Abstract
Using an in vivo model of erectile activity, the effects of sildenafil were studied in mice lacking neuronal or endothelial nitric oxide synthase (nNOS and eNOS, respectively).
Under pentobarbitone anaesthesia, intracavernous pressure (ICP) and mean arterial pressure (MAP) were monitored continuously in wild-type, nNOS−/− and eNOS−/− mice. The magnitude of erectile activity was quantified as the ratio of ICP to MAP.
No differences in basal ICP or MAP were observed amongst wild-type, eNOS−/− and nNOS−/− mice. Electrical stimulation of the cavernous nerve (ESCN; 4.0 V, 16 Hz, 1 ms, 30 s) evoked increases in ICP and ICP/MAP as well as penile tumescence. Responses to ESCN were reduced in nNOS−/−, but not in eNOS−/− mice.
L-NAME (50 mg kg−1, i.v.) significantly increased MAP and attenuated erectile responses in both wild-type and eNOS−/− mice.
Sildenafil (1 mg kg−1, i.v.) augmented electrically-evoked erectile activity in a voltage-dependent manner in wild-type mice and facilitated erectile responses in eNOS−/− mice. By contrast, sildenafil failed to augment the diminished erectile responses in mice lacking the nNOS isoform.
These data reveal the relative importance of nNOS, compared to eNOS, as the critical NOS isoform in the control of erectile function and illustrate that the nNOS isoform is required for sildenafil-induced facilitation of erectile responses in vivo in mice.
Keywords: Cavernous nerve, electrical stimulation, erectile function, nitric oxide synthase, phosphodiesterase inhibitors, sildenafil
Introduction
Penile erection (tumescence) is a highly coordinated reflex that can be triggered by either peripheral (tactile) or central activation (visual, olfactory, auditory or imaginative cues) of somatic pathways and, as such, is subject to modulation at many levels of the neuraxis. In the periphery, erectile function depends upon an intricate balance and coordination among parasympathetic and sympathetic nerves, neurotransmitters, blood vessels and cavernous muscles (Andersson & Wagner, 1995; Andersson, 2001). Tumescence results from the release of parasympathetic neurotransmitters, which relax smooth muscles and decrease vascular tone. The relaxation of cavernosal and arterial smooth muscle permits blood flow to the sinusoidal spaces of the corpora cavernosa, which physically reduces venous outflow and increases intracavernous pressure (ICP), resulting in penile engorgement and erection (Carrier et al., 1994; Andersson & Wagner, 1995; Lue, 2000; Andersson, 2001).
The haemodynamics of erectile function are modulated by neural input from brain and spinal cord pathways. At the level of the spinal cord, control of erectile activity emanates from the lumbosacral region from which neurons project through the pelvic nerve and pelvic plexus to the cavernous nerve, terminating at the level of the penis. The transmission of sensory input from the penis, through afferents in the dorsal and pudendal nerves, to the sacral spinal cord completes a reflex pathway in which reflexogenic erections can be elicited and maintained (Andersson & Wagner, 1995; Lue, 2000; Andersson, 2001).
Although several neurotransmitters and autocoids modulate erectogenesis at supraspinal, spinal and peripheral levels, nitric oxide (NO) is considered to play a critical role in the control of erectile function (Martinez-Pineiro et al., 1993; Andersson & Wagner, 1995; Lue, 2000). NO activates soluble guanylate cyclase in cavernosal smooth muscle to generate cGMP which, in turn, promotes vasorelaxation. That the NO – cGMP system modulates erectile function is supported by the inhibitory effects of NOS inhibitors and the stimulatory effects of phosphodiesterase inhibitors which augment cGMP-mediated vasorelaxation. Indeed, considerable evidence has shown that the availability of NO is dependent on the catalytic activity of nitric oxide synthase (NOS) and that inhibition of NOS, by inhibitors such as L-NG-nitro-arginine methyl ester (L-NAME) and L-NG-nitroarginine and L-NG-monomethylarginine, blocks cGMP production (Burnett et al., 1996) and inhibits erectile activity in vivo. Conversely, cGMP-specific phosphodiesterase (PDE) inhibitors, which prevent the hydrolysis of cGMP, allow for the accumulation of cGMP in the cells and potentiate the NO-cGMP axis (Jeremy et al., 1997; Gonzalez-Cadavid et al., 1999). Furthermore, type 5 PDE inhibitors, such as sildenafil, vardenafil and tadalafil (IC351) prolong penile tumescence in the presence of sexual stimulation and are amongst the most effective treatments for erectile dysfunction (Boolell et al., 1996; Jeremy et al., 1997; Goldstein et al., 1998; Andersson, 2001; Kim et al., 2001; Padma-Nathan et al., 2001; Stark et al., 2001).
Despite the overwhelming evidence illustrating the involvement of the NO – cGMP pathway in the control of penile erection (Ignarro, 1992; Martinez-Pineiro et al., 1993; Burnett, 1995; Jeremy et al., 1997), little is known about the contribution of specific NOS isoforms to erectile function. Of the three different isoforms of NOS, it was believed that only the constitutive isoforms are localized in tissues involved in erectile activity. More recent evidence now indicates that iNOS is expressed in penile cavernosal smooth muscle and urethral epithelium in the absence of pathology (Rajasekaran et al., 1998; Podlasek et al., 2001) though the importance of iNOS to erectile function remains unclear. Neuronal NOS (nNOS) is present in penile autonomic nerves (pelvic plexus, cavernous and dorsal nerves) and arterioles; whereas, endothelial NOS (eNOS) localizes to the endothelial layers of the dorsal arteries and veins of the penis and corporeal sinusoid space (Burnett et al., 1993; Burnett, 2000). Thus, the differential localization of nNOS and eNOS suggests that each may contribute uniquely to erectile function.
