Abstract
Choroidal blood vessels, located between the sclera and retina, constitute the principle source of blood flow to ocular structures. The choroid is innervated by vasoconstrictor sympathetic and vasodilator parasympathetic nerves.
We have shown previously that sympathetic denervation for 6 weeks leads to significant increases in choroidal thickness, percentage of choroid occupied by vascular lumina, and numbers of choroidal venules, large arterioles and outer retinal capillaries. Sympathetic deafferentation produces similar increases, indicating that loss of sympathetic nerve activity is responsible for increased vascularity after sympathectomy. Thus, sympathetic neurotransmission normally may be important in suppressing vascular proliferation in the adult rodent eye.
The aim of the present study was to determine whether sympathetic nerves act by way of adrenergic receptors to maintain normal choroidal vascular integrity.
The α-adrenoceptor antagonist, phentolamine (1 mg kg−1 day−1), the β-receptor antagonist, propranolol (1 mg kg−1 day−1), or saline vehicle was infused for 3 weeks using subcutaneously implanted osmotic minipumps.
In phentolamine treated rats, no significant changes were noted relative to saline infused controls. However, propranolol treatment resulted in increases in choroidal thickness, vascular luminal area, and numbers of large choroidal venules and both small and large arterioles, approximating the remodelling seen after chronic sympathectomy.
We conclude that sympathetic nerves play a role in maintaining normal choroidal vascular architecture through actions mediated primarily by β-adrenoceptors.
Keywords: α-adrenoreceptors, β-adrenoreceptors, morphometry, blood vessels, propranolol, phentolamine, eye
Introduction
Choroid blood vessels of the eye provide 80 – 95% of the blood to ocular structures, including the retina and ciliary processes (Bill, 1984). Choroidal vessels are innervated by sympathetic and parasympathetic nerves. Parasympathetic innervation of the choroid derives from the ipsilateral pterygopalatine ganglion (Ruskell, 1970). Parasympathetic nerve stimulation produces nitric oxide-mediated vasodilation, which appears to be selective for vessels in the anterior choroid (Steinle et al., 2000). Sympathetic nerves from the superior cervical ganglion project to the choroid and, when stimulated, elicit marked vasoconstriction throughout the choroid (Koss & Gherezghiher, 1993; Steinle et al., 2000). Therefore, autonomic nerves exert short-term regulatory effects on choroidal blood flow by way of changes in vascular smooth muscle tone.
Sympathetic nerves are also critical for maintaining the structural integrity of the choroidal vasculature in the adult rat. We have found previously that, 6 weeks following superior cervical ganglionectomy, choroidal thickness and vascularity are significantly increased, together with increases in numbers of venules and larger arterioles, and that this is further associated with a 4.5-fold increase in blood flow. Numbers of outer retinal capillaries were also increased (Steinle et al., 2002). These changes appear to be due to the absence of sympathetic nerve activity rather than the structural loss of sympathetic nerves, since similar changes in vascular architecture occurred after surgical removal of the preganglionic input to the superior cervical ganglion (Steinle et al., 2002). Therefore, sympathetic innervation plays an important role in regulating ocular vascular architecture, and this appears to be mediated by some aspect of sympathetic neurotransmission.
Sympathetic neurotransmission exerts its predominant effects via the α and β classes of adrenergic receptors, which comprise five major subtypes (α-1 and α-2, and β-1, β-2 and β-3) (Hieble et al., 1995). In the present study, we examined whether ocular sympathetic nerves may influence choroidal vascular architecture by acting on either the α or β class of adrenergic receptor by chronically administering an α or β antagonist and using morphometric analysis to determine whether vascular remodelling similar to that seen after ocular sympathetic denervation occurred.
Methods
Experimental preparations
Studies were conducted on 14 adult Sprague – Dawley rats (Harlan, Indianapolis, IN, U.S.A.) aged 60 days and weighing 180 – 200 g. Rats were anesthetized by intraperitoneal injection of a mixture of ketamine hydrochloride (27.5 mg kg−1, Sanofi Winthrop, New York, NY, U.S.A.), xylazine hydrochloride (2.5 mg kg−1, Rompun, Miles, Shawnee Mission, KS, U.S.A.), and atropine sulphate (0.24 mg kg−1, Vedco, St. Joseph, MO, U.S.A.). Osmotic mini-pumps (Alzet, Model Number 2ML4, Alza Corporation, Palo Alto, CA, U.S.A.) were implanted subcutaneously in a dorsal interscapular configuration. These pumps deliver solution at a rate of 2.5 μl h−1 and provide constant infusion for at least 28 days. Pumps were loaded with saline vehicle alone (n=5) or saline containing the α-adrenoceptor antagonist phentolamine (Sigma, St. Louis, MO, U.S.A.; n=5) or the β-adrenoceptor antagonist propranolol (Sigma, St. Louis, MO, U.S.A.; n=4). Both drugs were delivered at dosages of 1 mg day−1, which previous studies have shown to be effective for maintaining adrenoceptor blockade (Greenberg & Wilborn, 1982; Holmang et al., 1995; Sironi et al., 2000).
