Abstract
Systemic and regional cardiovascular changes were measured following bilateral microinjection of specific and selective opioid-receptor antagonists into the paraventricular nuclei of the hypothalamus (PVN) of awake, freely moving rats.
PVN microinjection of increasing doses of the specific opioid antagonist naloxone – methiodide (1 – 5.0 nmol), or a selective μ-opioid receptor antagonist, β-funaltrexamine (0.05 – 0.5 nmol), evoked important cardiovascular changes characterized by small and transient increases in heart rate (HR) and mean arterial pressure (MAP), vasoconstriction in renal and superior mesenteric vascular beds and vasodilation in the hindquarter vascular bed.
No significant cardiovascular changes were observed following PVN administration of the highly selective δ-opioid-receptor antagonist, ICI 174864 (0.1 – 1 nmol), or the selective κ-opioid-receptor antagonist, nor-binaltorphine (0.1 – 1 nmol).
Most of the cardiovascular responses to naloxone (3 nmol) and β-funaltrexamine (0.5 nmol) were attenuated or abolished by an i.v. treatment with a specific vasopressin V1 receptor antagonist.
These results suggest that endogenous opioid peptides and μ-type PVN opioid receptors modulate a tonically-active central depressor pathway acting on systemic and regional haemodynamic systems. Part of this influence could involve a tonic inhibition of vasopressin release.
Keywords: Paraventricular nucleus of the hypothalamus, blood pressure, regional blood flow, opioid receptor antagonist, naloxone, β-funaltrexamine
Introduction
The paraventricular nucleus of the hypothalamus (PVN) is an important integrating site for modulating autonomic and neuroendocrine cardiovascular responses (Sawchenko & Swanson, 1982a, 1982b; 1983; Jin & Rockhold, 1989; Kannan et al., 1989). The PVN also contains numerous enkephalin-containing neurons and nerve terminals, as well as opioid binding sites (Sar et al., 1979; Goodman et al., 1980; Sawchenko & Swanson, 1982a; Wamsley, 1983; Fallon & Leslie, 1986; Desjardins et al., 1990). Earlier studies have shown that activation of a μ-opioid receptor mediated pathway in this nucleus, using the μ-selective enkephalin analogue, [D-Ala2, MePhe4, Gly5ol]enkephalin (DAMGO) or dermorphin, causes dose-related increases in blood pressure, heart rate and sympathetic outflow as measured by a rise in the plasma level of catecholamines in conscious rats (Appel et al., 1986; Kiritsy-Roy et al., 1986; Bachelard & Pitre, 1995). Previous studies from this laboratory have demonstrated that the pressor response to DAMGO was secondary to α-adrenoceptor-mediated vasoconstriction in renal and superior mesenteric vascular beds and to β-adrenoceptor-mediated vasodilation in the hindquarter vascular bed (Bachelard & Pitre, 1995; Bachelard et al., 1997). In contrast to the cardiovascular responses observed after PVN injection of DAMGO, we reported no cardiovascular changes following PVN administration of increasing doses of the δ-selective enkephalin analogue, [D-Phe2,5]enkephalin (DPDPE) or the κ-selective enkephalin analogue, U50488H (Bachelard & Pitre, 1995). Therefore, these results suggest a role for opioid peptides and μ-opioid receptors in the central regulation of cardiovascular function, whereas the involvement of PVN δ- and κ-receptors in cardiovascular regulation are less obvious.
Opioid peptides are also associated to inhibitory modulation of vasopressin release. Thus, vasopressin cell bodies and dendrites of the magnocellular part of the PVN contains immunoreactive opioid axons (Goldsmith et al., 1991). Additional pharmacological evidence suggests that opioid peptides exert a tonic inhibitory control on vasopressin release (Yamada et al., 1989). Previous study showed that i.c.v. injection of the selective μ-agonist DALDA (H-Tyr-D-Arg-Phe-Lys-NH2), but not the selective κ- and δ-agonists U-69,593 and [D-Pen2,D-Pen5]-enkephalin decreased plasma levels of vasopressin (van de heijning et al., 1991). These results indicate that central μ-opioid receptors are preferably involved in that mechanism. Although μ-opioid agonists produced important cardiovascular and endocrinological changes, little is known about the endogenous influence of the PVN enkephalin-containing neurons and nerve terminals on autonomic regulation. Therefore, we first investigated the influence of the endogenous PVN opioid neurons and nerve terminals in the cardiovascular system by measuring the regional haemodynamic effects produced by PVN microinjection of highly selective opioid antagonists. Secondly, to verify if cardiovascular changes are produced in part by an increase in vasopressin release secondary to inhibition of endogenous opioid activity in the PVN, cardiovascular responses were reassessed after i.v. treatment with the selective vasopressin V1-receptor antagonist d(CH2)5(Tyr(Et))DAVP. To avoid the confounding effects of anaesthesia, these studies were carried out in conscious, unrestrained rats (van loon, 1984). Thus, blood pressure, heart rate, and regional haemodynamic responses to PVN administration of naloxone – methiodide were compared with the cardiovascular responses to the μ-opioid receptor antagonist, β-funaltrexamine (Takemori et al., 1981; Ward et al., 1982), the δ-opioid receptor antagonist, ICI-174864, (Cowan et al., 1985; Hirning et al., 1985), and the κ-opioid receptor antagonist, nor-binaltorphimine (Portoghese et al., 1987; Takemori et al., 1988).
Methods
The research and the care of animals conformed to the Guide for the care and use of laboratory animals as adopted by the National Institute of Health. Male Wistar rats (300 – 400 g; from Charles River, St-Constant, Québec, Canada) were anaesthetized with a mixture of ketamine – xylazine (100 and 10 mg kg−1, respectively, i.p., supplemented as required) and then positioned in a stereotaxic frame with the incisor bar set at 3.3 mm below the interaural line. The skull was exposed and cleaned, and two 23-gauge stainless steel guide cannulae targeted 2 mm dorsal to the PVN were obliquely implanted (angle of 10° relative to the vertical) according to the following coordinates: 1.4 mm caudal and ±1.75 mm lateral to the bregma and 6.3 mm ventral to the surface of the skull. The cannulae were secured to the skull with screws and dental cement. Patency of the guide cannulae was ensured by inserting 31-gauge stainless steel stylets fashioned to extend 0.5 mm beyond the end of the 23-gauge guides and maintained in place with a piece of silastic tubing. The reflected muscles and skin were replaced and sutured. After surgery the animals were treated with ampicillin (Polyflex, Ayerst, 150 mg kg−1, s.c.) and flunixin (Banamine, Schering, 1 mg kg−1, i.m.), housed in individual cages, and allowed to recover.
