Abstract
Myocardial injury caused by ischaemia and reperfusion comes from multiple pathogenic events, including endothelial damage, neutrophil extravasation into tissue, mast cell activation, and peroxidation of cell membrane lipids. These events are followed by myocardial cell alterations resulting eventually in cell necrosis. An enhanced formation of reactive oxygen species is widely accepted as a stimulus for tissue destruction and cardiac failure.
In this study, we have investigated the cardioprotective effects of M40403 in myocardial ischaemia-reperfusion injury. M40403 is a low molecular weight, synthetic manganese containing superoxide dismutase mimetic (SODm) that selectively removes superoxide anion. Ischaemia was induced in rat hearts in vivo by ligating the left anterior descending coronary artery. Thirty minutes after the induction of ischaemia, the ligature was removed and reperfusion allowed to occur for at least 60 min. M40403 (0.1–1 mg kg−1) was given intravenously 15 min before ischaemia.
The results obtained in this study showed that M40403 significantly reduced the extent of myocardial damage, mast cell degranulation and the incidence of ventricular arrhythmias. Furthermore, M40403 significantly attenuated, in a dose-dependent manner, neutrophil infiltration in the myocardium as well as the associated induction of lipid peroxidation. Calcium overload seen post-reperfusion of the ischaemic myocardium was also reduced by M40403.
Immunohistochemical analysis for nitrotyrosine revealed a positive staining in cardiac tissue taken after reperfusion: this was attenuated by M40403. Moreover reperfused cardiac tissue sections showed positive staining for P-selectin and for anti-intercellular adhesion molecule (ICAM-1) in the vascular endothelial cells. M40403 treatment markedly reduced the intensity and degree of P-selectin and ICAM-1 in these tissues. No staining for nitrotyrosine, P-selectin or ICAM-1 was found in cardiac tissue taken at the end of the ischaemic period.
Overall, M40403 treatment reduced the morphological signs of myocardial cell injury and significantly improved survival.
Taken together, these results clearly indicate that M40403 treatment exerts a protective effect against ischaemia-reperfusion-induced myocardial injury, supporting a key role for superoxide anion in reperfusion injuries. This suggests that synthetic enzymes of SOD such as M40403, offer a novel therapeutic approach for the treatment of ischaemic heart disease where superoxide anion plays a dominant role.
Keywords: Ischaemia-reperfusion, rat heart, superoxide anion, peroxynitrite, superoxide dismutase mimetic, M40403
Introduction
Cardiac ischaemia results in endothelial injury followed by myocardial dysfunction and damage. Paradoxically, reperfusion of ischaemic heart, although providing the cells with oxygen and trophic substances, further excacerbates tissue damage since it promotes the accumulation of neutrophils and formation of oxygen-derived free radicals (McCord, 1985; Lefer et al., 1991). In particular, endothelial cell injury caused by ischaemia enhances neutrophil adhesion and migration from the vasculature to the tissue (Bradford, 1976; Harlan, 1985; Salvemini et al., 1993; 1999a). Leukocyte-endothelial cell interaction involves a complex system of adhesion molecules including selectins, β2 integrins and the immunoglobulin superfamily (Geng et al., 1990; von andrian et al., 1991; Butcher, 1992). Neutrophil activation at sites of injury leads to the production of large amounts of oxygen-derived free radicals, the primary of which is the superoxide anion: overt production of superoxide anion extends endothelial and myocardial damage (Ratych et al., 1987; Johnson III et al., 1991; Lefer & Lefer, 1993). This process is in turn enhanced by platelet activation (Geng et al., 1990). Resident mast cells also contribute to the dysfunction of the ischaemic-reperfused heart through the release of superoxide (Mannaioni et al., 1991) and mediators such as histamine (Keller et al., 1988; Masini et al., 1990). These in turn contributes to the up-regulation of P-selectin and ICAM-1 expression on endothelial cells (Clark et al., 1995) and to the development of arrhythmias (Wolff & Levi, 1986; Bani et al., 1998). The release of histamine from mast cells is also amplified by the excessive release of superoxide and concomitant decrease in nitric oxide (NO), (Mannaioni et al., 1991). The formation of superoxide anion is kept under tight control by endogenous superoxide dismutase (SOD) enzymes (Fridovich, 1995; Cuzzocrea et al., 2001b). There are two forms of SOD: the Mn enzyme in mitochondria (SOD2) and Cu/Zn enzyme present in the cytosol (SOD1) or extracellular surfaces (SOD3). The importance of SOD2 is highlighted by the findings that in contrast to SOD1 (Reaume et al., 1996) and SOD3 (Carlsson et al., 1995), SOD2 knockout is lethal to mice (Lebovitz et al., 1996; Melov et al., 1999). In acute and chronic inflammation and in ischaemia and reperfusion injury, the production of superoxide anion is increased at a rate that overwhelms the capacity of the endogenous SOD enzyme defence system to remove them. The result of such imbalance results in superoxide-mediated damage. Excessive release of superoxide anion contributes to tissue damage seen post-reperfusion in several ischaemic organs including kidney (Morpurgo et al., 1996), stomach (Yoshikawa et al., 1990), intestine (Zimmermann et al., 1993; Salvemini et al., 1999a, b; Cuzzocrea et al., 2001a), skin (Goossens et al., 1990) and heart (Ambrosio & Flaherty, 1992; Grill et al., 1992). In the heart, a number of studies have shown that dismutation of superoxide with the native SOD enzyme (Burton, 1985; Ma et al., 1992; Hangaishi et al., 2001; Li et al., 2001) or with overexpression of the human manganese SOD enzyme (Chen et al., 1998; 2000) protects against myocardial ischaemia and reperfusion injury.
Some important tissue damaging and pro-inflammatory roles attributed to superoxide anion include: endothelial cell damage and increased microvascular permeability (Hardy et al., 1994; Okayama et al., 1999; Tanita et al., 1999; Koo et al., 2001), formation of chemotactic factors such as leukotrienes B4 (Fantone & Ward, 1982; Deitch et al., 1990), recruitment of neutrophils at sites of inflammation (Boughton-Smith et al., 1993; Salvemini et al., 1996; 1999a), release of histamine from mast cells (Mannaioni & Masini, 1988), lipid peroxidation and oxidation and DNA single-strand damage (Dix et al., 1996). In addition, superoxide anion by interacting with nitric oxide destroys the biological activity of this mediator (Gryglewski et al., 1986). This attenuates important anti-inflammatory and tissue protective properties of nitric oxide namely: maintenance of blood vessel tone and platelet reactivity (Furchgott & Zawadzki, 1980; Radomski et al., 1990), cytoprotective effect in numerous organs (including heart, intestine and kidney) (Moncada et al., 1991; Alderton et al., 2001), and release of anti-inflammatory and cytoprotective prostacyclin (via activation of the constitutive cyclo-oxygenase enzyme (Salvemini et al., 1993). Furthermore, in addition to destroying beneficial nitric oxide, the product formed from the reaction of superoxide anion with nitric oxide is peroxynitrite, a well-described, potent cytotoxic and pro-inflammatory molecule (Beckman et al., 1990; Ischiropoulos et al., 1992; Beckman & Crow, 1993; Crow & Beckman, 1995; Salvemini et al., 1999a). Substantial evidence now exists to suggest that peroxynitrite accounts for the majority of nitric oxide-driven inflammatory events (Salvemini et al., 1998), including myocardial injury post-ischaemia and reperfusion (Matheis et al., 1992; Villa et al., 1994; Naseem et al., 1995; Schulz & Wambolt, 1995). Under these conditions, a reduction in peroxynitrite generation, markedly reduces reperfusion injury, as shown by reduced cardiac lipid peroxidation (Masini et al., 2000) or improved myocardial mechanical performance (Schulz & Wambolt, 1995). Therefore, removal of superoxide protects nitric oxide and reduces formation of the cytotoxic peroxynitrite.
We have recently shown that selective removal of superoxide by SOD mimetics such as M40403 or M40401 exerts beneficial effects in models of ischaemia and reperfusion of the intestine as well as in models of acute inflammation (Salvemini et al., 1999a; Cuzzocrea et al., 2001a). These are stable, low molecular weight, manganese-containing, non-peptidic molecules possessing the function and catalytic rate of native SOD enzymes (Riley et al., 1996; Salvemini et al., 1999b; Aston et al., 2001). An important property of these SODm is that they catalytically remove superoxide anion at a high rate without interacting with other reactive species including nitric oxide, peroxynitrite, hydrogen peroxide, oxygen or hypochlorite (Riley et al., 1996; Salvemini et al., 1999b). Thus, SODm can serve as selective probes for deciphering the role of superoxide in biological systems where other such relevant biological oxidants may be present. It is important to realize that this property is not shared by other ‘so called and claimed classes of SOD mimetics' including several metalloporphyrins. This selectivity has not been demonstrated by other claimed SOD mimetic compounds such as the MnIII or FeIII porphyrins or the MnIII (Salen) which also react with other pertinent biological oxidants including nitric oxide and peroxynitrite (Patel & Day, 1999).
The aim of the study was to evaluate the role(s) of superoxide in myocardial ischaemia and reperfusion injury in rats and highlight potential mechanism(s) through which M40403 exerts its protective effects.
Methods
Animals
Eighty-four male albino rats, Wistar strain, weighing 250–300 g (Morini, Reggio Emilia, Italy) were used. They were quarantined for 7 days at 22–24°C with a 12 h light/12 h dark cycle before use. Standard laboratory chow (Rodentia, Bergamo, Italy) and water were available ad libitum. The experimental protocol was designed in compliance with the recommendations of the European Economic Community (86/609/CEE) for the care and use of laboratory animals and was approved by the animal care committee of the University of Florence (Italy). The rats were randomly distributed in seven groups composed of 12–16 animals.