Twenty-one days after pump implantation, rats were anesthetized with urethane (1.5 g kg−1). To confirm the efficacy of the adrenoceptor blockade regimens, rats from each group were assessed using trans-scleral laser Doppler flowmetry to record blood flow changes from anterior choroidal vessels by methods identical to those described previously (Steinle et al., 2000). The reduction in blood flow in response to topical administration of 1% norepinephrine was abolished in phentolamine-treated rats, and the increase in flow elicited by 1% isoproterenol was reduced by approximately 90%, thus confirming the efficacy of the antagonist regimens. All surgical procedures and subsequent experimental manipulations were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center.
Rats were perfused transcardially with Millonig's buffer containing 1% sodium nitrate, followed by Karnovsky's fixative (1% paraformaldehyde, 1.5% glutaraldehyde, 0.54% dextrose in Millonig's buffer, pH 7.3). Following fixation, the anterior portions of both eyes were removed by excising the cornea to the level of the scleral junction and discarded. Posterior portions were further fixed by immersion in fresh Karnovsky's fixative for 4 h at 4°C. After 4 h, each eye was dissected into four wedge-shaped pieces, with the narrow tip originating at the insertion of the optic nerve. Tissue pieces were then rinsed twice in buffer, post-fixed for 1 h in 1% buffered osmium tetroxide, dehydrated and embedded into flat molds.
Morphometric analysis of choroidal vasculature
Choroidal vascular features were measured in tissue sections oriented transversely relative to the antero-posterior axis of the eye. Plastic sections from both right and left eyes were cut at 1 μm thickness, stained with toluidine blue and viewed with a light microscope, and images were acquired with a digital camera.
Choroidal thickness was defined as the distance from the retinal pigmented epithelium to the outermost extent of the choroidal blood vessels, which was generally delimited by several layers of fibroblasts; for each of the four blocks from both right and left eyes, three measurements were obtained equidistant along the section, and all measurements for both eyes of a given rat were averaged.
To determine the percentage of choroidal area occupied by vascular lumina, a 4125 μm2 stereology grid with line intersects at 5 μm intervals was superimposed randomly over an image. Grid intersects overlying blood vessel lumina were counted and divided by total intersections overlying the choroid in 12 sections each from the right and left eyes. To evaluate the number of blood vessels within a given region of choroid or retina, all vessels located within a full-thickness linear segment of choroid or retina corresponding to 400 μm in length were counted. Minimal luminal diameters of all vessels were measured, and vessels were categorized based on their structure (Tomanek et al., 1986; Cimini & Weiss, 1988; Steinle et al., 2002). Vessels with flattened smooth muscle layers and lumen diameters between 10 μm and 100 μm were categorized as venules. These were further subdivided as small venules, with diameters of 10 – 14.9 μm, and large venules with diameters 15 μm or greater. Vessels with thickened smooth muscle layers and lumen diameters between 10 and 100 μm were categorized as arterioles. Small arterioles had diameters of 10 – 19.9 μm, while large arterioles had diameters greater than 20 μm. Vessels consisting of an endothelial cell without smooth muscle enclosure and with diameters of 5 – 10 μm were categorized as capillaries.
Data were compared by one-way ANOVA or t-test, with post hoc analysis by Student – Newman – Keuls test. A probability of <0.05 was taken to indicate statistically significant differences.
Results
The choroid is comprised of blood vessels surrounded by fibroblasts, nerves, and extracellular matrix (Figure 1). In rats receiving saline infusion, the choroidal thickness was 25±2 μm, with a vascular luminal area of 24±1% (Table 1). The choroid consisted of venules with mean diameters of 13.5±0.9 μm, and arterioles with diameters of 13.7±1.9 μm. The majority of capillaries were found along Bruch's membrane, adjacent to the retinal pigmented epithelium.