The encoding of each NOS isoform by distinct genes has been exploited to study the relative contribution of the NOS isoforms to erectile function. Mice with a null mutation of the nNOS gene are devoid of nNOS expression, yet possess erectile function sufficient for copulation and reproduction (Huang et al., 1993; Burnett et al., 1996). This result contradicts the notion that the primary source of NO involved in erectogenesis derives from nNOS activity (Giuliano et al., 1995; Gonzalez-Cadavid et al., 1999) and suggests that either nitrergic or non-nitrergic compensatory mechanisms maintain erectile function in the absence of nNOS or are upregulated to serve such a function in the nNOS null mice. The involvement of eNOS in normal erectogenesis is also unclear. Relaxation of human corpus cavernosum by transmural electrical stimulation does not require functional endothelium (Sezen & Burnett, 2000); however, eNOS is also expressed in corporal smooth muscle (Rajasekaran et al., 1998; Podlasek et al., 2001), implicating it in penile erection. Nonetheless, mice lacking this isoform are capable of adequate reproductive function (Huang et al., 1995) and exhibit a decreased latency to ejaculation (Kriegsfeld et al., 1999).
In this study, we examined mice with targeted deletions of either nNOS or eNOS to understand the contribution of each isoform to erectile function in the absence and presence of electrical stimulation of the cavernous nerve. Although it is well established that sildenafil enhances erectile function via actions on the NO – cGMP pathway (Boolell et al., 1996; Jeremy et al., 1997) little is known about the functional requirements for either eNOS or nNOS in sildenafil-induced erectogenesis. Therefore, we sought to further characterize the effects of sildenafil in nNOS and eNOS null mice.
Methods
Animals and housing
All experiments were approved by the Merck Research Laboratories-Rahway Institutional Animal Care and Use Committee. Male mice lacking either nNOS (B6:129S-NOS1tm1Plh, stock no. 002633) or eNOS (B6:129P2-NOS3tm1Unc, stock no. 002684) and their appropriate wild-type controls (B6129SF2/J and C57BL/6J, respectively) were obtained from Jackson Laboratories (Bar Harbor, ME, U.S.A.). Mice were housed 3 – 10 per cage on a normal 12 : 12 h light – dark cycle, given free access to food and water, and were 11 – 18 weeks of age at the time of study.
Surgical procedures
A murine model to monitor direct and electrical stimulation-evoked changes in intracavernosal pressure (ICP), essentially similar to that recently described (Mizusawa et al., 2001), was developed. Mice were anaesthetized with sodium pentobarbital (80 – 100 mg kg−1, i.p.) and placed on a heating pad. Using a 30 G needle connected to PE10 tubing filled with either drug or heparinized saline (20 units ml−1), the jugular vein and carotid artery were exposed and cannulated for intravenous compound administration and to measure arterial pressure, respectively. The cavernous nerve and penile body were exposed through a midline incision. Surrounding muscles were cauterized and removed to visualize the cavernous nerve, which arises from the ipsilateral pelvic ganglion and is situated dorsal to the prostate. A 30 G needle attached to PE10 tubing, filled with heparinized saline, was inserted into the base of the corpus cavernosum near the crura. Proper placement of the cannula was confirmed by observing a slight increase in pressure (∼5 mmHg) upon insertion of the cannula and a tumescence response of the penile body after a flush with heparinized saline.
Experimental protocol
Arterial and cavernosal cannulae were connected to pressure transducers and amplified to monitor mean arterial pressure (MAP) and intracavernous pressure (ICP) which were expressed in mmHg. Pressure data were acquired, digitized at 1 Hz, visualized, stored and analysed using the Po-Ne-Mah Life Science Suite software (Gould Instrument Systems, Valley View, OH, U.S.A.). Fluid supplements (0.05 ml heparinized saline) were administered every 30 min. The cavernous nerve was then isolated and placed on fixed position bipolar silver electrodes (Harvard Apparatus, Holliston, MA, U.S.A:) encased in plastic to allow stimulation of the nerve without additional stimulation of surrounding tissues. The stimulating electrodes, which are held and advanced by a micromanipulator, were attached to a Grass SD9 square wave stimulator (Grass Instruments, West Warwick, RI, U.S.A.) to deliver electrical impulses at constant stimulation parameters. ICP, MAP and ICP/MAP responses were recorded continuously for the duration of the experiment. After instrument calibration, MAP and ICP were monitored for at least 5 min in the absence of electrical stimulation to ensure stable activity. We examined responses to ESCN at different voltages. A 1.5 V stimulation (16 Hz, 1 ms, 30 s) augmented ICP/MAP ratio from a value of 0.05 at rest to a peak of ∼0.28 (data not shown); whereas, a 4.0 V stimulation increased the ICP/MAP ratio to a peak range of 0.5 – 0.7 (Figure 1). Based on these findings, a 4 V stimulus was used throughout the study. Following baseline recording, electrical stimulation (4.0 V, 16 Hz, 1 ms, for 30 s) was applied to the cavernous nerve (ESCN). Preliminary studies established that these parameters were optimal to evoke sub-maximal responses (60 – 75% of maximum) in wild-type mice of various genetic backgrounds without inducing fatigue in the nerve. Once baseline stimulation-evoked responses were determined, test compound or the appropriate vehicle was administered and direct effects on erectile activity were recorded for 15 min. Based on our experience with rat models of erectile function in which the pro-erectile effects of sildenafil were examined and preliminary studies in mice in which 0.1 mg kg−1, i.v. did not significantly augment erectile activity (n=8; data not shown), we chose a test dose of 1 mg kg−1, i.v. Electrical stimulation-evoked changes were monitored at 15, 45 and 60 min post dosage. Electrical stimulation was always presented in duplicate with a 5 min inter-stimulation-interval; the responses reported at each time point represent the mean of these two responses in each animal.