At least 7 days later, the rats were re-anaesthetized with a mixture of ketamine – xylazine (100 and 10 mg kg−1, respectively, i.p., supplemented as required) and had pulsed Doppler flow probes (Haywood et al., 1981) implanted around the left renal and superior mesenteric arteries and the lower abdominal aorta, according to the method previously developed by Gardiner & Bennett (1988) and as we previously described (Bachelard & Pitre, 1995; Bachelard et al., 1997). After operation, the rats were given s.c. injections of ampicillin (150 mg kg−1) and flunixin (1 mg kg−1) and allowed to recover for at least 7 days. After this period, the rats were re-anaesthetized with a mixture of ketamine – xylazine (100 and 10 mg kg−1, respectively, i.p., supplemented as required). The leads of the implanted probes were soldered to a six-way microconnector (Microtech Inc.), which was connected to a pulsed Doppler monitoring system (VF-1 mainframe, Crystal Biotech) to check the quality of the signals. The animal showing good-quality signals (signal: noise ratio >20 : 1) from all three probes had one catheter implanted in the right jugular vein (for drug administration) and one in the distal abdominal aorta via the femoral artery (for measurement of blood pressure and heart rate). The catheters were tunneled subcutaneously to emerge at the same point as the Doppler probe wires. The microconnector, soldered to the Doppler probe wires, was clamped in a custom-made harness worn by the rat, and the catheters were passed through a flexible, protecting spring attached to the harness. Experiments were not begun until at least 48 h after the final surgical intervention (catheter implantation).
Throughout the experiments, continuous recordings were made of instantaneous heart rate, and phasic and mean arterial blood pressures, and renal, mesenteric and hindquarter Doppler shift signals using a pulsed Doppler monitoring system (Crystal Biotech, Holliston, MA, U.S.A.), modified to operate with a pulse repetition frequency of 125 kHz (Gardiner et al., 1990a) and a Gould ES2000 electrostatic recorder. Changes in Doppler shift signals were calculated as percentage of the baseline level. The latter have been shown to be a reliable index of volume flow (Haywood et al., 1981; Wright et al., 1987) and are referred to as flows in the text. The Doppler shift and corresponding mean arterial blood pressure signals were used to calculate percentage changes in regional vascular conductances (Gardiner et al., 1990b). The rats were allowed free access to food and water for the duration of the experiment.
Before every experiment a 30 min baseline recording period was made. Bilateral injections were made directly into the PVN of awake, undisturbed, freely moving rats through 31-gauge stainless still injectors that extended 2 mm beyond the previously implanted guide cannulae. The injectors were connected via polyethylene tubing to two Hamilton microsyringes (5 μl) and inserted into the guide cannulae without handling the rats. All solutions for microinjections were freshly prepared. The injection volume was 0.2 μl, delivered by hand simultaneously into both sides for 1 min. At selected time points (which averaged ±20 s) heart rate, mean arterial blood pressure, and mean Doppler shifts were measured to represent the full profile of the effects of the opioid antagonist and related to the pre-drug baseline (absolute changes for the former two variables, percentages for the Doppler shifts). The observation period after each drug administration was 60 min.
At the end of the experiments, all animals received an injection of 0.2 μl of India ink to mark the placement of the cannula tip. The placements of the microinjection sites were verified histologically in serial coronal sections (50 μm, cut on a freezing microtome), mounted on glass slides, and stained with neutral red (Figure 1). Rats showing injection sites within a distance larger than 0.5 mm from the PVN (after post-mortem examination) were excluded from the study. These rats evoked no significant cardiovascular changes compared to vehicle values (data not shown).
Figure 1.
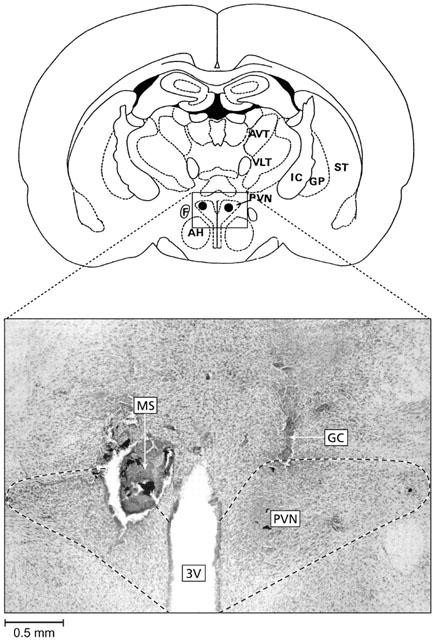
Representative micrograph showing the location of the injection sites (MS, marked with india ink) in the paraventricular nucleus of the hypothalamus (PVN, dashed line represent the limit of the nucleus). GC is the lesion evoked by the guide cannulae. AP=1.4 mm posterior to the bregma. A rat was considered successfully injected when both cannula tips were shown to be slightly above the PVN or within a distance of 0.5 mm of the PVN. 3V, third ventricle; AH, anterior hypothalamic area; AVT, anteroventral thalamic nucleus; F, fornix; GP, globus pallidus; IC, internal capsule; ST, striatum; VLT, ventrolateral thalamic nucleus.
Experimental protocols
Experiment 1: Cardiovascular responses to PVN injections of opioid receptor antagonists
Four separate groups of rats were used in this study, and each group received only one specific opioid receptor antagonist. The rats from each group were used on a maximum of 4 consecutive days, during which they received PVN bilateral injections of the vehicle (artificial cerebrospinal fluid, aCSF) (all groups), naloxone methiodide (group 1), β-funaltrexamine hydrochloride (β-FNA; group 2), nor-binaltorphimine dihydrochloride (nor-BNI; group 3), or ICI-174,864 (group 4) at doses ranging from 0.1 to 5.0 nmol (for naloxone methiodide), 0.05 and 0.5 nmol (for β-FNA), 0.1 and 1.0 nmol (for nor-BNI and ICI-174,864) per microinjection site. No rat received more than one PVN bilateral injection per day. The doses of the opioid receptor antagonists were chosen according to previous studies using similar doses and showing that they were effective in blocking the respective receptors (Drolet et al., 1991; Kiritsy-Roy et al., 1986). Preliminary study was performed for tachyphylaxis control. Results showed no desensitization to naloxone (3 nmol, n=6) when injected on 3 consecutive days (data not shown). All antagonists were dissolved in aCSF, pH 7.4 (vehicle), which served as the control injection. The composition of the aCSF (in mM) was NaCl, 125; NaHCO3, 27; KCl, 2.5; NaH2PO4, 0.5; Na2HPO4, 1.2; Na2SO4, 0.5; CaCl2, 1.0; MgCl2, 1.0; and glucose, 5.0. Cardiovascular variables were recorded for 60 min following each injection.