Figure 1.
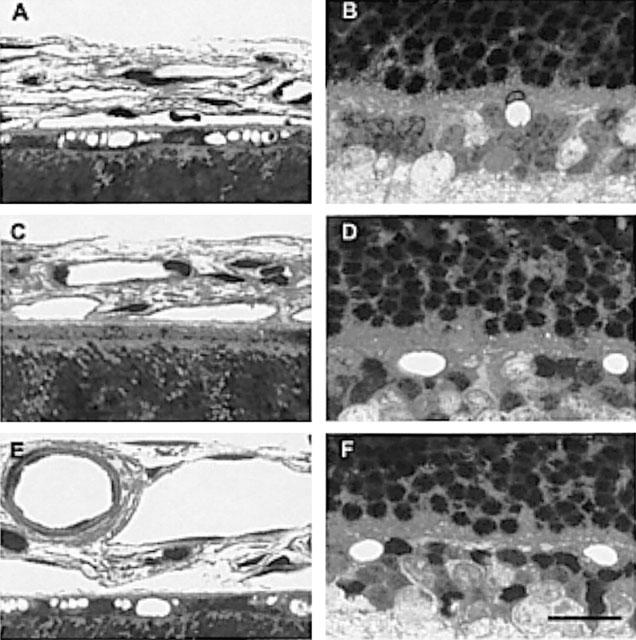
Photomicrographs through sections of the choroid (A,C,E) or retina (B,D,F) from eyes of adult rats infused for 3 weeks with saline (A,B), phentolamine (C,D), or propranolol (E,F). One μm epoxy sections were stained with Toluidine blue. Calibration bar=50 μm.
Table 1.
Thickness and percentage of vascular luminal area obtained from rats infused for 3 weeks with saline vehicle, phentolamine or propranolol
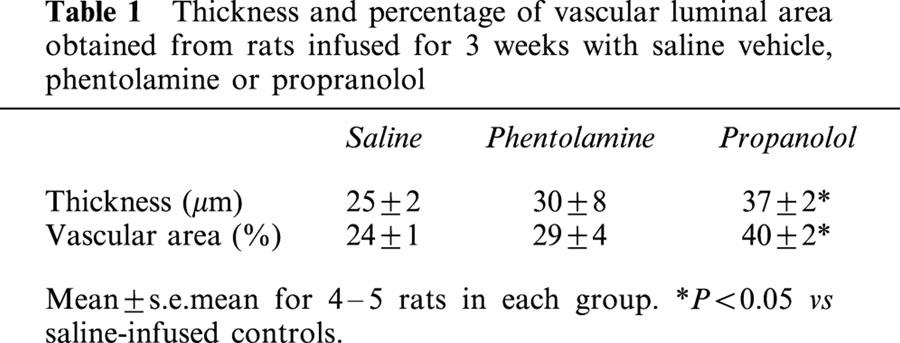
After phentolamine infusion, choroidal thickness and vascular luminal areas were similar to those of saline controls (Table 1). Phentolamine did not affect numbers of small or large choroidal venules (Figure 2) or small or large arterioles (Figure 3) per unit length of choroid. There were no differences in mean diameters of venules (13.9±0.9 μm) or arterioles (16.7±1.5 μm) as compared to saline infused rats. Retinal capillary numbers were not altered by phentolamine treatment (Figure 4).
Figure 2.
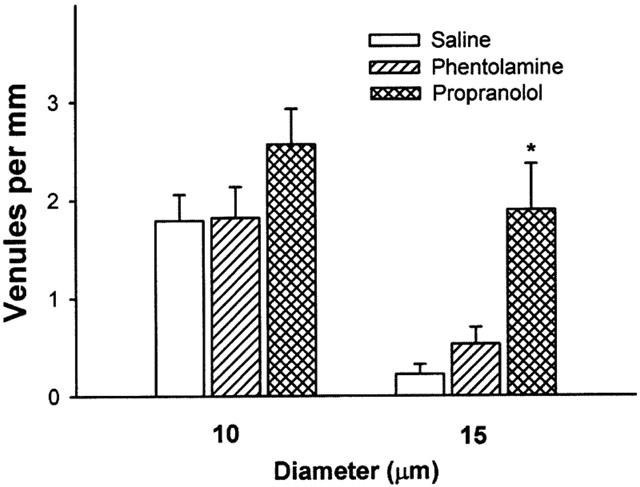
Numbers of small and large venules in a 1 mm segment of choroid from saline (saline), phentolamine (phentolamine) and propanolol (propanolol) treated rats. Small venules in the 10 μm grouping have diameters between 10 – 14.9, and larger venules in the 15 μm group have diameters of 15 μm or greater. Data are expressed as the mean±s.e.mean for 4 – 5 rats in each group. *P<0.01 vs saline infused rats.
