced significant increases in the percentages of time spent in open arms and, at 10 pmol only, also increased the frequency of open arm entries (F(7,44)=10.96 and F(7,44)=5.95, respectively; P<0.0001 in both cases), as well as the incidence of head-dipping behaviour (F(7,44)=2.18, P<0.05). The dose-response curves for each of these effects of N/OFQ were typically bell-shaped, such that no significant changes were detected following injection of 1 nmol of this peptide. Moreover, none of the doses of N/OFQ tested (0.1 pmol – 1 nmol) affected the number of closed arm entries (F(7,44)=1.15; P>0.05), but at 10 pmol the peptide significantly augmented the number of entries into and time spent in open arms, as well as reduced both the time animals spent in the closed arms and the incidence of stretch-attend postures (Table 1 shows results for 10 pmol dose only). The reference anxiolytic drug DZP (7 nmol, i.c.v.), like N/OFQ, also increased the percentage of time spent in open arms (Figure 3).
Figure 3.
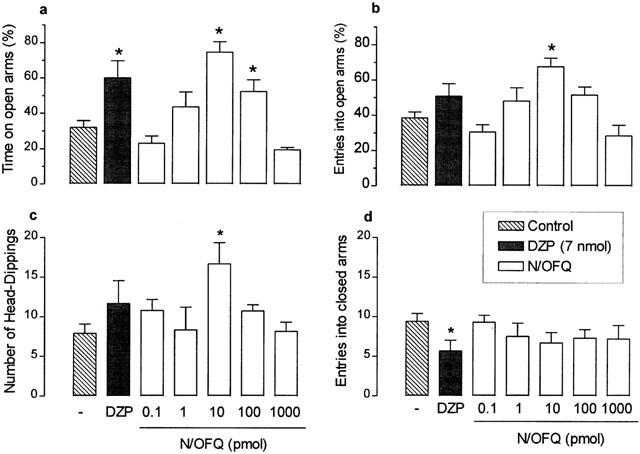
Effects of nociceptin/orphanin FQ (N/OFQ) on the percentages of time spent in (a) and entries into (b) open arms, and on the number of head-dippings (c) and entries into enclosed arms (d) in mice placed, for 5 min, in the elevated plus-maze 5 min after i.c.v. injection. Control mice were treated with vehicle only (PBS). Results obtained following similar injection of diazepam (DZP; 7 nmol) are also shown for comparison. Each value represents the mean±s.e.m. of 6 – 8 animals. *P<0.05 as compared to the PBS-treated control group (one-way ANOVA followed by Bonferroni's test).
Time-course of behavioural actions of NST, NST-C6 and N/OFQ
The doses of NST, NST-C6 or N/OFQ that induced the most prominent behavioural effects (i.e. at 1, 0.1 and 10 pmol, respectively) were selected to assess the time-courses of their actions on performance in the EPM. As shown in Figure 4, both NST and NST-C6 still exerted pronounced anxiogenic-like actions in animals placed in EPM up to 15 min after i.c.v. injection (per cent time spent in open arms F(3,28)=29.8 and F(3,37)=18.64; per cent frequency of open arm entries F(3,28)=24.3 and F(3,37)=10.83, respectively; P<0.0001 in all cases). The intensity of these effects at 15 min was similar to that seen 5 min after injection of either peptide, whereas no residual behavioural effects were detected in animals tested 30 min after administration. In contrast, the anxiolytic-like effects of N/OFQ were only evident in animals tested 5 min after injection (per cent time spent in open arms F(3,26)=8.85, P<0.005; per cent frequency of open arm entries F(3,26)=6.39, P<0.01), subsiding completely in animals tested in the apparatus 15 or 30 min after administration.
Figure 4.
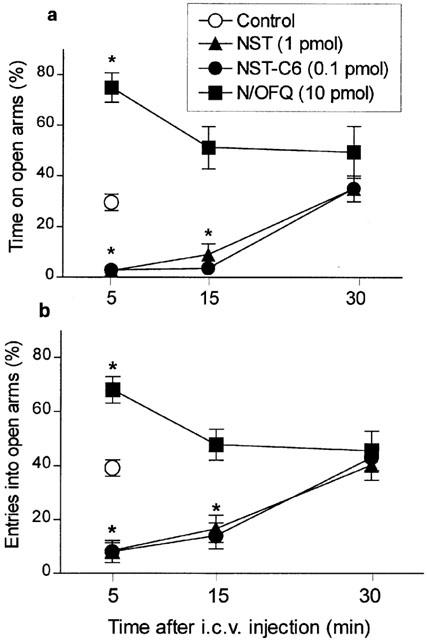
Time-course of the effects of nocistatin (NST, 1 pmol), nocistatin C-terminal hexapeptide (NST-C6, 0.1 pmol) and nociceptin/orphanin FQ (N/OFQ, 10 pmol) on the percentages of time spent in (a) and of entries into (b) open arms in mice placed, for 5 min, in the elevated plus-maze at the times after i.c.v. injection indicated. Vehicle-treated control mice were evaluated at 5 min after injection only. Each value represents the mean±s.e.m. of 6 – 8 animals. *P<0.05 as compared to PBS-treated control group (one-way ANOVA followed by Bonferroni's test).