Surgical procedure
The rats were anaesthetized by intraperitoneal injection of ketamine (Parke Davis, Milan, Italy; 150 mg kg−1). A cannula was inserted into the trachea and the animals were ventilated with air using a Palmer pump (U. Basile, Comerio, Italy). Subcutaneous peripheral limb electrodes were inserted and an electrocardiogram (ECG) was continuously recorded for the entire duration of the experiment. Body temperature was maintained at 38±1°C. The right carotid artery was cannulated and connected to a pressure transducer (P23XL, Spectramed Statham, Oxhnard, CA, U.S.A.) to monitor mean arterial blood pressure (MAP). Heart rate (HR) and MAP were continuously recorded by a two channel polygraph recorder (AD Instruments). All rats underwent thoracotomy at the fifth left intercostal space, the pericardium was opened and a loose 00 braided silk suture was placed around the left anterior descending coronary artery approximately 1 to 2 mm below its origin. To facilitate the successive removal of the suture, a small silicon ring was inserted in the silk thread below the knot. Then, the chest was closed by a silk suture to minimize heart displacement, taking care to leave the ends of the coronary suture threads emerging from the surgical wound. Rats were allowed to equilibrate for 20 min to enable ECG values to stabilize. Ischaemia was induced by tightening the threads of the coronary suture and was maintained for 30 min. Reperfusion was obtained by reopening the chest and cutting the ligature around the coronary artery. The duration of reperfusion was predetermined to 60 min. In the animals that did not survive the entire reperfusion period, reperfusion lasted until cessation of the cardiac activity as revealed in ECG recordings. In all the animals, the survival time was recorded. To exclude that premature mortality of rats was caused by the surgical procedures or individual abnormalities, rats showing ECG signs of impaired cardiac function during the stabilization period before induction of ischaemia or soon after the coronary artery ligature were excluded from the experiments. M40403 (0.1, 0.3 and 1 mg kg−1), or an equivalent volume (0.5 ml) of vehicle (26 mM sodium bicarbonate buffer, pH 8.0–8.1) were injected i.v. in the dorsal penile artery 15 min before ischaemia. All rats were sacrificed at 60 min post-reperfusion for biochemical analysis.
Experimental groups
Group 1
This group consisted of 12 sham-operated rats. These rats were injected with 0.5 ml of vehicle and then underwent the same surgical procedures as above but without the tightening of the coronary sutures. They were used as controls (sham-operated) for biochemical and morphological analyses.
Group 2
This group consisted of 12 sham-operated rats. These rats were injected with 0.5 ml of a solution containing 1 mg kg−1 of M40403 (the highest dose used). They were used as controls (sham operated plus M40403).
Group 3
This group consisted of 16 rats undergoing ischaemia and reperfusion in the absence of SODm (IR).
Group 4–6
These groups consisted of 12 rats treated with M40403 at 0.1, 0.3 and 1 mg kg−1 respectively 15 min before ischaemia and reperfusion.
Group 7
This group consisted of eight rats that underwent ischaemia alone for 30 min and were then sacrificed at this time point. This group was used to determine the effects of ischaemia on the biochemical parameters studied.
Determination area at risk and infarct size
Sixty minutes after reperfusion of the ischaemic myocardium, the left anterior descending coronary artery was re-ligated with a loose 00 braided silk suture in the same place of the previous ligature and 2 ml of Evans Blue (Sigma Co., St. Louis, MO, U.S.A.) was retrogradely injected with a thin catheter inserted into carotid artery to delineate the in vivo area at risk (AAR). At the end of reperfusion, or at the moment of cessation of the cardiac activity in the rats that did not survive the predetermined reperfusion period, the chest was re-opened and the hearts quickly removed. In all samples, the anterior left ventricular wall, 3-mm distal to the ligature (to exclude tissue areas damaged by the surgical procedure) was excised. This was cut into fragments, some of which, chosen at random, were used for morphological studies and the others for biochemical assays. The extension of the left ventricular myocardium undergoing damage caused by ischaemia-reperfusion was determined by the nitroblue tetrazolium dye exclusion method. The sham-operated hearts from the rats of group 1 and 2 were treated with the same method and were used as negative controls. On removal, the hearts were attached to a Langendorff's apparatus through a cannula introduced into the aorta and perfused with 10 ml of 1% nitroblue tetrazolium dissolved in a modified Tyrode solution, pH 7.4, at a constant pressure of 40 cm of water at 37°C for 20 min.
In this way, the aortic semilunar valve remains closed and the dye enters directly into the coronary arteries. Following this treatment, the normal myocardium shows an intense blue staining reaction because of the presence of dehydrogenase enzymes, whereas ischaemia-reperfusion-injured lesions remain unstained. Thus, the latter regions appear as clearly delineated, unstained zones. The hearts were detached from the cannula, weighed, fixed in buffered 4% formaldehyde for 12 h, and the ventricles sectioned in 1-mm transverse slices from the apex to the ligature. The 1-mm sections were placed in individual wells with the basal side exposed. Each slide was weighed and visualized under an Olympus microscope equipped with a CCTV television camera (Sony, Tokyo, Japan) interfaced with an Apple Macintosh LC III personal computer through a Videospigot card (Supermac, Sunnyvale, CA, U.S.A.). The left ventricular area, AAR, and the area of infarction for each slide were then determined by computer planimetry using 1.49 Image Analysis Program, National Institute of Health, Bethesda, MD, U.S.A.). The size of myocardial infarction was determined by the following equation (A1xWt1)+(A2xWt2)+(A3xWt3)+A4xWt4)+(A5xWt5), where A is per cent area of infarction by planimetry from subscripted numbers 1–5 representing sections and Wt is the weight of the same numbered sections. In each heart, the total volume of the damaged myocardium was calculated as the sum of the partial values of the different slices. To allow a comparison of the extension of myocardial injury between hearts of different size, the total volume of the damaged myocardium was divided by the heart weight (grams).
Analysis of electrocardiogram (ECG)
The development of disturbances of the cardiac rhythm, such as ventricular tachycardia (VT) and ventricular fibrillation (VF), which are known to be associated with myocardial ischaemia and reperfusion (Fujimoto et al., 1983), were evaluated in ECG recordings of all rats subjected to the study during both coronary artery occlusion and reperfusion as described (Gelvan et al., 1991). Ventricular tachycardia was recognized as three or more consecutive premature ventricular contractions, and ventricular fibrillation was recognized as irregular modulating baseline. A heart was considered to be in normal sinus rhythm when normal sinus complexes occurring at a regular rate were observed. The values are reported as the number of rats with heart arrhythmia over the total number of animals of each group. Electrocardiogram analysis, which allows to identify the moment of the cessation of cardiac activity, was also used to evaluate the survival period of the rats during the post-ischaemic reperfusion phase.
Immunohistochemical localization of nitrotyrosine
Tyrosine nitration was detected as previously described (Cuzzocrea et al., 1997) in cardiac sections by immunohistochemistry. Tissues were fixed in 10% buffered formalin and 8 μm sections were prepared from paraffin embedded tissues. After deparaffinization, endogenous peroxidase was quenched with 0.3% H2O2 in 60% methanol for 30 min. The sections were permeabilized with 0.1% Triton X-100 in phosphate buffered saline for 20 min. Non specific adsorption was minimized by incubating the section in 2% normal goat serum in phosphate buffered saline for 20 min. Endogenous biotin or avidin binding sites were blocked by sequential incubation for 15 min with avidin and biotin (DBA, Milan, Italy). The sections were then incubated overnight with 1 : 1000 dilution of primary anti-nitrotyrosine antibody (DBA, Milan, Italy) or with control solutions. Controls included buffer alone or non-specific purified rabbit IgG. Specific labelling was detected with a biotin-conjugated goat anti-rabbit IgG and avidin-biotin peroxidase complex (DBA, Milan, Italy).
Immunohistochemical analysis of P-selectin and ICAM-1
P-selectin and ICAM-1, localization was detected as previously described (Gauthier et al., 1994) in cardiac sections by immunohistochemistry. Acetone-fixed frozen sections were permeabilized with 0.1% Triton X-100 in phosphate buffered saline for 20 min and incubated in 2% normal serum for 1 h in order to minimize non-specific adsorption. Sections were then incubated overnight at 4°C with rabbit anti-human polyclonal antibody directed at P-selectin (CD62P) which react with rat or mouse anti-rat antibody directed at ICAM-1 (CD54) at a dilution 1 : 500. Controls included buffer alone or non-specific purified IgG. Antibody binding sites were visualized using an avidin-biotin peroxidase complex immunoperoxidase technique (Vector Laboratories) with diaminobenzidine.
Evaluation of myeloperoxidase activity
Myeloperoxidase (MPO) activity can be used as a marker for neutrophil accumulation in tissues. MPO activity was evaluated according to Bradley et al. (1982). Frozen samples of left ventricular tissue weighing approximately 100 mg were homogenized in 1.5 ml of 50 mmol l−1 potassium phosphate buffer, pH 6. One millilitre of the homogenate was centrifuged at 10,000×g for 10 min, and the pellet was suspended in 1 ml of potassium phosphate buffer (50 mmol l−1), pH 6, containing 0.5% hexadecyl-trimethylammonium bromide (Sigma) to negate peroxidase activity of haemoglobin and myoglobin (Miller et al., 1993) and to solubilize membrane-bound MPO. The suspensions were treated with three cycles of freezing-thawing, sonicated on ice for 10 s, and centrifuged at 12,000×g for 10 min. MPO activity was determined in the supernatants. Briefly, 0.1 ml of the supernatant was mixed with 2.9 ml of potassium phosphate buffer (50 nmol l−1), pH 6, containing 0.19 mg ml−1 of -dianisidine chloride and 0.0005% H2O2 as a substrate for MPO. Oxidized -dianisidine forms a stable chromophore absorbing at a 460-nm wavelength. The absorbance was determined spectrophotometrically over 2 min. The values of tissue MPO activity were obtained by comparison with standard concentrations of -dianisidine in the presence of excess H2O2. One unit of MPO activity is defined as that required to degrade 1 micromol of hydrogen peroxide per minute at 25°C. Protein concentration was determined with the Bradford method (Bradford, 1976). The results are expressed as mU mg −1 of proteins.
