Abstract
Effects of A-322312 (α1B-adrenoceptor (AR) antagonist), A-119637 (α1D-AR antagonist), prazosin (non-selective α1-AR antagonist), and yohimbine (α2-AR antagonist) were studied in rat corpus cavernosum (CC) and cavernous artery (Acc) preparations. Effects of intracavernous (i.c.) or intraperitoneal (i.p.) administration of α1-AR antagonists on apomorphine-induced erections were investigated.
A-119637 attenuated electrically induced contractions in isolated CC (−logIC50; 8.12±0.15), and relaxed noradrenaline (NA)-contracted preparations by more than 90% at 10−7 M. At the same concentration, the −logEC50 value for NA in Acc was altered from 6.79±0.07 to 4.86±0.13. In the CC and Acc, prazosin similarily inhibited contractile responses.
Inhibitory effects of A-322312 (10−7 M) in electrically activated CC were 32.3±5.1%, whereas no effect on concentration-response curves for NA was observed in the Acc. Yohimbine (10−8 M and 10−7 M), enhanced electrically-induced contractions in isolated CC by 20 to 50%. At 10−6 M, inhibitory effects of yohimbine were obtained.
A-119637 (0.3 μmol kg−1, i.p.) tripled the number of erections, and produced a 6 fold increase in the duration of apomorphine-induced erectile responses. A-322312, prazosin, or yohimbine did not enhance erections induced by apomorphine. None of the α1-AR antagonists significantly increased ICP upon i.c. administration. Decreases in blood pressure were seen with A-119637 and prazosin.
The present findings show that there is a functional predominance of the α1D-AR subtype in the rat erectile tissue, and that blockade of this receptor facilitates rat penile erection induced by a suboptimal dose of apomorphine.
Keywords: Intracavernous pressure, corpus cavernosum, cavernous artery, α1-subtype, electrical stimulation, nerves, prazosin
Introduction
The trabecular smooth muscles and vasculature of the corpus cavernosum (CC) receive a dense adrenergic innervation. These nerves release noradrenaline (NA) which, by acting on postjunctional α-adrenoceptors, play an important role for smooth muscle contractility during penile flaccidity and detumescence (Andersson, 2001, Traish et al., 2000).
In the human CC, both α1 and α2-adrenoceptors have been demonstrated, and current information favour the view of a functional predominance of α1-adrenoceptors (Andersson, 2001). Three distinct α1-adrenoceptor (AR) subtypes have been cloned and pharmacologically characterized (Guimaraes & Moura, 2001). In the human CC, mRNAs encoding for α1A, α1B and α1D-ARs, with a higher expression of the α1A-AR subtype (Dausse et al., 1998), have been demonstrated. These three subtypes were also found in rat erectile tissues by in situ hybridization (Veronneau-Longueville et al., 1998). Based on pharmacological findings (Muramatsu et al., 1995), the presence of a fourth α1-AR subtype, denoted α1L, with low affinity for prazosin, has also been proposed for the CC (Davis et al., 1998). It is not clear which α1-AR subtype is of main importance for contractility in penile smooth musculature. In the rat corpus cavernosum, α1A-ARs, but not α1B-ARs, have been suggested to be responsible for α1-AR agonist-induced contractions (Tong & Cheng, 1997). Other investigators have proposed a functional relevance for the α1B-, and/or the α1L-AR subtype in the contractility of the erectile tissue (Davis et al., 1999; Sironi et al., 2000). Functional experiments and investigations in animals' models are so far inconclusive, and since the cavernous smooth muscle expresses mixtures of α1-AR subtypes, agonists may produce contractions by interaction with more than one receptor subtype (Traish et al., 1995b).
In cavernosal resistance arteries, postjunctional α1-ARs also appear to mediate the main smooth muscle contractile effects (Simonsen et al., 1997a), but a contribution of α2-ARs cannot be excluded (Hedlund & Andersson, 1985). In penile erectile tissues and resistance arteries, presynaptic stimulation of α2-ARs, has been suggested to decrease transmitter release from adrenergic and/or cholinergic nerves, (Hedlund et al., 1984; Molderings et al., 1989; Simonsen et al., 1997b).
Apomorphine, a dopamine receptor agonist, has been reported to produce significant and durable erections with a low incidence of adverse effect, and the drug has recently been introduced as a sublingual preparation for treatment of erectile dysfunction (Heaton, 2001).
Clinical studies on oral or intracavernous α-AR antagonists as alternative treatments for erectile dysfunction have been performed (Buvat et al., 1998; Andersson et al., 2000; Lue, 2000, Andersson & Stief, 2001). In particular, oral administration of phentolamine, a non-selective α1 and α2-AR antagonist, is attracting some attention. This treatment is beneficial for some patients, however, circulatory side-effects can occur at a high dose (Becker et al., 1998; Goldstein, 2000). In order to obtain better efficacy and to diminish side effects, it is desirable to increase the selectivity of the drugs for the target tissues.
If a combination of an α-AR antagonist and apomorphine produces better efficacy than with either drug alone in the treatment of erectile dysfunction has not yet been tested. The aims of the present study were therefore to investigate the effects of some α1-AR subtype selective antagonists on rat CC and cavernous artery (Acc) in vitro, and on apomorphine-induced erection in vivo.
Methods
Animals
Ninety-three male Sprague – Dawley rats (280 – 340 g) were used. The rats were kept and cared for in standard cages under clean conditions in separate quarters in a 1200 – 1200 h light-darkness cycle with free access to water and pellets. The Animal Ethics Committee of Lund University approved the experiments performed.