Experiment 2: Effect of pretreatment with a vasopressin V1-receptor antagonist on cardiovascular responses to naloxone methiodide or β-FNA bilaterally injected into the PVN
The vasopressin V1-receptor antagonist d(CH2)5(Tyr(Et))DAVP was administered i.v. as a bolus (10 μg kg−1, 0.1 ml), followed by continuous infusion (10 μg kg−1 h−1, 0.3 ml h−1) (Gardiner et al., 1989) to a group of 21 rats. This treatment was found to produce a stable antagonism of the vasopressin V1-receptor, as the pressor response to vasopressin (20 ng) was retested at regular interval (120 min) during the infusion. Fifteen minutes after the onset of the infusion, a first subgroup of rats (n=12) were given a bilateral microinjection of aCSF (0.2 μl), followed 180 min later by a bilateral microinjection of naloxone methiodide (3 nmol), and a second subgroup of rats (n=9) received a bilateral microinjection of aCSF (0.2 μl), followed 180 min later by a bilateral microinjection of β-FNA (0.5 nmol) into the PVN.
Drugs
The drugs used were naloxone methiodide (RBI, Natick, MA, U.S.A.), nor-BNI (nor-binaltorphimine dihydrochloride; RBI), ICI-174,864 (RBI), β-FNA (β-funaltrexamine hydrochloride; RBI) and the vasopressin V1-receptor antagonist d(CH2)5(Tyr(Et))DAVP ([1-(β-Mercapto-β,β-cyclopenta-methylene propionyl), 2-(O-Ethyl)-Tyrosine]-Arg8-Vasopressin; Bachem California, Torrance, U.S.A.).
The vasopressin V1 receptor antagonist was dissolved in 0.5 ml of glacial acetic acid, diluted to a working concentration with isotonic saline.
Data analysis
The data analysis for this paper was generated using SAS/STAT software, Version 8.02 of the SAS System for Windows (©1999 SAS Institute Inc., Cary, NC, U.S.A.). Results were analysed for statistical significance by two-way analysis of variance (ANOVA) with repeated measures. Since the test for sphericity was rejected, the procedure Mixed of SAS with the unstructured covariance-matrix to take into account of the correlation between the observations was used in all the analysis. When a significant interaction between treatment and time was detected, contrast to test for the effect of time at each dose was used. When no interaction between treatment and time was detected, the comparison between the dose and the control (aCSF) was done averaging over time. The stepdown Bonferronni (see Multitest Procedure of SAS) correction was used to adjust the P-value for these comparisons. Differences were considered statistically significant at P<0.05.
Results
The baseline values (prior to any drug administration) for cardiovascular variables are given in Table 1. Control bilateral injection of aCSF (0.2 μl) into the PVN had no significant effect on any measured or calculated variables (Figures 2, 3, 4, 5, 6 and 7).
Table 1.
Baseline values of heart rate (HR), mean arterial blood pressure (MAP), regional Doppler shift and regional vascular conductance in conscious, unrestrained rats
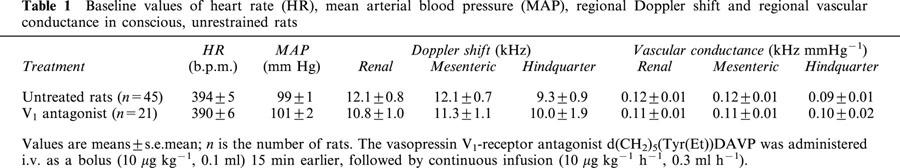
Figure 2.
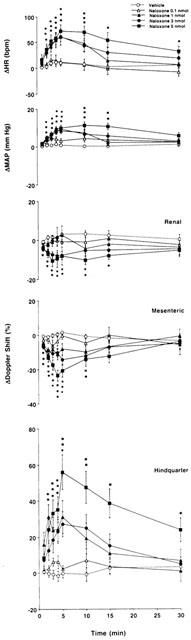
Cardiovascular changes elicited by bilateral microinjection of the vehicle (aCSF, n=12) or increasing doses of naloxone – methiodide (0.1 – 5 nmol, n=12) into the PVN of conscious, unrestrained rats. Values are means±s.e.mean shown by vertical lines. A significant interaction between treatment and time was found for all measured cardiovascular variables. *P<0.05 when the cardiovascular responses to naloxone – methiodide at the dose of 1, 3 or 5 nmol are compared with those elicited by the control injection of a CSF.
Figure 3.
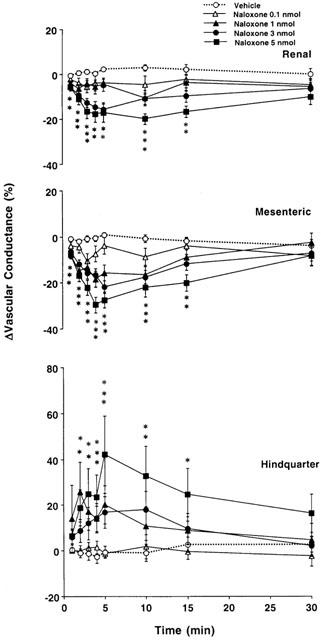
Changes in regional vascular conductances elicited by bilateral microinjection of the vehicle (aCSF, n=12) or increasing doses of naloxone – methiodide (0.1 – 5 nmol, n=12) into the PVN of conscious, unrestrained rats. These data were derived from the data shown in Figure 2. Values are means±s.e.mean shown by vertical lines. A significant interaction between treatment and time was found for all calculated cardiovascular variables. *P<0.05 when the cardiovascular responses to naloxone – methiodide at the dose of 1, 3 or 5 nmol are compared with those elicited by the control injection of aCSF.
Figure 4.
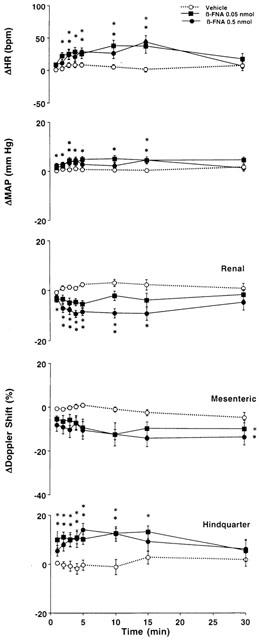
Cardiovascular changes elicited by bilateral microinjection of the vehicle (aCSF, n=15) or increasing doses of β-FNA (0.05 – 0.5 nmol, n=15) into the PVN of conscious, unrestrained rats. Values are means±s.e.mean shown by vertical lines. A significant interaction between treatment and time for ΔHR, ΔMAP and ΔRenal and Hindquarter Doppler Shift was found. *P<0.05 when the cardiovascular responses to β-FNA at the dose of 0.05 or 0.5 nmol are compared with those elicited by the control injection of aCSF. No significant interaction was observed for ΔMesenteric Doppler Shift, the comparison between β-FNA (at each dose) and the control (aCSF) was done averaging over time.
Figure 5.