Determination of malonyldialdehyde production
Malonyldialdehyde (MDA) is an end-product of peroxidation of cell membrane lipids caused by oxygen-derived free radicals and is considered a reliable marker of myocardial cell damage (Rao et al., 1983). MDA levels in cardiac tissues was determined by measurement of the chromogen generated from the reaction of MDA with 2-thiobarbituric acid as described previously (Aruoma et al., 1989). Approximately 100 mg of myocardial tissue were homogenized with 1 ml of 50 mmol l−1 Tris-HCl buffer containing 180 mmol l−1 KCl and 10 mmol l−1 EDTA, final pH 7.4, using a tissue homogenizer (Ing. Terzano, Milan, Italy). 0.5 ml of 2-thiobarbituric acid (1% w v−1) in 0.05 mol l−1 NAOH and 0.5 ml of HCl (25% w v−1 in water) were added to 0.5 ml of sample. The mixture was placed in test tubes, sealed with screw caps, and heated in boiling water for 10 min. After cooling, the chromogen was extracted in 3 ml of 1 butanol, and the organic phase was separated by centrifugation at 2000×g for 10 min. The absorbance of the organic phase was read spectrophotometrically at a 532-nm wavelength. Protein concentration was determined according to the Bradford (1976) assay. The values are expressed as nmoles of thiobarbituric acid-reactive substances (MDA equivalents) per mg of protein, using a standard curve of 1,1,3,3-tetramethoxypropane.
Evaluation of calcium content
Excessive calcium influx is a critical event, accompanying irreversible injury in myocardial ischaemia-reperfusion (Bourdillon & Poole-Wilson, 1981). The calcium content of the myocardial tissue was measured by atomic absorption spectrometry as described previously (Bani et al., 1998). Briefly, tissue fragments weighing approximately 30 mg were rinsed thoroughly in calcium-free-buffered solution, dried in an oven at 80°C, and digested overnight with 65% HNO3 (100 μl 10 mg−1 of dry tissue). After addition of 32% HCl (150 μl 10 mg−1 dry tissue) the samples were dried at 45°C under nitrogen. At the moment of the assay the samples were suspended in 50 μl of 32% HCl and added with lanthanum chloride (LaCl 7H2O) to provide a final concentration of 1% lanthanum. The amounts of calcium in the samples were read in an atomic absorption spectrophotometer (Perkin-Elmer, Uberlingen, Germany) at a 422 nm wavelength. The relevant values were determined by comparison with a standard curve obtained with increasing concentrations of CaCl2 and expressed as ng of calcium per mg of tissue, dry weight.
Computer-assisted mast cell morphometry
Evaluation of light transmittance across mast cells was performed according to the methods described previously (Bianchi & Mugnai, 1991; Masini et al., 1997). The mast cells were viewed by a CCTV television camera (Sony) applied to a Reichert-Jung Microstar IV light microscope (Cambridge Instruments Inc., Buffalo, NY, U.S.A.) with a ×100 oil immersion objective and interfaced with an Apple Macintosh LCIII personal computer through a Videospigot card (Supermac). The card allows for the light transmitted across the microscopic slide to be determined within a range of 256 grey levels, which are comprised between 0 (black level) and 255 (white level). The card also allows for a digitized image of mast cells to be reproduced on the basis of the values estimated. Measurements of transmittance were carried out using a 1.49 Image analysis program (National Institute of Health, Bethesda, MD, U.S.A.). In each experimental group, the transmittance of 100 different mast cells, 10 from each animal of the group (five per fragment), was analysed and the mean transmittance value (mean±s.e.m.) was then calculated.
Light microscopy for morphological observations
For histopathological examination, biopsies of left ventricular tissues were taken at the end of the reperfusion period. The tissues were fixed in Dietric solution (14.25% ethanol. 1.85% formaldehyde, 1% acetic acid) for 1 week at room-temperature, dehydrated by graded ethanol and embedded in Paraplast (Sherwood Medical, Mahwash, NJ, U.S.A.). From each sample, 7 μm thick slices were obtained and stained with ematossylin eosin and studied using light microscopy (Dialux 22 Leitz).
Statistical analysis
The reported data are expressed as mean±s.e.m. For ECG analysis, significance of difference between the experimental groups was assayed by the χ2 test. In the biochemical and morphometric assay, and in the evaluation of survival of the rats during reperfusion, the distribution of the measured values in the different experimental groups was assessed to be gaussian. Statistical analysis was performed by either one-way analysis of variance (ANOVA) test followed by Student-Newman-Keuls multiple comparison test or by Student's t-test for unpaired values. Calculation were carried out using a GraphPad Prism 2.0 statistical program (GraphPad Software, San Diego, CA, U.S.A.). P<0.05 was considered significant.
Materials
M40403 was synthesized by MetaPhore, as described previously (Salvemini et al., 1999b). Primary anti-nitrotyrosine, anti-ICAM and anti P-Selectin antibodies were from Upstate Biotech (DBA, Milan, Italy). All other reagents and compounds used were obtained from Sigma Chemical Company (Sigma, Milan, Italy).
Results
Extension of myocardial infarct size caused by regional ischaemia and reperfusion
The results of the computer-assisted morphometry on the ischaemic reperfused hearts stained with nitroblue tetrazolium showed that M40403 dose-dependently reduced the extension of myocardial damage (Figure 1). The mean values for the area at risk (AAR) ranged from 56±3% to 59±4% and were similar in all animal groups studied (P=ns, data not shown). The summary data for the infarct size, expressed as per cent of AAR, following 30 min coronary occlusion and 60 min of reflow are shown in Figure 2. M40403 did not modify the extension of at risk area per left ventricle (LV), P=ns), while the portion of this area rendered necrotic was significantly reduced (P<0.01) in the animals treated with M40403 at the dose of 1 mg kg−1. Sham-operation did not result in a significant degree of infarction (data not shown).
Figure 1.
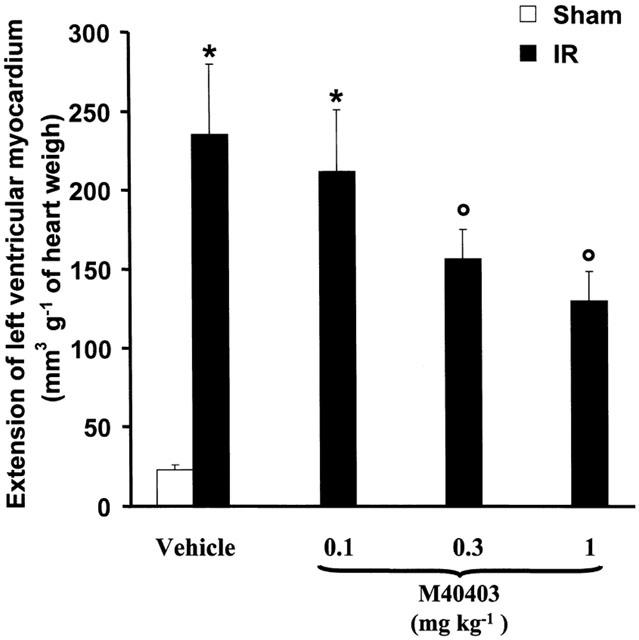
Extension of left ventricular myocardium with ischaemic reperfusion-induced injury as evaluated by computer-assisted morphometry on heart stained with nitroblue tetrazolium. Significance of difference between groups (one-way ANOVA: each group is the mean±s.e.m. of at least 12 experiments; *n.s.; °P<0.01).
Figure 2.
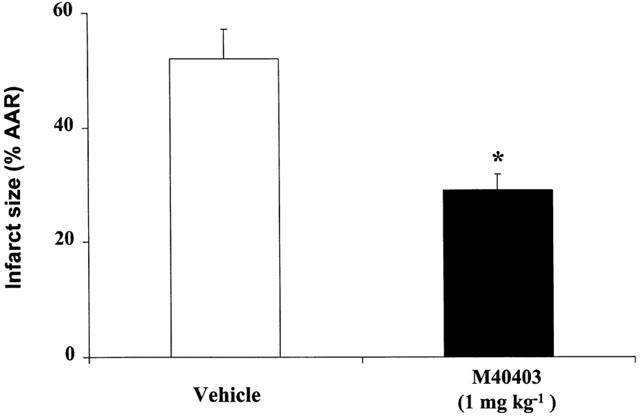
Graph of infarct size expressed as per cent of area at risk (AAR) after 30 min of ischaemia and 60 min of reperfusion. Each group is the mean±s.e.m. of at least nine experiments. Legend for each bar is shown underneath. *P<0.001.
Haemodynamic parameters: ECG analysis and duration of survival during the post ischaemic reperfusion phase
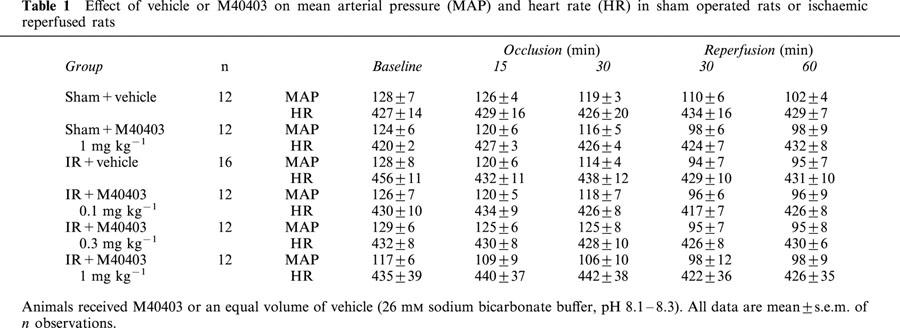
Mean values for MAP and HR measured during the course of the experiments are reported in Table 1. Baseline haemodynamic data were similar (P=ns) in all groups studied. In sham operated rats, infusion of vehicle for M40403, 1 mg kg−1 had no significant haemodynamic effects (Table 1). In rats subjected to coronary occlusion and reperfusion, mean values for MAP significantly decreased throughout the experimental period, but there was no alteration in heart rate. The mean values of MAP and HR of rats and subjected to IR and treated with M40403 were not different from the IR group treated with vehicle (Table 1). Examination of ECG recordings from the rats subjected to myocardial ischaemia and reperfusion showed that M40403 (1 mg kg−1) reduced the occurrence of ventricular arrhythmias. During ischaemia, VT occurred in six out of 16 control animals, whereas no ventricular arrhythmias were found in the 12 rats treated with 1 mg kg−1 M40403 (P<0.003). During reperfusion, VT and VF occurred in 12 out of the 16 rats treated with the vehicle, whereas these arrhythmias were present in seven out of the 12 rats treated with 0.1 mg kg−1 of M40403 (P<0.01), in five out of the 12 rats treated with 0.3 mg kg−1 of M40403 and only in one of the 12 rats treated with the highest of M40403. In the sham operated groups treated with vehicle or with M40403 at the dose of 1 mg kg−1, VT was observed only in one animal, starting 45 min from placement of the silk thread around the left coronary artery.