Functional in vitro experiments
Thirty-one rats were sacrificed by carbon monoxide asphyxia followed by exsanguination. The penises were removed and placed in chilled Krebs solutions. As previously described (Hedlund et al., 1999), the tunica albuginea was carefully opened, and the erectile tissue was microsurgically dissected free. Silk ligatures were applied at both ends of the strip preparations, which were then suspended between two metal prongs in thermostatically-controlled organ baths (5 ml, 37°C) containing Krebs solution, routinely changed every 20 min, and bubbled with a mixture of 95% O2 and 5% CO2 (pH 7.4). The cavernous artery (Acc) was carefully dissected from the CC specimens and cleaned from surrounding erectile tissue. Ring-preparations with an outer diameter of approximately 250 μm and a length of 0.7 mm were prepared and mounted on metal prongs (Ø70 μm) in vessel organ baths (2.5 ml, 37°C) also containing Krebs solution and aerated with 95% O2 and 5% CO2 (pH 7.4). Isometric tension was recorded by means of Grass Instruments FT03C force-transducer connected to a Grass 7D polygraph (Grass Instruments Co, MA, U.S.A.).
Electrical field stimulation was performed with two platinum electrodes, placed in parallel to the strips in the organ baths. Single square-wave pulses at supramaximum voltage and with a duration of 0.5 ms were delivered by a Grass S48 stimulator. The train duration was 5 s and the train interval 120 s.
During an equilibration period of 45 min., tension was adjusted until mean stable tensions of 1.6±0.1 mN (n=49, N=31), and 0.82±0.06 (n=21, N=9) were obtained for CC and Acc, respectively. Contractile abilities of the preparations were established by adding a K+ solution (124 mM) to the organ baths. Mean responses to K+ amounted to 14.5±2.4 mN for CC and 1.3±0.09 mN for Acc. Concentration-response curves for noradrenaline (NA; 10−9 – 10−4 M) were determined by cumulative administration of the agonist to preparations of Acc. Preparations were then pre-treated (25 min) with antagonists (10−9 – 10−6 M), and the effect of the respective drug on NA-induced contractions was evaluated at each concentration. In CC, effects of drugs were investigated on stable and reproducible contractions induced by NA (3×10−6 M) or by electrical field stimulation. Frequency-response relationships were investigated at supramaximum voltage in all preparations stimulated electrically. When investigating the effect of drugs on electrically induced contractions, a frequency producing activities that corresponded to approximately 70% of maximal contraction was chosen. A preparation was regarded as stable when the amplitude of three consecutive electrically induced contractions did not differ by more than 5%. The investigated drugs were then added cumulatively. The degree of inhibition was expressed as a percentage of the contraction elicited prior to the addition of the lowest concentration of the drugs.
In vivo studies
The rats were anaesthetized with pentobarbital sodium (Apoteket, Umeå, Sweden; 50 mg kg−1) and ketamine (Parke Davis, Barcelona, Spain; 10 mg kg−1) given intraperitoneally. During the experiment, the rats breathed spontaneously. Through a small abdominal incision, a heparinized (100 IE ml−1) polyethylene catheter (PE-10, Parsippany, NJ, U.S.A.) was introduced into the femoral artery to measure systemic blood pressure. With a midline incision in the perineum, the base of the penis, enclosed by striated muscles, was exposed. The ischiocavernous muscle covering the crus CC was divided on one side, and entrance to the underlying tunica albuginea was given. A 25-gauge needle attached to a heparinized polyethylene catheter (PE-50), was inserted into the crus CC. For intracavernous (i.c.) administration of drugs, a 27-gauge-needle attached to a heparinized polyethylene catheter (PE-10) was placed into the other crus CC. Continuous direct measurements of mean arterial and intracavernous pressures (ICP) were performed with transducers (P23 DC, Statham Instrument Inc., CA, U.S.A.) and registered on a Grass Polygraph 7E (Grass Instrument Co, Mass, U.S.A.).
A stabilizing period of 20 – 30 min was allowed before registration of basal intracavernous pressure (BICP) and arterial blood pressures. Drugs were given intraperitoneally (i.p.) or i.c. in volumes of 200 μl and 50 μl, respectively.
Apomorphine 25 μg kg−1 was given subcutaneously (s.c.) 20 min after i.p. drug administration, or 5 min after i.c. administration. The observation period after injection of apomorphine was 30 min. The total number of responses (over 37 mmHg), total duration of responses (the added duration of all responses), time to the first response (TFR), peak ICP (PICP), PICP/blood pressure and area under the curve (AUC) were analysed in each rat. Statistical analyses were then performed on the animals included in each experimental group.
Drugs and solutions
A saline solution, containing 154 mM NaCl, and a Krebs solution of the following composition were used (mM): NaCl 119, KCl 4.6, CaCl2 1.5, MgCl2 1.2, NaHCO3 15, NaH2PO4 1.2, glucose 5.5. A high K+ solution (124 mM) was used, in which the NaCl in the normal Krebs solution was replaced by equimolar amounts of KCl. The following drugs were used: A-322312 (Patane et al., 1998), 4-amino-2-[4-[1-(benzyloxycarbonyl)-2(S)-[[(1,1-diethylethyl)amino]carbonyl]-piperazinyl]-6,7-dimethoxyqinazoline, α1B-AR antagonist, A-119637 (Carroll et al., 2001), α1D-AR antagonist, (Abbott Laboratories, Abbott Park, IL, U.S.A.), prazosin, yohimbine, and apomorphine (Sigma Chemical Co. St Louis, MO, U.S.A.). A-322312 and A-119637 were dissolved with 90% of saline and 10% of Cremphore EL (Sigma Chemical Co. St Louis, MO, U.S.A.), and kept as stock solutions (10−2 M). Prazosin and apomorphine were dissolved in distilled water and saline, respectively.
Calculations
The results are given as mean values±standard error of the mean. One way ANOVA was used for comparison between groups, and were followed by Bonferroni correction. Student's paired two-tailed t-test was used for statistical comparison between before and after giving a drug. A probability of P<0.05 was accepted as significant. Small n denotes the number of strip preparation, and capital N denotes the number of individuals. All statistical calculations are based on N. The values for the negative logarithm of the drug concentration producing half-maximal excitation (−logEC50), or relaxation/inhibition (−logIC50) were determined graphically for each curve by linear interpolation.