Figure 1.
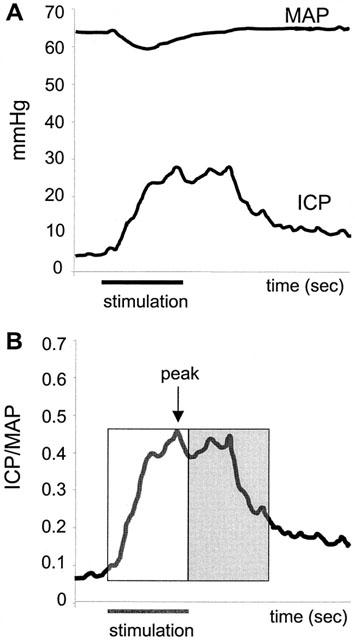
(A) Intracavernous pressure (ICP) and mean arterial pressure (MAP) changes in the mouse corpus cavernosum in response to ESCN (4 V). Data collected every second. Horizontal bar depicts 30 s stimulation. (B) The ICP/MAP ratio is a derived value to normalize for arterial pressure. Horizontal bar depicts 30 s stimulation. Graphical representation of segments of data analysed to depict the magnitude and duration of evoked responses. Open box represents the AUC during 30 s stimulation, while the shaded box represents the AUC 30 s post stimulation. Peak ICP/MAP (or magnitude) is the highest ICP/MAP achieved in response to ESCN.
Biological Agents
Sildenafil was synthesized at Merck-Frosst (Kirkland, Canada) and was dissolved in a vehicle consisting of ethanol, polyethylene glycol (PEG400) and sterile water (1 : 4 : 5 ratio). L-NG-nitro-arginine-methyl-ester hydrochloride (L-NAME, Sigma-Aldrich, St. Louis, MO, U.S.A.) was prepared in sterile saline.
Data analyses
Digitized data were collected and stored in an Excel file using Po-Ne-Mah Life Science Suite software. For each time point, responses evoked by duplicate electrical stimulations were averaged, and the mean values were used for comparison. Response segments were used to evaluate changes in ICP, MAP and the ratio of ICP/MAP (to normalize for any blood pressure effects) in response to ESCN in the presence and absence of compound or vehicle (Figure 1A). The magnitude and duration of each response evoked by ESCN was determined by calculating the Area Under the Curve (AUC) for the 30 s during (‘stim') or 30 s after (‘post') stimulation. The magnitude of the response was also quantified by determining the maximal ICP/MAP ratio (‘peak') attained over the combined 60 s stimulation and post-stimulation period (Figure 1B). Mice were excluded from the study and subsequent analyses for the following reasons; overstimulation (>10) of the cavernous nerve, improper cannula placement in corpus cavernosum/failure to respond to electrical stimulation of either cavernous nerve, or low blood pressure (<40 mmHg).
Statistics
Student's two-tailed, paired t-tests or, where appropriate, repeated measures ANOVA followed by Dunnett's post hoc analyses, were conducted to compare responses between baseline and treatment groups. P<0.05 was considered statistically significant. Data were presented as mean±standard error of the mean (s.e.mean). All statistical analyses were performed using Prism software (GraphPad Software, San Diego, CA, U.S.A.).
Results
Figure 1 illustrates the quantitative measurement of intracavernous pressure (ICP) and arterial pressure (MAP) before, during and after electrical stimulation of the cavernous nerve (ESCN). ESCN in C57BL/6J mice produces a transient decrease in MAP and sustained increases in ICP that persist beyond the period of stimulation. The ratio of ICP to MAP (Figure 1B) provides an index of erectile activity that controls for changes in MAP.
Sildenafil (1 mg kg−1, i.v.) did not directly affect the ICP/MAP ratio in the absence of electrical stimulation. However, sildenafil significantly prolonged the responses evoked by ESCN by 27% over baseline 15 min after treatment (P < 0.05; Figure 2A,B). The enhancement by sildenafil was manifest only in the post-stimulation period as the magnitude of the response during the stimulation was unchanged (−2%, P>0.05; Figure 2B). The peak ICP/MAP ratio increased to 0.65 (±0.03) from 0.59 (±0.05), but this effect was not statistically significant. By 45 min post dosage, the simulation-evoked and post-stimulation ICP responses returned to pre-sildenafil levels (Figure 2B).