Figure 3.
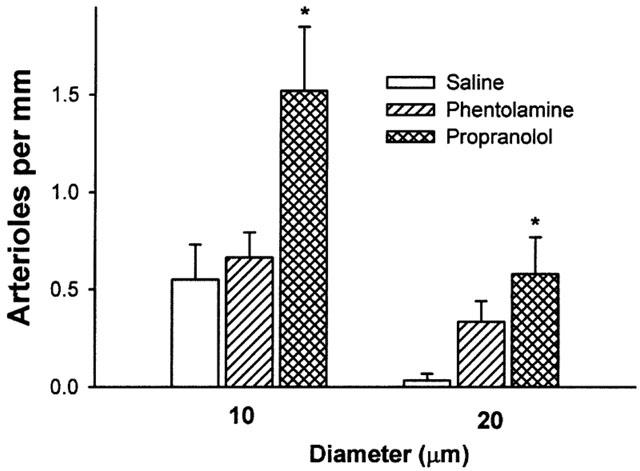
Numbers of small and large arterioles in a 1 mm segment of choroid from saline (saline), phentolamine (phentolamine) and propanolol (propanolol) treated rats. Small arterioles in the 10 μm grouping have diameters between 10 – 19.9 μm and large arterioles in the 20 μm grouping have diameters of 20 μm or greater. Data are expressed as mean±s.e.mean for 4 – 5 rats. *P<0.05 vs saline infused controls.
Figure 4.
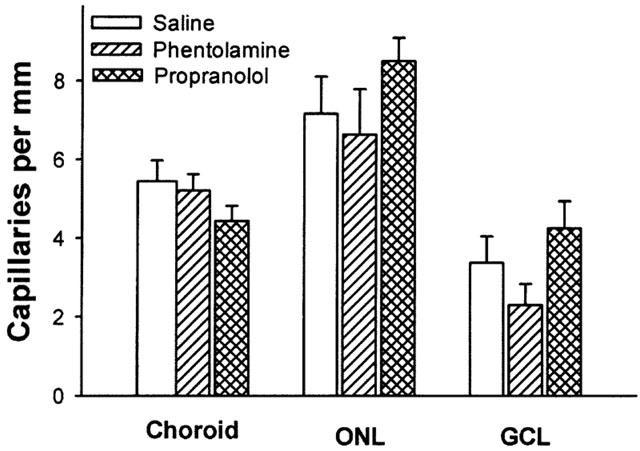
Numbers of capillaries in a 1 mm segment of choroid or retina from saline (saline), phentolamine (phentolamine) and propanolol (propanolol) treated rats. Data are expressed as mean±s.e.mean. ONL=outer nuclear layer; GCL=ganglion cell layer.
Propranolol infusion increased choroidal thickness by 48% relative to saline treated animals (P=0.003, Table 1) and vascular luminal area was increased by 67% (P=0.002, Table 1). Propranolol treatment also increased numbers of large choroidal venules (P=0.001 vs saline; Figures 1 and 2), small arterioles (P=0.015 vs saline), and large arterioles (P=0.019 vs saline infusion, Figures 1 and 3). Mean diameters of venules (14.4±1.0 μm) and arterioles (16.5±1.7 μm) were not different from those of saline infused controls. Choroidal and retinal capillaries were not changed by propranolol treatment (Figure 4).