Effects of co-injections of NST or NST-C6 together with N/OFQ on behaviour in the EPM test
Animals given co-injections of either NST (1 pmol) or NST-C6 (0.1 pmol) together with N/OFQ (10 pmol) and tested in the plus-maze 5 min after administration failed to exhibit alterations in the percentages of time spent in open arms or in the frequency of open arm entries, when compared to performance of their respective vehicle-treated control groups (Figure 5). Co-injection of either NST or NST-C6 with N/OFQ also did not affect locomotor activity (i.e. number of closed arm entries) or the incidence of any of the ethological parameters recorded (results not shown).
Figure 5.
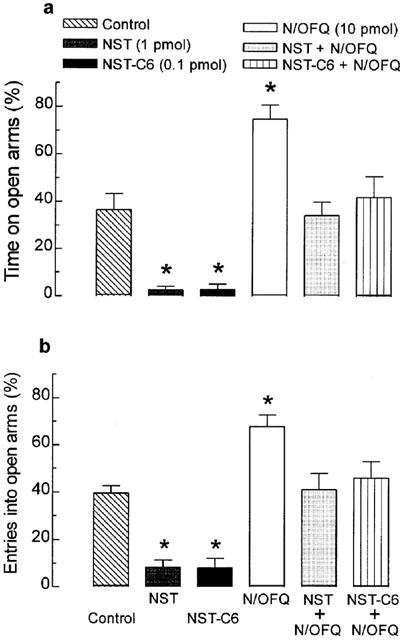
Effects of single or combined treatment with nocistatin (NST, 1 pmol), nocistatin C-terminal hexapeptide (NST-C6, 0.1 pmol) and/or nociceptin/orphanin FQ (N/OFQ, 10 pmol) on the percentages of time spent in (a) and of entries into (b) open arms in mice placed, for 5 min, in the elevated plus-maze 5 min after i.c.v. (co-injection). Control mice were treated with vehicle only (PBS). Each value represents the mean±s.e.m. of 6 – 8 animals. *P<0.05 as compared to the PBS-treated control group (non-paired two-tailed Student's t-test).
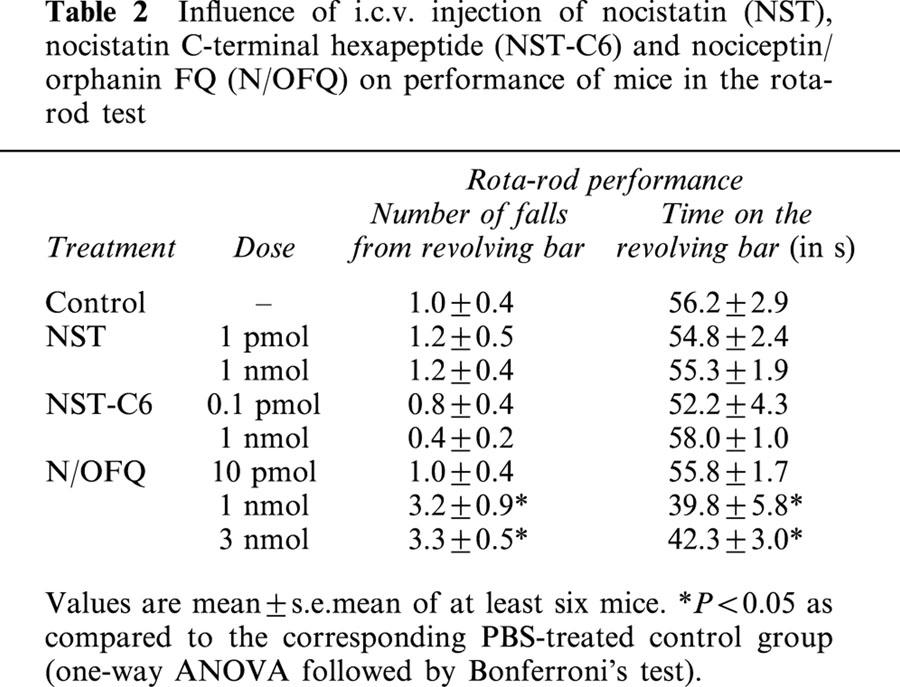
Influence of NST, NST-C6 and N/OFQ on performance in the rota-rod test
None of the doses of NST or NST-C6 tested affected motor performance of mice submitted to the rota-rod test (Table 2 shows only results obtained with the maximally-effective anxiogenic-like doses and highest doses tested). Likewise, N/OFQ also failed to decrease motor performance at doses causing anxiolytic-like effects (10 or 100 pmol), but at 1 or 3 nmol this peptide significantly increased the number of falls from and decreased the time spent on the revolving bar. Furthermore, at both of these higher doses, N/OFQ induced sporadic muscle jerks and severe alterations in the locomotor activity, as has been previously described (Reinscheid et al., 1995).