Figure 2.
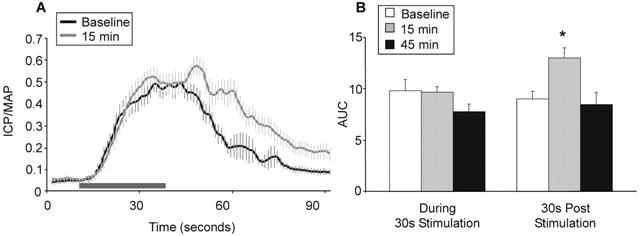
(A) Facilitatory effects of sildenafil (1 mg kg−1, i.v.) on ICP/MAP in C57BL/6J mice (n=8) in response to ESCN (4 V) pre and 15 min post sildenafil (mean±s.e.mean). The duration of electrically-evoked response was prolonged 15 min after sildenafil administration. Horizontal bar depicts 30 s stimulation. (B) Mean AUC effects during and post stimulation in response to sildenafil treatment (±s.e.mean). *Statistically significant response from baseline, P<0.05, students two-tailed t-test.
The involvement of NOS in erectile activity was initially addressed using a non-specific NOS inhibitor. L-NAME (50 mg kg−1, i.v.) significantly attenuated, but did not completely abolish, responses to ESCN in C57BL/6J mice (Figure 3). In the absence of stimulation, L-NAME had no direct effect on basal ICP. Responses during and post stimulation were reduced by 84 and 64%, respectively, 15 min after L-NAME treatment and remained at this level for at least 45 min. MAP markedly increased by ca. 30 mmHg within 1 – 2 min following L-NAME administration (Figure 3A). However, the increases in MAP alone did not account for the decrease in ICP/MAP ratio since ICP responses themselves were reduced by L-NAME treatment.
Figure 3.
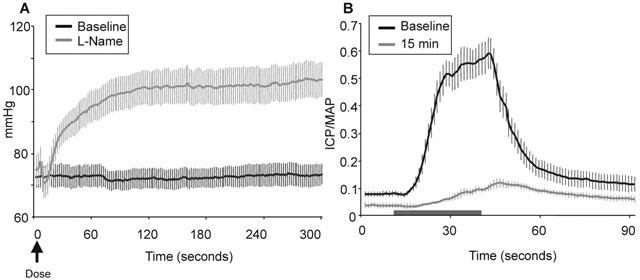
Effects of L-NAME (50 mg kg−1, i.v.) in C57BL/6J mice (n=8) in the absence and presence of electrical stimulation of the cavernous nerve (mean±s.e.mean). (A) Direct mean arterial pressure (mmHg) effects in the absence of electrical stimulation. (B) ICP/MAP evoked effects in response to ESCN (4 V). Normal erectile responses to electrical stimulation (4 V) were attenuated, but not completely abolished, by treatment with L-NAME. These diminished effects are not a result of the increased MAP, whereas, ICP was also attenuated as was ICP/MAP. Horizontal bar depicts 30 s stimulation.
Since non subtype-selective inhibition of NOS greatly reduced erectile activity, we next evaluated the responsiveness of eNOS−/− and nNOS−/− mice to ESCN in the presence and absence of sildenafil or vehicle (Figure 4). Prior to sildenafil administration, the responses to ESCN during and post stimulation were comparable in wild-type and eNOS−/− mice. Consistent with the observations in C57BL/6J mice, sildenafil had no direct effect on ICP and did not augment the magnitude of the response during electrical stimulation (Table 1), but it significantly enhanced the duration of the responses evoked by ESCN (Figure 5A; P<0.01). In contrast to the effects in wild-type mice, the onset of the sildenafil-induced increase in erectile activity were significantly delayed and sustained, lasting for at least 60 min (Figure 4C). MAP was also significantly elevated by approximately 12% at 45 and 60 min post sildenafil (P<0.01). Vehicle treatment had no significant effect in eNOS−/− mice, but L-NAME (50 mg kg−1, i.v.) significantly attenuated both the magnitude (ICP/MAP from 0.50±0.08 to 0.34±0.09; P<0.05) and duration of the ESCN evoked responses in these mice (Figure 5A).
Figure 4.
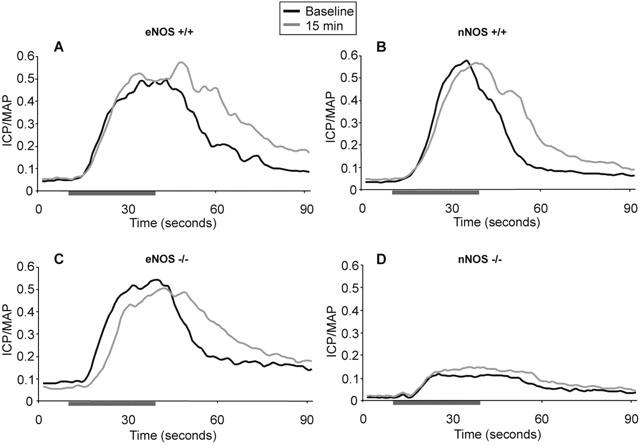
Mean ESCN responses in eNOS and nNOS−/− and+/+mice (n=8/ group) pre and 15 min post sildenafil (1 mg kg−1, i.v.) administration. Prolongation of the duration of ESCN effects observed with eNOS and nNOS+/+mice and eNOS−/− mice, but lacking in nNOS−/− mice. Horizontal bar depicts 30 s stimulation. For clarity, s.e.mean has been omitted, for values see Table 1. (A) Effects of sildenafil in eNOS+/+mice pre and 15 min post dosage. C57BL/6J mice. (B) Effects of sildenafil in nNOS+/+mice. Hybrid mix B6129SF2/J. (C) Effects of sildenafil on eNOS−/− mice. Prolongation of evoked effects along with a delayed onset of evoked responses following sildenafil treatment. (D) Effects of sildenafil on nNOS−/− mice. Significant attenuation of ESCN evoked effects in nNOS−/− mice both pre and post sildenafil treatment.