Table 1.
Effect of vehicle or M40403 on mean arterial pressure (MAP) and heart rate (HR) in sham operated rats or ischaemic reperfused rats
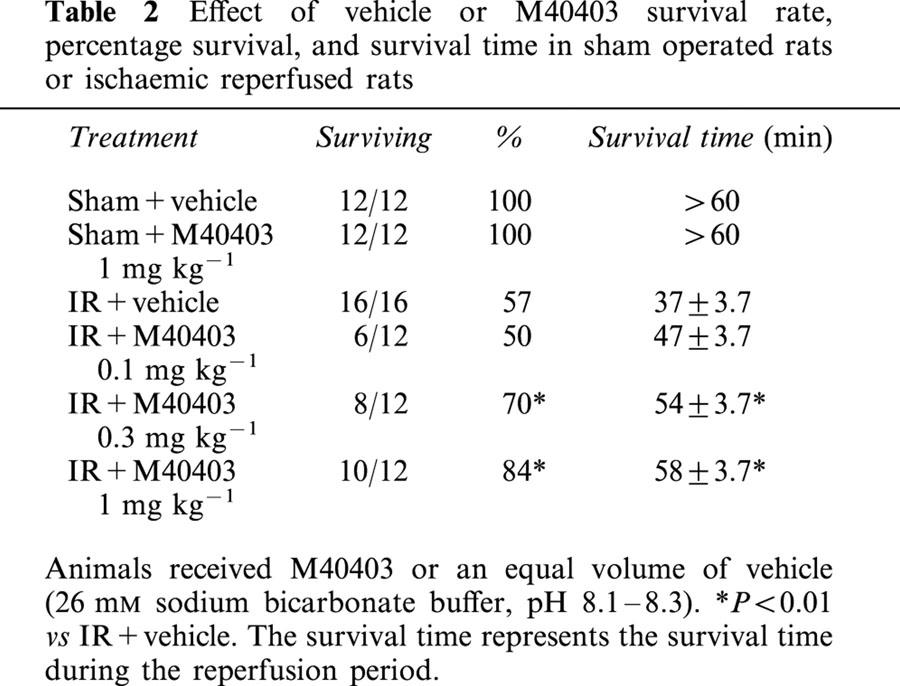
During ischaemia, none of the animals died; while in the post-ischaemic reperfusion period, some animals died in both the M40403-treated and untreated groups. However, the number of animals that survived was higher and the duration of survival was significantly longer in the M40403-treated groups than in the untreated one (Table 2). All sham-operated rats survived the entire experimental period (90 min).
Table 2.
Effect of vehicle or M40403 survival rate, percentage survival, and survival time in sham operated rats or ischaemic reperfused rats
Computer-assisted morphometry of mast cells
Morphometric analysis of cardiac mast cells showed that light transmittance across mast cells, which is inversely related to the amount of secretory granules, was significantly higher in the ischaemic-reperfused hearts in comparison with the sham-operated ones (Figure 3). Conversely, in the rats treated with the highest dose (1 mg kg−1), the light transmittance across cardiac mast cells was nearly similar to the sham-operated ones (Figure 3).
Figure 3.
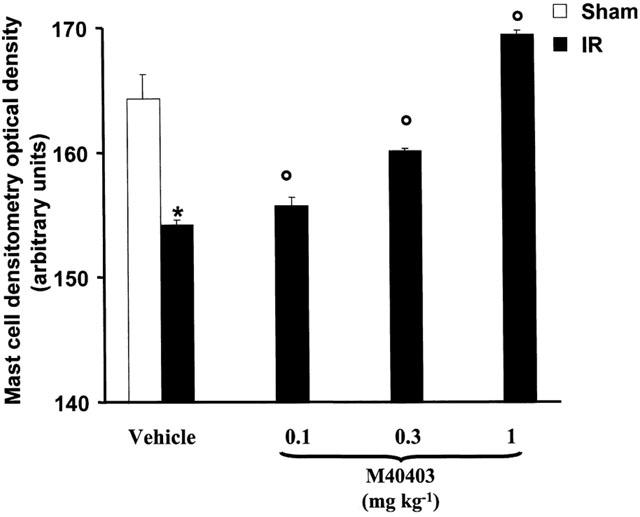
Mast cell densitometry evaluated as light transmittance across left ventricular mast cells. Compared with sham-operated heart (group 2), the ischaemic-reperfused hearts show a significant increase in light transmittance indicating a decrease in intracellularly secretory granules. This effect is dose dependently reverted by M40403 treatments. Values are mean±s.e.m. of eight experiments. P<0.001 vs sham operated hearts, P<0.05 one-way ANOVA.
Biochemical studies
The results of the biochemical assays for the markers of ischaemia-reperfusion-induced myocardial injury showed that in sham operated rats malonyldialdehyde (MDA) production, an index of peroxidation of cell membrane lipids and calcium content, was very low. In the ischaemic-reperfused hearts, MDA production, and calcium content were significantly elevated (Figures 4 and 5). These parameters were significantly reduced in a dose dependent manner by M40403 (Figures 4 and 5).
Figure 4.

Reperfusion of the ischaemic myocardium leads to enhanced release of calcium which was inhibited in a dose dependent manner by M40403 (0.1–1 mg kg−1). Values are mean±s.e.m. of eight experiments. *n.s.; °P<0.01 one-way ANOVA.
Figure 5.
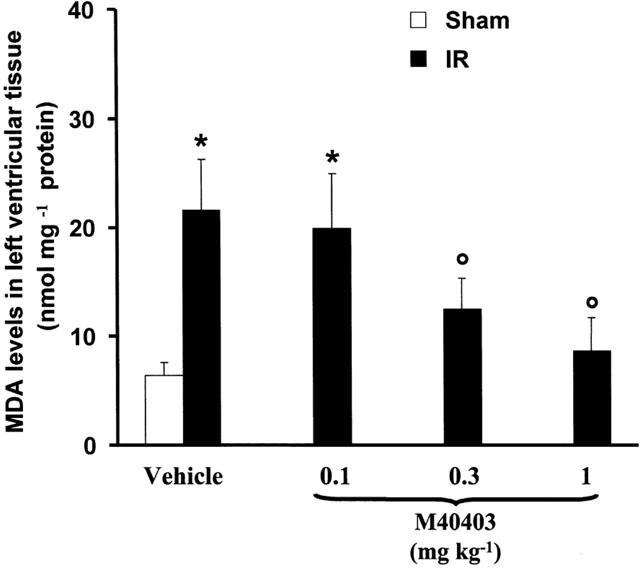
Reperfusion of the ischaemic myocardium leads to enhanced release of calcium which was inhibited in a dose dependent manner by M40403 (0.1–1 mg kg−1). Values are mean±s.e.m. of eight experiments. *n.s.; °P<0.01 one-way ANOVA.
Immunohistology measurements of nitrotyrosine
In sham-operated hearts, little if any positive staining for nitrotyrosine was observed (Figure 6A). In the post-ischaemic myocardium, however, strong positive staining for nitrotyrosine was observed within myocytes (Figure 6B). M40403 treatment (1 mg kg−1) reduced the degree of immunostaining for nitrotyrosine (Figure 6C) in the reperfused heart.
Figure 6.
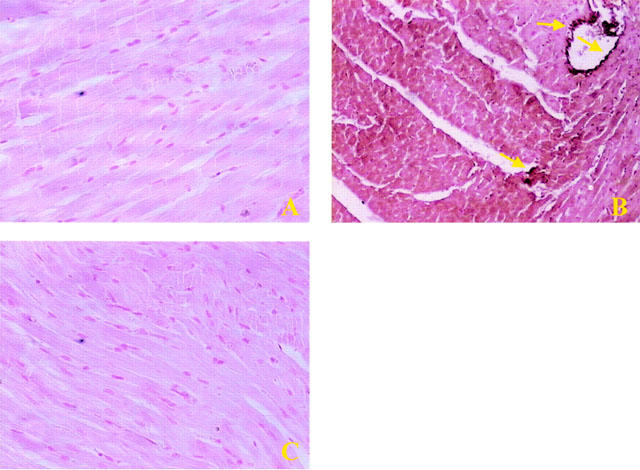
No positive staining for nitrotyrosine (A) was found in the left ventricular section from sham-operated rats. Sixty minutes after reperfusion immunohistochemical analysis for nitrotyrosine (B) show positive staining localized in the vascular wall (see arrows) and in the cardiomyocyte in the injured area. The intensity of the positive staining for nitrotyrosine (C) was significantly reduced in the left ventricular section from M40403-treated rats. Original magnification: ×150. Figure is representative of at least three experiments performed on different experimental days.
Histological changes, myeloperoxidase activity and immunohistochemical localization of ICAM-1 and P-selectin
At 60 min after reperfusion, neutrophil infiltration and activation of vascular endothelial cells were assessed by the measurement of tissue myeloperoxidase activity and by ICAM-1 and P-selectin immunostaining. As shown in Figure 7, myeloperoxidase activity significantly increased (P<0.001) in left ventricular tissue of ischaemic-reperfused rats. Histological examination of cardiac tissue (see representative sections in Figure 8) revealed pathological changes that correlated closely with the increase in myeloperoxidase activity. In particular, in sham-operated hearts, the myocardium had a normal appearance without intracellular oedema or blood vessel dilation and neutrophils adherent to the coronary endothelium or in the intramuscular connective tissue (Figure 8A). In the ischaemic-reperfused hearts, neutrophil infiltration was observed (Figure 8B). Intracellular oedema was also seen (Figure 8B). In contrast, in the ischaemic-reperfused hearts from rats treated with M40403, (1 mg kg−1), neutrophil infiltration was an occasional finding and the increase in myeloperoxidase activity induced by ischaemia-reperfusion was significantly reduced (P<0.01) (Figure 7). Overall, organ injury was significantly attenuated (Figure 8C).
Figure 7.