Discussion
Ocular vascular architecture after α-adrenergic receptor blockade
α-adrenergic receptors are distributed widely on vascular smooth muscle cells (Ping & Faber, 1993). α-adrenoceptor activation can elicit smooth muscle cell contraction (Docherty, 1998), and can influence other aspects of cellular dynamics such as mitogenic state (Hu et al., 1999). Therefore, diminished α-adrenoceptor input to vascular smooth muscle of the choroid could provide a potential mechanism for the changes in vascular architecture following sympathetic denervation. In addition, α-adrenoceptor-mediated vascular smooth muscle contraction also regulates total peripheral resistance, and therefore arterial blood pressure (Docherty, 1998). Indeed, infusion of the α-receptor antagonist prazosin reduces blood pressure by approximately 40 mmHg in rats (Ziada et al., 1989), leading to increased cardiac output and organ blood flow. This is accompanied by increased shear stress, which is believed to elicit vascular remodelling under some conditions (Ichioka et al., 1998; Dawson & Hudlicka, 1989; Ziada et al., 1989). In the present experiments, however, chronic phentolamine infusion did not produce significant changes in choroidal vasculature. This suggests that neither diminished activation of α adrenoceptor-mediated mitogenic effects on vascular cells nor changes in shear stress resulting from increased organ blood flow are likely to explain the increased choroidal vascularity after sympathectomy. Thus we consider that decreased α adrenoceptor activation is unlikely to be a primary mechanism through which vascular remodelling occurs after sympathectomy.
β adrenoceptor blockade elicits changes in choroidal vascularity similar to those of sympathectomy
Unlike α-adrenoceptor blockade, long-term propranolol administration produced changes in choroidal vasculature that closely resembled those seen following sympathectomy. Choroidal thickness and percentage vascular area were significantly increased, as were numbers of large venules and both small and large arterioles. In contrast to chronic sympathectomy, however, there was no significant increase in numbers of retinal capillaries. Whether this is due to differences in lengths of treatment regimens (6 weeks for sympathectomy vs 3 weeks of adrenoceptor blockade) or to effects elicited by sympathetic denervation that occur independent of the β receptor is unclear. Nonetheless, the present findings suggest that the bulk of the choroidal vascular remodelling after sympathetic denervation can be emulated by rendering β adrenoceptors inactive.
Choroidal vascular remodelling after β-adrenoceptor blockade is not likely to be due to non-specific effects of propranolol on vascular resistance and shear stress. In normotensive rats, propranolol produces only small effects on blood pressure, which remain in the normal range (Owens, 1987). Similarly, using laser Doppler flowmetry to measure blood flow in the posterior choroid and vortex veins, we were unable to detect any apparent change after acute intravenous administration of propranolol (Steinle & Smith, unpublished). Moreover, since the increased blood flow and shear stress that would have occurred during chronic α adrenoceptor antagonist infusion in the present study failed to elicit vascular remodelling, it seems unlikely that vascular remodelling following chronic propranolol infusion could have occurred secondary to changes in choroidal blood flow.
It seems more plausible that propranolol may be mediating changes in choroidal vascularity by way of a local mechanism that involves β adrenoceptors located in the eye and perhaps directly on the choroidal blood vessels. β adrenoceptors are known to play a role determining intraocular pressure, and administration of β blocking agents to patients with glaucoma can produce clinically significant reductions in intra-ocular pressure, possibly through reduced aqueous humor formation (Lesar, 1987). Similarly, sympathetic denervation can decrease intra-ocular pressure in experimental animals (Schmid et al., 1999). However, while intraocular pressure is likely to have been reduced in both our sympathectomized and propranolol-treated rats, it is unclear whether or why this would lead to altered choroidal vascularity.
Alternatively, β adrenoceptor blockade could produce choroidal vascular remodelling through actions directly on choroidal blood vessels. While the distribution of β adrenoceptors within the choroidal blood vessels has not been described, it is known that these receptors are present on both smooth muscle cells (Bevan, 1983) and endothelial cells (Wang et al., 2000). β adrenergic receptors have been shown to modulate several angiogenic factors, although studies to date have only documented a role in increasing their release (Wen et al., 1996; Fredriksson et al., 2000; Jin et al., 2000). It is possible, however, that β adrenoceptors may also exert inhibitory effects on the release of other angiogenic factors, and that this action could predominate in the choroid. Alternatively, β adrenoceptors may regulate angiostatic factor production from choroidal endothelial or smooth muscle cells, whose tonic release is necessary in order to maintain normal choroidal vascular architecture.
The finding that long-term β adrenoceptor blockade leads to increased choroidal vascularity is interesting in light of the common use of this class of drugs. β blockers frequently are used for the treatment of open-angle glaucoma (Lee, 1988), although the effects of topical applications of these agents on ocular vascularity have not to our knowledge been investigated. On the other hand, many patients receive β adrenoceptor blockers as a component of antihypertensive therapeutic regimens. It is interesting, therefore, that an association appears to exist between anti-hypertenisve medication and increased neovascularization in patients with age-related macular degeneration (Hyman et al., 2000). Clearly, additional investigations are warranted to determine if long-term β adrenoceptor antagonist administration may influence ocular vascularity in humans.