Table 1.
ESCN responses pre and post sildenafil (1 mg kg−1, i.v.) in mice lacking NOS isoforms and their appropriate wild-type controls
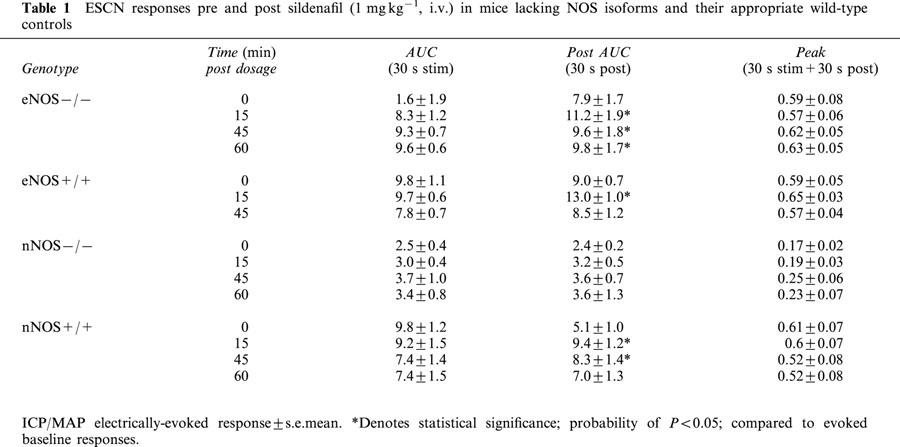
Figure 5.
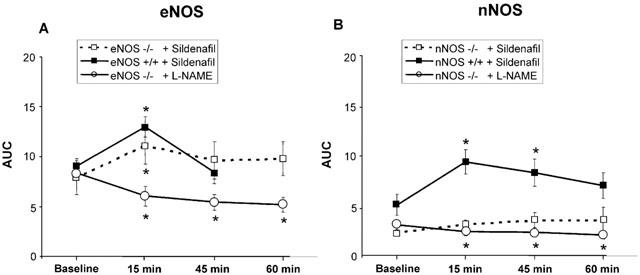
Post AUC effects of sildenafil (1 mg kg−1, i.v.) or L-NAME (50 mg kg−1, i.v.) in eNOS and nNOS null and wild-type mice in response to ESCN (30 s post stimulation). ESCN responses represent mean±s.e.mean. (A) Sildenafil induced effects in eNOS null (−/−, open square) and wild-type (+/+, filled square) mice (n=8). Significant prolongation of evoked effects in both −/− and+/+mice 15 min post dosage, continuing out to 60 min in −/− mice. L-NAME administration significantly attenuates ESCN responses in eNOS−/− mice (n=6, open circle). (B) Sildenafil induced effects in nNOS null (−/−, open square) and wild-type (+/+, filled square) mice (n=8). Significant prolongation of evoked effects in wild-type mice 15 and 45 min post dosage. Sildenafil failed to overcome the attenuation of erectile activity in mice lacking nNOS. L-NAME administration further reduced ESCN responses in nNOS−/− mice (n=6, open circle). *Statistical significance represents a probability of P<0.05.
Unlike eNOS−/− mice, nNOS−/− mice exhibited dramatically reduced responses to ESCN (Figures 4D and 5B). The mean ICP/MAP ratio in nNOS−/− mice following ESCN ranged from 0.1 to 0.35, compared to approximately 0.5 to 0.6 in wild-type mice (Table 1). Sildenafil only modestly and non-significantly enhanced the response to ESCN in the nNOS−/− mice (Figure 4D). By contrast, sildenafil significantly prolonged the post-stimulation responses in nNOS wild-type mice 15 and 45 min after administration (Figure 5B; P<0.05). MAP in nNOS−/− mice decreased significantly by 20% 15 min after sildenafil administration (P<0.01), but returned to baseline by 45 min. Similarly, nNOS wild-type mice showed transient albeit significant reduction in MAP by 15% at 15, but not 45, min post dosage (P<0.001). Vehicle administration did not elicit changes in ICP or MAP in wild-type or nNOS−/− mice 15 min post dosage. L-NAME administration (50 mg kg−1, i.v.) further reduced ESCN responses in nNOS−/− mice (ICP/MAP from 0.14±0.03 to 0.10±0.02; Figure 5B; P=0.05).