Table 2.
Influence of i.c.v. injection of nocistatin (NST), nocistatin C-terminal hexapeptide (NST-C6) and nociceptin/orphanin FQ (N/OFQ) on performance of mice in the rota-rod test
Discussion
The results of the current study demonstrate, to the best of our knowledge for the first time, that NST is a remarkably potent anxiogenic-like compound in mice. In addition, we have found that NST-C6, a sequence which is fully conserved in the distinct NST isoforms of all species studied to date (Okuda-Ashitaka & Ito, 2000), shares the agonistic properties of the full-length bovine peptide in the EPM test. Conversely, we also confirmed the previously reported anxiolytic-like profile of action of N/OFQ in mice in the EPM test (Jenck et al., 1997). As NST and N/OFQ are both derived from ppN/OFQ, these findings strongly suggest that anxiety levels in the mouse can be modulated, in opposing directions, depending on how the precursor is processed or on which of the two systems is more prevalent.
Very little is known about the processing mechanisms of ppN/OFQ. However, pro-hormone convertase 2 appears to play a key role in N/OFQ generation, as hypothalamic or amygdala extracts from mice gene-targeted to produce defective enzyme contain only about 10 – 30% of the N/OFQ seen in wild-type controls (Allen et al., 2001). Furthermore, although ppN/OFQ and biosynthetic intermediates were undetectable in extracts from hypothalamus of wild-type mice, both entities were present in those from defective pro-hormone convertase 2 animals. Unfortunately, the influence of this genetic manipulation on production of NST was not examined. It is believed that the main sites of cleavage of ppN/OFQ by pro-hormone convertase 2 lie between Lys-Arg residues, especially those which separate NST from N/OFQ and the later peptide from the remaining N-terminal ppN/OFQ fragment, which also contains the bioactive nociceptin II sequence (Reinscheid et al., 2000; Mathis et al., 2001). However, the relative bio-availability of endogenous NST and N/OFQ in different brain areas and elsewhere can only be estimated once the various processing steps, including the separation of NST from the rest of the C-terminal fragment of ppN/OFQ and the mechanisms of breakdown of both peptides, are understood.
The anxiogenic-like profiles of action of NST (and NST-C6) and the anxiolytic-like ones of N/OFQ were detectable using three distinct behavioural parameters, namely the percentages of time spent on and of entries into the open arms, as well as the incidence of head-dipping behaviour. All three peptides appeared to influence anxiety in a selective fashion, since neither affected the number of entries into the closed arms of the EPM, a parameter which is loaded with locomotor activity in factorial analysis studies (Wall & Messier, 2000), even though NST-6C and N/OFQ also affected in opposing ways the time animals spent in closed arms. Moreover, neither NST nor NST-C6 modified motor performance on the rota-rod test over the full range of doses examined, including those causing peak anxiogenic-like effects. On the other hand, N/OFQ significantly depressed performance in this test only at doses ⩾1 nmol, a finding which is in agreement with previous reports (Reinscheid et al., 1995; Rizzi et al., 2001a). Indeed, overall, the influences of both NST-C6 and N/OFQ on anxiety levels were more clear cut than those of the two reference anxiogenic and anxiolytic drugs tested, i.e. PTZ and DZP, at least at the doses of 200 and 7 nmol (i.c.v.), respectively.
It is noteworthy that the dose-response curves demonstrating the anxiogenic- and anxiolytic-like effects of NST (and NST-C6) and N/OFQ were U- and bell-shaped, respectively. Similarly, the curve for i.c.v. NST for reversal of the antagonism of morphine-induced analgesia by N/OFQ in the rat is also bell-shaped (Zhao et al., 1999), as is that for the allodynic effect of i.t. N/OFQ in mice (Okuda-Ashitaka et al., 1998). Bell-shaped curves have also been reported for anxiolytic-like effects of i.c.v. N/OFQ (and also for p.o. DZP) in rats submitted to the elevated plus-maze test and in mice exposed to a novel environment (following pre-treatment with urocortin) or submitted to light – dark aversion or conflict tests (Jenck et al., 1997).
