Abstract
To assess the role of nucleotide receptors in endothelial-smooth muscle signalling, changes in perfusion pressure of the rat arterial mesenteric bed, the luminal output of nitric oxide (NO) and guanosine 3′,5′ cyclic monophosphate (cGMP) accumulation were measured after the perfusion of nucleotides.
The rank order of potency of ATP and analogues in causing relaxation of precontracted mesenteries was: 2-MeSADP=2-MeSATP>ADP>ATP=UDP=UTP>adenosine. The vasodilatation was coupled to a concentration–dependent rise in NO and cGMP production. MRS 2179 selectively blocked the 2-MeSATP-induced vasodilatation, the NO surge and the cGMP accumulation, but not the UTP or ATP vasorelaxation.
mRNA encoding for P2Y1, P2Y2 and P2Y6 receptors, but not the P2Y4 receptor, was detected in intact mesenteries by RT–PCR. After endothelium removal, only P2Y6 mRNA was found.
Endothelium removal or blockade of NO synthase obliterated the nucleotides-induced dilatation, the NO rise and cGMP accumulation. Furthermore, 2-MeSATP, ATP, UTP and UDP contracted endothelium-denuded mesenteries, revealing additional muscular P2Y and P2X receptors.
Blockade of soluble guanylyl cyclase reduced the 2-MeSATP and UTP-induced vasodilatation and the accumulation of cGMP without interfering with NO production.
Blockade of phosphodiesterases with IBMX increased 15–20 fold the 2-MeSATP and UTP-induced rise in cGMP; sildenafil only doubled the cGMP accumulation. A linear correlation between the rise in NO and cGMP was found.
Endothelial P2Y1 and P2Y2 receptors coupled to the NO/cGMP cascade suggest that extracellular nucleotides are involved in endothelial-smooth muscle signalling. Additional muscular P2Y and P2X receptors highlight the physiology of nucleotides in vascular regulation.
Keywords: Extracellular ATP, endothelium signalling, P2Y1 receptors, P2Y2 receptors, nitric oxide, cGMP production
Introduction
Following the discovery of a role for nitric oxide (NO) in endothelial-smooth muscle signalling, the physiology of NO as an endothelial messenger has been widely studied in health and disease (Moncada, 1997; Hanafy et al., 2001). In the vascular system, NO is coupled to the synthesis of guanosine 3′,5′ cyclic monophosphate (cGMP), which is a key intermediate in the relaxation of vascular smooth muscles (Carvajal et al., 2000; Lucas et al., 2000). The notion that NO and cGMP are integrand components of endothelial-smooth muscle signalling, gave rise to the concept of the NO/cGMP cascade (Moncada et al., 1991; Moncada, 1997). Although the seminal observations of Furchgott & Zawadzki (1980) used acetylcholine (ACh) as a potent and powerful stimulus of NO release, the role of ACh in the physiology of blood flow regulation is limited to a few vascular territories. Bradykinin (Cherry et al., 1982) or shear stress (Busse & Fleming, 1998) appear as two determinant and physiologically relevant stimuli involved in blood pressure homeostasis. A role for extracellular nucleotides and adenosine in the control of blood pressure regulation is beginning to emerge (Boarder & Hourani, 1998). ATP, ADP, UTP and adenosine are released from endothelial cells and platelets (Kunapuli & Daniel, 1998), lowering blood pressure by the relaxation of small blood vessels and the microcirculation (Drury & Szent Györgyi, 1929; Burnstock, 1990; Lewis et al., 2000).
Nucleotide P2Y and P2X receptors have been described in vascular beds (Ralevic & Burnstock, 1998). While the former receptors are G-protein coupled and are mainly localized in the endothelium of blood vessels (Pirotton et al., 1996), the latter are ionic channels that are abundantly expressed in vascular smooth muscle cells (Huidobro-Toro & Valdecantos, 2000; Khakh et al., 2001; Briones et al., 2001). In a collection of pioneering papers, Ralevic & Burnstock (1988, 1991, 1996) and Windscheif et al. (1994) used the arterial mesenteric bed of rodents to study the pharmacology of ATP and related nucleotides in the vascular system. They characterized ATP-mediated relaxant and contractile mechanisms, identifying in the endothelial cells of this vascular tree two nucleotide receptors that were tentatively classified as P2Y and P2U. Furthermore, the blockade of NO synthase with Nω-nitro-L-arginine methyl ester attenuated the ATP, UTP and ACh mediated vasodilatation, implying the involvement of NO in the nucleotide-induced relaxations. An extension of these protocols in isolated perfused human placental cotyledons also showed that the endothelium of this human vascular bed contains several nucleotide receptors which account for the relaxations and contractions mediated by endogenous and exogenous nucleotides (Ralevic et al., 1997).
To contribute further to the understanding of the role of P2Y receptors in endothelial-smooth muscle signalling, we have identified endothelial P2Y receptor subtypes involved in vasorelaxation and we have characterized their coupling to the NO/cGMP cascade by measuring the production of NO and cGMP. We chose the arterial rat mesenteric bed as an experimental model. Preliminary results indicated that nucleotides such as ATP and 2-methylthioATP (2-MeSATP) vasodilate this territory causing a rise in the luminally accessible NO (Buvinic & Huidobro-Toro, 2000). The use of drugs plus molecular biology procedures was critical to correlate the presence of the receptor with their functional role. MRS 2179, a competitive P2Y1 receptor antagonist (Boyer et al., 1998), and selective inhibitors of the L-arginine cascade, allowed us to classify endothelial P2Y receptors and link them to the NO/cGMP pathway allowing us to determine the interdependence of these intracellular messengers in their vasodilatation (Buvinic & Huidobro-Toro, 2001). The results presented herein demonstrate that the rat arterial mesenteric bed is endowed with endothelial P2Y1 and P2Y2 receptors, which relax in a concentration–dependent manner the vascular bed through a surge of NO and a subsequent rise in tissue cGMP.
Methods
Perfusion of the rat arterial mesenteric bed
Adult male Sprague–Dawley rats (250–300 g) bred in our Animal Reproduction Laboratories were anaesthetized with 40 mg kg−1 sodium pentobarbital. The abdominal cavity was excised at the midline; the superior mesenteric artery was cannulated with polyethylene tubing and perfused with Tyrode buffer bubbled with 95% O2/5% CO2 at 37°C. Perfusion of the arterial bed was performed using a peristaltic pump at a flow of 2 ml min−1. The mesenteric bed was excised from the intestines and placed in a dish specially designed to collect the perfusates (Donoso et al., 1996). A pressure transducer was placed close to the entrance of the artery; the transducer was connected to a Grass polygraph. Fluctuations in the perfusion pressure recorded were interpreted as changes in the resistance of the arterial mesenteric bed. All mesenteries were perfused for 20 min prior to drug applications.
Quantification of the vasodilatation
To assess the vasodilatation induced by (μM) ATP 0.1–, ADP 0.01–10, UTP (uridine 5′ triphosphate), 0.1–100, UDP (uridine 5′ diphosphate), 0.1–100, 2-MeSADP (2 methylthioADP), 0.001–3, 2-MeSATP 0.0001–1 μM, α,β-methyleneATP 0.001–1 μM, adenosine 10–100, ACh 0.01–10 isoproterenol 1–100 μM), mesenteries were pre-contracted with 10 μM noradrenaline (NA). When the perfusion pressure was stable, the agonists were perfused for 6 min, maintaining the NA in the buffer. The return to basal perfusion pressure was achieved by perfusion with drug-free buffer for additional 15 min. The vasodilatations were expressed as a percentage of the contraction obtained with NA. Each agonist was assessed in a minimum of three separate mesenteries. Four or five different agonist concentrations were assessed per mesentery. To compare the relative vasodilatation agonist potencies, the negative logarithm of the agonist-concentration that produced 50% of the maximal response (pD2) was estimated.
Samples for luminal NO and tissue cGMP determinations
To quantify the luminally accessible NO and the tissue cGMP production induced by (μM) 2-MeSATP 0.001–1, UTP 0.01–10, adenosine 10–100 the agonists were perfused for 6 min, without pre-contracting the mesentery. The perfusate was collected every minute; the luminal NO was quantified by chemiluminescence, as detailed in a next paragraph. After a 6 min agonist application, the mesenteries were homogenized in 3 ml 10% trichloroacetic acid and centrifuged 30 min at 3000 r.p.m. and 4°C. The aqueous phase was extracted four times with four volumes of ethyl ether each run. The samples were dried in a speed-vac and stored at −20°C for less than a week until the RIA for cGMP was performed (Figueroa et al., 2001).