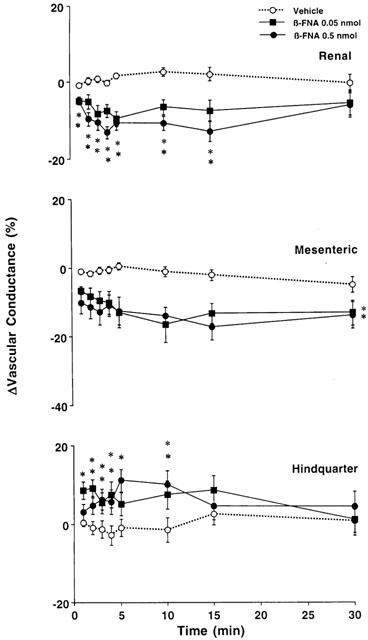
Changes in regional vascular conductances elicited by bilateral microinjection of the vehicle (aCSF, n=15) or increasing doses of β-FNA (0.05 – 0.5 nmol, n=15) into the PVN of conscious, unrestrained rats. These data were derived from the data shown in Figure 4. Values are means±s.e.mean shown by vertical lines. A significant interaction between treatment and time for ΔRenal and Hindquarter Vascular Conductances was found. *P<0.05 when the cardiovascular responses to β-FNA at the dose of 0.05 or 0.5 nmol are compared with those elicited by the control injection of aCSF. No significant interaction was observed for ΔMesenteric Vascular Conductance, the comparison between β-FNA (at each dose) and the control (aCSF) was done averaging over time.
Figure 6.
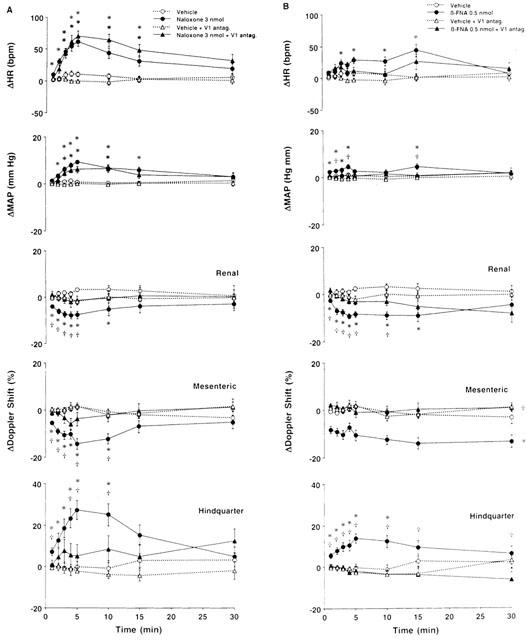
(A) Cardiovascular responses to naloxone – methiodide (3 nmol) or vehicle (aCSF) bilaterally injected into the PVN of conscious rats, in the absence (n=12 for both central injections) or presence (n=12 for naloxone – methiodide; n=11 for aCSF) of intravenous treatment with a vasopressin V1-receptor antagonist (10 μg kg−1, i.v. bolus, 10 kg−1 h−1, infusion). (B) Cardiovascular responses to β-FNA (0.5 nmol) or vehicle (aCSF) bilaterally injected into the PVN of conscious rats, in the absence (n=15 for both central injections) or presence (n=9 for both central injections) of intravenous treatment with the vasopressin V1-receptor antagonist. Values are means±s.e.mean shown by vertical lines. (A) A significant interaction between treatment and time was found for all measured cardiovascular variables; (B) A significant interaction between treatment and time was found for ΔHR, ΔMAP and ΔRenal and Hindquarter Doppler Shift. *P<0.05 when the cardiovascular responses to naloxone – methiodide or β-FNA in the absence or presence of the vasopressin V1-receptor antagonist are compared with their respective untreated or intravenously treated control (aCSF) group. †P<0.05, when the cardiovascular responses to naloxone – methiodide or β-FNA in the presence of the vasopressin V1-receptor antagonist are compared with those elicited in the absence of the vasopressin V1-receptor antagonist. No significant interaction was observed for ΔMesenteric Doppler Shift, the comparison between β-FNA and the control (aCSF) (in the absence or presence of the vasopressin V1 receptor antagonist) was done averaging over time.
Figure 7.
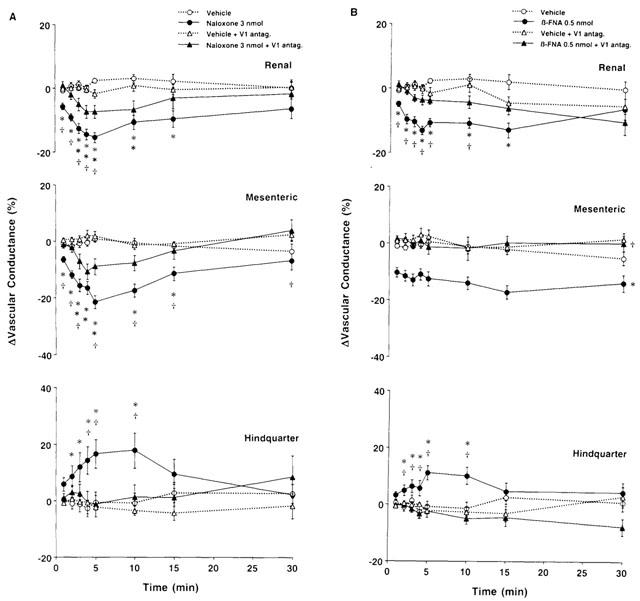
(A) Changes in regional vascular conductances elicited by bilateral microinjection of naloxone – methiodide (3 nmol) or vehicle (aCSF) into the PVN of conscious rats, in the absence (n=12 for both central injections) or presence (n=12 for naloxone – methiodide; n=11 for aCSF) of intravenous treatment with a vasopressin V1-receptor antagonist (10 μg kg−1, i.v. bolus, 10 kg−1 h−1, infusion). (B) Changes in regional vascular conductances elicited by bilateral microinjection of β-FNA (0.5 nmol) or vehicle (aCSF) into the PVN of conscious rats, in the absence (n=15 for both central injections) or presence (n=9 for both central injections) of intravenous treatment with the vasopressin V1-receptor antagonist. Values are means±s.e.mean shown by vertical lines. (A) A significant interaction between treatment and time was found for all calculated cardiovascular variables; (B) A significant interaction between treatment and time was found for ΔRenal and ΔHindquarter Vascular Conductances. *P<0.05 when the cardiovascular responses to naloxone – methiodide or β-FNA in the absence or presence of the vasopressin V1-receptor antagonist are compared with their respective untreated or intravenously treated control (aCSF) group. †P<0.05, when the cardiovascular responses to naloxone – methiodide or β-FNA in the presence of the vasopressin V1-receptor antagonist are compared with those elicited in the absence of the vasopressin V1-receptor antagonist. No significant interaction was observed for ΔMesenteric Vascular Conductance, the comparison between β-FNA and the control (aCSF) (in the absence or presence of the vasopressin V1 receptor antagonist) was done averaging over time.
Haemodynamic responses to PVN injections of naloxone – methiodide and the selective μ-opioid-receptor antagonist β-FNA
Important cardiovascular responses to PVN injections of increasing doses of naloxone – methiodide (group 1, n=12) or β-FNA (group 2, n=15) were observed in conscious unrestrained Wistar rats and are presented in Figures 2, 3 and 4.