In summary, the increase in ocular choroidal vascularity seen following chronic sympathetic denervation is replicated in part by sustained β adrenoceptor blockade. This suggests that the mechanism leading to increased ocular vascularity involves the diminished β adrenoceptor activation that occurs following sympathetic denervation. It therefore appears that sympathetic nerves play an important role in preventing abnormal vascular remodelling in the adult rodent eye by tonic activation of β adrenoceptors.
Acknowledgments
This work was supported by NIH grant 33025 with core support from HD02825. The authors would like to thank Taline Manougian for her assistance with tissue sectioning.
References
- BEVAN J.A. The human adrenergic neurovascular mechanism. Gen. Pharmacol. 1983;14:21–26. doi: 10.1016/0306-3623(83)90057-5. [DOI] [PubMed] [Google Scholar]
- BILL A. Circulation in the eye. Handbook of Physiology Part 2 in Microcirculation. 1984. pp. 1001–1035.
- CIMINI C.M., WEISS H.R. Microvascular morphometry and perfusion in renal hypertension-induced cardiac hypertrophy. Am. J. Physiol. 1988;255:H1384–H1390. doi: 10.1152/ajpheart.1988.255.6.H1384. [DOI] [PubMed] [Google Scholar]
- DAWSON J.M., HUDLICKA O. The effects of long term administration of prazosin on the microcirculation in skeletal muscles. Cardiovasc. Res. 1989;23:913–920. doi: 10.1093/cvr/23.11.913. [DOI] [PubMed] [Google Scholar]
- DOCHERTY J.R. Subtypes of functional alpha1- and alpha2- adrenoceptors. Eur. J. Pharmacol. 1998;361:1–15. doi: 10.1016/s0014-2999(98)00682-7. [DOI] [PubMed] [Google Scholar]
- FREDRIKSSON J.M., LINDQUIST J.M., BRONNIKOV G.E., NEDERGAARD J. Norepinephrine induces vascular endothelial growth factor gene expression in brown adipocytes through a beta–adrenoreceptor/cAMP/protein kinase A pathway involving Src but independently of Erk1/2. J. Biol. Chem. 2000;275:13802–13811. doi: 10.1074/jbc.275.18.13802. [DOI] [PubMed] [Google Scholar]
- GREENBERG S., WILBORN W.M. Effect of chronic administration of clonidine and propranolol on the myocardium of spontaneously hypertensive rats. Arch. Int. Pharmacodyn. Ther. 1982;255:141–161. [PubMed] [Google Scholar]
- HIEBLE J.P., BONDINELL W.E., RUFFOLO R.R. , JR Alpha- and beta-adrenoceptors: from the gene to the clinic. 1. Molecular biology and adrenoceptor subclassification. J. Med. Chem. 1995;38:3415–3444. doi: 10.1021/jm00018a001. [DOI] [PubMed] [Google Scholar]
- HOLMANG A., JENNISCHE E., BJORNTORP P. The effects of long-term hyperinsulinaemia on insulin sensitivity in rats. Acta Physiol. Scand. 1995;153:67–73. doi: 10.1111/j.1748-1716.1995.tb09835.x. [DOI] [PubMed] [Google Scholar]
- HU Z.W., SHI X.Y., LIN R.Z., CHEN J., HOFFMAN B.B. Alpha1-Adrenergic receptor stimulation of mitogenesis in human vascular smooth muscle cells: role of tyrosine protein kinases and calcium in activation of mitogen-activated protein kinase. J. Pharmacol. Exp. Ther. 1999;290:28–37. [PubMed] [Google Scholar]
- HYMAN L., SCHACHAT A.P., HE Q., LESKE C. Hypertension, cardiovascular disease, and age-related macular degeneration. Arch. Ophthalmol. 2000;117:351–358. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- ICHIOKA S., SHIBATA M., KOSAKI K., SATO Y., HARII K., KAMIYA A. In vivo measurement of morphometric and hemodynamic changes in the microcirculation during angiogenesis under chronic alpha1-adrenergic blocker treatment. Microvasc. Res. 1998;55:165–174. doi: 10.1006/mvre.1998.2069. [DOI] [PubMed] [Google Scholar]