The abilities of both NST and N/OFQ to modulate anxiety correlate well with histochemical, autoradiographical and analytical evidence showing high levels of expression of ppN/OFQ, N/OFQ, NST peptides and NOP binding (receptor) sites in several brain areas related to emotionality. In situ hybridization for ppN/OFQ mRNA in mouse brain has revealed particularly intense labelling of neurones of the septum and septo-hippocampal and central amygdaloid nuclei, as well as less pronounced levels in hippocampus (Boom et al., 1999; Köster et al., 1999). These findings are in good agreement with the labelling of both ppN/OFQ mRNA and mature N/OFQ seen in rat brain (Neal et al., 1999a) and with the detection of relatively high levels of mature NST in bovine septum and hippocampus (Lee et al., 1999). The distribution of NOP receptor mRNA or receptor-like binding sites in the brain appears to be somewhat more widespread than that of ppN/OFQ mRNA or of N/OFQ, but also encompasses the septum, hippocampus and amygdala (Neal et al., 1999b). On the other hand, there are as yet no reports on the relative distribution of NST binding sites throughout the brain, although selective binding of bovine NST has been demonstrated to occur in membranes obtained from mouse brain and spinal cord (Okuda-Ashitaka et al., 1998).
There is evidence that endogenous N/OFQ and NST might be physiologically active, at least in certain conditions. Analgesia induced by morphine or stress in mice, or by electro-acupuncture in rats, is potentiated by i.c.v. injections of either the highly selective NOP receptor antagonist [Nphe1]nociceptin(1-13)NH2 (Rizzi et al., 2000; 2001b) or of an antibody against N/OFQ (Tian & Han, 2000), respectively. On the other hand, i.t. administration of an antibody against NST markedly potentiates (by over 100 fold) i.t. N/OFQ-induced allodynia in mice (Okuda-Ashitaka et al., 1998). However, the evidence for a physiological role of ppN/OFQ-derived peptides in anxiety is controversial. Mice null-mutated for ppN/OFQ display increased anxiety-related behaviour when exposed to a novel environment and impaired adaptation to repeated stress (Köster et al., 1999), whereas animals lacking the NOP receptor fail to exhibit any differences in behaviour in the EPM test, relative to wild-type controls (Mamiya et al., 1998). Several putative reasons could account for such discrepant findings, including possible contribution of other ppN/OFQ-derived peptides (NST and nociceptin II) towards the modulation of anxiety, mediation of the anxiolytic-like actions of N/OFQ via an as yet uncharacterized additional receptor type and/or differences in the genetic background of each knockout lineage, among others. In light of such considerations, it is unclear if the anxiogenic-like effect of NST-C6 seen in the current study reflects functional antagonism of an ongoing anxiolytic-like influence of endogenous N/OFQ, or is brought about by a N/OFQ- independent mechanism.
The maximally effective anxiogenic-like doses of NST (1.0 pmol) and NST-C6 (0.1 pmol) were 10 and 100 fold lower, respectively, than that causing the maximal anxiolytic-like effect of N/OFQ (10 pmol). Furthermore, we also observed that the behavioural effects of both NST and NST-C6 were longer-lasting than those of N/OFQ. Considering that ppN/OFQ contains only single copies of the NST and N/OFQ sequences (Mollereau et al., 1996) and that levels of mature NST and N/OFQ in brain are similar (Lee et al., 1999; Quigley et al., 1998), this could be simplistically interpreted to indicate that NST may be physiologically more important than N/OFQ in modulating anxiety. However, this may well depend on their relative bio-availabilities (i.e. rates of formation and degradation) in the appropriate brain areas.
Mice co-injected with maximally effective i.c.v. doses of either NST or NST-C6 together with N/OFQ failed to display any behavioural alterations in the EPM test. It thus seems that the effects of NST and N/OFQ on anxiety are mutually exclusive. Similar antagonistic profiles of action of NST have been observed against the effects of N/OFQ on food intake (Olszewski et al., 2000), learning and memory (Hiramatsu & Inoue, 1999), formalin-evoked pain (Yamamoto & Sakashita, 1999), prostaglandin E2-induced allodynia (Okuda-Ashitaka et al., 1998) and glutamate release (Nicol et al., 1998). It is important to mention that NST can per se inhibit carrageenan/kaolin-induced hyperalgesia (Nakagawa et al., 1999) and food intake (Olszewski et al., 2000), but it is unknown if these effects are due to antagonism of endogenous N/OFQ actions. Moreover, the firing rate of most rat thalamic neurones in vivo is decreased by N/OFQ, and these same cells are excited by NST (Albrecht et al., 2001). Nevertheless, this same study also detected a small proportion of neurones which were excited by N/OFQ, but unresponsive to NST. Thus, although most effects of N/OFQ, including its effects on anxiety, are amenable to antagonism by NST, some clearly are not.