Results
Functional in vitro experiments
Electrical field stimulation produced reproducible and frequency-dependent contractions of rat CC preparations. Submaximal stimulation (18 Hz, 20 V) induced tetrodotoxin (10−6 M)-sensitive contractile responses, which amounted to 11.7±2.2 mN (n=29, N=26).
A-119637 (N=6) exhibited concentration-dependent inhibitory activity on electrically induced contractions (Figure 1). At concentrations of 10−8 M and 10−7 M, mean inhibitory effects of 60.4±9.6% (P<0.001) and 93.4±3.7% (P<0.001) were obtained, respectively. The −logIC50 value for A-119637 was 8.12±0.15. A-322312 (N=7) had less inhibitory effects which amounted to 12.6±2.3% at 10−8 M and 32.3±5.1% (P<0.05) at 10−7 M. A −logIC50 value of 6.66±0.08 was obtained for the drug. Prazosin (N=6) inhibited the responses by 20.7±3.3% at a concentration of 10−8 M, and 92.3±1.2% at 10−7 M. The −logIC50 value for prazosin amounted to 8.33±0.15. Yohimbine (N=5) attenuated electrically induced contractions at 10−6 M, producing inhibitory effects of 44.3±9.2%. At lower concentration, contractions were enhanced by 18.9±8.8% (10−8 M) and 49.2±14.1% (10−7 M).
Figure 1.
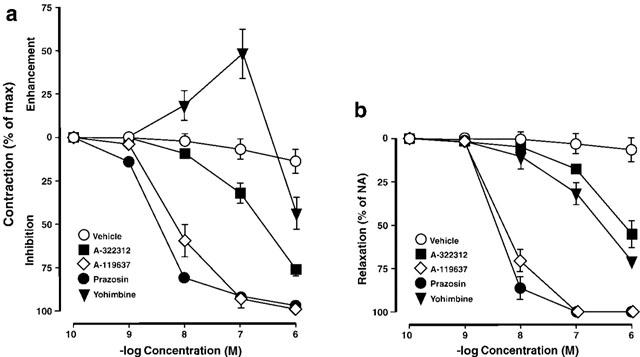
(A) Effects of α-adrenoceptor antagonists or vehicle (10−10 M – 10−6 M; N=7) on contractions induced by electrical field stimulation (18 Hz, 20 V), or (B) by noradrenaline (NA; 3×10−6 M, N=6) in the rat isolated corpus cavernosum. Effects are expressed as per cent enhancement/inhibition/relaxation of contraction, and values are given as mean±standard error of the mean.
Noradrenaline-activated (3×10−6 M) CC (N=5) was effectively relaxed by prazosin and A-119637 in a concentration-dependent manner (Figure 1). Relaxant effects of 87.0±2.7% and 72.4±4.3% were obtained at 10−8 M, respectively, and at higher concentrations, the NA-induced contractions were completely relaxed. A-322312 and yohimbine had less relaxant actions (Figure 1), and at 10−7 M, effects amounted to 19.4±1.8% and 26.9±3.4%, respectively. The −logIC50 for prazosin, A-119637, A-322312, and yohimbine in NA-activated CC preparations amounted to 8.47±0.04, 8.32±0.05, 6.18±0.13, and 6.52±0.05, respectively.
In the Acc, NA induced stable and reproducible concentration-dependent contractile responses with a −logEC50 value of 6.79±0.07 (n=20, N=7). Contractions produced by NA were concentration-dependently attenuated by prazosin and A-199637 (Figure 2; n=5, N=5), and the −logEC50 were changed to 4.68±0.12 (10−7 M; P<0.05), and 4.86±0.13 (10−7 M; P<0.05), respectively. In the presence of A-322312 or yohimbine, no effect on dose-response curves for NA was observed (−logEC50: 6.87±0.13 and 6.89±0.17 ns).
Figure 2.
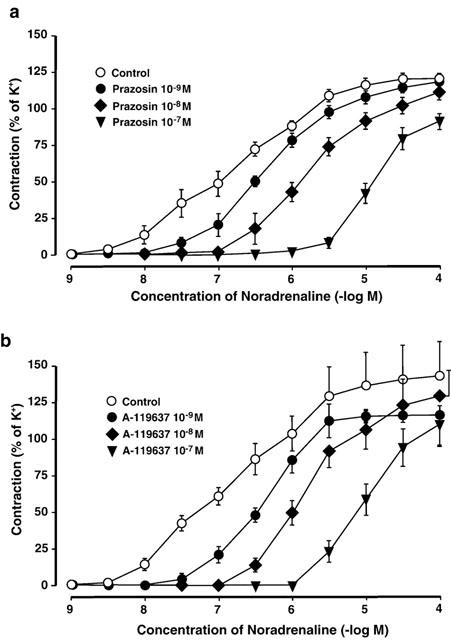
(A) Effects of prazosin (10−9 – 10−7 M), or (B) A-119637 (10−9 – 10−7 M), on contractions induced by noradrenaline (NA; 10−9 – 10−4 M, N=5) in the rat isolated cavernous artery (Acc). NA-induced contractile effects are expressed as per cent of K+ (124 mM) contraction, and values are given as mean±standard error of the mean.
In vivo studies
A mean BICP of 10.5±0.4 mmHg (N=62), and a mean arterial blood pressure of 120.8±2.0 mmHg (N=62) were recorded at the beginning of experiments. No erections occurred spontaneously during the stabilization period. After i.p. administration of drugs or vehicle, and before administration of apomophine, erections were observed in seven out of 31 rats.