In the first group of rats we found that the lowest dose of naloxone methiodide bilaterally injected into the PVN (0.1 nmol on each side) did not have effects significantly different from those of aCSF. With the 1 nmol dose of naloxone – methiodide there were significant increases in mean arterial blood pressure accompanied by a tachycardia and a fall in superior mesenteric flow, while an increase in hindquarter flow was observed (Figure 2). Renal flow did not change significantly. These effects were associated with falls in renal and superior mesenteric vascular conductances, and an increase in hindquarter vascular conductance compared to measurements following aCSF (Figure 3).
Bilateral injection of the highest doses of naloxone – methiodide (3 and 5 nmol) into the PVN produced more pronounced cardiovascular effects, characterized by long-lasting increases in blood pressure and marked increases in heart rate, compared with the effects of control bilateral injection of aCSF. Moreover, there were falls in renal and superior mesenteric blood flows, and an increase in hindquarter flow following bilateral injection of naloxone – methiodide into the PVN (Figure 2). These responses were associated with falls in renal and superior mesenteric vascular conductances, and increases in hindquarter vascular conductance (Figure 3).
In the second group of rats, we found that the cardiovascular changes elicited by bilateral injection of β-FNA into the PVN were similar to those previously observed in the group of rats receiving naloxone – methiodide. Thus, slight but significant increases in mean arterial blood pressure accompanied by tachycardias, falls in renal and superior mesenteric blood flows and increases in hindquarter flow were noted following bilateral injection of 0.05 and 0.5 nmol of β-FNA into the PVN, when compared with the effects of control injection of aCSF (Figure 4). These cardiovascular changes were associated with significant falls in renal and superior mesenteric vascular conductances, and increases in hindquarter vascular conductance (Figure 5).
Haemodynamic responses to PVN injections of the selective δ-opioid receptor antagonist, ICI 174,864
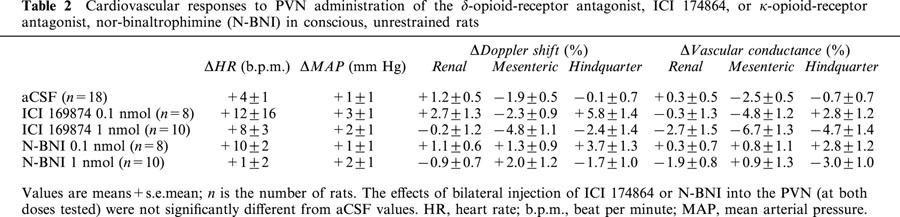
The effects of bilateral injection of ICI 174,864, at the dose of 0.1 nmol (n=8) or 1 nmol (n=10) into the PVN (on each side) were not significantly different from aCSF values (Table 2).
Table 2.
Cardiovascular responses to PVN administration of the δ-opioid-receptor antagonist, ICI 174864, or κ-opioid-receptor antagonist, nor-binaltrophimine (N-BNI) in conscious, unrestrained rats
Haemodynamic responses to PVN injections of the selective κ-opioid receptor antagonist nor-Binaltrophimine (nor-BNI)
The effects of bilateral injection of nor-BNI, at the dose of 0.1 nmol (n=8) or 1 nmol (n=10) into the PVN (on each side) were not significantly different from aCSF values (Table 2).
Haemodynamic responses to PVN injection of naloxone – methiodide (3 nmol) and β-FNA (0.5 nmol) in the presence of vasopressin V1-receptor antagonist
Fifteen minutes after pretreatment with the vasopressin V1-receptor antagonist, no significant changes were seen on blood pressure, heart rate, renal, superior mesenteric and hindquarter flows, or vascular conductances (Table 1).
Figures 6 and 7 show that in the presence of the vasopressin V1-receptor antagonist, control injection of aCSF (0.2 μl, n=12 in A and n=9 in B) into the PVN had no significant effect on any measured or calculated variables. However, we found that in rats receiving intravenous infusion of the vasopressin V1-receptor antagonist, bilateral injection of naloxone – methiodide (3 nmol, n=12) into the PVN produced slight but significant increases in mean arterial blood pressure and heart rate, compared with aCSF (Figure 6A). The blood pressure response to naloxone – methiodide was found to be significantly reduced in rats treated with the vasopressin V1-receptor antagonist, whereas the heart rate response was not significantly different from that evoked by naloxone – methiodide in the absence of the vasopressin V1-receptor antagonist. In the presence of the V1-receptor antagonist, there were no significant changes in renal, superior mesenteric or hindquarter flows, compared with aCSF. Thus, the decreases in renal and superior mesenteric flows and the increase in hindquarter flow observed in untreated rats were completely inhibited in the presence of the vasopressin V1-receptor antagonist (Figure 6A). The pressor and heart rate responses to naloxone – methiodide in the presence of the V1-receptor antagonist were associated with significant falls in renal and superior mesenteric vascular conductances and are presented in Figure 7A. These effects were found to be significantly reduced when compared with those evoked in the absence of the vasopressin V1-receptor antagonist. Furthermore, the hindquarter vasodilation observed in untreated animals was abolished by the treatment (Figure 7A).
Figure 6B shows that in rats receiving i.v. infusion of the vasopressin V1-receptor antagonist, bilateral injection of β-FNA (0.5 nmol, n=9) into the PVN had no effect on heart rate of blood pressure. These responses were different from those seen in rats not receiving the vasopressin V1-receptor antagonist, in that the heart rate and blood pressure responses were abolished. Moreover, we found that the decreases in renal and superior mesenteric flows and vascular conductances, as well as the increases in hindquarter flow and vascular conductance observed in untreated rats following bilateral injection of β-FNA into the PVN were significantly inhibited in the presence of the vasopressin V1-receptor antagonist, as shown in Figures 6B and 7B.
Discussion
This study focuses on the regional haemodynamic responses to PVN administration of specific and selective opioid-receptor antagonists in conscious, unrestrained rats. This study is a follow-up of our previous study indicating a role for opioid peptides and μ-opioid receptors in central cardiovascular regulation (Bachelard & Pitre, 1995; Bachelard et al., 1997). Thus, the present study shows that bilateral injection of naloxone – methiodide into the PVN of conscious rats causes increases in blood pressure and heart rate. These results are consistent with those of previous reports indicating that intravenous injection of naloxone induces increases in blood pressure in dog and human through a central mechanism (Janssen & Lutherer, 1980; Bouloux, 1987). Furthermore, the present study elucidates the peripheral mechanisms of these changes. Thus, we found that the pressor response to bilateral injection of naloxone – methiodide into the PVN was accompanied by decreases in renal and superior mesenteric vascular conductances and increases in hindquarter vascular conductance. Together, the results suggest the presence of a tonically-active central depressor pathway involving endogenous opioids. This is consistent with previous reports showing that morphine, β-endorphin and [D-Ala2] Met-enkephalin induced a dose-dependent and naloxone-reversible reduction in neuronal activity of a high proportion of PVN neurons, when applied to rat hypothalamic slice preparation (Muehlethaler et al., 1980; Pittmann et al., 1980). Thus, the cardiovascular responses observed following PVN administration of naloxone – methiodide could possibly result from the inhibition of a tonic inhibitory influence exerted by opioid peptides on PVN neurons. Although naloxone was injected at nmolar range, it produced the same cardiovascular changes than the highly selective μ-opioid receptor antagonist β-FNA, suggesting its specificity to opioid system.