Blockade of the P2Y1 nucleotide-receptor with MRS 2179
To assess the concentration of MRS 2179, a selective and competitive P2Y1 receptor antagonist (Boyer et al., 1998), required to antagonise the vasorelaxation evoked by 30 nM 2-MeSATP or 1 μM UTP, 0.001–1 μM MRS 2179 was perfused 1 min before and during the application of the nucleotides. The changes in perfusion pressure were monitored continually. Dilations induced by (μM) 2-MeSATP 0.001–3, UTP 0.1–10., ADP 0.1 and ATP 1 were performed in presence of 100 nM MRS 2179. The apparent estimated antagonist KB (pA2 antilogue) using the single concentration procedure was derived from the following expression: pA2=−log[antagonist]+log(IC50 ratio−1) as described by Valenzuela & Huidobro-Toro (1991).
Determinations of NO release and tissue cGMP production induced by 300 nM 2-MeSATP and 1 μM UTP were assessed in the absence and presence of 30 nM MRS 2179.
mRNA determinations by RT–PCR
Rat mesenteries were manually defatted; the total RNA was extracted following Chomczynski & Sacchi (1987). Mesenteries with and without endothelium were prepared by perfusing several times for 45 s pulse with 0.1% saponin. The reverse–transcription reaction was performed with 2 μg of total RNA using an oligo-dt primer. The PCR was carried out using forward and reverse primers specific for each P2Y receptor subtype. After an initial denaturation for 5 min at 94°C, amplifications using 1/10 of the RT products were carried out for 35 cycles as follows: denaturation at 94°C for 40 s, annealing at 60°C for 1 min and extension at 72°C for 1 min. After completion of the cycles, a final 10 min extension at 72°C was carried out. To amplify a 663 bp fragment of the rat P2Y1 receptor, the forward-CTGCCTGAGTTGGAAAGA and reverse-TCCCAGTGCCAGAGTAGA primers were used. To amplify a 538 bp fragment of the rat P2Y2 receptor we used: forward-ACCCGCACCCTCTATTAC and reverse-CTTAGATACGATTCCCCA (Erlinge et al., 1998). To amplify a 415 bp fragment of the rat P2Y4 receptor, forward-CACCGATACCTGGGTATCTG and reverse-CAGACAGCAAAGACAGTCA primers were used (Harper et al., 1998). The fragment corresponding to the rat P2Y6 receptor was amplified with the forward-CGCTTCCTCTTCTATGCCAACC and reverse-CCATCCTGGCGGCACAGGCGGC primers (Adrian et al., 2000). Rat mRNA determination of the platelet-endothelial cell adhesion molecule (CD31) and the myosin alkali light chain (MALC) were assessed in the same mesentery preparations used to identify the P2Y receptor subtype. The former served as an endothelial marker, while the latter was used to characterize the vascular smooth muscle. Primers designed to amplify the region 131–481 of CD31 and the 241–448 region of the MALC were used. PCR products were analysed by electrophoresis in 1% agarose; to confirm the identity of the corresponding PCR products, the putative bands having the estimated size were isolated for direct sequencing using an ABI-Prism Sequencing Analyzer. Amplifications without the RT step were made to exclude possible contamination with genomic DNA.
Removal of endothelium cell layer and pharmacological blockade of the NO/cGMP pathway
General protocols
Two groups of rats were used to perform the following protocols: (1) Determination of the luminal release of NO and tissue cGMP induced by a 6 min perfusion with 0.3–1 μM 2-MeSATP or UTP (1–10 μM) in non-contracted mesenteries, and (2) Determination of the vasorelaxation evoked by 0.001–1 μM 2-MeSATP or 0.1–100 μM UTP in NA-pre-contracted tissues. Each group was composed of five subgroups in which we assessed the effect of: (i) Endothelium denudation; (ii) Inhibition of NOS with 100 μM Nω-Nitro-L-arginine (L-NNA), iii. Blockade of soluble guanylyl cyclase with 3 μM 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ); (iv) Blockade of phosphodiesterase V with 10 nM sildenafil; (v) Blockade of all phosphodiesterases with 500 μM 3-isobutyl-1-methylxanthine (IBMX). Results compare the mean changes in luminal NO, tissue cGMP production and the nucleotide-induced vasodilatation in the absence and presence of NO/cGMP cascade blockers or endothelium removal.
To perform the protocols assessing the influence of endothelial denudation, blockade of the endothelial NOS, or the soluble guanylyl cyclase, the concentration of Na required to pre-contract the mesenteries was lowered 10 times. This reduction in the concentration of NA was required to achieve the same magnitude of pre-contraction as in the intact tissues.
Endothelium removal
Endothelium denudation was achieved by perfusing with 0.1% saponin for 55 s followed by drug-free buffer perfusion for the next 30 min (Peredo & Enero, 1993; Donoso et al., 1996). ACh (0.1–100 μM) and isoproterenol (0.001–100 μM) were used as positive controls of the endothelium-dependent and endothelium-independent vasodilators.
NOS blockade
Endothelial NOS was blocked perfusing the mesenteries with 100 μM L-NNA for 45 min (Boric et al., 1999), prior to nucleotide testing.
Soluble guanylyl cyclase blockade
The soluble guanylyl cyclase was inhibited by perfusing the mesenteries with 3 μM ODQ for 20 min (Garthwaite et al., 1995; Buvinic & Huidobro-Toro, 2001).
Phosphodiesterase blockade
To block all phosphodiesterases, mesenteries were 30 min perfused with 500 μM IBMX (Taylor et al., 1999). To specifically block type V phosphodiesterase, mesenteries were perfused for 25 min with 10 nM sildenafil (Ballard et al., 1998; Buvinic & Huidobro-Toro, 2001).
Analytical techniques
Quantification of the NO by chemiluminescence
The sample NO content was quantified using a Sievers 280 analyser. To reduce the nitrites in each sample, the instrument reaction chamber was filled with 8 ml of glacial acetic acid containing 100 mg of potassium iodide at room temperature. Fifty μl of a sample was injected to the chamber; a stream of N2 carried the resulting NO to a cell in which the specific chemiluminescence generated by the NO-ozone reaction was quantified (Figueroa et al., 2001). Calibration of the equipment was performed weekly with 10–1000 nM sodium nitrite. The equipment allows detecting 0.5–1 pmol NO (10–20 pmol ml−1). Background buffer readings were subtracted to determine the net NO release. Results are expressed as the integrated area of the NO peak produced by the agonists over basal values (Δ NO, pmol).
Quantification of the cGMP by radioimmunoassay
The nucleotide was quantified using a 10 fmol-threshold RIA for acetylated cGMP. As a tracer 2′-O-succinylguanosine 3′, 5′-cyclic monophosphate tyrosyl methyl ester was used; the nucleotide was labelled locally with 125I (Figueroa et al., 2001). Results are expressed as the production of tissue cGMP induced by agonist over the basal values of cGMP produced in mesenteries perfused with drug-free buffer (ΔcGMP, pmol g−1). As pharmacological blockade of the NO/cGMP cascade alters the basal production of cGMP in the mesenteric bed (Buvinic & Huidobro-Toro, 2001), the expression of the nucleotide-induced cGMP accumulation over respective basal values were used to standardize our results.
Drug sources
The composition of the Krebs–Ringer buffer was (mM): NaCl 118, KCl 5.4, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 23.8 and glucose 11.1. The buffer's reagents were obtained from Merck, Chile. 2-MeSADP, 2-MeSATP, ATP, ADP, UTP, UDP, ((-methyleneATP, MRS 2179, adenosine, NA, ACh, isoproterenol, saponin, L-NNA, ODQ and IBMX were purchased from Sigma Chemicals (St. Louis, U.S.A.). 2-MeSATP and other nucleotides were also purchased from RBI (Natick, MA, U.S.A.). Dr. Mark Currie, from the Medical University of South Carolina, donated the specific anti-cGMP antibody. Pfizer Central Research (Sandwich, U.K.) generously donated sildenafil citrate.
Statistical analysis
One- and two-way ANOVA's, linear correlation analysis and Student's ‘t-test' were used throughout. Dunnett's for multiple comparisons with a single control were used when appropriate. P values less than 0.05 were considered statistically significant.