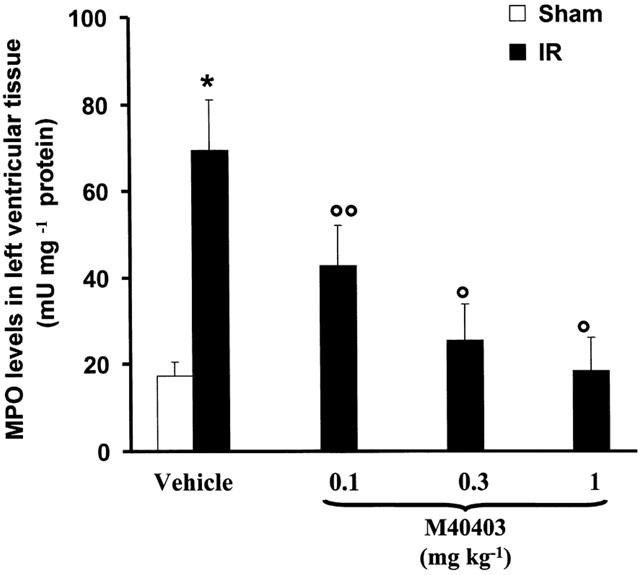
Reperfusion of the ischaemic myocardium leads to neutrophil recruitment into the myocardium (as evidenced by increased MPO levels). Neutrophil recruitment was in turn inhibited in a dose dependent manner by M40403 (0.1–1 mg kg−1). Values are mean±s.e.m. of eight experiments. *n.s.; °P<0.01 one-way ANOVA.
Figure 8.
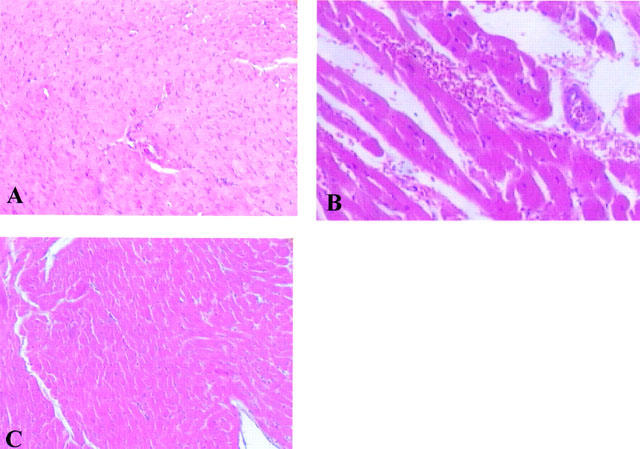
Left ventricular section from sham rats demonstrating the normal architecture of the myocardiac tissue (A). In the ischaemic-reperfused hearts, a neutrophil infiltration was observed (B). Intracellular oedema was also seen. Myocardium from M40403-treated rats shows reduced tissue injury (C). Original magnification: ×150 (for A and C)×300 (for B). Figure is representative of at least three experiments performed on different experimental days.
Staining of cardiac tissue sections obtained from sham-operated rats with anti-ICAM-1 antibody showed a specific staining along vessels (arrow), demonstrating that ICAM-1 is constitutively expressed (Figure 9A). After 1 h of reperfusion, the staining intensity substantially increased in the vessels (Figure 9B; see arrows). Sections from M40403-treated rats did not reveal any up-regulation of the constitutive ICAM-1, which was normally expressed in the endothelium along the vascular wall (Figure 9C; see arrow). Left ventricular tissue sections obtained from rats undergoing 30 min of ischaemia followed by 1 h reperfusion showed positive staining for P-selectin localized in the vessels (Figure 9E; see arrows). No staining was observed in sham-operated rats (Figure 9D) and in tissue obtained at 1 h after reperfusion from M40403-treated rats (Figure 9F). To verify the binding specificity for ICAM-1 or P-selectin, some sections were also incubated with only the primary antibody (no secondary) or with only the secondary antibody (no primary). In these situations no positive staining was found in the sections, indicating that the immunoreaction was positive in all the experiments carried out.
Figure 9.
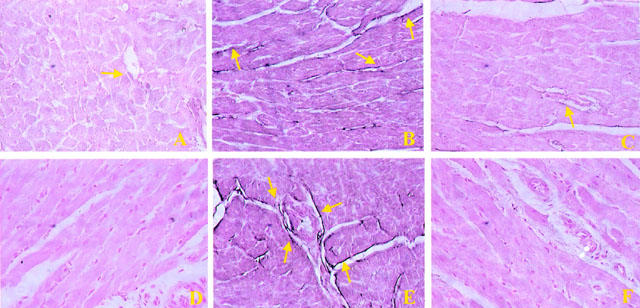
Staining of left ventricular section obtained from sham-operated rats with anti-ICAM-1 antibody showed a specific staining along vessels (arrow), demonstrating that ICAM-1 is constitutively expressed (A), no P-selectin staining was seen in sham animals. (D). Section obtained from IR shocked-rats showed intense positive staining (see arrows) for ICAM-1 (B) and for P-selectin (E) on vascular wall. The degree of endothelial staining for ICAM-1 (C) and for P-selectin (F) was markedly reduced in tissue section obtained from M40403-treated rats. Original magnification:×150. Figure is representative of at least three experiments performed on different experimental days.
Biochemical parameters in hearts taken from animals that underwent ischaemia alone
No increase in levels of MPO activity, MDA production and calcium contents were seen in tissues obtained from animals that underwent a period of ischaemia (30 min) alone (group 7, data not shown).
Discussion
The results of this study show that, M40403 exerts a significant cardioprotective effect in the ischaemic and reperfused rat heart. The biochemical and histological alterations observed in this study were dependent upon reperfusion of the ischaemic myocardium since no alterations were observed in hearts that underwent a period of ischaemia alone. This is consistent with numerous reports that indicate that reperfusion is the key trigger of many of the events that lead to myocardial dysfunction associated with ischaemia and reperfusion injury (Braunwald & Kloner, 1985). Superoxide anions are in fact formed in excess during reperfusion of the ischaemic organ and not during the ischaemic phase (Cuzzocrea et al., 1997; 2001a, b; Salvemini et al., 1999a, b). M40403 exerts its beneficial effects by removing superoxide generated during reperfusion (Salvemini et al., 1999a, b).
Systemic administration of M40403 results in a marked reduction of the myocardial areas damaged by post-ischaemic reperfusion as well as in a substantial reduction in the occurrence of severe ventricular arrhythmias. The current study also shows that M40403 acts at multiple levels in the cascade of events that lead to myocardial injury. These events include endothelial dysfunction, up-regulation of adhesion molecules such as ICAM-1 and P-selectin; (Geng et al., 1990; Dreyer et al., 1991; Lawrence & Springer, 1991; Butcher, 1992) neutrophil accumulation in the myocardium (Smith III et al., 1988; Reynolds & McDonagh, 1989; Bani et al., 1998), lipid peroxidation (Bani et al., 1998), platelet and mast cell activation (Keller et al., 1988; May et al., 1991; Bani et al., 1998), generation of peroxynitrite (Matheis et al., 1992; Naseem et al., 1995; Schulz & Wambolt, 1995; Wang & Zweier, 1996) and calcium overload that eventually leads to myocardial cell damage and death (Duncan et al., 1992).
M40403 decreased the recruitment of neutrophils from the circulation to the myocardium, as indicated by the decrease in myeloperoxidase activity, a marker of neutrophil accumulation in tissues (Mullane et al., 1985). This effect was confirmed histologically. Endothelial cells appear to be one of the major regulators of neutrophil traffic, regulating the process of neutrophil chemoattraction, adhesion, and migration into the tissue. P-selectin is rapidly released during the early phase of reperfusion, after exposure to certain stimuli, such as histamine, or complement, allowing the leukocytes to roll along the endothelium (Geng et al., 1990; Lorant et al., 1991; Patel et al., 1991; Lorant et al., 1993). ICAM-1, which is constitutively expressed on the surface of endothelial cells, is then involved in neutrophil adhesion (Wertheimer et al., 1992; Farhood et al., 1995). Reperfusion of the ischaemic tissue induces the appearance of P-selectin on the endothelial vascular wall and up-regulates the expression of ICAM-1. ICAM-1 and P-selectin expression was attenuated by M40403 providing an explanation as to the possible mechanism through which M40403 blocks neutrophil infiltration.
Moreover, inhibition of neutrophil infiltration by M40403 correlated well with an inhibition of lipid peroxidation (as measured by MDA) supporting the notion that release of superoxide from activated neutrophils is responsible at least in part to peroxidation of lipid membranes in the myocardium (Rao et al., 1983).
In cardiac ischaemia and reperfusion injury, superoxide, produced during reperfusion, rapidly reacts with nitric oxide to form peroxynitrite (Matheis et al., 1992; Naseem et al., 1995; Schulz & Wambolt, 1995). A possible and most likely mechanism by which M40403 reduces ischaemia-reperfusion injury is by reducing peroxynitrite formation by simply removing superoxide before it reacts with nitric oxide. This has two fundamentally important consequences: sparing of the beneficial nitric oxide and removal of toxic peroxynitrite. The release of nitric oxide from the constitutive enzyme has numerous beneficial and cytoprotective roles in the heart (Gaballa & Goldman, 1999; Gaballa et al., 1999) and nitric oxide donors exert beneficial effects in myocardial ischaemia and reperfusion injury (Johnson III et al., 1991). Peroxynitrite has several pro-inflammatory and cytotoxic effects (Squadrito et al., 1995) and its removal by agents such as FeTMPS, a porphyrin-containing molecule which increases the rate of isomerization of peroxynitrite to nitrate (Stern et al., 1996) is cytoprotective and anti-inflammatory (Stern et al., 1996; Salvemini et al., 1996; Misko et al., 1998; Cuzzocrea et al., 2000). Furthermore, peroxynitrite has been implicated in myocardial ischaemia-reperfusion injury (Matheis et al., 1992; Naseem et al., 1995; Schulz & Wambolt, 1995). Peroxynitrite also nitrates tyrosine residues in proteins and nitrotyrosine formation, detected by immunofluorescence, has been used as a marker of endogenous formation of peroxynitrite (Beckman, 1996) or as an indicator of ‘increased nitrosative stress' (Eiserich et al., 1998). We have found that nitrotyrosine is indeed present in heart sections taken after ischaemia and reperfusion (but not in sections that underwent ischaemia alone) and that M40403 reduced the staining in these tissues. Based on these findings, we conclude that the reperfusion of ischaemic myocardium evoked, at least in part, a superoxide-driven peroxynitrite formation that was, in turn responsible for the formation of nitrotyrosine.