Bilateral injection of the selective μ-opioid receptor antagonist, β-FNA, produced a significant tachycardia and a slight pressor response accompanied by vasoconstriction in renal and superior mesenteric vascular beds and vasodilation in hindquarter vascular bed. Although β-FNA displayed a short lasting κ-agonist activity (Qi et al., 1990), a recent work from our laboratory showed that PVN microinjection of the selective κ-agonist U69593 (0.01 – 5 nmol) induced no significant cardiovascular changes compared to vehicle values (Bachelard & Pitre, 1995). Thus, the cardiovascular effects observed in that study following administration of β-FNA are likely induced by inhibition of μ-opioid receptors into the PVN. The magnitude of the cardiovascular responses elicited by PVN administration of β-FNA was significantly smaller than that observed with naloxone – methiodide. This difference might result from the fact that higher doses of naloxone – methiodide (0.1 – 5 nmol) were used, comparatively to β-FNA (0.05 – 0.5 nmol), or alternatively to pharmacodynamic features of the antagonists. Moreover, considering the relatively high doses of naloxone – methiodide used and the fact that, contrarily to β-FNA, naloxone is not a μ-specific opioid antagonist, one can not exclude the possibility that this antagonist was acting on other subtypes of opioid receptors (e.g. δ- and κ-opioid receptors) to modulate cardiovascular responses. However, no significant cardiovascular changes was observed after PVN injection of the highly selective δ-opioid receptor antagonist, ICI 174,864, or a highly selective κ-opioid receptor antagonist, nor-BNI. Therefore, it is suggested that most of the cardiovascular responses to PVN administration of naloxone – methiodide are due to an inhibition of μ-opioid receptor.
Part of the cardiovascular responses observed after bilateral injection of naloxone – methiodide or β-FNA in the PVN are possibly vasopressin-mediated. This possibility is supported by neuroanatomical studies demonstrating the presence of opioid peptide-containing nerve terminals on vasopressin neurons in the PVN and supraoptic nucleus (Goldsmith et al., 1991). Moreover, several studies have provided evidence for an inhibitory role of endogenous opioid peptides on the release of vasopressin from the hypothalamo-neurohypophysial system into the plasma (Grossman et al., 1980; Arnauld et al., 1983; Yamada et al., 1989; Goldsmith et al., 1991; Otake et al., 1991; van de heijning et al., 1991). Although plasma levels of vasopressin was not measured in the present study, the results of our experiments carried out in the presence of the vasopressin V1-receptor antagonist indicate that vasopressin might contribute to the cardiovascular responses to PVN injection of naloxone – methiodide or β-FNA. Thus, in the presence of the vasopressin V1-receptor antagonist, the renal and superior mesenteric vasoconstrictor responses and the hindquarter vasodilator response to naloxone – methiodide or β-FNA were significantly inhibited by the treatment. It is likely that vasopressin was primarily responsible for the renal and superior mesenteric vasoconstrictor response to naloxone – methiodide or β-FNA, since the vasoconstrictor effect of intravenously injected vasopressin is particularly marked in those vascular beds (Gardiner et al., 1988). However, the factors underlying the hindquarter vasodilator responses to naloxone – methiodide remain unknown at the present stage. It is unlikely that vasopressin was primarily responsible for this response, since a previous study has reported vasoconstrictor effects in that vascular bed following intravenous injection of vasopressin in conscious rats (Gardiner et al., 1988). Moreover, in the present study the heart rate responses to PVN injection of naloxone – methiodide were not altered by the treatment with the vasopressin V1-receptor antagonist, and the pressor response was not completely inhibited by the treatment, indicating a non-vasopressin-mediated pressor and heart rate response. Further studies are required to elucidate the mechanism of these effects.
In the present study, we found that microinjection of aCSF (0.2 μl) into the PVN had no effect on blood pressure, heart rate or regional blood flows, indicating that displacement of the tissue by the volume injected was not a cause of the observed effects. However, considering the volume of injection (0.2 μl) and the doses of naloxone – methiodide (0.1 – 5.0 nmol) or β-FNA (0.05 – 0.5 nmol) used, one cannot exclude the possibility that the injections could have diffused to opioid-sensitive sites outside the PVN in concentrations sufficiently high to affect circulatory regulation by adjacent brain networks. However, the possibility that the cardiovascular responses to PVN administration of naloxone – methiodide were due to leakage of the antagonist from its central site of injection into the periphery has been excluded since the antagonist used, a quaternary salt of naloxone, does not cross the blood brain barrier (Iorio & Frigeni, 1984; Milne et al., 1990).
In a previous study, we found that PVN administration of a selective μ-opioid-receptor agonist, DAMGO, induced important cardiovascular effects characterized by increases in heart rate and blood pressure, while no effect was observed following PVN administration of selective δ- or κ-opioid-receptor agonists (Bachelard & Pitre, 1995). We determined that the pressor response was secondary to alpha adrenoceptor-mediated vasoconstriction in renal and superior mesenteric vascular beds and to beta adrenoceptor-mediated vasodilation in the hindquarter vascular bed (Bachelard & Pitre, 1995; Bachelard et al., 1997). Although those cardiovascular changes appear to be similar to the ones observed in the present study, using specific opioid-receptor antagonists, the haemodynamic profiles and characteristics of the cardiovascular responses are quite different. Indeed, in the present study we found that the maximum cardiovascular changes after the administration of naloxone – methiodide or β-FNA into the PVN are rapidly achieved (e.g. within 4 – 5 min) and the effects are short-lasting (subsiding in 10 – 15 min). In the previous study, using the μ-selective agonist DAMGO (1 nmol), we found that the maximum effects, occurred much later (e.g. within 30 – 45 min after PVN administration) and the cardiovascular responses were long-lasting (more than 1 h) (Bachelard et al., 1997). Moreover, by using specific antagonists intravenously injected we found that the cardiovascular responses to PVN injection of DAMGO were likely due to activation of the sympatho – adrenomedullary axis, while the involvement of circulating vasopressin or angiotensin II has been excluded (Bachelard et al., 1997). In the present study, we found evidence of vasopressin-mediated vasoconstrictor effects in the renal and superior mesenteric vascular beds. Therefore, cardiovascular responses to microinjection of opioid agonists and antagonists into the PVN could originate from two different mechanisms. Indeed, the anatomical localization of opioid receptors on vasopressin cell bodies of the PVN (van de heijning et al., 1991) and the very quick and short lasting effect of the antagonists suggest a local action with these drugs. Contrarily to opioid antagonists which blocked a physiological activity, an approach using opioid agonists leads to pharmacological effects which could activate neurons not accessible to endogenous opioids.