Discussion
To elucidate further the involvement and relative importance of neuronal and endothelium-derived NO, electrically-induced erectile activity was quantified in mice lacking eNOS or nNOS and in their wild-type controls. Blockade of NOS activity with the non-specific NOS inhibitor, L-NAME, nearly abolished normal erectile responses to electrical stimulation in C57BL/6J mice, confirming that NOS is essential for modulating erectile function (Martinez-Pineiro et al., 1993; Andersson & Wagner, 1995; Lue, 2000). The inhibitory effects of L-NAME and facilitatory effects of sildenafil presented here were consistent with that previously reported (Mizusawa et al., 2001) and illustrate the appropriateness of this model for evaluating the contribution of specific NOS isoforms and PDE inhibitors to erectile function. Moreover, vehicle treatment did not lead to an increase in either spontaneous (i.e. in the absence of electrical stimulation) or stimulation-evoked changes in the ratio of ICP to MAP, indicating that the stimulation protocol used in these studies did not itself promote erectile activity.
Since both eNOS−/− and nNOS−/− mice possess erectile function sufficient for reproduction, the relative contributions of each isoform to erectogenesis is unclear. In the absence of electrical stimulation, basal ICP was indistinguishable amongst the mice examined here suggesting that the loss of individual isoforms does not exert pronounced effects on nitrergic tone within erectogenic pathways. In this study, we found that mice with a targeted deletion of the eNOS isoform exhibited normal erectile activity in response to ESCN and responded to sildenafil in a similar manner to wild-type mice. Furthermore, L-NAME (50 mg kg−1, i.v.) significantly attenuated, but did not eliminate, the responses evoked by ESCN which illustrates that a residual nitrergic component is present in these mice. Interestingly, in contrast to the effects in wild-type mice, the time course of the sildenafil-induced increase in erectile activity was significantly enhanced in eNOS−/−, lasting for at least 60 min. It is possible that the prolonged duration associated with sildenafil reflects a compensatory increase in a factor on which sildenafil acts or simply that the pharmacokinetic profile of sildenafil is altered in eNOS−/− mice. Regardless, taken together these results indicate that eNOS is not critical for normal erectogenesis under the conditions examined here and that eNOS is not required for the facilitatory actions of sildenafil. However, it is important to note that lower levels of nerve stimulation may reveal a greater contribution of the eNOS pathway and the potential modulatory role of the endothelium than that detected here.
Among the previously reported effects of disrupting expression of eNOS that could confound studies of erectile activity, hypertension was reported to be 17 – 26% depending on anaesthetic (halothane and urethane, respectively; Huang et al., 1995). Under sodium pentobarbital anaesthesia, we recorded a 14% increase in MAP in eNOS−/− compared to wild-type and found that the hypertensive phenotype was exacerbated by sildenafil treatment.
In contrast to our findings in eNOS−/− mice, mice lacking the nNOS isoform exhibited markedly reduced responses to ESCN. That increases in ICP were not completely lost is consistent with previous studies which reported that nNOS−/− mice are fertile and capable of sustaining minimal erectile function (Huang et al., 1993). Contrary to previous findings (Burnett et al., 1996) in which nNOS−/− mice exhibited visible full erections in response to ESCN (2 V, 5 ms, 16 Hz, 60 s), we found that a 4 V stimulation was insufficient to elicit full erection or even visible tumescence. It is not clear to what extent differences in anaesthetic levels or other methodological variables could account for the disparate findings. In the nNOS−/− mice, we tested a range of stimulus intensities (1.5 to 6 V) and found comparable changes in ICP to those produced by a 4 V stimulation. Moreover, sildenafil failed to augment electrically-evoked responses in nNOS null mice; whereas responses to sildenafil treatment in the appropriate wild-type control mice were as robust as those seen in C57BL/6J mice. These data strongly suggest that the lack of effect of sildenafil in nNOS null mice was due to the targeted disruption of nNOS and not merely to the genetic background on which these mice were bred. In aggregate, these data suggest that nNOS is essential for optimal activity within the penile NO-cGMP pathway and, in turn, for normal erectile function. Our results are consistent with other reports in which disruption of processes downstream from the NO-cGMP pathway attenuates erectogenesis. For example, mice deficient in cGMP-dependent kinase I have a diminished capacity for reproduction (Hedlund et al., 2000). The dramatic albeit transient reduction in MAP in the nNOS wild-type and null mice following sildenafil treatment may be attributed to their hybrid genotype since we have observed similar decreases in MAP in their progeny strain (129SV) and in strains not backcrossed six or more generations with C57BL/6J (unpublished observations).
Given the identified importance of nNOS in erectile function, what endows nNOS−/− mice with erectile activity sufficient for reproduction? One previous suggestion was that eNOS upregulates, in the absence of nNOS, and this compensation permits minimal erectile function in nNOS null mutants (Burnett et al., 1996). While this possibility is plausible, our results show that the contribution of eNOS to erectile function, at least under normal conditions, is minimal. Moreover, based on the demonstrated importance of nNOS, one would predict that eNOS would not only need to be upregulated to compensate for the loss of nNOS, but also neuronally localized (Huang et al., 1993). Although the involvement of the constitutive NOS isoforms in erectile function is better understood than that of iNOS, the localization of iNOS in erectile tissues (e.g. corpora cavernosa endothelial lining and smooth muscle; Rajasekaran et al., 1998; Podlasek et al., 2001), suggests that iNOS activity could contribute to the residual erectile function observed in the nNOS−/− mice.