- JIN Y., SHEIKH F., DETILLIEUX K.A., CATTINI P.A. Role for early growth response-1 protein in alpha(1)-adrenergic stimulation of fibroblast growth factor-2 promoter activity in cardiac myocytes. Mol. Pharmacol. 2000;57:984–990. [PubMed] [Google Scholar]
- KOSS M.C., GHEREZGHIHER T. Adrenoceptor subtypes involved in neurally evoked sympathetic vasoconstriction in the anterior choroid of cats. Exp. Eye Res. 1993;57:441–447. doi: 10.1006/exer.1993.1146. [DOI] [PubMed] [Google Scholar]
- LEE M.E. Beta adrenergic blocking agents for open-angle glaucoma. J. Am. Optom. Assoc. 1988;59:567–571. [PubMed] [Google Scholar]
- LESAR T.S. Comparison of ophthalmic beta-blocking agents. Clin. Pharm. 1987;6:451–463. [PubMed] [Google Scholar]
- OWENS G.K. Influence of blood pressure on development of aortic medial smooth muscle hypertrophy in spontaneously hypertensive rats. Hypertension. 1987;9:178–187. doi: 10.1161/01.hyp.9.2.178. [DOI] [PubMed] [Google Scholar]
- PING P., FABER J.E. Characterization of alpha-adrenoceptor gene expression in arterial and venous smooth muscle. Am. J. Physiol. 1993;265:H1501–H1509. doi: 10.1152/ajpheart.1993.265.5.H1501. [DOI] [PubMed] [Google Scholar]
- RUSKELL G.L. The orbital branches of the pterygopalatine ganglion and their relationship with internal carotid nerve branches in primates. J. Anat. 1970;106:323–339. [PMC free article] [PubMed] [Google Scholar]
- SCHMID G.F., PAPASTERGIOU G.I., LIN T., RIVA C.A., LATIES A.M., STONE R.A. Autonomic denervations influence ocular dimensions and intraocular pressure in chicks. Exp. Eye Res. 1999;68:573–581. doi: 10.1006/exer.1998.0649. [DOI] [PubMed] [Google Scholar]
- SIRONI G., COLOMBO D., POGGESI E., LEONARDI A., TESTA R., RAMPIN O., BERNABE J., GIULIANO F. Effects of intracavernous administration of selective antagonists of alpha(1)-adrenoceptor subtypes on erection in anesthetized rats and dogs. J. Pharmacol. Exp. Ther. 2000;292:974–981. [PubMed] [Google Scholar]
- STEINLE J.J., KRIZSAN-AGBAS D., SMITH P.G. Regional regulation of choroidal blood flow by autonomic innervation in the rat. Am. J. Physiol. 2000;279:R202–R209. doi: 10.1152/ajpregu.2000.279.1.R202. [DOI] [PubMed] [Google Scholar]
- STEINLE J.J., PIERCE J.D., CLANCY R.L., SMITH P.G.Increased ocular blood vessel frequencies and sizes following chronic sympathectomy in rat Exp. Eye Res. 2002. in press [DOI] [PubMed]
- TOMANEK R.J., PALMER P.J., PEIFFER G.L., SCHREIBER K.L., EASTHAM C.L., MARCUS M.L. Morphometry of canine coronary arteries, arterioles, and capillaries during hypertension and left ventricular hypertrophy. Circ. Res. 1986;58:38–46. doi: 10.1161/01.res.58.1.38. [DOI] [PubMed] [Google Scholar]
- WANG H.Z., HONG S.J., WU K.Y. Change of calcium and cAMP concentration by adrenoceptor agents in cultured porcine corneal endothelial cells. J. Ocul. Pharmacol. Ther. 2000;16:299–309. doi: 10.1089/jop.2000.16.299. [DOI] [PubMed] [Google Scholar]
- WEN R., CHENG T., LI Y., CAO W., STEINBERG R.H. Alpha 2-adrenergic agonists induce basic fibroblast growth factor expression in photoreceptors in vivo and ameliorate light damage. J. Neurosci. 1996;16:5986–5992. doi: 10.1523/JNEUROSCI.16-19-05986.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIADA A., HUDLICKA O., TYLER K.R. The effect of long-term administration of alpha 1-blocker prazosin on capillary density in cardiac and skeletal muscle. Pflugers Arch. 1989;415:355–360. doi: 10.1007/BF00370888. [DOI] [PubMed] [Google Scholar]