In summary, by using specific and some highly selective opioid receptor antagonists, we demonstrated that PVN administration of naloxone – methiodide and β-FNA produced important cardiovascular responses, in conscious, unrestrained rats. Thus, bilateral injection of both antagonists into the PVN induced tachycardia and increases in blood pressure, accompanied by vasoconstriction in renal and superior mesenteric vascular beds and vasodilation in the hindquarter vascular bed. On the other hand, no significant cardiovascular changes were observed following PVN administration of highly selective δ- or κ-opioid-receptor antagonists. Therefore, these results suggest the presence of a tonically-active central depressor pathway involving endogenous opioid peptides and μ-type PVN opioid receptors. By using a specific vasopressin V1 receptor antagonist intravenously administered, we found that part of this influence may involve a tonic inhibition of vasopressin release.
Acknowledgments
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC). H. Bachelard is a chercheur-boursier of the Fonds de la Recherche en Santé du Québec (FRSQ). The authors wish to thank Ms Hélène Crépeau, from the Service de consultation statistique, Département de mathématiques et de statistique, University Laval, for statistical assistance and professional advice.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- β-FNA
β-funaltrexamine
- b.p.m.
beat per minute
- DAMGO
[D-Ala2, MePhe4,Gly5-ol]enkephaline
- DPDPE
[D-Phe2,5]enkephaline
- HR
heart rate
- MAP
mean arterial pressure
- nor-BNI
nor-Binaltorphimine
- PVN
paraventricular nucleus of the hypothalamus
References
- APPEL N.M., KIRITSY-ROY J.A., VAN LOON G.R. μ-opioid receptors at discrete hypothalamic and brainstem sites mediate opioid peptide induced increases in central sympathetic out flow. Brain Res. 1986;378:8–20. doi: 10.1016/0006-8993(86)90281-7. [DOI] [PubMed] [Google Scholar]
- ARNAULD E., CIRINO M., LAYTON B.S., RENAULD L.P. Contrasting actions of amino acids, acetylcholine, noradrenaline and leucine enkephalin on the excitability of supraoptic vasopressin-secreting neurons. Neuroendocrinol. 1983;36:187–196. doi: 10.1159/000123455. [DOI] [PubMed] [Google Scholar]
- BACHELARD H., PITRE M. Regional haemodynamic effects of μ-, δ-, and κ-opioid agonists microinjected into the hypothalamic paraventricular nuclei of conscious, unrestrained rats. Br. J. Pharmacol. 1995;115:613–621. doi: 10.1111/j.1476-5381.1995.tb14976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACHELARD H., PITRE M., LESSARD A. Mechanisms of the regional hemodynamic effects of a mu-opioid receptor agonist microinjected into the hypothalamic paraventricular nuclei of conscious unrestrained rats. J. Pharmacol. Exp. Ther. 1997;280:460–470. [PubMed] [Google Scholar]
- BOULOUX P.M.G.Cardiovascular responses to stress: the role of opioid peptides Baillière's Clinical endocrinology and metabolism 1987London: Baillière Tindall; 439–465.ed. Grossman, A., pp [DOI] [PubMed] [Google Scholar]
- COWAN A., ZHU X.Z., PORRECA F. Studies in vivo with ICI 174864 and [D-Pen2, D-Pen5]enkephalin. Neuropeptides. 1985;5:311–314. doi: 10.1016/0143-4179(85)90015-0. [DOI] [PubMed] [Google Scholar]
- DESJARDINS G.C., BRAWER J.R., BEAUDET A. Distribution of μ, δ and κ opioid receptors in the hypothalamus of the rat. Brain Res. 1990;536:114–123. doi: 10.1016/0006-8993(90)90015-4. [DOI] [PubMed] [Google Scholar]
- DROLET G., MORILAK D.A., CHALMERS J. Endogenous opioids tonically inhibit the depressor neurones in the caudal ventrolateral medulla of rabbits: mediation through δ- and κ-receptors. Neuropharmacol. 1991;30:383–390. doi: 10.1016/0028-3908(91)90064-i. [DOI] [PubMed] [Google Scholar]
- FALLON J.H., LESLIE F.M. Distribution of dynorphin and enkephalin peptides in the rat brain. J. Comp. Neurol. 1986;249:293–336. doi: 10.1002/cne.902490302. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., BENNETT T. Regional haemodynamic responses to adrenoceptor antagonism in conscious rats. Am. J. Physiol. 1988;255:H813–H824. doi: 10.1152/ajpheart.1988.255.4.H813. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., BENNETT T., COMPTON A.M. Regional haemodynamic effects of neuropeptide Y, vasopressin and angiotensin II in conscious, unrestrained, Long Evans and Brattleboro rats. J. Auton, Nerv. Syst. 1988;24:15–27. doi: 10.1016/0165-1838(88)90131-2. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., BENNETT T. Regional haemodynamic effects of vasopressin infusion in conscious, unrestrained, Brattleboro rats. Br. J. Pharmacol. 1989;97:147–152. doi: 10.1111/j.1476-5381.1989.tb11935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., BENNETT T., HARTLEY L.J. Can pulsed Doppler technique measure changes in aortic blood flow in conscious rats. Am. J. Physiol. 1990a;259:H448–H456. doi: 10.1152/ajpheart.1990.259.2.H448. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., BENNETT T., PALMER R.M.J., MONCADA S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990b;15:486–492. doi: 10.1161/01.hyp.15.5.486. [DOI] [PubMed] [Google Scholar]
- GOLDSMITH P.C., BOGGAN J.E., THIND K.K. Opioid synapses on vasopressin neurons in the paraventricular and supraoptic nuclei of juvenile monkeys. Neuroscience. 1991;45:709–719. doi: 10.1016/0306-4522(91)90283-t. [DOI] [PubMed] [Google Scholar]
- GOODMAN R.R., SNYDER S.H., KUHAR M.J., YOUNG W.S. III: Differentiation of delta and mu opiate receptor localization by light microscopic autoradiography. Proc. Natl. Acad. Sci. U.S.A. 1980;77:6239–6243. doi: 10.1073/pnas.77.10.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSMAN A., BESSER G.M., MILLES J.J., BAYLIS P.H. Inhibition of vasopressin release in man by an opiate peptide. Lancet. 1980;ii:1108–1110. doi: 10.1016/s0140-6736(80)92542-8. [DOI] [PubMed] [Google Scholar]