Previous data indicate that low levels of nNOS catalytic activity remain in mice devoid of nNOS expression (Huang et al., 1993). A source for this nNOS activity is PnNOS, an nNOS subtype variant that is localized in the corporal bodies, pelvic ganglia and the cavernous nerve (Gonzalez-Cadavid et al., 2000). Gonzalez-Cadavid and colleagues have demonstrated that PnNOS activity may depend on differing transcriptional and post-transcriptional processing in the central nervous system versus the peripheral nervous system. Two PnNOS mRNA variants have been identified in the mouse. The first full-length transcript, termed nNOSa, encodes a 155-Kda protein; while the second, nNOSb, encodes a 135-Kda protein lacking the (PIN) protein inhibitor binding region. Since the nNOSb transcript survives the obliteration of alternative splicing and thus bypasses the blockade of gene expression and inhibition by PIN, nNOS null mice express the nNOSb, and not the nNOSa variant. As such, activity associated with the nNOSb variant may account for part of the retained fertility of these mice (Gonzalez-Cadavid et al., 2000).
In the current study, we identified the presence of residual responses to ESCN in nNOS−/− mice, and found that L-NAME attenuated, but did not eliminate ESCN responses in wild-type and eNOS−/− mice. L-NAME reduced responses in eNOS−/− mice to a level comparable to that observed in nNOS−/− mice. In nNOS−/− mice, a modest response to ESCN was detected following L-NAME treatment. These findings in the L-NAME treated eNOS−/− and nNOS−/− mice suggest that non-nitrergic mechanisms may contribute to the residual erectile function, although we cannot exclude the possibility that L-NAME does not inhibit nNOSb activity.
Conclusions
The NO-cGMP pathway has been confirmed as being a key mediator of murine erectile activity (Mizusawa et al., 2001). In the current study, we identified nNOS as the essential NOS isoform in the regulation of cGMP-mediated vasorelaxation and erectile function. Elimination of nNOS, but not eNOS, impaired normal erectile function and sildenafil did not ameliorate this deficit. The lack of efficacy of sildenafil in nNOS deficient mice suggests that human patient populations with erectile dysfunction (ED) whose aetiology is associated with disease states in which nNOS levels decrease may not respond as well to sildenafil, and possibly all PDE5 inhibitors, as patients whose nNOS activity is within a normal range.
Acknowledgments
The authors wish to thank Drs S. Sezen and A. Burnett for their assistance with the development of this model.
Abbreviations
- cGMP
3′5′-cyclic guanosine monophosphate
- eNOS
endothelial nitric oxide synthase
- ESCN
electrical stimulation of the cavernous nerve
- GTP
5′-guanosine triphosphate ICP, intracavernosal pressure
- iNOS
inducible nitric oxide synthase
- L-NAME
L-NG-nitro-arginine methyl ester hydrochloride
- MAP
mean arterial pressure
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- PDE
phosphodiesterase
- PIN
protein inhibitor binding region
- PnNOS
penile neuronal nitric oxide synthase
References
- ANDERSSON K.E. Pharmacology of penile erection. Pharmacol. Rev. 2001;53:417–450. [PubMed] [Google Scholar]
- ANDERSSON K.E., WAGNER G. Physiology of penile erection. Physiol. Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- BOOLELL M., ALLEN M.J., BALLARD S.A., GEPI-ATTEE S., MUIRHEAD G.J., NAYLOR A.M., OSTERLOH I.H., GINGELL C. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int. J. Impot. Res. 1996;8:47–52. [PubMed] [Google Scholar]
- BURNETT A.L. Role of nitric oxide in the physiology of erection. Biol. Reprod. 1995;52:485–489. doi: 10.1095/biolreprod52.3.485. [DOI] [PubMed] [Google Scholar]
- BURNETT A.L. Lecture 2: nitric oxide synthase and heme oxygenase knockout mice-what have we learned. Int. J. Impot. Res. 2000;12:S42–S44. doi: 10.1038/sj.ijir.3900560. [DOI] [PubMed] [Google Scholar]
- BURNETT A.L., NELSON R.J., CALVIN D.C., LIU J.X., DEMAS G.E., KLEIN S.L., KRIEGSFELD L.J., DAWSON V.L., DAWSON T.M., SNYDER S.H. Nitric oxide-dependent penile erection in mice lacking neuronal nitric oxide synthase. Mol. Med. 1996;2:288–296. [PMC free article] [PubMed] [Google Scholar]
- BURNETT A.L., TILLMAN S.L., CHANG T.S., EPSTEIN J.I., LOWENSTEIN C.J., BREDT D.S., SNYDER S.H., WALSH P.C. Immunohistochemical localization of nitric oxide synthase in the autonomic innervation of the human penis. J. Urol. 1993;150:73–76. doi: 10.1016/s0022-5347(17)35401-0. [DOI] [PubMed] [Google Scholar]