Results
Nucleotide-induced vasodilatation; effect of endothelium removal and MRS 2179 blockade
Atp and related structural nucleotides vasodilate pre-contracted mesenteries with the following relative rank order of potencies: 2-MeSADP=2-MeSATP>ADP>ATP=UDP=UTP>adenosine (Table 1). ACh and isoproterenol, were 10 and 1000 fold less potent that 2-MeSATP, respectively (Table 1). Furthermore, in precontracted preparations, α,β-methyleneATP, a non-hydrolyzable P2X agonist, contracted this territory with a pD2 of 7.5±0.1 (n=3). Altogether, these results reveal the presence of vasodilatator and vasomotor nucleotide receptors in the mesenteric arterial bed.
Table 1.
Relative affinity of several nucleotides and other agonists to vasodilate the mesenteric bed
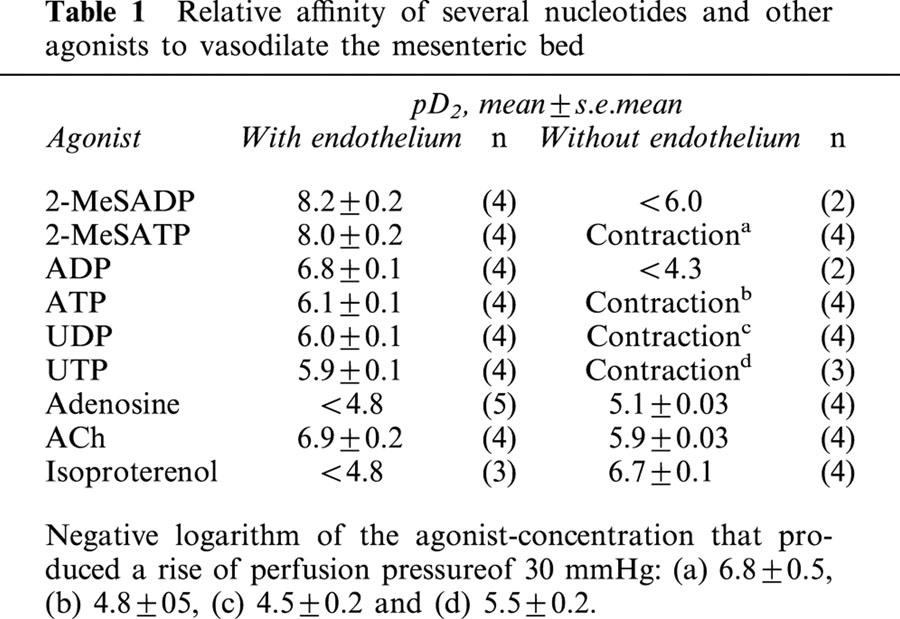
Following endothelium removal, 2-MeSATP, ATP, UTP and UDP evoked concentration-dependent vasocontractions reaching up to 50 mmHg (Figure 1); the concentration of these agonists that raised 30 mmHg the perfusion pressure of pre-contracted mesenteries is listed in Figure 1. In contrast, the dilatations evoked by 2-MeSADP or ADP were markedly attenuated by endothelium removal without eliciting vasocontractions (Table 1). Likewise, the ACh-induced vasodilatation was reduced by endothelium removal; however, the adenosine and isoproterenol-induced relaxations increased by this procedure, raising significantly their pD2 (Table 1). To maintain the magnitude of the precontraction required to test the potency of the vasodilators, the endothelium denuded preparations were pre-contracted with 10 fold less NA, due to their increased reactivity to vasocontractile agents.
Figure 1.
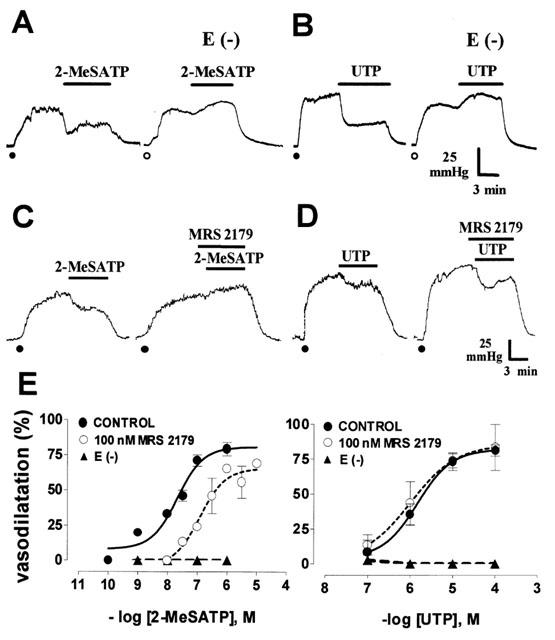
The 2-MeSATP and UTP-induced vasodilatations are endothelium dependent, but only the 2-MeSATP relaxation is antagonised by MRS 2179. Representative recordings show that 30 nM 2-MeSATP or 1 μM UTP induced sustained relaxations that are endothelium dependent (A and B); after removal of the endothelium E (−), these nucleotides consistently vasoconstricted this territory. While the 2-MeSATP vasodilatation was antagonized by 100 nM MRS 2179 (C), the 1 μM UTP-induced relaxation was not blocked by this drug (D). The recordings shown in A–D are derived from separate mesenteries; in each case, the nucleotide was assessed before and after endothelium removal, or perfusion with MRS 2179. Closed dots denote mesentery pre-contraction with 10 μM noradrenaline, while the open dots indicate a precontraction with 1 μM noradrenaline in endothelium-denuded preparations. (E) shows 2-MeSATP and UTP concentration-response vasorelaxations with and without endothelium (n=3–6 per agonist) or perfusion with 100 nM MRS 2179 (n=3–8, applied a min before and during perfusion with the nucleotide). Symbols represent mean values; bars, the s.e.mean.
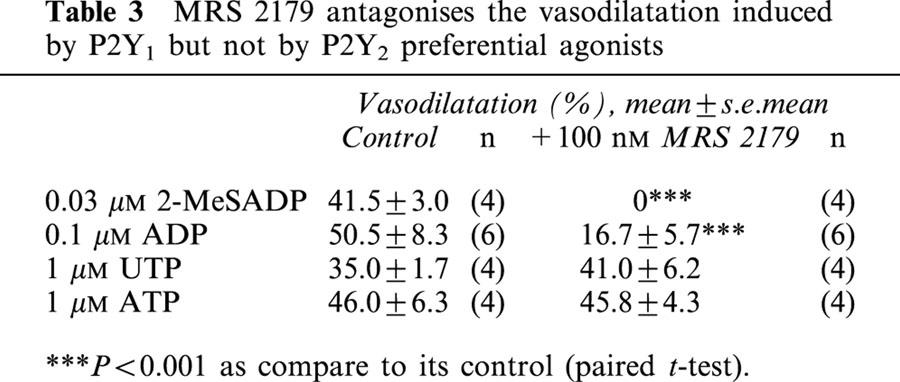
MRS 2179 antagonized in a concentration-dependent manner the 2-MeSATP-induced relaxation, without altering the UTP response (Figure 1C–D and Table 2). One hundred nM MRS 2179 shifted 12 fold to the right the relaxation curve of 2-MeSATP; the pD2 changed from 7.9±0.1 to 6.8±0.1 (P<0.01, Figure 1E). The estimated KB from this assay was 8.03. MRS 2179 did nor alter the UTP pD2 (5.9±0.1 vs 5.8±0.04). MRS 2179 also blocked the ADP-induced dilatation but not the ATP-induced relaxation (Table 3), results which are compatible with the selectivity of MRS 2179 as a P2Y1 receptor antagonist. Application of 1–1000 nM MRS 2179 alone did not change the perfusion pressure of the rat arterial mesenetric bed.
Table 2.
MRS 2179 antagonises the vasodilatation induced by 2-MeSATP but not by UTP
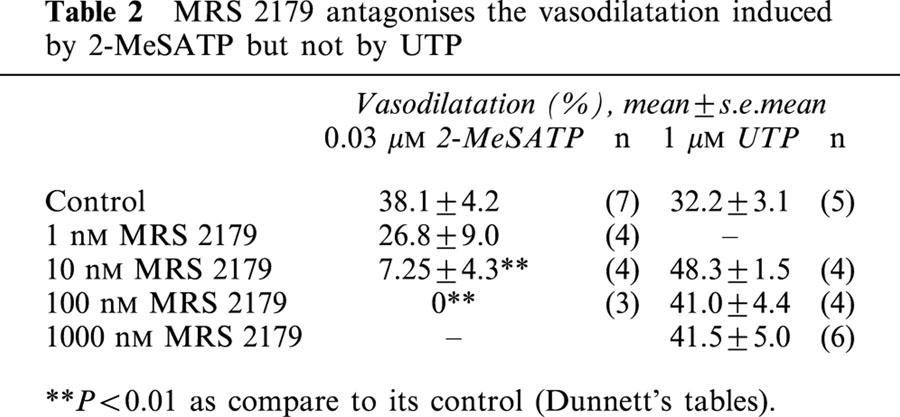
Table 3.