It is important to mention that the current results were obtained using bovine NST hepta-decapeptide, which markedly differs from the murine 41-residue isoform. Thus, one cannot rule out entirely the possibility that murine NST might display distinct biological properties in this (and other) species not shared by its bovine counterpart. However, the fact that the murine and bovine isoforms of NST have been shown to be roughly equipotent at inhibiting spinal allodynia induced by i.t. injections of either N/OFQ or PGE2 (Okuda-Ashitaka et al., 1998), allied to our finding that the anxiogenic-like effects seen with bovine NST were mimicked closely by NST-C6, a sequence which is fully conserved in all NST isoforms known, would seem to argue against major differences in their bioactivity. Nonetheless, this issue remains to be adequately addressed by studies which effectively compare the binding characteristics of the various NST isoforms (and NST-C6) to specific binding (receptor) sites, as well as their biological properties. On the other hand, our finding that NST-C6 was actually slightly more potent (3 – 10 fold) than NST in causing anxiogenic-like effects in the EPM test confirms previous evidence reported by Okuda-Ashitaka et al. (1998), obtained in a very different behavioural paradigm, that this fragment appears to retain the agonistic properties of bovine NST. Together, both studies present functional evidence which highlights the usefulness of NST-C6 as a valuable additional pharmacological tool to study the NST system.
In conclusion, the results of the current study reveal remarkably potent anxiogenic-like actions of NST and confirm the anxiolytic-like properties of N/OFQ in mice submitted to the elevated plus-maze test. They also indicate a potential role of endogenous NST in controlling central functions related to fear and anxiety. Since ppN/OFQ is the common precursor of both NST and N/OFQ, the balance between processing of either peptide may well contribute to modulation of anxiety. This hypothesis is currently under investigation in our laboratory. Finally, although receptors for NST have not yet been characterized, it seems pertinent to speculate that their blockade may constitute a potential target for a new class of anxiolytic drugs, and that NST-C6 might be an interesting pharmacological tool to study actions related to the NST system.
Acknowledgments
This study was supported by the Brazilian National Council of Research (CNPq), who supplied fellowships to E.C. Gavioli. G.A. Rae and T.C.M. De Lima are recipients of research grants and fellowships from CNPq.
Abbreviations
- DZP
diazepam
- EPM
elevated plus-maze
- N/OFQ
nociceptin/orphanin FQ
- NST
nocistatin
- NST-C6
nocistatin C-terminal hexapeptide
- PBS
phosphate-buffered saline
- ppN/OFQ
prepronociceptin/orphanin FQ
- PTZ
pentylenetetrazol
References
- ALBRECHT D., BLUHDORN R., SIEGMUND H., BERGER H., CALO' G. Inhibitory action of nociceptin/orphanin FQ on functionally different thalamic neurons in urethane-anaesthetized rats. Br. J. Pharmacol. 2001;134:333–342. doi: 10.1038/sj.bjp.0704264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLEN R.G., PENG B., PELLEGRINO M.J., MILLER E.D., GRANDY D.K., LUNDBLAD J.R., WASHBURN C.L., PINTAR J.E. Altered processing of pro-orphanin FQ/nociceptin and pro-opiomelanocortin-derived peptides in the brains of mice expressing defective pro-hormone convertase 2. J. Neurosci. 2001;21:5864–5870. doi: 10.1523/JNEUROSCI.21-16-05864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOM A., MOLLEREAU C., MEUNIER J.C., VASSART G., PARMENTIER M., VANDERHAEGHEN J.J., SCHIFFMANN S.N. Distribution of the nociceptin and nocistatin precursor transcript in the mouse central nervous system. Neuroscience. 1999;91:991–1007. doi: 10.1016/s0306-4522(98)00683-6. [DOI] [PubMed] [Google Scholar]