In the presence of intraperitoneally given vehicle, submaximal stimulation by apomorphine (25 μg kg−1, s.c., N=7) produced 1.1±0.4 erections with a mean duration of 1.1±0.3 min (Figures 3 and 4). Administration of A-119637 (0.3 μmol kg−1, i.p., N=6) increased the number of apomorphine-induced (25 μg kg−1, s.c.) erections to 3.3±0.8, and also prolonged the duration of the erectile responses to a mean value of 6.2±1.7 min. (Figures 3 and 4; P<0.01). No significant differences in corresponding increases in ICP was obtained with A-322312 (0.3 μmol kg−1, i.p., N=6), and 1.5±0.5 erections with a duration of 2.0±0.7 min was obtained with the drug. At the same concentration, s.c. apomorphine induced 2.2±0.5 erectile responses with a duration of 3.5±1.0 min in the presence of prazosin (0.3 μmol kg−1, i.p., N=6). The mean AUC values amounted to 43.5±17.6 mmHg min−1 for vehicle, 126.4±34.5 mmHg min−1 for prazosin, 79.4±28.0 mmHg min−1 for A-322312 and 266.2±76.9 mmHg min−1 (P<0.05) for A-119637 (Figure 5). Intraperitoneal yohimbine (0.3 μmol kg−1, i.p., N=6) did not significantly affect changes in ICP in response to apomorphine (25 μg kg−1, s.c.), and 2.0±0.5 responses with a mean duration of 2.4±0.7 min, and with a mean value for AUC of 104.1±34.4 mmHg min−1 were obtained.
Figure 3.
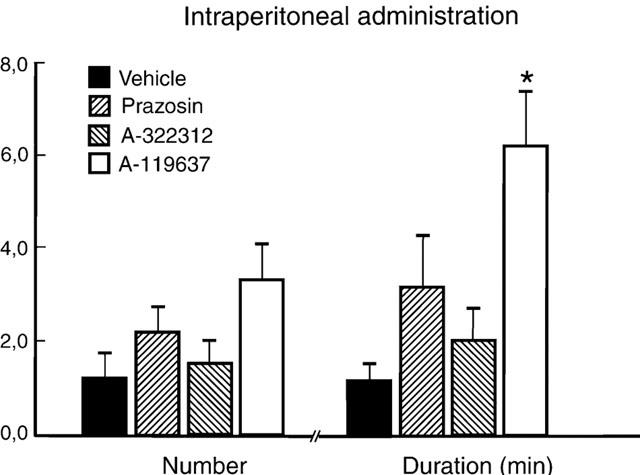
Number and duration of erectile responses induced by intraperitoneal administration of α1-adrenoceptor antagonists (0.3 μmol kg−1) or vehicle (N=6 each) during submaximal stimulation with apomorphine (25 μg kg−1, s.c.). The results are presented as mean values±standard error of the mean. *P<0.05.
Figure 4.
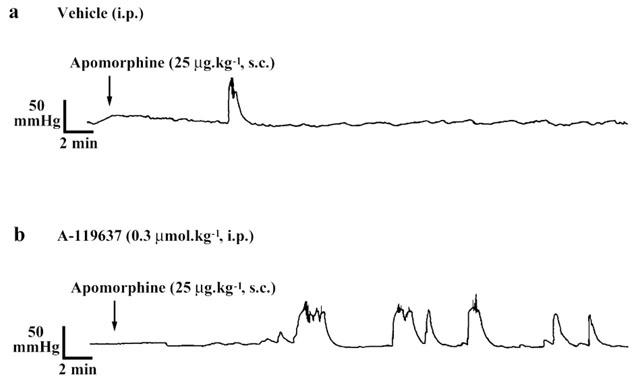
A tracing showing intracavernous pressure changes by subcutaneous apomorphine (25 μg kg−1) in rats pretreated with intraperitoneal α1D-adrenoceptor antagonists (A-119637, 0.3 μmol kg−1) or vehicle.
Figure 5.
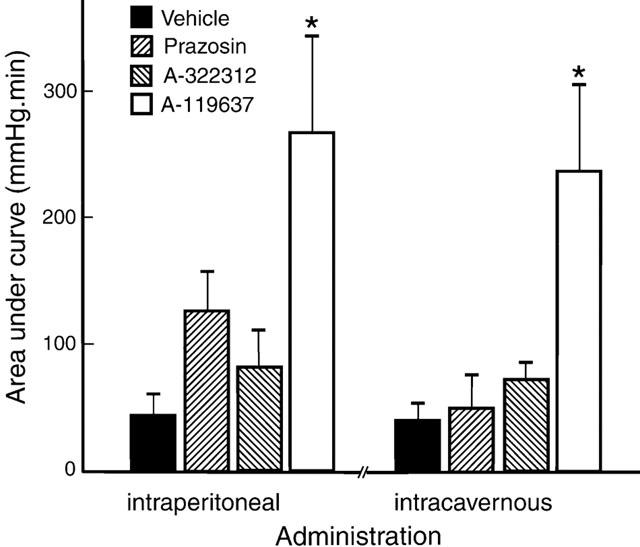
Area under the curve of erectile responses induced by intraperitoneal or intracavernous administration of α1-adrenoceptor antagonists or vehicle during submaximal stimulation with apomorphine (25 μg kg−1, s.c.). The results are presented as mean values±standard error of the mean. *P<0.05.
Intraperitoneal administration of A-119637 decreased mean arterial blood pressure from 122.4±4.3 mmHg to 111.3±5.4 mmHg (P<0.01). Similar effects were seen with prazosin given i.p., and a reduction in blood pressure from 115.0±2.9 mmHg to 98.4±5.4 mmHg (P<0.01) 5.4 mmHg (P<0.01) was obtained. Vehicle, A-322312, or yohimbine did not significantly affect mean arterial blood pressure upon i.p. administration.
None of the α1-AR antagonists significantly increased ICP upon i.c. administration (0.3 μg kg−1). After pretreatment with vehicle (i.c.), apomorphine (25 μg kg−1, s.c.) produced 0.9±0.3 erections with a duration of 1.0±0.4 min (N=7). Corresponding values for prazosin, A-322312, A-119637, and yohimbine (N=6, 0.3 μmol kg−1) were 1.2±0.5 and 1.9±0.9 min, 1.2±0.3 and 2.0±1.0 min, 3.2±0.7 (P<0.05) and 6.0±1.3 min (P<0.05), and 1.5±0.7 and 1.8±0.8 min, respectively (Figure 6).