Superoxide and peroxynitrite can also cause DNA single-strand damage that is the obligatory trigger for PARP activation (Inoue & Kawanishi, 1995; Salgo et al., 1995), which ultimately leads to cell injury (Docherty et al., 1999). Furthermore, recent studies have demonstrated the PARP−/− mice are significantly protected from myocardial ischaemia when compared with PARP+/+ mice (Zingarelli et al., 1998; Yang et al., 2000).
Calcium leakage occurs during reperfusion (Masini et al., 1989a, b) and a loss of calcium homeostasis with an excess calcium influx is thought to be a critical event underlying irreversible myocardial injury (Hayashi, 2000). M40403, was found in these studies to prevent calcium overload in the myocardial tissue and to strongly reduce myocyte alterations like those previously described in conditions of excess intracellular calcium (Bani et al., 1998).
An additional contribution to the cardioprotective effect of M40403 against ischaemia-reperfusion injury may rely on its effect on cardiac mast cells. In fact, resident mast cells undergo degranulation during post-ischaemic reoxygenation of the heart, releasing powerful mediators, such as histamine, serotonin and leukotrienes (Keller et al., 1988; Masini et al., 1990). These substances can affect vascular resistance and permeability, thus increasing tissue oedema, up-regulate adhesion molecules and induce cardiac arrhythmias (Wolff & Levi, 1986; Bani et al., 1998; Masini et al., 1999). The results of the current study show that M40403, inhibits ischaemia-reperfusion-induced mast cell degranulation and hence the release of granule-stored mediators, including histamine, thus contributing to the myocardial salvage. Our own studies had shown that superoxide anion is able to evoke mast cell degranulation and subsequent histamine release (Mannaioni & Masini, 1988).
The protective effects of M40403 were supported by histological examination and accompanied by overall improved survival rate.
In conclusion, the results of the present study clearly indicate that superoxide anion generated during the reperfusion phase plays a critical role in the development of cardiac reperfusion injury. M40403, a low molecular weight SOD mimetic, which removed superoxide anion selectively without interfering with other reactive oxygen species, may offer a novel therapeutic approach for the treatment of ischaemic heart disease.
Acknowledgments
We would like to thank Dr Mark R. Payne, Department of Pediatric Cardiology, Wake Forest University School of Medicine, Winston-Salem, NC, U.S.A. for useful discussions and critical evaluation of this manuscript.
Abbreviations
- ICAM-1
intracellular adhesion molecules
- IR
ischaemia-reperfusion
- MDA
malonyldialdehyde
- MPO
myeloperoxidase
- O2−
superoxide anion
- ONOO−
peroxynitrite
- SODm
superoxide dismutase mimetic
References
- ALDERTON W.K., COOPER C.E., KNOWLES R.G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMBROSIO G., FLAHERTY J.T. Effects of the superoxide radical scavenger superoxide dismutase, and of the hydroxyl radical scavenger mannitol, on reperfusion injury in isolated rabbit hearts. Cardiovasc. Drugs Ther. 1992;6:623–632. doi: 10.1007/BF00052564. [DOI] [PubMed] [Google Scholar]
- ARUOMA O.I., HALLIWELL B., LAUGHTON M.J., QUINLAN G.J., GUTTERIDGE J.M. The mechanism of initiation of lipid peroxidation. Evidence against a requirement for an iron(II)-iron(III) complex. Biochem. J. 1989;258:617–620. doi: 10.1042/bj2580617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASTON K., RATH N., NAIK A., SLOMCZYMSKA U., SCHALL O.F., RILEY D.P. Computer-aided design (CAD) of Mn(II) complexes: superoxide dismutase mimetics with catalytic activity exceeding the native enzyme. Inorg. Chem. 2001;40:1779–1789. doi: 10.1021/ic000958v. [DOI] [PubMed] [Google Scholar]
- BANI D., MASINI E., BELLO M.G., BIGAZZI M., SACCHI T.B. Relaxin protects against myocardial injury caused by ischemia and reperfusion in rat heart. Am. J. Pathol. 1998;152:1367–1376. [PMC free article] [PubMed] [Google Scholar]
- BECKMAN J.S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., BECKMAN T.W., CHEN J., MARSHALL P.A., FREEMAN B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKMAN J.S., CROW J.P. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem. Soc. Trans. 1993;21:330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- BIANCHI S., MUGNAI L. Mast cell fixation and staining in image analysis. Eur. J. Basic Appl. Histochem. 1991;35:161–174. [PubMed] [Google Scholar]
- BOUGHTON-SMITH N.K., EVANS S.M., LASZLO F., WHITTLE B.J., MONCADA S. The induction of nitric oxide synthase and intestinal vascular permeability by endotoxin in the rat. Br. J. Pharmacol. 1993;110:1189–1195. doi: 10.1111/j.1476-5381.1993.tb13940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOURDILLON P.D., POOLE-WILSON P.A. Effects of ischemia and reperfusion on calcium exchange and mechanical function in isolated rabbit myocardium. Cardiovasc. Res. 1981;15:121–130. doi: 10.1093/cvr/15.3.121. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRADLEY P.P., PRIEBAT D.A., CHRISTENSEN R.D., ROTHSTEIN G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- BRAUNWALD E., KLONER R. Myocardial reperfusion: a double edged sword. J. Clin. Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K.P. Superoxide dismutase enhances recovery following myocardial ischemia. Am. J. Physiol. 1985;248:H637–H643. doi: 10.1152/ajpheart.1985.248.5.H637. [DOI] [PubMed] [Google Scholar]
- BUTCHER E.C. Leukocyte-endothelial cell adhesion as an active, multi-step process: a combinatorial mechanism for specificity and diversity in leukocyte targeting. Adv. Exp. Med. Biol. 1992;323:181–194. doi: 10.1007/978-1-4615-3396-2_23. [DOI] [PubMed] [Google Scholar]
- CARLSSON L.M., JONSSON J., EDLUND T., MARKLUND S.L. Mice lacking extracellular superoxide dismutase are more sensitive to Hyperoxia. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Z., OBERLEY T.D., HO Y., CHUA C.C., SIU B., HAMDY R.C., EPSTEIN C.J., CHUA B.H. Overexpression of CuZnSOD in coronary vascular cells attenuates myocardial ischemia/reperfusion injury. Free Radic. Biol. Med. 2000;29:589–596. doi: 10.1016/s0891-5849(00)00363-4. [DOI] [PubMed] [Google Scholar]
- CHEN Z., SIU B., HO Y.S., VINCENT R., CHUA C.C., HAMDY R.C., CHUA B.H. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J. Mol. Cell Cardiol. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- CLARK W.M., LAUTEN J.D., LESSOV N., WOODWARD W., COULL B.M. Time course of ICAM-1 expression and leukocyte subset infiltration in rat forebrain ischemia. Mol. Chem. Neuropathol. 1995;26:213–230. doi: 10.1007/BF02815139. [DOI] [PubMed] [Google Scholar]
- CROW J.P., BECKMAN J.S. Reactions between nitric oxide, superoxide, and peroxynitrite: footprints of peroxynitrite in vivo. Adv. Pharmacol. 1995;34:17–43. doi: 10.1016/s1054-3589(08)61079-0. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., MAZZON E., DUGO L., CAPUTI A.P., ASTON K., RILEY D.P., SALVEMINI D. Protective effects of a new stable, highly active SOD mimetic, M40401 in splanchnic artery occlusion and reperfusion. Br. J. Pharmacol. 2001a;132:19–29. doi: 10.1038/sj.bjp.0703775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUZZOCREA S., MISKO T.P., COSTANTINO G., MAZZON E., MICALI A., CAPUTI A.P., MACARTHUR H., SALVEMINI D. Beneficial effects of peroxynitrite decomposition catalyst in a rat model of splanchnic artery occlusion and reperfusion. FASEB J. 2000;14:1061–1072. doi: 10.1096/fasebj.14.9.1061. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., RILEY D.P., CAPUTI A.P., SALVEMINI D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 2001b;53:135–159. [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., COSTANTINO G., SZABO A., SALZMAN A.L., CAPUTI A.P., SZABO C. Beneficial effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase in a rat model of splanchnic artery occlusion and reperfusion. Br. J. Pharmacol. 1997;121:1065–1074. doi: 10.1038/sj.bjp.0701234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEITCH E.A., BRIDGES W., BERG R., SPECIAN R.D., GRANGER D.N. Hemorrhagic shock-induced bacterial translocation: the role of neutrophils and hydroxyl radicals. J. Trauma. 1990;30:942–951. doi: 10.1097/00005373-199008000-00002. [DOI] [PubMed] [Google Scholar]
- DIX T.A., HESS K.M., MEDINA M.A., SULLIVAN R.W., TILLY S.L., WEBB T.L. Mechanism of site-selective DNA nicking by the hydrodioxyl (perhydroxyl) radical. Biochemistry. 1996;35:4578–4583. doi: 10.1021/bi952010w. [DOI] [PubMed] [Google Scholar]
- DOCHERTY J.C., KUZIO B., SILVESTER J.A., BOWES J., THIEMERMANN C. An inhibitor of poly (ADP-ribose) synthetase activity reduces contractile dysfunction and preserves high energy phosphate levels during reperfusion of the ischaemic rat heart. Br. J. Pharmacol. 1999;127:1518–1524. doi: 10.1038/sj.bjp.0702705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREYER W.J., MICHAEL L.H., WEST M.S., SMITH C.W., ROTHLEIN R., ROSSEN R.D., ANDERSON D.C., ENTMAN M.L. Neutrophil accumulation in ischemic canine myocardium. Insights into time course, distribution, and mechanism of localization during early reperfusion. Circulation. 1991;84:400–411. doi: 10.1161/01.cir.84.1.400. [DOI] [PubMed] [Google Scholar]