- CALO' G., GUERRINI R., RIZZI A., SALVADORI S., REGOLI D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br. J. Pharmacol. 2000;129:1261–1283. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CICCOCIOPPO R., PANOCKA I., POLIDORI C., REGOLI D., MASSI M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology. 1999;141:220–224. doi: 10.1007/s002130050828. [DOI] [PubMed] [Google Scholar]
- COLE J.C., RODGERS R.J. Ethological evaluation of the effects of acute and chronic buspirone treatment in the murine elevated plus-maze test: comparison with haloperidol. Psychopharmacology. 1994;114:288–296. doi: 10.1007/BF02244851. [DOI] [PubMed] [Google Scholar]
- COX B.M., CHAVKIN C., CHRISTIE M.J., CIVELLI O., EVANS C., HAMON M.D., HOELLT V., KIEFFER B., KITCHEN I., MCKNIGHT A.T., MEUNIER J.C., PORTOGHESE P.S.Opioid receptors The IUPHAR Compendium of Receptor Characterization and Classification 2000London: IUPHAR Media Ltd; 321–333.ed. Girdlestone, D. pp [Google Scholar]
- ERB K., LIEBEL J.T., TEGEDER I., ZEILHOFER H.U., BRUNE K., GEISSLINGER G. Spinally delivered nociceptin/orphanin FQ reduces flinching behaviour in the rat formalin test. Neuroreport. 1997;8:1967–1970. doi: 10.1097/00001756-199705260-00034. [DOI] [PubMed] [Google Scholar]
- FLORIN S., SUADEAU C., MEUNIER J.C., COSTENTIN J. Nociceptin stimulates locomotion and exploratory behavior in mice. Eur. J. Pharmacol. 1996;317:9–13. doi: 10.1016/s0014-2999(96)00707-8. [DOI] [PubMed] [Google Scholar]
- FLORIN S., SUAUDEAU C., MEUNIER J.C., COSTENTIN J. Orphan neuropeptide NocII, a putative pronociceptin maturation product, stimulates locomotion in mice. Neuroreport. 1997;8:705–707. doi: 10.1097/00001756-199702100-00025. [DOI] [PubMed] [Google Scholar]
- GRISEL J.E., MOGIL J.S., BELKNAP J.K., GRANDY D.K. Orphanin FQ acts as a supraspinal, but not a spinal, anti-opioid peptide. Neuroreport. 1996;7:2125–2129. doi: 10.1097/00001756-199609020-00012. [DOI] [PubMed] [Google Scholar]
- HALEY T.J., MCCORMICK W.G. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Brit. J. Pharmacol. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRAMATSU M., INOUE K. Effects of nocistatin on nociceptin-induced impairment of learning and memory in mice. Eur. J. Pharmacol. 1999;367:151–155. doi: 10.1016/s0014-2999(99)00003-5. [DOI] [PubMed] [Google Scholar]
- INOUE M., SHIMOHIRA I., YOSHIDA A., ZIMMER A., TAKESHIMA H., SAKURADA T., UEDA H. Dose-related opposite modulation by nociceptin/orphanin FQ of substance P nociception in the nociceptors and spinal cord. J. Pharmacol. Exp. Ther. 1999;291:308–313. [PubMed] [Google Scholar]
- JENCK F., MOREAU J.L., MARTIN J.R., KILPATRICK G.J., REINSCHEID R.K., MONSMA F.J., NOTHACKER H.P., CIVELLI O. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14854–14858. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENCK F., WICHMANN J., DAUTZENBERG F.M., MOREAU J.L., OUAGAZZAL A.M., MARTIN J.R., LUNDSTROM K., CESURA A.M., POLI S.M., ROEVER S., KOLCZEWSKI S., ADAM G., KILPATRICK G. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4938–4943. doi: 10.1073/pnas.090514397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSTER A., MONTKOWSKI A., SCHULZ S., STUBE E.M., KNAUDT K., JENCK F., MOREAU J.L., NOTHACKER H.P., CIVELLI O., REINSCHEID R.K. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10444–10449. doi: 10.1073/pnas.96.18.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURSEN S.E., BELKNAP J.P. Intracerebro-ventricular injections in mice. Some methodological refinements. J. Pharmacol. Meth. 1986;16:155–157. doi: 10.1016/0160-5402(86)90038-0. [DOI] [PubMed] [Google Scholar]
- LEE T.L., FUNG F.M., CHEN F.G., CHOU N., OKUDA-ASHITAKA E., ITO S., NISHIUCHI Y., KIMURA T., TACHIBANA S. Identification of human, rat and mouse nocistatin in brain and human nocistatin in brain and human cerebrospinal fluid. Neuroreport. 1999;10:1537–1541. doi: 10.1097/00001756-199905140-00026. [DOI] [PubMed] [Google Scholar]