MRS 2179 antagonises the vasodilatation induced by P2Y1 but not by P2Y2 preferential agonists
Endothelial localization of the P2Y1 and P2Y2 receptor mRNA
mRNA for the P2Y1 and P2Y2 receptors was detected by RT–PCR only in mesenteries with an intact endothelial cell layer (Figure 2), confirming the endothelial localization of these receptor subtypes. Furthermore, while the mRNA for the P2Y4 receptor was not found, the mRNA for the P2Y6 receptor was detected in mesenteries with and without endothelium. Using selected primers, we observed the expected single bands of 663, 538 and 368 bp for the P2Y1 P2Y2 and P2Y6 receptor cDNA fragments, respectively (Figure 2). Sequencing confirmed more than 99% identity of these products with the corresponding cDNA for the rat P2Y receptor subtypes.
Figure 2.
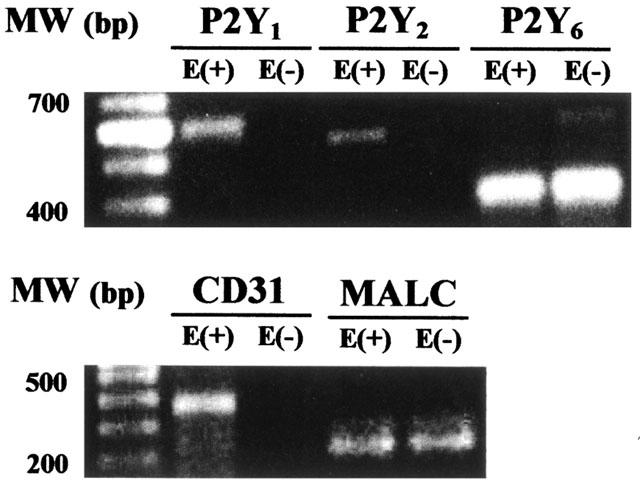
Identification of P2Y1, P2Y2 and P2Y6 receptors mRNA by RT–PCR. Gels show PCR products corresponding to P2Y1, P2Y2 or P2Y6 receptors based on their estimated molecular weight (MW, bp). When total tissue mRNA is extracted from mesenteries lacking the endothelial cell layer (E−), no PCR products for these receptors were observed, except for the P2Y6 receptor subtype. Upon endothelium denudation, the PCR products for CD31, an endothelial cell marker, were not evidenced. In contrast, the smooth muscle PCR product for myosin alkali light chain (MALC) is observed with (E+) and without endothelium (E−). Identical results were attained in duplicate protocols.
The mRNA for CD31, an endothelial marker, was only identified in mesenteries with intact endothelium, while the PCR products for the myosin alkali light chain (MALC, a smooth muscle marker) were observed in mesenteries mRNA extracts with and without the endothelium (Figure 2).
As controls, protocols performed in the absence of cDNA, did not yield PCR products. Likewise, protocols carried out in the absence of the RT-step did not yield PCR products (data not shown), confirming the absence of genomic DNA contamination.
Activation of the NO/cGMP cascade by selective P2Y1 and P2Y2 agonists
Luminally accessible NO and tissue cGMP production induced by nucleotides
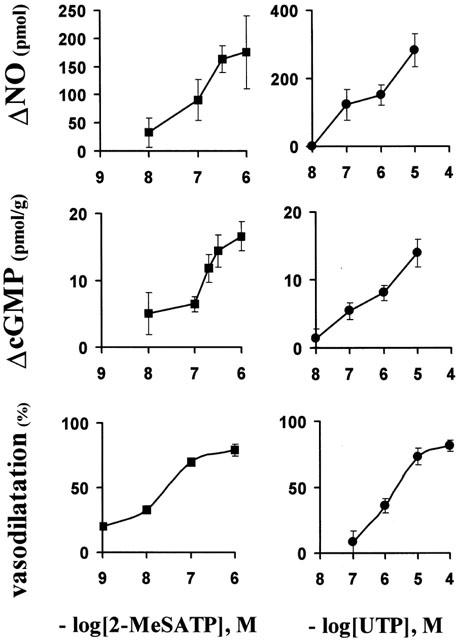
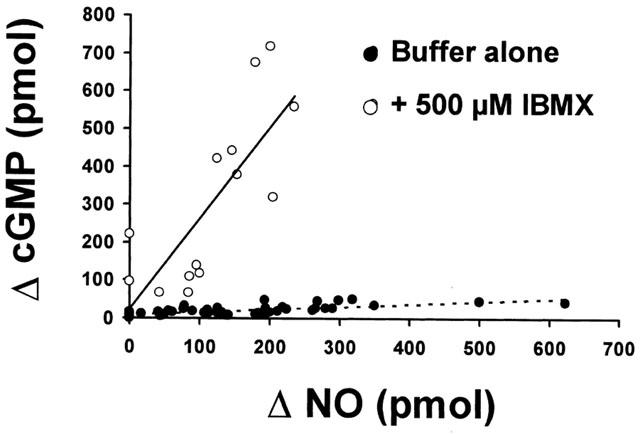
Both 2-MeSATP and UTP induced concentration-dependent rises in the luminally accessible NO and tissue cGMP content (Figure 3). As a demonstration of the physiological interdependence between NO and cGMP production elicited by these nucleotides, a significant correlation between these messengers was found with varying concentrations of 2-MeSATP and UTP (r=0.74, P<0.01, Figure 4). In the presence of IBMX, a non-selective phosphodiesterases blocker, the correlation between No and cGMP production exhibited a striking increase in the slope of the correlation curve (0.072 vs 2.41, Figure 4), presumably suggesting that the fast cGMP hydrolysis hinders the real magnitude of the cascade amplification.
Figure 3.
2-MeSATP and UTP concentration-dependent vasodilatations and their corresponding productions of NO and cGMP. Concentration-dependent rise in the luminally accessible NO production (upper panels); increase in tissue cGMP production (middle panels) and vasodilatations (lower panels). Symbols represent the mean values; bars, the s.e.mean (n=3–16 per concentration of nucleotides).
Figure 4.
Correlation between tissue production of cGMP and the luminally accessible NO induced by several concentrations of 2-MeSATP and UTP. Closed circles represent NO and cGMP determinations in mesenteries perfused with varying concentrations of these nucleotides in a drug-free buffer. The correlation coefficent was 0.74 (P<0.01, n=49); the slope of this curve is 0.072. To prevent cGMP degradation, we next assessed NO and cGMP determinations in mesenteries perfused with 500 μM IBMX (open circles). The correlation coefficient (r) between NO and cGMP production was 0.77 (P<0.01, n=14); the slope 2.4. Each symbol represents a different mesentery, in which both NO and cGMP were determined following a single nucleotide application.
To rule out the possible participation of adenosine as a metabolic subproduct in the effect of ATP and related purines, perfusion with 10–100 μM adenosine did not increase the production of NO nor caused a rise in the tissue cGMP (n=4–6, ANOVA P>0.05, data not shown). Likewise, to rule out the role of metabolic byproducts of UTP, the perfusion with 10 μM UDP, which causes 67±4% vasodilatation, elicited a rise of 67±15 pmol NO vs 282±46 pmol NO (P<0.01) evoked by 10 μM UTP.
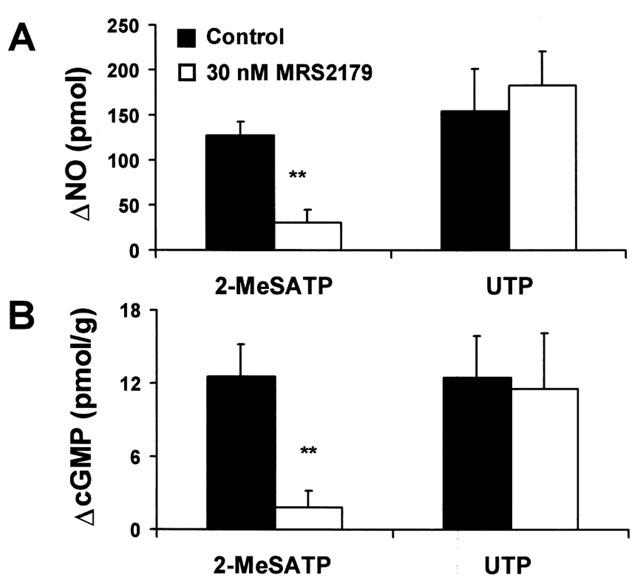
MRS 2179 blocks the surge of NO and cGMP production evoked by 2-MeSATP
Consonant with the lack of 2-MeSATP vasodilatation observed in mesenteries perfused with the P2Y1 receptor antagonist, perfusion with 30 nM MRS 2179 resulted in a reduction in the luminal NO released or cGMP production elicited by 300 nM 2-MeSATP (P<0.01, Figure 5). In contrast, neither the NO production nor the rise in tissue cGMP evoked by 1 μM UTP was significantly altered by 30 nM MRS 2179 (Figure 5), a result consistent with the previous data showing the selectivity of MRS 2179 for the P2Y1 receptor. Additionally, 30 nM MRS 2179 per se, did not nor alter the basal release of luminal NO nor the tissue production of cGMP, nor modified the perfusion pressure of the mesenteries.