- DUNCAN E., ONODERA T., ASHRAF M. Production of hydroxyl radicals and their disassociation from myocardial cell injury during calcium paradox. Free Radic. Biol. Med. 1992;12:11–18. doi: 10.1016/0891-5849(92)90053-j. [DOI] [PubMed] [Google Scholar]
- EISERICH J.P., HRISTOVA M., CROSS C.E., JONES A.D., FREEMAN B.A., HALLIWELL B., VAN D.V. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- FANTONE J.C., WARD P.A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am. J. Pathol. 1982;107:395–418. [PMC free article] [PubMed] [Google Scholar]
- FARHOOD A., MCGUIRE G.M., MANNING A.M., MIYASAKA M., SMITH C.W., JAESCHKE H. Intercellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. J. Leukoc. Biol. 1995;57:368–374. [PubMed] [Google Scholar]
- FRIDOVICH I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- FUJIMOTO T., PETER T., HAMAMOTO H., MANDEL W.J. Electrophysiologic observations on ventricular tachyarrhythmias following reperfusion. Am. Heart J. 1983;105:201–209. doi: 10.1016/0002-8703(83)90514-8. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F., ZAWADZKI J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GABALLA M.A., GOLDMAN S. Overexpression of endothelium nitric oxide synthase reverses the diminished vasorelaxation in the hindlimb vasculature in ischemic heart failure in vivo. J. Mol. Cell Cardiol. 1999;31:1243–1252. doi: 10.1006/jmcc.1999.0956. [DOI] [PubMed] [Google Scholar]
- GABALLA M.A., RAYA T.E., HOOVER C.A., GOLDMAN S. Effects of endothelial and inducible nitric oxide synthases inhibition on circulatory function in rats after myocardial infarction. Cardiovasc. Res. 1999;42:627–635. doi: 10.1016/s0008-6363(98)00343-5. [DOI] [PubMed] [Google Scholar]
- GAUTHIER T.W., DAVENPECK K.L., LEFER A.M. Nitric oxide attenuates leukocyte-endothelial interaction via P-selectin in splanchnic ischemia-reperfusion. Am. J. Physiol. 1994;267:G562–G568. doi: 10.1152/ajpgi.1994.267.4.G562. [DOI] [PubMed] [Google Scholar]
- GELVAN D., SALTMAN P., POWELL S.R. Cardiac reperfusion damage prevented by a nitroxide free radical. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4680–4684. doi: 10.1073/pnas.88.11.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENG J.G., BEVILACQUA M.P., MOORE K.L., MCINTYRE T.M., PRESCOTT S.M., KIM J.M., BLISS G.A., ZIMMERMAN G.A., MCEVER R.P. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990;343:757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- GOOSSENS D.P., RAO V.K., HARMS B.A., STARLING J.R. Superoxide dismutase and catalase in skin flaps during venous occlusion and reperfusion. Ann. Plast. Surg. 1990;25:21–25. doi: 10.1097/00000637-199007000-00005. [DOI] [PubMed] [Google Scholar]
- GRILL H.P., ZWEIER J.L., KUPPUSAMY P., WEISFELDT M.L., FLAHERTY J.T. Direct measurement of myocardial free radical generation in an in vivo model: effects of postischemic reperfusion and treatment with human recombinant superoxide dismutase. J. Am. Coll. Cardiol. 1992;20:1604–1611. doi: 10.1016/0735-1097(92)90457-x. [DOI] [PubMed] [Google Scholar]
- GRYGLEWSKI R.J., PALMER R.M., MONCADA S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- HANGAISHI M., NAKAJIMA H., TAGUCHI J., IGARASHI R., HOSHINO J., KUROKAWA K., KIMURA S., NAGAI R., OHNO M. Lecithinized Cu, Zn-superoxide dismutase limits the infarct size following ischemia-reperfusion injury in rat hearts in vivo. Biochem. Biophys. Res. Commun. 2001;285:1220–1225. doi: 10.1006/bbrc.2001.5319. [DOI] [PubMed] [Google Scholar]
- HARDY M.M., FLICKINGER A.G., RILEY D.P., WEISS R.H., RYAN U.S. Superoxide dismutase mimetics inhibit neutrophil-mediated human aortic endothelial cell injury in vitro. J. Biol. Chem. 1994;269:18535–18540. [PubMed] [Google Scholar]
- HARLAN J.M. Leukocyte-endothelial interactions. Blood. 1985;65:513–525. [PubMed] [Google Scholar]
- HAYASHI H. Pathogenesis and the role of Ca2+ overload during myocardial ischemia/reperfusion. Nagoya J. Med. Sci. 2000;63:91–98. [PubMed] [Google Scholar]
- INOUE S., KAWANISHI S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett. 1995;371:86–88. doi: 10.1016/0014-5793(95)00873-8. [DOI] [PubMed] [Google Scholar]
- ISCHIROPOULOS H., ZHU L., CHEN J., TSAI M., MARTIN J.C., SMITH C.D., BECKMAN J.S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- JOHNSON G., III, TSAO P.S., LEFER A.M. Cardioprotective effects of authentic nitric oxide in myocardial ischemia with reperfusion. Crit. Care Med. 1991;19:244–252. doi: 10.1097/00003246-199102000-00021. [DOI] [PubMed] [Google Scholar]
- KELLER A.M., CLANCY R.M., BARR M.L., MARBOE C.C., CANNON P.J. Acute reoxygenation injury in the isolated rat heart: role of resident cardiac mast cells. Circ. Res. 1988;63:1044–1052. doi: 10.1161/01.res.63.6.1044. [DOI] [PubMed] [Google Scholar]
- KOO D.D., WELSH K.I., WEST N.E., CHANNON K.M., PENINGTON A.J., ROAKE J.A., MORRIS P.J., FUGGLE S.V. Endothelial cell protection against ischemia/reperfusion injury by lecithinized superoxide dismutase. Kidney Int. 2001;60:786–796. doi: 10.1046/j.1523-1755.2001.060002786.x. [DOI] [PubMed] [Google Scholar]
- LAWRENCE M.B., SPRINGER T.A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- LEBOVITZ R.M., ZHANG H., VOGEL H., CARTWRIGHT J., JR, DIONNE L., LU N., HUANG S., MATZUK M.M. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFER A.M., LEFER D.J. Pharmacology of the endothelium in ischemia-reperfusion and circulatory shock. Annu. Rev. Pharmacol. Toxicol. 1993;33:71–90. doi: 10.1146/annurev.pa.33.040193.000443. [DOI] [PubMed] [Google Scholar]
- LEFER A.M., TSAO P.S., LEFER D.J., MA X.L. Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J. 1991;5:2029–2034. doi: 10.1096/fasebj.5.7.2010056. [DOI] [PubMed] [Google Scholar]
- LI Q., BOLLI R., QIU Y., TANG X.L., GUO Y., FRENCH B.A. Gene therapy with extracellular superoxide dismutase protects conscious rabbits against myocardial infarction. Circulation. 2001;103:1893–1898. doi: 10.1161/01.cir.103.14.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORANT D.E., PATEL K.D., MCINTYRE T.M., MCEVER R.P., PRESCOTT S.M., ZIMMERMAN G.A. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: a juxtacrine system for adhesion and activation of neutrophils. J. Cell Biol. 1991;115:223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORANT D.E., TOPHAM M.K., WHATLEY R.E., MCEVER R.P., MCINTYRE T.M., PRESCOTT S.M., ZIMMERMAN G.A. Inflammatory roles of P-selectin. J. Clin. Invest. 1993;92:559–570. doi: 10.1172/JCI116623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA X.L., JOHNSON G., III, LEFER A.M. Low doses of superoxide dismutase and a stable prostacyclin analogue protect in myocardial ischemia and reperfusion. J. Am. Coll. Cardiol. 1992;19:197–204. doi: 10.1016/0735-1097(92)90073-v. [DOI] [PubMed] [Google Scholar]
- MANNAIONI P.F., MASINI E. The release of histamine by free radicals. Free Radic. Biol. Med. 1988;5:177–197. doi: 10.1016/0891-5849(88)90080-9. [DOI] [PubMed] [Google Scholar]
- MANNAIONI P.F., MASINI E., PISTELLI A., SALVEMINI D., VANE J.R. Mast cells as a source of superoxide anions and nitric oxide-like factor: relevance to histamine release. Int. J. Tiss. Reac. 1991;13:271–278. [PubMed] [Google Scholar]
- MASINI E., BANI D., BELLO M.G., BIGAZZI M., MANNAIONI P.F., SACCHI T.B. Relaxin counteracts myocardial damage induced by ischemia-reperfusion in isolated guinea pig hearts: evidence for an involvement of nitric oxide. Endocrinology. 1997;138:4713–4720. doi: 10.1210/endo.138.11.5520. [DOI] [PubMed] [Google Scholar]
- MASINI E., BANI D., SARDI I., BARONTI R., BANI-SACCHI T., BIGAZZI M., MANNAIONI P.F. Dual role of nitric oxide in myocardial ischemia-reperfusion. Inflamm. Res. 2000;49 Suppl 1:S78–S79. doi: 10.1007/PL00000194. [DOI] [PubMed] [Google Scholar]
- MASINI E., BIANCHI S., GAMBASSI F., PALMERANI B., PISTELLI A., CARLOMAGNO L., MANNAIONI P.F. Ischemia reperfusion injury and histamine release in isolated and perfused guinea-pig heart: pharmacological interventions. Agents Actions. 1990;30:198–201. doi: 10.1007/BF01969037. [DOI] [PubMed] [Google Scholar]
- MASINI E., GAMBASSI F., GIANNELLA E., PALMERANI B., PISTELLI A., CARLOMAGNO L., MANNAIONI P.F. Ischemia-reperfusion injury and histamine release in isolated guinea-pig heart: the role of free radicals. Agents Actions. 1989a;27:154–157. doi: 10.1007/BF02222225. [DOI] [PubMed] [Google Scholar]
- MASINI E., GIANNELLA E., PALMERANI B., PISTELLI A., GAMBASSI F., MANNAIONI P.F. Free radicals induce ischaemia-reperfusion injury and histamine release in the isolated guinea pig heart. Int. Arch. Allergy Appl. Immunol. 1989b;88:132–133. doi: 10.1159/000234765. [DOI] [PubMed] [Google Scholar]
- MASINI E., SALVEMINI D., NDISANG J.F., GAI P., BERNI L., MONCINI M., BIANCHI S., MANNAIONI P.F. Cardioprotective activity of endogenous and exogenous nitric oxide on ischaemia reperfusion injury in isolated guinea pig hearts. Inflamm. Res. 1999;48:561–568. doi: 10.1007/s000110050504. [DOI] [PubMed] [Google Scholar]