- LISTER R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- MAMIYA T., NODA Y., NISHI M., TAKESHIMA H., NABESHIMA T. Enhancement of spatial attention in nociceptin/orphanin FQ receptor-knockout mice. Brain Res. 1998;783:236–240. doi: 10.1016/s0006-8993(97)01406-6. [DOI] [PubMed] [Google Scholar]
- MARTIN W.J., MALMBERG A.B., BASBAUM A.I. Pain: nocistatin spells relief. Curr. Biol. 1998;16:R525–R527. doi: 10.1016/s0960-9822(07)00338-7. [DOI] [PubMed] [Google Scholar]
- MATHIS J.P., ROSSI G.C., PELLEGRINO M.J., JIMENEZ C., PASTERNAK G.W., ALLEN R.G. Carboxyl terminal peptides derived from prepro-orphanin FQ/nociceptin (ppOFQ/N) are produced in the hypothalamus and possess analgesic bioactivities. Brain Res. 2001;895:89–94. doi: 10.1016/s0006-8993(01)02035-2. [DOI] [PubMed] [Google Scholar]
- MEUNIER J.C., MOLLEREAU C., TOLL L., SUADEAU C., MOISAND C., ALVINERIE P., BUTOUR J.L., GUILLEMOT J.C., FERRARA P., MONSERRAT B., MAZARGUIL H., VASSART G., PARMENTIER M., COSTENTIN J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- MOGIL J.S., GRIESEL J.E., ZHANGS G., BELKNAP J.K., GRANDY D.K. Functional antagonism of mu, kappa, and delta-opioid antinociception by orphanin FQ. Neurosci. Lett. 1996;214:131–134. doi: 10.1016/0304-3940(96)12917-7. [DOI] [PubMed] [Google Scholar]
- MOLLEREAU C., SIMONS M.J., SOULARUE P., LINERS F., VASSART G., MEUNIER J.C., PARMENTIER M. Structure, tissue distribution, and chromosomal localization of the prepronociceptin gene. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8666–8670. doi: 10.1073/pnas.93.16.8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY N.P., LEE Y., MAIDMENT N.T. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- NAKAGAWA T., KANEKO M., INAMURA S., SATOH M. Intracerebroventricular administration of nocistatin reduces inflammatory hyperalgesia in rats. Neurosci. Lett. 1999;265:64–66. doi: 10.1016/s0304-3940(99)00202-5. [DOI] [PubMed] [Google Scholar]
- NAKANO H., MINAMI T., ABE K., ARAI T., TOKUMURA M., IBII N., OKUDA-ASHITAKA E., MORI H., ITO S. Effect of intrathecal nocistatin on the formalin-induced pain in mice versus that of nociceptin/orphanin FQ. J. Pharmacol. Exp. Ther. 2000;292:331–336. [PubMed] [Google Scholar]
- NEAL C.R., JR, MANSOUR A., REINSCHEID R., NOTHACKER H.P., CIVELLI O., WATSON S.J., JR Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J. Comp. Neurol. 1999a;406:503–547. [PubMed] [Google Scholar]
- NEAL C.R., JR, MANSOUR A., REINSCHEID R., NOTHACKER H.P., CIVELLI O., AKIL H., WATSON S.J., JR Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with 125I-[14Tyr]-orphanin FQ binding. J. Comp. Neurol. 1999b;412:563–605. [PubMed] [Google Scholar]
- NICOL B., LAMBERT D.G., ROWBOTHAM D.J., OKUDA-ASHITAKA E., ITO S., SMART D., MCKNIGHT A.T. Nocistatin reverses nociceptin inhibition of glutamate release from rat brain slices. Eur. J. Pharmacol. 1998;356:2–3. doi: 10.1016/s0014-2999(98)00545-7. [DOI] [PubMed] [Google Scholar]
- OKUDA-ASHITAKA E., MINAMI T., TACHIBANA S., YOSHIHARA Y., NISHIUCHI Y., KIMURA T., ITO S. Nocistatin, a peptide that blocks nociceptin action in pain transmission. Nature. 1998;392:286–289. doi: 10.1038/32660. [DOI] [PubMed] [Google Scholar]
- OKUDA-ASHITAKA E., TACHIBANA S., HOUTANI T., MINAMI T., MASU Y., NISHI M., TAKESHIMA H., SUGIMOTO T., ITO S. Identification and characterization of an endogenous ligand for opioid receptor homologue ROR-C: its involvement in allodynic response to innocuous stimulus. Mol. Brain Res. 1996;43:96–104. doi: 10.1016/s0169-328x(96)00165-9. [DOI] [PubMed] [Google Scholar]
- OKUDA-ASHITAKA E., ITO S. Nocistatin: a novel neuropeptide encoded by the gene for the nociceptin/orphanin FQ precursor. Peptides. 2000;21:1101–1109. doi: 10.1016/s0196-9781(00)00247-3. [DOI] [PubMed] [Google Scholar]
- OLSZEWSKI P.K., SHAW T.J., GRACE M.K., BILLINGTON C.J., LEVINE A.S. Nocistatin inhibits food intake in rats. Brain Res. 2000;872:181–187. doi: 10.1016/s0006-8993(00)02535-x. [DOI] [PubMed] [Google Scholar]