Figure 6.
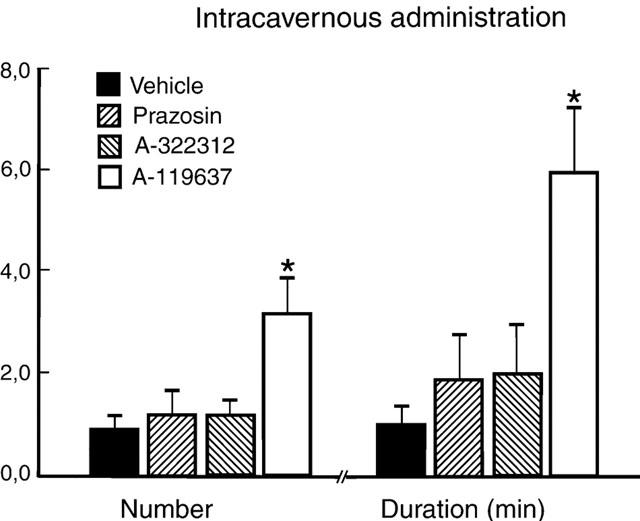
Number and duration of erectile responses induced by intracaverrnous administration of α1-adrenoceptor antagonists (0.3 μmol kg−1, N=6 each) or vehicle (N=7) during submaximal stimulation with apomorphine (25 μg kg−1, s.c.). The results are presented as mean values±standard error of the mean. *P<0.05.
The mean AUC values were 39.0±14.9 mmHg min−1 for vehicle, 49.1±26.9 mmHg min−1 for prazosin, 69.9±27.4 mmHg min−1 for A-322312, 234.8±55.4 mmHg min−1 (P<0.05) for A-119637 and 97.5±52.7 for yohimbine (Figure 5). Intracavernous administration of prazosin or A-119637 decreased blood pressure from 124.9±3.6 mmHg to 94.7±2.0 mmHg (P<0.01), and from 133.5±7.5 mmHg to 107.6±6.6 mmHg (P<0.01), whereas vehicle, A-322312, or yohimbine did not change blood pressure significantly.
There was no difference in mean values for TFR, BICP, PICP and PICP/blood pressure between vehicle, A 322312, A 119637, or prazosin after intraperitoneal or intracavernous administrations of either agent. Summarized values for vehicle and drugs amounted to 11.7±1.1 min. for TFR (N=50), 10.9±0.4 mmHg for BICP (N=62), 79.7±2.6 mmHg for PICP (N=50) and 0.79±0.02 for PICP/blood pressure (N=50).
Discussion
The present results suggest an important role for the α1D-AR subtype in mediating nerve-induced contractions of the isolated rat CC. A-119637, the α1D-AR antagonist studied, was almost as potent and effective as prazosin, the non-selective α1-AR antagonist used. In further support of a postjunctional α1D-AR mediated contraction in the rat CC, A-119637 exhibited pronounced relaxant effects of NA-activated preparations, similar to those obtained with prazosin. The α1B-AR antagonist, A-322312, was less potent and efficacious than A-119637 or prazosin in antagonizing nerve- or agonist-induced contractile activity, confirming previous findings in the rat CC of a minor postjunctional α1B-AR contractile function (Tong & Cheng, 1997). In contrast to our findings of a functional predominance of α1D-AR, Tong & Cheng (1997) concluded that the α1A-AR subtype was responsible for α1-AR agonist-induced contractile responses. The reason for this is unclear, but may be explained by e.g. the use of different α1-AR agonists or other differences in experimental approach. Under the present experimental conditions, both nerve-mediated, and NA-induced contractile activities were attenuated by A-119637.
In the rat isolated Acc, similar to findings in the CC, the contractile effect of NA seems to be mediated mainly by the α1D-AR subtype. In comparison to other arteries, although regional differences do occur, the α1D- and α1A-AR subtypes are those mainly implicated to be involved in the contractions evoked by α1-AR agonists (Guimaraes & Moura, 2001). Using high concentrations of A-119637, i.e. above 10−7 M (unpublished data), NA did not produce maximal contractile responses. In binding studies and investigations of functional in vitro activity, A-119637 was shown to have high affinities for the rat and human α1D-AR, and in rat tissues, an approximately 100 fold selectivity for the α1D-AR over the α1A- and α1B-ARs (Carroll et al., 2001). Thus, at high concentrations of A-119637, possible contributory effects on the α1A- AR subtype cannot be excluded. As found for the CC, postjunctional α1B-AR activity, but also α2-AR mediated effects, appear to be of minor importance for the modulation of smooth muscle tone in the Acc.
In vivo, we found that per se none of the antagonists used had any proerectile effects when injected i.c. at the presently investigated doses. This is not unexpected. Normal penile erection is a combined event with reduction in sympathetic tone and subsequent decrease in α-AR mediated functions, and simultaneously, increased firing of parasympathetic cholinergic nerves to increase NO/cGMP-mediated effects. Manipulation of one of the signal systems that postjunctionally regulates smooth muscle contractility may not induce erection. A combined effort to promote cavernous smooth muscle relaxation is probably necessary to obtain an optimal erectile response.