Figure 5.
MRS 2179 blocks the 2-MeSATP-induced production of NO and cGMP, without altering the same responses elicited by UTP. Rise of the luminal accessible NO released (A) and the tissue production of cGMP (B) evoked by 0.3 μM 2-MeSATP or 1 μM UTP (n=3–6) before and after application of 30 nM MRS 2179. The same mesentery was used to determine the luminal outflow of NO and the corresponding tissue content of cGMP; separate mesenteries were used to examine the effect of the nucleotides in the absence and in the presence of 30 nM MRS 2179. Columns refer to the mean values; bars, to the s.e.mean. **P<0.01.
Endothelium removal, NOS inhibition or soluble guanylyl cyclase blockade obliterated the luminal outflow of NO, the production of tissue cGMP and vasorelaxation
Consonant with the lack of vasodilatation induced by selective P2Y1 and P2Y2 agonists in preparations devoid of endothelium, the luminal outflow of NO evoked by 1 μM 2-MeSATP or 10 μM UTP was reduced 70% and more than 95% respectively (Figure 6 upper panel, P<0.05, n=4 for each nucleotide). Likewise, endothelium removal annulled the rise in tissue cGMP evoked by both agonists (Figure 6, middle panel) and the subsequent nucleotide-induced relaxation (Figure 6, lower panel).
Figure 6.
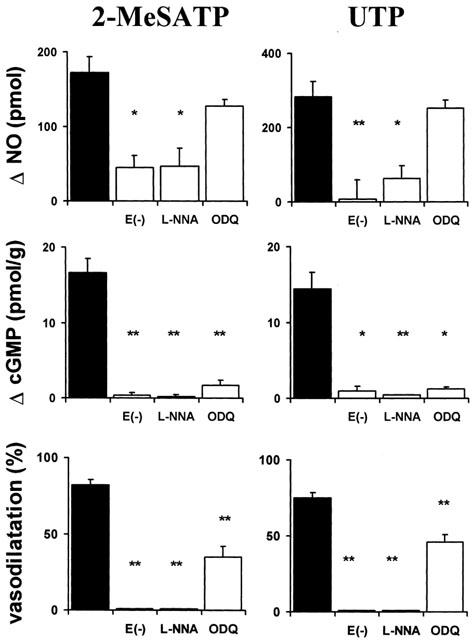
Endothelium removal, blockade of NOS or soluble guanylyl cyclase obliterate the 2-MeSATP or UTP-induced vasodilatation and the surge in luminal NO and tissue cGMP production. Separate mesenteries were perfused with either 1 μM 2-MeSATP or 10 μM UTP. A single mesentery was used to measure luminal accessible NO and its corresponding increase in cGMP production in three separate experimental conditions: removal of the endothelial cell layer (E(−) n=4); blockade of NOS with L-nitro arginine (L-NNA n=4); inhibition of soluble guanylyl cyclase (ODQ n=4). Closed columns represent the effect of the nucleotides in drug-free buffer (controls, n=12). Columns indicate mean values; bars, to the s.e.mean. *P<0.05; **P<0.01.
NOS blockade reduced significantly NO, cGMP production and the vasorelaxation elicited by 2-MeSATP or UTP (Figure 6, P<0.01, n=4, for each agonist).
ODQ did not significantly modify the luminal release of NO induced by 2-MeSATP or UTP, while it blunted the rise in tissue cGMP (Figure 6). The corresponding vasodilatation was reduced (57 and 40%, for 2-MeSATp and UTP respectively, Figure 6, P<0.01, n=4), though not to the same extent as for the cGMP production.
Additionally, all three procedures: the removal of the endothelial cell layer, the blockade of the NOS, and the inhibition of soluble guanyl cyclase, decreased significantly the basal production of NO and tissue cGMP (data not shown). Consequently, the rise in luminal NO released and the cGMP production elicited by the nucleotides under these experimental conditions are expressed as the net difference between basal and nucleotide stimulated messenger production (ΔNO and cGMP production).
Blockade of tissue phosphodiesterases
Sildenafil further increased the rise in tissue cGMP accumulation evoked by 300 nM 2-MeSATP (10.1±1.6 vs 18.0±1.5 pmol g−1, P<0.01, n=4) or 1 μM UTP (7.0±3.1 vs 19.7±4.5 pmol g−1, P<0.01, n=4). The nucleotide-induced rise in luminal accessible NO was not modified by sildenafil (Table 4). IBMX increased 15–20 fold the production of cGMP induced by 300 nM 2-MeSATP (12.6±2.7 vs 182.4±49.4 pmol g−1, P<0.01, n=4) and 1 μM UTP (8.1±1.1 vs 153.9±59.3 pmol g−1, P<0.01, n=4), without altering the luminally accessible NO produced by these nucleotides (Table 4). The relatively larger production of cGMP as compared to the production of NO may be a reflection of the cascade amplification. Consonat with the above findings, both sildenafil and IBMX consistently increased the basal production of cGMP in the mesenteries without modifying the basal production of luminal NO (data not shown).
Table 4.
Phosphodiesterase inhibitors increase the accumulation of tissue cGMP elicited by 2-MeSATP and UTP
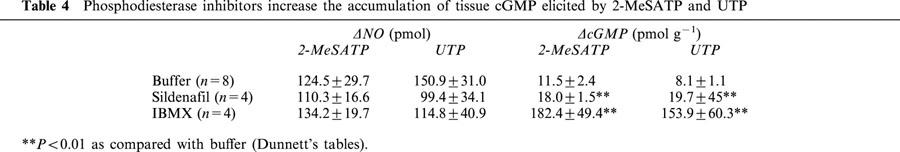
Ten nM Sildenafil or 500 μM IBMX blunted the NA-induced vasoconstriction, unabling the determinations of the vasorelaxations in the presence of phosphodiesterase inhibitors.
Discussion
Results presented in this report allow the identification and pharmacological characterization of endothelial P2Y1 and P2Y2 receptors, which bring about vasodilatation through the activation of the NO/cGMP pathway. Consequently, varying concentrations of 2-MeSATP and UTP, used as prototype ligands for the P2Y1 and P2Y2 receptors respectively, show a significant correlation between NO and cGMP production, consonant with the interdependence between these intracellular messengers and vasodilatation. Furthermore, the blockade of the NO/cGMP cascade at several steps, and the consequent measurements of the NO and cGMP produced, confirmed that these P2Y receptor subtypes are functionally coupled to this pathway. A schematic model of this working hypothesis is presented in Figure 7. The scheme also details the additional presence of P2Y, P2X and adenosine receptors in vascular smooth muscle that may also play a part in the vasomotor tone.
Figure 7.
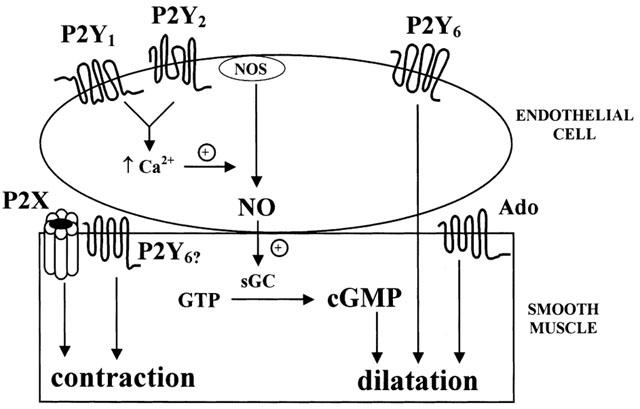
Schematic model shows nucleotide receptors in the endothelium and the smooth muscle of the rat arterial mesenteric bed. Locally released nucleotides (ATP, ADP or UTP) act on endothelial P2Y1 and P2Y2 receptors promoting a concentration-dependent surge of NO triggered by the release of intracellular stored calcium activating nitric oxide synthase (NOS). The tridimensional spread of the gas diffuses to the adjacent smooth muscle where it activates soluble guanylyl cyclase (sGC) accumulating cGMP. The cyclic nucleotide ensues the relaxation of the vascular smooth muscles via cGMP-dependent kinases. The P2Y6 receptor has also been identified both in the endothelial layer as in the smooth muscle. At present it is not clear whether the latter receptor mediates the contractions ensued by pyrimidines nucleotides. The model also depicts the presence of muscular P2X receptors. Adenosine receptors (Ado) dilate the rat arterial mesenteric bed, via a NO/cGMP independent muscular pathway.