- POMONIS J.D., BILLINGTON C.J., LEVINE S. Orphanin FQ, agonist of orphan opioid receptor ORL1, stimulates feeding in rats. Neuroreport. 1996;8:369–371. doi: 10.1097/00001756-199612200-00072. [DOI] [PubMed] [Google Scholar]
- QUIGLEY D.I., MCDOUGALL J., DARLAND T., ZHANG G., RONNEKLIEV O., GRANDY D.K., ALLEN R.G. Orphanin FQ is the major OFQ1-17-containing peptide produced in the rodent and monkey hypothalamus. Peptides. 1998;19:133–139. doi: 10.1016/s0196-9781(97)00268-4. [DOI] [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MONSMA F.J., CIVELLI O. Orphanin FQ: a neuropeptide that activates an opioid-like G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H., CIVELLI O. The orphanin FQ/nociceptin gene: structure, tissue distribution of expression and functional implications obtained from knockout mice. Peptides. 2000;21:901–906. doi: 10.1016/s0196-9781(00)00226-6. [DOI] [PubMed] [Google Scholar]
- RIBEIRO S.J., DE LIMA T.C.M. Naloxone-induced changes in tachykinin NK3 receptor modulation of experimental anxiety in mice. Neurosci. Lett. 1998;258:1–4. doi: 10.1016/s0304-3940(98)00880-5. [DOI] [PubMed] [Google Scholar]
- RIZZI A., BIGONI R., MARZOLA G., GUERRINI R., SALVADORI S., REGOLI D., CALO' G. The nociceptin/orphanin FQ receptor antagonist, [Nphe1]NC(1-13)NH2, potentiates morphine analgesia. Neuroreport. 2000;11:2369–2372. doi: 10.1097/00001756-200008030-00007. [DOI] [PubMed] [Google Scholar]
- RIZZI A., BIGONI R., MARZOLA G., GUERRINI R., SALVADORI S., REGOLI D., CALO G. Characterization of the locomotor activity-inhibiting effect of nociceptin/orphanin FQ in mice. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001a;363:161–165. doi: 10.1007/s002100000358. [DOI] [PubMed] [Google Scholar]
- RIZZI A., MARZOLA G., BIGONI R., GUERRINI R., SALVADORI S., MOGIL J.S., REGOLI D., CALO' G. Endogenous nociceptin signalling and stress-induced analgesia. Neuroreport. 2001b;12:3009–3013. doi: 10.1097/00001756-200110080-00006. [DOI] [PubMed] [Google Scholar]
- RODGERS R.J., DALVI A. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- SAKURADA T., KATSUYAMA S., SAKURADA S., INOUE M., TAN-NO K., KISARA K., SAKURADA C., UEDA H., SASAKI J. Nociceptin-induced scratching, biting and licking in mice: involvement of spinal NK1 receptors. Br. J. Pharmacol. 1999;127:1712–1718. doi: 10.1038/sj.bjp.0702698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDIN J., GEORGIEVA J., SCHOTT P.A., OGREN S.O., TERENIUS L. Nociceptin/orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur. J. Neurosci. 1997;9:194–197. doi: 10.1111/j.1460-9568.1997.tb01367.x. [DOI] [PubMed] [Google Scholar]
- TEIXEIRA R.M., SANTOS A.R.S., CALIXTO J.B., RAE G.A., DE LIMA T.C.M. Effects of central administration of tachykinin receptor agonists and antagonists on plus-maze behavior in mice. Eur. J. Pharmacol. 1996;131:7–14. doi: 10.1016/0014-2999(96)00390-1. [DOI] [PubMed] [Google Scholar]
- TIAN J.H., HAN J.S. Functional studies using antibodies against orphanin FQ/nociceptin. Peptides. 2000;21:1047–1050. doi: 10.1016/s0196-9781(00)00242-4. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., SAKASHITA Y. Effects of nocistatin and its interaction with nociceptin/orphanin FQ on the rat formalin test. Neurosci. Lett. 1999;262:179–182. doi: 10.1016/s0304-3940(99)00073-7. [DOI] [PubMed] [Google Scholar]
- WALL P.M., MESSIER C. Ethological confirmatory factor analysis of anxiety-like behaviour in the murine elevated plus-maze. Behav. Brain Res. 2000;114:199–212. doi: 10.1016/s0166-4328(00)00229-1. [DOI] [PubMed] [Google Scholar]
- ZHAO C.S., LI B.S., ZHAO G.Y., LIU H.X., LUO F., WANG Y., TIAN J.H., CHANG J.K., HAN J.S. Nocistatin reverses the effect of orphanin FQ/nociceptin in antagonizing morphine analgesia. Neuroreport. 1999;10:297–299. doi: 10.1097/00001756-199902050-00017. [DOI] [PubMed] [Google Scholar]