- MATHEIS G., SHERMAN M.P., BUCKBERG G.D., HAYBRON D.M., YOUNG H.H., IGNARRO L.J. Role of L-arginine-nitric oxide pathway in myocardial reoxygenation injury. Am. J. Physiol. 1992;262:H616–H620. doi: 10.1152/ajpheart.1992.262.2.H616. [DOI] [PubMed] [Google Scholar]
- MAY G.R., CROOK P., MOORE P.K., PAGE C.P. The role of nitric oxide as an endogenous regulator of platelet and neutrophil activation within the pulmonary circulation of the rabbit. Br. J. Pharmacol. 1991;102:759–763. doi: 10.1111/j.1476-5381.1991.tb12246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCORD J.M. Oxygen-derived free radicals in postischemic tissue injury. N. Engl. J. Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- MELOV S., COSKUN P., PATEL M., TUINSTRA R., COTTRELL B., JUN A.S., ZASTAWAY T.H., DIZDAROGLU M., GOODMAN S.I., HUANG T.T., MIZIORKO H., EPSTEIN C.J., WALLACE D.C. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:846–851. doi: 10.1073/pnas.96.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER M.J.S., SADOWSKA-KROWICKA H., CHOTINARUENOL S., KAKKIS J.L., CLARK D.A. Amelioration of chronic ileitis by nitric oxide synthase inhibition. J. Pharmacol. Exp. Ther. 1993;264:11–16. [PubMed] [Google Scholar]
- MISKO T.P., HIGHKIN M.K., VEENHUIZEN A.W., MANNING P.T., STERN M.K., CURRIE M.G., SALVEMINI D. Characterization of the cytoprotective action of peroxynitrite decomposition catalysts. J. Biol. Chem. 1998;273:15646–15653. doi: 10.1074/jbc.273.25.15646. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M., HIGGS E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MORPURGO E., CADROBBI R., MORPURGO M., RIGOTTI P., SCHIAVON F., SCHIAVON O., CALICETI P., ANCONA E., VERONESE F.M. Protective effect of superoxide dismutase and polyethylene glycol-linked superoxide dismutase against renal warm ischemia/reperfusion injury. Transplantation. 1996;62:1221–1223. doi: 10.1097/00007890-199611150-00006. [DOI] [PubMed] [Google Scholar]
- MULLANE K.M., KRAEMER R., SMITH B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J. Pharmacol. Methods. 1985;14:157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- NASEEM S.A., KONTOS M.C., RAO P.S., JESSE R.L., HESS M.L., KUKREJA R.C. Sustained inhibition of nitric oxide by NG-nitro-L-arginine improves myocardial function following ischemia/reperfusion in isolated perfused rat heart. J. Mol. Cell Cardiol. 1995;27:419–426. doi: 10.1016/s0022-2828(08)80038-7. [DOI] [PubMed] [Google Scholar]
- OKAYAMA N., GRISHAM M.B., KEVIL C.G., EPPIHIMER L.A., WINK D.A., ALEXANDER J.S. Effect of reactive oxygen metabolites on endothelial permeability: role of nitric oxide and iron. Microcirculation. 1999;6:107–116. [PubMed] [Google Scholar]
- PATEL K.D., ZIMMERMAN G.A., PRESCOTT S.M., MCEVER R.P., MCINTYRE T.M. Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J. Cell Biol. 1991;112:749–759. doi: 10.1083/jcb.112.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATEL M., DAY B.J. Metalloporphyrin class of therapeutic catalytic antioxidants. Trends Pharmacol. Sci. 1999;20:359–364. doi: 10.1016/s0165-6147(99)01336-x. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M., MONCADA S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl. Acad. Sci. U.S.A. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAO P.S., COHEN M.V., MUELLER H.S. Production of free radicals and lipid peroxides in early experimental myocardial ischemia. J. Mol. Cell Cardiol. 1983;15:713–716. doi: 10.1016/0022-2828(83)90260-2. [DOI] [PubMed] [Google Scholar]
- RATYCH R.E., CHUKNYISKA R.S., BULKLEY G.B. The primary localization of free radical generation after anoxia/reoxygenation in isolated endothelial cells. Surgery. 1987;102:122–131. [PubMed] [Google Scholar]
- REAUME A.G., ELLIOTT J.T., HOFFMAN E.K., KOWALL N.W., FERRANTE R.J., SIWEK D.F., WILCOX H.M., FLOOD D.G., BIAL M.F., BROWN JR, R.H., SCOTT R.W., SNIDER W.D. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- REYNOLDS J.M., MCDONAGH P.F. Early in reperfusion, leukocytes alter perfused coronary capillary and vascular resistance. Am. J. Physiol. 1989;256:H982–H989. doi: 10.1152/ajpheart.1989.256.4.H982. [DOI] [PubMed] [Google Scholar]
- RILEY D.P., HENKE S.L., LENNON P.J., WEISS R.H., NEUMANN W.L., RIVERS W.J., ASTON K.W., SAMPLE K.R., RAHMAN H., LING C.S., SHIEH J.L., BUSCH D.H. Synthesis, characterization and stability of Manganese (II) C-substituted 1,4,7,10,13,-Pentaazacyclopentadecane complexes exhibiting superoxide dismutase activity. Inorg. Chem. 1996;35:5213–5231. [Google Scholar]
- SALGO M.G., BERMUDEZ E., SQUADRITO G.L., PRYOR W.A. Peroxynitrite causes DNA damage and oxidation of thiols in rat thymocytes [corrected] Arch. Biochem. Biophys. 1995;322:500–505. doi: 10.1006/abbi.1995.1493. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., JENSEN M.P., RILEY D.P., MISKO T.P. Therapeutic manipulations of peroxynitrite. Drug News and Perspectives. 1998;11:204–214. [PubMed] [Google Scholar]
- SALVEMINI D., MISKO T.P., MASFERRER J.L., SEIBERT K., CURRIE M.G., NEEDLEMAN P. Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., RILEY D.P., LENNON P.J., WANG Z.Q., CURRIE M.G., MACARTHUR H., MISKO. T.P. Protective effects of a superoxide dismutase mimetic and peroxynitrite decomposition catalysts in endotoxin-induced intestinal damage. Br. J. Pharmacol. 1999a;127:685–692. doi: 10.1038/sj.bjp.0702604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., WYATT P.S., BOURDON D.M., MARINO M.H., MANNING P.T., CURRIE M.G. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflmmation. Br. J. Pharmacol. 1996;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., ZWEIER J.L., SAMOUILOV A., MACARTHUR H, MISKO T.P., CURRIE M.G., CUZZOCREA S., SIKORSKI J.A., RILEY D.P. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999b;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- SCHULZ R., WAMBOLT R. Inhibition of nitric oxide synthesis protects the isolated working rabbit heart from ischaemia-reperfusion injury. Cardiovasc. Res. 1995;30:432–439. [PubMed] [Google Scholar]
- SMITH E.F., III, EGAN J.W., BUGELSKI P.J., HILLEGASS L.M., HILL D.E., GRISWOLD D.E. Temporal relation between neutrophil accumulation and myocardial reperfusion injury. Am. J. Physiol. 1988;255:H1060–H1068. doi: 10.1152/ajpheart.1988.255.5.H1060. [DOI] [PubMed] [Google Scholar]
- SQUADRITO G.L., JIN X., PRYOR W.A. Stopped-flow kinetic study of the reaction of ascorbic acid with peroxynitrite. Arch. Biochem. Biophys. 1995;322:53–59. doi: 10.1006/abbi.1995.1435. [DOI] [PubMed] [Google Scholar]
- STERN M.K., JENSEN M.P., KNAMEN K. Peroxynitrite decomposition catalysts. J. Am. Chem. Soc. 1996;118:8735–8736. [Google Scholar]
- TANITA T., SONG C., KUBO H., HOSHIKAWA Y., CHIDA M., SUZUKI S., ONO S., FUJIMURA S. Superoxide anion mediates pulmonary vascular permeability caused by neutrophils in cardiopulmonary bypass. Surg. Today. 1999;29:755–761. doi: 10.1007/BF02482321. [DOI] [PubMed] [Google Scholar]
- VILLA L.M., SALAS E., DARLEY-USMAR V.M., RADOMSKI M.W., MONCADA S. Peroxynitrite induces both vasodilatation and impaired vascular relaxation in the isolated perfused rat heart. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12383–12387. doi: 10.1073/pnas.91.26.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON ANDRIAN U.H., CHAMBERS J.D., MCEVOY L.M., BARGATZE R.F., ARFORS K.E., BUTCHER E.C. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG P., ZWEIER J.L. Measurement of nitric oxide and peroxynitrite generation in the postischemica heart. Evidence for peroxynitrite-mediated reperfusion injury. J. Biol. Chem. 1996;271:29223–29230. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- WERTHEIMER S.J., MYERS C.L., WALLACE R.W., PARKS T.P. Intercellular adhesion molecule-1 gene expression in human endothelial cells. Differential regulation by tumor necrosis factor-alpha and phorbol myristate acetate. J. Biol. Chem. 1992;267:12030–12035. [PubMed] [Google Scholar]
- WOLFF A.A., LEVI R. Histamine and cardiac arrhythmias. Circ. Res. 1986;58:1–16. doi: 10.1161/01.res.58.1.1. [DOI] [PubMed] [Google Scholar]
- YANG Z., ZINGARELLI B., SZABO C. Effect of genetic disruption of poly (ADP-ribose) synthetase on delayed production of inflammatory mediators and delayed necrosis during myocardial ischemia-reperfusion injury. Shock. 2000;13:60–66. doi: 10.1097/00024382-200013010-00011. [DOI] [PubMed] [Google Scholar]
- YOSHIKAWA T., NAITO Y., UEDA S., TAKAHASHI S., OYAMADA H., YONETA T., SUGINO S., KONDO M. Ischemia-reperfusion injury and free radical involvement in gastric mucosal disorders. Adv. Exp. Med. Biol. 1990;264:401–410. doi: 10.1007/978-1-4684-5730-8_64. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN B.J., ARNDT H., KUBES P., KURTEL H. Reperfusion injury in the small intestine. 1993Berlin: Springer-Verlag; 322–335.ed. Schlag, G. & Redl, H. pp [Google Scholar]
- ZINGARELLI B., SALZMAN A.L., SZABO C. Genetic disruption of poly (ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ. Res. 1998;83:85–94. doi: 10.1161/01.res.83.1.85. [DOI] [PubMed] [Google Scholar]