The relative agonist potency of several structurally related nucleotides, the use of MRS 2179, and the yield of RT–PCR products allowed us to identify the P2Y1, P2Y2 and P2Y6 receptors in this bed. In support of the presence of the P2Y1 receptor, the metabolically more stable ATP analogues 2-MeSATP or 2-MeSADP proved about 100 fold more potent as a vasodilator than ATP, in accordance with the relative agonist potency defined for this receptor subtype (Dixon, 2000). Furthermore, MRS 2179 a new and selective P2Y1 receptor antagonist (Nandanan et al., 2000), competitively and selectively blocked both the vasorelaxation and the rise in luminal NO and tissue cGMP evoked by 2-MeSATP or ADP, without interfering with the UTP or ATP-induced relaxation, further supporting the presence of P2Y1 receptors. Functional P2Y2 receptors were also identified in this bed. Three arguments support the latter conclusion: (i) the equipotent vasodilator activity of UTP and ATP, (ii) the resistance of UTP and ATP to 100 nM MRS 2179 antagonism; (iii) the identification of the mRNA for the P2Y2 receptor subtype and the lack of mRNA for the P2Y4 receptor, a receptor also characterized by a high affinity for UTP. Furthermore, we detected mRNA for the P2Y6 receptor in endothelial cells, which may account for the UDP-mediated vasodilatation. Therefore, the combined characterization of the mRNA plus the functional vasodilatation induced by the activation of these receptors supports the conclusion that the arterial vascular bed expresses P2Y1 P2Y2 and P2Y6 receptors, which are linked to endothelium-dependent vasodilatations.
Two arguments support the preponderant endothelial localization of the P2Y1, P2Y2 and P2Y6 receptors. First, we consistently observed that in the absence of the endothelium, the vasorelaxations-induced by 2-MeSADP and ADP were attenuated while ATP, 2-MeSATP, UTP UDP evoked concentration-dependent contractions. Secondly, the RT–PCR fragments corresponding to the rat P2Y1 and P2Y2 receptors, which were characterized in the intact mesentery, were not found in mesenteries devoid of the endothelial cell layer. An exception to this finding is the dual localization of the P2Y6 receptor in the endothelium and the smooth muscle. The vascular reactivity indicates that the shedding of the endothelial layer does not alter the smooth muscle integrity; the use of endothelial and smooth muscle markers further confirm the validity of our experimental procedures.
Functionally, the vasodilatation mediated by the endothelial P2Y1 and P2Y2 receptors is linked to the NO/cGMP pathway. Several arguments favour this interpretation. We demonstrate, for the first time, that 2-MeSATP and UTP cause concentration-dependent increments in the luminal release of NO and smooth muscle accumulation of cGMP, which are interdependent and causally related to the vasodilatation. In the presence of IBMX there was a notable increase in the slope of the NO vs cGMP curve; the cGMP/NO ratio was 2.19, an initial indication of the potential amplification of this cascade. We are aware of the potential pitfalls of our model and analytical determinations. Among these are the knowledge that the NO produced is spread tridimensionally (Wood & Garthwaite, 1994) yet we only measure the luminally accessible pool, that we determine only nitrites, and that cGMP is rapidly hydrolyzed by the tissue phosphodiesterases. We are aware that endothelial removal, or blockade of NOS with L-NNA interferes with the NO cascade significantly attenuating the production of NO and cGMP induced by nucleotides and the subsequent vasorelaxation. After ODQ treatment, the nucleotide-induced cGMP production is greatly obliterated and the vasodilatation is significantly decreased. However, we are aware that the decrease in the vasodilator response is only partially blocked. This last observation is not totally unexpected since NO may also act independently of cGMP accumulation, either through potassium channels (Homer & Wanstall, 2000) or indirectly modifying a number of relevant macromolecules (Davis et al., 2001).
Selective inhibition of phosphodiesterase V or the blockade of all phosphodiesterases, a pharmacological manoeuvre that does not interfere with NO production, augments markedly the nucleotide-induced accumulation of cGMP. We anticipated that in the presence of phosphodiesterase inhibitors the nucleotide-induced vasorelaxations should be markedly increased. However, we did not observe this vasodilatation after 1–100 nM sildenafil or 500 μM IBMX treatment because the arterial mesenteric bed did not contract in the presence of up to 1000 μM NA. It appears that the over-accumulation of cGMP interferes with the contractile machinery blocking non-selectively and non-competitively the vasomotor responses evoked by potassium, NA, angiotensin II and serotonin (Buvinic & Huidobro-Toro, 2001; Aedo & de la Cerda, unpublished observations).
The observation that UDP and UTP vasodilate with a similar pD2, yet under equivalent concentration of these nucleotides the corresponding NO surge evoked by UDP is significantly lower, casts doubts on a role for the NO/cGMP pathway in UDP-induced vasodilatation. Notwithstanding UTP may be hydrolyzed to UDP, these nucleotides appear to vasodilate via a separate mechanism. In this regard, we cannot discard that while UTP may activate preponderantly the P2Y2 receptor, UDP may bring about vasodilatation via the combined activation of the P2Y2 and the P2Y6 receptor. The use of UDPβS (uridine 5′-O-thiodiphosphate) or UTPγS (uridine 5′-O-thiotriphosphate), two novel metabolically stable pyrimidines recently reported by Malmsjö et al. (2000a,b) will help to clarify this issue. These nucleotides appear to discriminate between P2Y receptor subtypes.
To properly assess the role of the endothelium in the nucleotide's-induced vasodilatation, we used other vasodilators such as Ach, isoproterenol and adenosine. Both ACh and the nucleotides decrease vasodilatation upon endothelial removal. However, this did not occur with isoproterenol and adenosine, which act predominantly on receptors localized in the vascular smooth muscle. Endothelium denudation favours the diffusion of these agents to the smooth muscle and thus increasing their vasodilator potencies. In the case of Ach, which like the nucleotides utilizes mainly endothelial receptors, the removal of the endothelium did not abolish the dilation, since apparently ACh acts presynaptically releasing CGRP, a well-established vasodilator (Shiraki et al., 2001). As a further control, we also assessed the putative interference of adenosine in the nucleotide response. Our results consistently show that the adenosine-induced vasodilatation is neither dependent on the endothelium or on the NO/cGMP pathway. In this vascular bed, adenosine receptors appear to be mainly localised in the smooth muscle and lead to vasodilatation via a non-cGMP dependent mechanism, as shown schematically in Figure 7.
An intriguing question is emerging, which relates to the nature of the nucleotide-induced contractions in endothelial-denuded mesenteries. It is plausible that vascular smooth muscle P2X receptors mediate the contractions evoked by ATP, 2-MeSATP or α,β-methyleneATP (Kennedy, 1996; Huidobro-Toro & Valdecantos, 2000; Gitterman & Evans, 2000). Upon closer examination of the MRS 2179-induced rightward displacements of the 2-MeSATP concentration-response curves, this agonist did not reach more than 60% relaxation. This finding could indicate that concentrations of 2-MeSATP above 1 μM might elicit some degree of vasoconstriction, counteracting the endothelial-mediated vasodilatation. This explanation cannot account for the vasomotor response evoked by UTP or UDP, since these pyrimidine nucleotides have not been reported to act on P2X receptors. García Velasco et al. (1995) and Rubino et al. (1999) argue that UTP contracts the rat aorta and pulmonary vessels via a receptor refractile to α,β-methyleneATP desensitization, blockade by PPADS or blockade by suramin. It is therefore plausible that these pyrimidines either activate muscular P2Y receptors, such as the P2Y6 receptor subtype reported herein, or they act on a new nucleotide receptor. In support of the involvement of muscular P2Y receptors linked to vasomotor activity, recent reports show that activation of P2Y2 receptors leads to contraction of human coronaries (Malmsjö et al., 2000b), while in isolated rat mesenteric artery biopsies the activation of the P2Y2/P2Y4 and P2Y6 receptors leads to concentration-dependent vasomotor effects (Malmsjö et al., 2000a). The lack of contractile responses elicited by ADP or 2-MeSADP in mesenteries without endothelium, rules out the participation of muscular P2Y1 receptors, a finding confirmed by the lack of the mRNA encoding this receptor subtype. Likewise, the lack of vasomotor responses evoked by these two nucleotides, allows discarding that they act through P2X receptor activation.