As previously suggested (Heaton et al., 1991, Mizusawa et al., 2001), an optimal (or maximal) number of erectile responses is obtained at 100 μg kg−1 apomorphine. When evaluating the effect of systemic α-AR antagonists on centrally evoked erections, a dose of 25 μg kg−1 (s.c.) apomorphine was used. This dose was chosen on the assumption that if the central and peripheral signals are operating at optimal levels, an enhancement of the erectile responses would be difficult to detect. With this experimental set-up, A-119637 tripled the number of erections and produced a 6 fold increase in the duration of the increases in intracavernous pressure. None of the other investigated α-AR antagonists significantly affected apomorphine-induced erectile responses. These findings are in agreement with the presently demonstrated in vitro data, and suggest that in the rat CC, the α1D-AR is functionally predominant. In contrast to our results, Sironi et al. (2000), investigating effects of α-AR antagonists on changes in ICP in response to stimulation of the cavernous nerve, concluded that in the CC from rats and dogs, α1B-and α1L-ARs, but not the α1D-AR subtype, are relevant for erectile function. Corresponding to the results in this study, Sironi et al. (2000) found no enhancing action of prazosin on nerve-induced changes in ICP, and concluded that hypotensive effects of the drug masked such possible effects. However, we found that both prazosin and A119637 lowered systemic blood pressures. The difference in effect on apomorphine-induced increases in ICP by prazosin and A119637 may be that prazosin, in addition to being an non-subtype selective antagonist at α1-ARs, also has affinities, although lower, for α2B- and α2c-ARs (Bylund & Ray-Prenger, 1989; Gavin et al., 1997). In agreement with the results on isolated cavernous tissue and the Acc, the effect of A119637 is attributed to an action at presumably peripheral sites. No information is available on the ability of A119637 to cross the blood – brain-barrier, and we can therefore not completely exclude a central site of action of the drug. In the present study, systemic administration of 0.3 μg kg−1 of the respective α-AR antagonist was used when investigating effects on submaximal apomorphine stimulation. Upon intracavernous injection of cumulative doses up to 1 mg kg−1 (Sironi et al., 2000), conclusions concerning selectivity may be questioned.
In the rat CC, α1A, α1B and α1D-AR subtypes were all expressed as demonstrated by Veronneau-Longueville et al. (1998) using in situ hybridization with specific oligonucleotide probes. In other species the results have been varying. In porcine CC, α1A-ARs may be predominant (Wagner & Wei, 1992) and in rabbit CC, contractions induced by phenylephrine were shown to be mediated by the α1B-AR (Furukawa et al., 1996). In the human CC, it has not been established which subtype is functionally predominant. Using RNAse protection assay, previous investigators (Traish et al., 1995a, Goepel et al., 1999) found α1A and α1D mRNA to be more abundant than α1B mRNA. Furthermore, Dausse et al. (1998) found α1D and α1B mRNA signals to represent approximately 50 and 60%, respectively, of the corresponding values obtained for α1A. Although α1D -ARs have been demonstrated in human CC smooth muscle (Traish et al., 1995a), CC from patients undergoing sex change surgery was found to predominantly express α1A, α1B and α2A protein, whereas α1D was expressed only at the in mRNA level (Goepel et al., 1999). Dausse et al. (1998) found predominance of α1A mRNA in human CC. Functionally, Traish et al. (1995b) found evidence for a role of α1A, α1B as well as α1D-ARs, and suggested the NA-induced contraction was mediated by stimulation of two or three receptor subtypes. Interestingly, Davis et al. (1998) suggested that the α1L-AR, which probably is a conformational state of the α1A-AR (Daniels et al., 1999), was the predominant subtype in human CC. Thus, there are differences in results obtained when estimating the relative functional contribution of a given α-AR on cavernous smooth muscle contractility. Further complicating the interpretation of data obtained in separate investigations, there seems to be a lack of correlation between the α-AR mRNAs detected and expression of protein for a specific α-AR, and the functional role for the respective α-AR in penile erectile tissue.
Prejunctional α2-ARs have been functionally demonstrated in the majority of vascular tissues investigated (Guimaraes & Moura, 2001). Modulation of prejunctional α2-AR functions has been shown to decrease stimulus-evoked release of noradrenaline from nerves in the corpus cavernosum (Molderings et al., 1989) and corpus spongiosum (Hedlund et al., 1984). In penile resistance arteries, stimulation of prejunctional α2-ARs have been reported to attenuate nitrergic neurotransmission (Simonsen et al., 1997b). In the present study, α2-AR blockade by yohimbine produced biphasic effects on nerve-induced contractions in rat isolated CC. At concentrations approximately corresponding to Ki values of yohimbine for some human and rat α2-ARs (Smith et al., 1995), the drug-enhanced contractions in response to activation of nerves in the rat CC. This effect probably represents the presence of prejunctional α2-ARs otherwise activated by stimulation-evoked release of noradrenaline. Radioligand binding and in vitro studies have shown that yohimbine has a 6 – 140 fold higher selectivity for α2- than α1-ARs.
In the present study, the inhibitory action on nerve-induced contractions and the relaxant effects on agonist activated by the drug at high concentrations, may be attributed to stimulation of α1-ARs. In addition, activation of α2-ARs on endothelial cells has been shown to stimulate the release of nitric oxide (Vanhoutte & Miller, 1989), promoting smooth muscle relaxation. If this also is valid for erectile vascular tissue needs to be further clarified.
A main finding in the present study was that when systemically given in doses which per se had little effect on erections, the α1D-AR antagonist A-119637 significantly enhanced the effect of apomorphine, given in a suboptimal dose (25 μg kg−1). This makes it tempting to speculate that a combination of a low dose of apomorphine and an α1D-AR antagonist would be an interesting pharmacological principle for treatment of erectile dysfunction. Apomorphine is believed to produce erection by enhancing central signals regulating penile erection (Heaton et al., 1991). If the target system(s) in the erectile tissues is defective, the drug should not work. It is assumed that in a majority of patients with erectile dysfunction, the NO/cGMP system is not working optimally (Andersson et al., 2000). A balance between contractile and relaxant factors regulates smooth muscle tone in the erectile tissue. Removal of an important contractile component would promote smooth muscle relaxation, particularly if the central signals are enhanced.
However, before any clinically relevant conclusions can be drawn, the significance of the present data for the human has to be established, and possible effects of blood pressure of the α1D-AR antagonist must be considered.