- CARRIER S., ZVARA P., LUE T.F. Erectile dysfunction. Endocrinol. Metab. Clin. North Am. 1994;23:773–782. [PubMed] [Google Scholar]
- GIULIANO F., RAMPIN O., BERNABE J., ROUSSEAU J.P. Neural control of penile erection in the rat. J. Auton. Nerv. Syst. 1995;55:36–44. doi: 10.1016/0165-1838(95)00025-s. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I., LUE T.F., PADMA-NATHAN H., ROSEN R.C., STEERS W.D., WICKER P.A. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N. Engl. J. Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- GONZALEZ-CADAVID N.F., BURNETT A.L., MAGEE T.R., ZELLER C.B., VERNET D., SMITH N., GITTER J., RAJFER J. Expression of penile neuronal nitric oxide synthase variants in the rat and mouse penile nerves. Biol. Reprod. 2000;63:704–714. doi: 10.1095/biolreprod63.3.704. [DOI] [PubMed] [Google Scholar]
- GONZALEZ-CADAVID N.F., IGNARRO L.J., RAJFER J. Nitric oxide and the cyclic GMP system in the penis. Mol. Urol. 1999;3:51–59. [PubMed] [Google Scholar]
- HEDLUND P., ASZODI A., PFEIFER A., ALM P., HOFMANN F., AHMAD M., FASSLER R., ANDERSSON K.E. Erectile dysfunction in cyclic GMP-dependent kinase I-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2349–2354. doi: 10.1073/pnas.030419997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG P.L., DAWSON T.M., BREDT D.S., SNYDER S.H., FISHMAN M.C. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- HUANG P.L., HUANG Z., MASHIMO H., BLOCH K.D., MOSKOWITZ M.A., BEVAN J.A., FISHMAN M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- IGNARRO L. Nitric oxide as the physiological mediator of penile erection. J. NIH Res. 1992;4:59–62. [Google Scholar]
- JEREMY J.Y., BALLARD S.A., NAYLOR A.M., MILLER M.A., ANGELINI G.D. Effects of sildenafil, a type-5 cGMP phosphodiesterase inhibitor, and papaverine on cyclic GMP and cyclic AMP levels in the rabbit corpus cavernosum in vitro. Br. J. Urol. 1997;79:958–963. doi: 10.1046/j.1464-410x.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- KIM N.N., HUANG Y.H., GOLDSTEIN I., BISCHOFF E., TRAIS A.M. Inhibition of cyclic GMP hydrolysis in human corpus cavernosum smooth muscle cells by vardenafil, a novel, selective phosphodiesterase type 5 inhibitor. Life Sci. 2001;69:2249–2256. doi: 10.1016/s0024-3205(01)01308-x. [DOI] [PubMed] [Google Scholar]
- KRIEGSFELD L.J., DEMAS G.E., HUANG P.L., BURNETT A.L., NELSON R.J. Ejaculatory abnormalities in mice lacking the gene for endothelial nitric oxide synthase (eNOS−/−) Physiol. Behav. 1999;67:561–566. doi: 10.1016/s0031-9384(99)00100-6. [DOI] [PubMed] [Google Scholar]
- LUE T.F. Erectile dysfunction. N. Engl. J. Med. 2000;342:1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- MARTINEZ-PINEIRO L., TRIGO-ROCHA F., HSU G.L., VON HEYDEN B., LUE T.F., TANAGHO E.A. Cyclic guanosine monophosphate mediates penile erection in the rat. Eur. Urol. 1993;24:492–499. doi: 10.1159/000474357. [DOI] [PubMed] [Google Scholar]
- MIZUSAWA H., HEDLUND P., HAKANSSON A., ALM P., ANDERSSON K.E. Morphological and functional in vitro and in vivo characterization of the mouse corpus cavernosum. Br. J. Pharmacol. 2001;132:1333–1341. doi: 10.1038/sj.bjp.0703938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PADMA-NATHAN H., MCMURRAY J.G., PULLMAN W.E., WHITAKER J.S., SAOUD J.B., FERGUSON K.M., ROSEN R.C. On-demand IC351 (Cialis) enhances erectile function in patients with erectile dysfunction. Int. J. Impot. Res. 2001;13:2–9. doi: 10.1038/sj.ijir.3900631. [DOI] [PubMed] [Google Scholar]
- PODLASEK C.A., GONZALEZ C.M., ZELNER D.J., JIANG H.B., MCKENNA K.E., MCVARY K.T. Analysis of NOS isoform changes in a post radical prostatectomy model of erectile dysfunction. Int. J. Impot. Res. 2001;13:S1–S15. doi: 10.1038/sj.ijir.3900772. [DOI] [PubMed] [Google Scholar]
- RAJASEKARAN M., MONDAL D., AGRAWAL K., CHEN I.L., HELLSTROM W., SIKKA S. Ex vivo expression of nitric oxide synthase isoforms (eNOS/iNOS) and calmodulin in human penile cavernosal cells. J. Urol. 1998;160:2210–2215. doi: 10.1097/00005392-199812010-00088. [DOI] [PubMed] [Google Scholar]
- SEZEN S.F., BURNETT A.L. Intracavernosal pressure monitoring in mice: responses to electrical stimulation of the cavernous nerve and to intracavernosal drug administration. J. Androl. 2000;21:311–315. [PubMed] [Google Scholar]
- STARK S., SACHSE R., LIEDL T., HENSEN J., ROHDE G., WENSING G., HORSTMANN R., SCHROTT K.M. Vardenafil increases penile rigidity and tumescence in men with erectile dysfunction after a single oral dose. Eur. Urol. 2001;40:181–190. doi: 10.1159/000049770. [DOI] [PubMed] [Google Scholar]