Relevant to the physiology of the endothelial nucleotide receptors is that both platelet degranulation or endothelial cells release adenine and pyrimidine nucleotides into the vascular lumen (Anderson & Parkinson, 1997; Connolly & Duley, 1999). Furthermore, nerve terminals and microglia have been reported to release these nucleotides (Harden & Lazarowski, 1999), which upon reaching the vascular wall may also participate in the local regulation of blood flow. Therefore, the release of nucleotides to the vascular lumen either by hypoxia or following platelet activation may bring about endothelial P2Y1 and P2Y2 receptor agonism, causing concentration-dependent vasodilatations, which couple to the NO/cGMP pathway. Consequent to the intense vasoconstriction mediated by maintained sympathetic discharges which co-release ATP, NA and neuropeptide Y, we may now raise the possibility that the release of endothelial nucleotides, may evoke a compensatory vasodilatation. The mechanism(s) that allow the vascular smooth muscle to coordinate information from the nerve terminals and the endothelial cells will provide a better understanding of the endothelial-smooth muscle signalling.
In summation, the presence of at least three distinct populations of endothelial P2Y plus muscular P2Y and various P2X receptor subtypes, highlights the notion that extracellular nucleotides may play a role in the fine regulation of blood flow. The use of more selective nucleotide receptor antagonists will help to understand their physiology and involvement in vascular disease, revealing opportunities for clinical interventions.
Acknowledgments
Partially funded by FONDAP-Biomedicina grant 13980001; the Millenium Institute for Fundamental and Applied Biology (MIFAB) contributed to the funding of the Center for Cell Regulation and Pathology. S.Buvinic is supported by a CONICYT doctoral fellowship.We thank Pfizer Laboratories (Sandwich, Kent, U.K.) for the sample of sildenafil citrate. To Profs. J.L. Boyer and G. Owen for helpful discussions and editorial assistance.
Abbreviations
- ACh
acetylcholine
- CD31
platelet-endothelial cell adhesion molecule
- cGMP
guanosine 3′,5′cyclic monophosphate
- IBMX
3-isobutyl-1-methylxanthine
- L-NNA
Nω-Nitro-L-arginine
- MALC
myosin alkali light chain
- 2-MeSADP
2 methylthioADP
- 2-MeSATP
2 methylthioATP
- MRS 2179
N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate
- NA
noradrenaline
- NO
nitric oxide
- NOS
nitric oxide synthase
- ODQ
1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one
- UDP
uridine 5′ diphosphate
- UTP
uridine 5′ triphosphate
References
- ADRIAN K., BERNHARD M.K., BREITINGER H., OGILVIE A. Expression of purinergic receptors (ionotropic P2X1-7 and metabotropic P2Y1-11) during myeloid differentiation of HL60 cells. Biochim. Biophys. Acta. 2000;1492:127–138. doi: 10.1016/s0167-4781(00)00094-4. [DOI] [PubMed] [Google Scholar]
- ANDERSON C.M., PARKINSON F.E. Potential signalling roles for UTP and UDP: sources, regulation and release of uracil nucleotides. TiPS. 1997;18:387–392. doi: 10.1016/s0165-6147(97)01106-1. [DOI] [PubMed] [Google Scholar]
- BALLARD S.A., GINGELL C.J., TANG K., TURNER L.A., PRICE M.E., NAYLOR A.M. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozyme. J. Urol. 1998;159:2164–2171. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- BOARDER M.R., HOURANI S.M. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends. Pharmacol. Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- BORIC M.P., FIGUEROA X.F., DONOSO M.V., PAREDES A., POBLETE I., HUIDOBRO-TORO J.P. Rise in endothelium-derived NO after stimulation of rat perivascular sympathetic mesenteric nerves. Am. J. Physiol. 1999;277:H1027–1035. doi: 10.1152/ajpheart.1999.277.3.H1027. [DOI] [PubMed] [Google Scholar]
- BOYER J.L., MOHANRAM A., CAMAIONI E., JACOBSON K.A., HARDEN T.K. Competitive and selective antagonism of P2Y1 receptors by N6-methyl 2′-deoxyadenosine 3′, 5′-biphosphate. Br. J. Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIONES R., MIRANDA R., HUIDOBRO TORO J.P. Distribution and pharmacology of P2X receptors in human blood vessels. Biol. Res. 2001;34:R53–. [Google Scholar]
- BURNSTOCK G. Dual control of local blood flow by purines. Ann. N. Y. Acad. Sci. 1990;603:1–17. doi: 10.1111/j.1749-6632.1990.tb37659.x. [DOI] [PubMed] [Google Scholar]
- BUSSE R., FLEMING I. Pulsatile stretch and shear stress: physical stimuli determining the production of endothelium-derived relaxing factors. J. Vasc. Res. 1998;35:73–84. doi: 10.1159/000025568. [DOI] [PubMed] [Google Scholar]
- BUVINIC S., HUIDOBRO-TORO J.P. Release of endothelial nitric oxide by purinergic mechanisms in the rat mesentery. J. Physiol. 2000;523:107P–. [Google Scholar]
- BUVINIC S., HUIDOBRO-TORO J.P. Basal tonic release of nitric oxide coupled to cGMP production regulates the vascular reactivity of the mesenteric bed. Eur. J. Pharmacol. 2001;424:221–227. doi: 10.1016/s0014-2999(01)01165-7. [DOI] [PubMed] [Google Scholar]
- CARVAJAL J.A., GERMAIN A.M., HUIDOBRO-TORO J.P., WEINER C.P. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J. Cell. Physiol. 2000;184:409–420. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- CHERRY P.D., FURCHGOTT R.F., ZAWADZKI J.V., JOTHIANANDAN D. Role of endothelial cells in relaxation of isolated arteries by bradykinin. Proc. Natl. Acad. Sci. U.S.A. 1982;79:2106–2110. doi: 10.1073/pnas.79.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- CONNOLLY G.P., DULEY J.A. Uridine and its nucleotides: biological actions, therapeutic potentials. TiPS. 1999;20:218–225. doi: 10.1016/s0165-6147(99)01298-5. [DOI] [PubMed] [Google Scholar]
- DAVIS K.L., MARTIN E., TURKO I.V., MURAD F. Novel effects of nitric oxide. Annu. Rev. Pharmacol. Toxicol. 2001;41:203–236. doi: 10.1146/annurev.pharmtox.41.1.203. [DOI] [PubMed] [Google Scholar]
- DIXON C.J. Evidence that 2-methylthioATP and 2-methylthioADP are both agonists at the rat hepatocyte P2Y(1) receptor. Br. J. Pharmacol. 2000;130:664–668. doi: 10.1038/sj.bjp.0703350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONOSO M.V., FAUNDEZ H., ROSA G., FOURNIER A., EDVINSSON L., HUIDOBRO-TORO J.P. Pharmacological characterization of ETA receptor in the vascular smooth muscle comparing its analogous distribution in the rat mesenteric artery and in the rat mesenteric bed. Peptides. 1996;17:1145–1153. doi: 10.1016/s0196-9781(96)00188-x. [DOI] [PubMed] [Google Scholar]
- DRURY A.N., SZENT-GYÖRGYI A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J. Physiol. (Lond) 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLINGE D., HOU M., WEBB T.E., BARNARD E.A., MÖLLER S. Phenotype changes of the vascular smooth muscle cell regulate P2 receptor expression as measured by quantitative RT-PCR. Biochem. Biophys. Res. Commun. 1998;248:864–870. doi: 10.1006/bbrc.1998.9083. [DOI] [PubMed] [Google Scholar]
- FIGUEROA X.F., POBLETE M.I., BORIC M.P., MENDIZABAL V.E., ADLER-GRASCHINSKY E., HUIDOBRO-TORO J.P. Clonidine-induced nitric oxide-dependent vasorelaxation mediated by endothelial alpha(2)-adrenoceptor activation. Br. J. Pharmacol. 2001;134:957–968. doi: 10.1038/sj.bjp.0704320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R.F., ZAWADZKI J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GARCÍA-VELASCO G., SÁNCHEZ M., HIDALGO A., GARCIA DE BOTO M.J. Pharmacological dissociation of UTP- and ATP-elicited contractions and relaxations in isolated rat aorta. Eur. J. Pharmacol. 1995;294:521–529. doi: 10.1016/0014-2999(95)00576-5. [DOI] [PubMed] [Google Scholar]