Acknowledgments
This work was supported by the Swedish Medical Research Council (grant no 6837), the Royal Physiographic Society, the Foundation of Crafoord, Magnus Bergvall, Åke Wiberg, Thelma Zoéga, and the Medical Faculty, University of Lund, Sweden.
Abbreviations
- Acc
cavernous artery
- AR
adrenoceptor
- AUC
area under curve
- BICP
basal intracavernous pressure
- CC
corpus cavernosum
- ICP
intracavernous pressure
- NA
noradrenaline
- PICP
peak intracavernous pressure
- TFR
time to first response
References
- ANDERSSON K.-E. Pharmacology of penile erection. Pharmacol. Rev. 2001;53:417–450. [PubMed] [Google Scholar]
- ANDERSSON K.-E., BURNETT A.L., CHEN K.K., CHRIST G.J., RAMPIN O., STIEF C.Current Research and Future Therapies 1st International Consultation on Erectile Dysfunction 2000Plymouth, UK: Plymbridge Distributors Ltd; 139–203.eds Jardin A. Wagner G. pp [Google Scholar]
- ANDERSSON K.-E., STIEF C. Oral α adrenoceptor blockade as a treatment of erectile dysfunction. World. J. Urol. 2001;19:9–13. doi: 10.1007/pl00007093. [DOI] [PubMed] [Google Scholar]
- BECKER A.J., STIEF C.G., MACHTENS S., SCHULTHEISS D., HARTMANN U., TRUSS M.C., JONAS U. Oral phentolamine as treatment for erectile dysfunction. J. Urol. 1998;159:1214–1216. [PubMed] [Google Scholar]
- BUVAT J., COSTA P., MORLIER D., LECOCQ B., STEGMANN B., ALBRECHT D. Double-blind multicenter study comparing alprostadil α-cyclodextrin with moxisylyte chlorhydrate in patients with chronic erectile dysfunction. J. Urol. 1998;159:116–119. doi: 10.1016/s0022-5347(01)64030-8. [DOI] [PubMed] [Google Scholar]
- BYLUND D.B., RAY-PRENGER C. Alpha-2A and alpha-2B adrenergic receptor subtypes: attenuation of cyclic AMP production in cell lines containing only one receptor subtype. J. Pharmacol. Exp. Ther. 1989;251:640–644. [PubMed] [Google Scholar]
- CARROLL W.A., SIPPY K.B., ESBENSHADE T.A., BUCKNER S.A., HANCOCK A.A., MEYER M.D. Two Novel and Potent 3-[(o-Methoxyphenyl)piperazinylethyl]-5-phenylthieno[2,3-d]pyrimidine-2,4-diones Selective for the α1D Receptor. Bioorg. Med. Chem. Lett. 2001;11:1119–1121. doi: 10.1016/s0960-894x(01)00159-7. [DOI] [PubMed] [Google Scholar]
- DAUSSE J.P., LERICHE A., YABLONSKY F. Patterns of messenger RNA expression for α1-adrenoceptor subtypes in human corpus cavernousum. J. Urol. 1998;160:597–600. [PubMed] [Google Scholar]
- DANIELS D.V., GEVER J.R., JASPER J.R., KAVA M.S., LESNICK J.D., MELOY T.D., STEPAN G., WILLIAMS T.J., CLARKE D.E., CHANG D.J., FORD A.P. Human cloned alpha 1A-adrenoceptor isoforms display alpha 1L-adrenoceptor pharmacology in functional studies. Eur. J. Pharmacol. 1999;370:337–343. doi: 10.1016/s0014-2999(99)00154-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B.J., CHAPPLE C.R., CHESS-WILLIAMS R. α1-adrenoceptor subtypes in human penile tissue. Br. J. Pharmacol. 1998;125:13P. [Google Scholar]
- DAVIS B.J., CHAPPLE C.R., CHESS-WILLIAMS R. The α1I-adrenoceptor mediates contraction in human erectile tissue (abstract 406) Eur. Urol. 1999;35:102. [Google Scholar]
- FURUKAWA K., CHESS-WILLIAMS R., UCHIYAMA T. α1B-adrenoceptor subtype mediating the phenylephrine-induced contractile response in rabbit corpus cavernosum penis. Jpn. J. Pharmacol. 1996;71:325–331. doi: 10.1254/jjp.71.325. [DOI] [PubMed] [Google Scholar]
- GAVIN K.T., COLGAN M.-P., MOORE D., SHANIK G., DOCHERTY J.R. α2C-Adrenoceptors mediate contractile responses to noradrenaline in the human saphenous vein. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;355:406–411. doi: 10.1007/pl00004961. [DOI] [PubMed] [Google Scholar]
- GOEPEL M., KREGE S., PRICE D.T., MICHELOTTI G.A., SCHWINN D.A., MICHEL M.C. Characterization of α-adrenoceptor subtypes in the corpus cavernosum of patients undergoing sex change surgery. J. Urol. 1999;162:1793–1799. [PubMed] [Google Scholar]
- GOLDSTEIN I. Oral phentolamine: an α-1, α-2 adrenergic antagonist for the treatment of erectile dysfunction. Int. J. Impot. Res. 2000;12:S75–S80. [PubMed] [Google Scholar]
- GUIMARAES S., MOURA D. Vascular adrenoceptors: An update. Pharmacol. Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- HEATON J.P. Key issues from the clinical trials of apomorphine SL. World J. Urol. 2001;19:25–31. doi: 10.1007/s003450000174. [DOI] [PubMed] [Google Scholar]
- HEATON J.P., VARRIN S.J., MORALES A. The characterization of a bioassay of erectile function in a rat model. J. Urol. 1991;145:1099–1102. doi: 10.1016/s0022-5347(17)38543-9. [DOI] [PubMed] [Google Scholar]