- HAYWOOD J.R., SHAFFER R.A., FASTENOW C., FINK G.D., BRODY M.J. Regional blood flow measurement with pulsed Doppler flowmeter in conscious rats. Am. J. Physiol. 1981;241:H273–H278. doi: 10.1152/ajpheart.1981.241.2.H273. [DOI] [PubMed] [Google Scholar]
- HIRNING L.D., MOSBERG H.I., HURST R., HRUBY V.J., BURKS T.F., PORRECA F. Studies in vitro with ICI 174,864, [D-Pen2,D-Pen5]-enkephalin (DPDPE) and [D-Ala2,NMePhe4,Gly-ol]-enkephalin (DAGO) Neuropeptides. 1985;5:383–386. doi: 10.1016/0143-4179(85)90034-4. [DOI] [PubMed] [Google Scholar]
- IORIO M.A., FRIGENI V. Narcotic agonist/antagonist properties of quaternary diastereoisomers derived from oxymorphone and naloxone. Eur. J. Med. Chem.-Chim. Ther. 1984;19:301–303. [Google Scholar]
- JANSSEN H.F., LUTHERER L.O. Ventriculocisternal administration of naloxone protects against severe hypotension during endotoxic shock. Brain Res. 1980;194:608–612. doi: 10.1016/0006-8993(80)91251-2. [DOI] [PubMed] [Google Scholar]
- JIN C., ROCKHOLD R.W. Effects of paraventricular hypothalamic microinfusions of kainic aid on cardiovascular and renal excretory function in conscious rats. J. Pharmacol. Exp. Ther. 1989;251:969–975. [PubMed] [Google Scholar]
- KANNAN H., HAYASHIDA Y., YAMASHITA H. Increases in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am. J. Physiol. 1989;256:R1325–R1330. doi: 10.1152/ajpregu.1989.256.6.R1325. [DOI] [PubMed] [Google Scholar]
- KIRITSY-ROY J.A., APPEL N.M., BOBBITT F.G., VAN LOON G.R. Effect of μ-opioid receptor stimulation in the hypothalamic paraventricular nucleus on basal and stress-induced catecholamine secretion and cardiovascular responses. J. Pharmac. Exp. Ther. 1986;239:814–822. [PubMed] [Google Scholar]
- MILNE R.J., CODDINGTON J.M., GAMBLE G.D. Quaternary naloxone blocks morphine analgesia in spinal but not intact rats. Neurosci. Lett. 1990;114:259–264. doi: 10.1016/0304-3940(90)90573-r. [DOI] [PubMed] [Google Scholar]
- MUEHLETHALER M., GAEHWILER B.H., DREIFUSS J.J. Enkephalin inhibition of hypothalamic paraventricular neurons. Brain Res. 1980;197:264–268. doi: 10.1016/0006-8993(80)90457-6. [DOI] [PubMed] [Google Scholar]
- OTAKE K., KONDO K., OISO Y. Possible involvement of endogenous opioid peptides in the inhibition of arginine vasopressin release by γ-aminobutyric acid in conscious rats. Neuroendocrinology. 1991;54:170–174. doi: 10.1159/000125865. [DOI] [PubMed] [Google Scholar]
- PITTMANN Q.J., HATTON J.D., BLOOM F.E. Morphine and opiate peptides reduce paraventricularneuronal activity: studies on rat hypothalamic slice preparation. Proc. Natl. Acad. Sci. U.S.A. 1980;77:5527–5531. doi: 10.1073/pnas.77.9.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTOGHESE P.S., LIPKOWSKI A.W., TAKEMORI A.E. Binaltorphimine and nor-binaltorphimine, potent and selective κ opioid receptor antagonists. Life Sci. 1987;40:1287–1292. doi: 10.1016/0024-3205(87)90585-6. [DOI] [PubMed] [Google Scholar]
- QI J.A., HEYMAN J.S., SHELDON R.J., KOSLO R.J., PORRECA F. Mu antagonist and kappa agonist properties of beta-funaltrexamine (beta-FNA) in vivo: long-lasting spinal analgesia in mice. J. Pharmacol. Exp. Ther. 1990;252 3:1006–1011. [PubMed] [Google Scholar]
- SAR M., STUMPF W.E., MILLER R.J., CHANG K.J., CUATRECASAS P. Immunohistochemical localisation of enkephalin in rat brain and spinal cord. J. Comp. Neurol. 1979;182:17–38. doi: 10.1002/cne.901820103. [DOI] [PubMed] [Google Scholar]
- SAWCHENKO P.E., SWANSON L.W. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J. Comp. Neurol. 1982a;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- SAWCHENKO P.E., SWANSON L.W. The organisation of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. Rev. 1982b;4:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- SWANSON L.W., SAWCHENKO P.E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Ann. Rev. Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- TAKEMORI A.E., HO B.Y., NAESETH J.S., PORTOGHESE P.S. Nor-Binaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J. Pharmacol. Exp. Ther. 1988;246:255–258. [PubMed] [Google Scholar]
- TAKEMORI A.E., LARSON D.L., PORTOGHESE P.S. The irreversible narcotic antagonistic and reversible agonistic properties of the fumarate methyl ester derivative of naltrexone. Eur. J. Pharmacol. 1981;70:445–451. doi: 10.1016/0014-2999(81)90355-1. [DOI] [PubMed] [Google Scholar]
- VAN DE HEIJNING B.J.M., KOEKKOEK-VAN DEN HERIK I., VAN WIMERSMA GREIDANUS T.B. The opioid receptor subtypes μ and κ, but not δ, are involved in the control of the vasopressin and oxytocin release in the rat. Eur. J. Pharmacol. 1991;209:199–206. doi: 10.1016/0014-2999(91)90170-u. [DOI] [PubMed] [Google Scholar]
- VAN LOON G.R.Endogenous opioid peptides in the regulation of arterial blood pressure Hypertension and the Brain 127 1984Mt Kisco, New York: Futura Press; eds. Kotchen, T.A., Guthrie, G.P., pp [Google Scholar]
- WAMSLEY J.K. Opioid receptors: autoradiography. Pharmacol. Rev. 1983;35:69–83. [PubMed] [Google Scholar]
- WARD S.J., PORTOGHESE P.S., TAKEMORI A.E. Pharmacological characterization in vivo of the novel opiate, β-funaltrexamine. J. Pharmacol. Exp. Ther. 1982;220:494–498. [PubMed] [Google Scholar]
- WRIGHT C.E., ANGUS J.A., KORNER P.I. Vascular amplifier properties in renovascular hypertension in conscious rabbits. Hindquarters responses to constrictor and dilator stimuli. Hypertension. 1987;9:122–131. doi: 10.1161/01.hyp.9.2.122. [DOI] [PubMed] [Google Scholar]
- YAMADA T., NAKAO K., ITOH H., SHIRAKAMI G., SUGAWARA A., SAITO Y., MUKOYAMA M., ARAI H., HOSODA K., SHIONO S., EIGYO M., MATUSHITA A., IMURA H. Effects of naloxone on vasopressin secretion in conscious rats: evidence for inhibitory role of endogenous opioid peptides on vasopressin secretion. Endocrinology. 1989;125:785–790. doi: 10.1210/endo-125-2-785. [DOI] [PubMed] [Google Scholar]