- GARTHWAITE J., SOUTHAM E., BOULTON C.L., NIELSEN E.B., SCHMIDT K., MAYER B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one. Mol. Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- GITTERMAN D.P., EVANS R.J. Properties of P2X and P2Y receptors are dependent on artery diameter in the rat mesenteric bed. Br. J. Pharmacol. 2000;131:1561–1568. doi: 10.1038/sj.bjp.0703760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAFY K.A., KRUMENACKER J.S., MURAD F. NO, nitrotyrosine, and cyclic GMP in signal transduction. Med. Sci. Monit. 2001;7:801–819. [PubMed] [Google Scholar]
- HARDEN T.K., LAZAROWSKI E.R. Release of ATP and UTP from astrocytoma cells. Prog. Brain. Res. 1999;120:135–143. doi: 10.1016/s0079-6123(08)63551-7. [DOI] [PubMed] [Google Scholar]
- HARPER S., WEBB T.E., CHARLTON S.J., NG L.L., BOARDER M.R. Evidence that P2Y4 nucleotide receptors are involved in the regulation of rat aortic smooth muscle cells by UTP and ATP. Br. J. Pharmacol. 1998;124:703–710. doi: 10.1038/sj.bjp.0701895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOMER K.L., WANSTALL J.C. Cyclic GMP-independent relaxation of rat pulmonary artery by spermine NONOate, a diazeniumdiolate nitric oxide donor. Br. J. Pharmacol. 2000;131:673–682. doi: 10.1038/sj.bjp.0703613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUIDOBRO-TORO J.P., VALDECANTOS P. Purinergic vasocontractile mechanisms in human chorionic vessels. J. Physiol. 2000;523:102P–. [Google Scholar]
- KENNEDY C. ATP as cotransmitter in perivascular sympathetic nerves. J. Auton. Pharmacol. 1996;16:337–340. doi: 10.1111/j.1474-8673.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- KHAKH B.S., BURNSTOCK G., KENNEDY C., KING B.F., NORTH A., SÉQUÉLA P., VOIGT M., HUMPHREY P.P.A. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- KUNAPULI S.P., DANIEL J.L. P2 receptors subtypes in the cardiovascular system. Biochem. J. 1998;336:513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS C.J., ENNION S.J., EVANS R.J. P2 purinoceptor-mediated control of rat cerebral (pial) microvasculature; contribution of P2X and P2Y receptors. J. Physiol. 2000;527:315–324. doi: 10.1111/j.1469-7793.2000.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCAS K.A., PITARI G.M., KAZEROUNIAN S., RUIZ-STEWART I., PARK J., SCHULZ S., CHEPENIK K.P., WALDMAN S.A. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol. Rev. 2000;52:375–413. [PubMed] [Google Scholar]
- MALMSJÖ M., ANDER M., HARDEN T.K., PENDERGAST W., EDVINSSON L., ERLINGE D. The stable pyrimidines UDPβS and UTPγS discriminate between the P2 receptors that mediate vascular contraction and relaxation of the rat mesenteric artery. Br. J. Pharmacol. 2000a;131:51–56. doi: 10.1038/sj.bjp.0703536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALMSJÖ M., HOU M., HARDEN T.K., PENDERGAST W., PANTEV E., EDVINSSON L., ERLINGE D. Characterization of contractile P2 receptors in human coronary arteries by use of stable pyrimidines uridine 5′-O-thiodiphosphate and uridine 5′-O-3-thiotriphosphate. JPET. 2000b;293:755–760. [PubMed] [Google Scholar]
- MONCADA S. Nitric oxide in the vasculature: physiology and pathophysiology. Ann. N. Y. Acad. Sci. 1997;811:60–67. doi: 10.1111/j.1749-6632.1997.tb51989.x. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M., HIGGS E.A. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- NANDANAN E., JANG S.Y., MORO S., KIM H.O., SIDDIQUI M.A., RUSS P., MARQUEZ V.E., BUSSON R., HERDEWIJN P., HARDEN K., BOYER J.L., JACOBSON K.A. Synthesis, biological activity, and molecular modeling of ribose-modified deoxyadenosine biphosphate analogues as P2Y1 receptor ligands. J. Med. Chem. 2000;43:829–842. doi: 10.1021/jm990249v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEREDO H.A., ENERO M.A. Effect of endothelium removal on basal and muscarinic cholinergic stimulated rat mesenteric vascular bed prostanoid synthesis. Prostaglandins Leukot. Essent. Fatty Acids. 1993;48:373–378. doi: 10.1016/0952-3278(93)90117-f. [DOI] [PubMed] [Google Scholar]
- PIROTTON S., COMMUNI D., MOTTE S., JANSSENS R., BOEYNAEMS J.M. Endothelial P2-purinoceptors: subtypes and signal transduction. J. Auton. Pharmacol. 1996;16:353–356. doi: 10.1111/j.1474-8673.1996.tb00052.x. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Actions mediated by P2-purinoceptor subtypes in the isolated perfused mesenteric bed of the rat. Br. J. Pharmacol. 1988;95:637–645. doi: 10.1111/j.1476-5381.1988.tb11686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Effects of purines and pyrimidines on the rat mesenteric arterial bed. Circ. Res. 1991;69:1583–1590. doi: 10.1161/01.res.69.6.1583. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Discrimination by PPADS between endothelial P2Y- and P2U- purinoceptors in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 1996;118:428–434. doi: 10.1111/j.1476-5381.1996.tb15420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- RALEVIC V., BURRELL S., KINGDOM J., BURNSTOCK G. Characterization of P2 receptors for purine and pyrimidine in human placental cotyledons. Br. J. Pharmacol. 1997;121:1121–1126. doi: 10.1038/sj.bjp.0701262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBINO A., ZIABARY L., BURNSTOCK G. Regulation of vascular tone by UTP and UDP in isolated rat intrapulmonary arteries. Eur. J. Pharmacol. 1999;370:139–143. doi: 10.1016/s0014-2999(99)00150-8. [DOI] [PubMed] [Google Scholar]
- SHIRAKI H., KAWASAKI H., TEZUCA S., NAKATSUMA A., NAWA H., ARAKI H., GOMITA Y., KUROSAKI Y. Adrenergic nerves mediate acetylcholine-induced endothelium-independent vasodilatation in the rat mesenteric resistance artery. Eur. J. Pharmacol. 2001;419:231–242. doi: 10.1016/s0014-2999(01)00981-5. [DOI] [PubMed] [Google Scholar]
- TAYLOR M.S., GAO H., GARDNER J.D., BENOIT J.N. Effects of IBMX on norepinephrine-induced vasoconstriction in small mesenteries arteries. Am. J. Physiol. 1999;276:G909–G914. doi: 10.1152/ajpgi.1999.276.4.G909. [DOI] [PubMed] [Google Scholar]
- VALENZUELA R., HAO LI C. , HUIDOBRO-TORO J.P. Pharmacological characterisation of the inhibitory activity of βh-endorphin (βh-EP), [Arg9,19,24,28,29]-βh-EP, [Gln8, Gly31]-βh-EP-Gly-Gly-NH2, in the neuroeffector junction of the mouse vas deferens. J. Pharm. Pharmacol. 1991;43:594–597. doi: 10.1111/j.2042-7158.1991.tb03544.x. [DOI] [PubMed] [Google Scholar]
- WINDSCHEIF U., RALEVIC V., BÄUMERT H.G., MUTSCHLER E., LAMBRECHT G., BURNSTOCK G. Vasoconstrictor and vasodilator responses to various agonists in the rat perfused mesenteric arterial bed: selective inhibition by PPADS of contractions mediated via P2X-purinoceptors. Br. J. Pharmacol. 1994;113:1015–1021. doi: 10.1111/j.1476-5381.1994.tb17094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD J., GARTHWAITE J. Models of the diffusional spread of nitric oxide: implications for neural nitric oxide signalling and its pharmacological properties. Neuropharmacology. 1994;33:1235–1244. doi: 10.1016/0028-3908(94)90022-1. [DOI] [PubMed] [Google Scholar]