- HEDLUND H., ANDERSSON K.-E. Comparison of the responses to drugs acting on adrenoceptors and muscarinic receptors in human isolated corpus cavernosum and cavernous artery. J. Auton. Pharmacol. 1985;5:81–88. doi: 10.1111/j.1474-8673.1985.tb00568.x. [DOI] [PubMed] [Google Scholar]
- HEDLUND H., ANDERSSON K.-E., MATTIASON A. pre- and postjunctional adreno- and muscarinic receptor functions in the isolated human corpus spongiosum urethrae. J. Auton. Pharmacol. 1984;4:241–249. doi: 10.1111/j.1474-8673.1984.tb00101.x. [DOI] [PubMed] [Google Scholar]
- HEDLUND P., ALM P., ANDERSSON K.-E. NO synthase in cholinergic nerves and NO-induced relaxation in the rat isolated corpus cavernosum. Br. J. Pharmacol. 1999;126:349–360. doi: 10.1038/sj.bjp.0702556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUE T.F. Erectile dysfunction. N. Engl. J. Med. 2000;342:1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- MIZUSAWA H., HEDLUND P., ANDERSSON K.-E. α-MSH- and oxytocin-induced penile erections and intracavernous pressure in the rat. J. Urol. 2001;167:757–760. doi: 10.1016/S0022-5347(01)69140-7. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G.J., GÖTHERT M., VAN AHLEN H., PORST H. Noradrenaline release in human corpus cavernosum and its modulation via presynaptic α2-adrenoceptors. Fundam. Clin. Pharmacol. 1989;102:261–267. doi: 10.1111/j.1472-8206.1989.tb00684.x. [DOI] [PubMed] [Google Scholar]
- MURAMATSU I., OHMURA T., HASHIMOTO S., OSHITA M. Functional subclassification of vascular α1-adrenoceptors. Pharmacol. Commun. 1995;6:23–28. [Google Scholar]
- PATANE M.A., SCOTT A.L., BROTEN T.P., CHANG R.S., RONSOM R.W., DISALVO J., FORRAY C., BOCK M.G. 4-amino-2-[4-[1-(benzyloxycarbonyl)-2(S)-[[(1,1-dimethylethyl)amino]carbonyl]-piperazinyl]-6,7-dimethoxyquinazoline (L-765,314): a potent and selective α1B adrenergic receptor antagonist. J. Med. Chem. 1998;41:1205–1208. doi: 10.1021/jm980053f. [DOI] [PubMed] [Google Scholar]
- SIMONSEN U., PRIETO D., HERNANDEZ M., SAENZ DE TEJADA I., GARCIA-SACRISTAN A. Adrenoceptor-mediated regulation of the contractility in horse penile resistance arteries. J. Vasc. Res. 1997a;34:90–102. doi: 10.1159/000159206. [DOI] [PubMed] [Google Scholar]
- SIMONSEN U., PRIETO D., HERNANDEZ M., SAENZ DE TEJADA I., GARCIA-SACRISTAN A. Prejunctional alpha 2-adrenoceptors inhibit nitrergic neurotransmission in horse penile resistance arteries. J. Urol. 1997b;157:2356–2360. [PubMed] [Google Scholar]
- SIRONI G., COLOMBO D., POGGESI E., LEONARDI A., TESTA R., RAMPIN O., BERNABE J., GIULIANO F. Effects of intracavernous administration of selective antagonists of α1-adrenoceptor subtypes on erection in anesthetized rats and dogs. J. Pharmacol. Exp. Ther. 2000;292:974–981. [PubMed] [Google Scholar]
- SMITH K., GAVIN K., DOCHERTY J.R. Investigation of the subtype of α2-adrenoceptor mediating prejunctional inhibition of cardioacceleration in the pithed rat heart. Br. J. Pharmacol. 1995;115:316–320. doi: 10.1111/j.1476-5381.1995.tb15879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONG Y.C., CHENG J.T. Subtyping of α1-adrenoceptors responsible for the contractile response in the rat corpus cavernousum. Neurosci. Lett. 1997;228:159–162. doi: 10.1016/s0304-3940(97)00388-1. [DOI] [PubMed] [Google Scholar]
- TRAISH A.M., GUPTA S., TOSELLI P., DE TAJADA I.S., GOLDSTEIN I., MORELAND R.B. Identification of α1-adrenergic receptor subtypes in human corpus cavernosum tissue and in cultured trabecular smooth muscle cells. Receptor. 1995a;5:145–157. [PubMed] [Google Scholar]
- TRAISH A., KIM N.N., MORELAND R.B., GOLDSTEIN I. Role of alpha adrenergic receptors in erectile function. Int. J. Impot. Res. 2000;12 Suppl 1:S48. [PubMed] [Google Scholar]
- TRAISH A.M., NETSUWAN N., DALEY J., PADMAN-NATHAN H., GOLDSTEIN I., DE TAJADA I.S. A heterogeneous population of α1 andrenergic receptors mediates contraction of human corpus cavernosum smooth muscle to norepinephrine. J. Urol. 1995b;153:222–227. doi: 10.1097/00005392-199501000-00081. [DOI] [PubMed] [Google Scholar]
- VANHOUTTE P.M., MILLER V.M. Alpha 2-adrenoceptors and endothelium-derived relaxing factor. Am. J. Med. 1989;87:15–55. doi: 10.1016/0002-9343(89)90496-8. [DOI] [PubMed] [Google Scholar]
- VERONNEAU-LONGUEVILLE F., RAMPIN O., JARDIN A., BENOIT G., GIULIANO F. Expression of α1 adrenoceptor subtypes in rat corpus cavernousum. Int. J. Impot. Res. 1998;10:187–194. doi: 10.1038/sj.ijir.3900348. [DOI] [PubMed] [Google Scholar]
- WAGNER G., WEI M.Q. Subclassification of α1-adrenoceptors in porcine smooth muscle of corpus cavernosum. Int. J. Impot. Res. 1992;4 Suppl 2:A32. [Google Scholar]


