Abstract
The present study examines the effect of PAT (peptide analogue of thymulin) in two rat models of inflammatory hyperalgesia induced by either i.pl. (1.25 μg in 50 μl saline) or i.p. (50 μg in 100 μl) injections of endotoxin ET.
Pretreatment with PAT (1, 5 or 25 μg in 100 μl saline, i.p.) decreased, in a dose dependent manner, both mechanical hyperalgesia, determined by the paw pressure (PP) test and thermal hyperalgesia determined by the hot plate (HP), the paw immersion (PI) and the tail flick (TF) tests.
Compared to the tripeptides K(D)PT and K(D)PV, known to antagonize interleukin (IL)-1β or IL-1β and PGE2 mechanisms, PAT, at lower dosages, exerted stronger anti-hyperalgesic effects.
When compared with the effect of a steroidal (dexamethasone) and a non-steroidal (indomethacin) anti-inflammatory drugs (NSAID), PAT demonstrated equal analgesic actions.
Pretreatment with PAT, reduced significantly the increased concentration of IL-1β, IL-6, TNF-α and NGF due to i.pl. injection of ET.
Injection of i.p. ET produced sickness behaviour characterized by hyperalgesia and fever. Pretreatment with PAT prevented the hyperalgesia and maintained the body temperature within the normal range and was accompanied by a down-regulation of the levels of pro-inflammatory cytokines and PGE2 in the liver.
PAT, in all doses used, did not result in any evident changes in the physiological parameters or in the normal behaviour of the rats.
The anti-hyperalgesic and anti-inflammatory effects of PAT can be attributed, at least partially, to the down-regulation of pro-inflammatory mediators.
Keywords: Inflammation, hyperalgesia, analgesics, septic shock, cytokines, NGF, thymulin
Introduction
Both chronic pain and acute pain are often the byproduct of inflammatory reactions to injuries and diseases. Most treatments aim primarily to relieve pain by reducing the inflammatory reactions. There are two major classes of anti-inflammatory drugs that have been used for the treatment of inflammation and the resulting pain: the non-steroidal anti-inflammatory drugs (NSAID) and the corticosteroids. Both of these drugs act by inhibiting the production of prostaglandins (PG), especially PGE2, which plays a key role in inflammation (Vane et al., 1994). Cyclo-oxygenase (COX) is the pivotal enzyme in prostaglandin biosynthesis and exists in two isoforms: COX-1 (constitutive and responsible for physiological functions) and COX-2 (inducible and involved mainly in inflammation). Inhibition of COX-1 has been associated with ulcerogenic side effects, whereas targeting of COX-2 is considered to result in therapeutic effects (Mitchell & Warner, 1999). Corticosteroids, which also inhibit the synthesis of PGE2, are well known for their immunosuppressive effects (Barnes & Adcock, 1993; Gold et al., 2001). Therefore, an ideal anti-inflammatory drug with analgesic effect would be a molecule that does not interfere with COX-1 and does not suppress the immune system. Recently, there has been interest in the development of peptides that might have properties of an ideal anti-inflammatory and analgesic drug (Hamilton et al., 1999; Huber et al., 2000).
Thymulin is a peptide hormone derived from thymic epithelial cells, shown to bind to specific receptors on human lymphoblastoid T-cell lines (Pleau et al., 1980) and to be mainly involved in several immune functions (Safieh-Garabedian et al., 1992). Recently, several reports have indicated that, besides its immunoblotting role, thymulin is capable of interacting directly and/or indirectly with the nervous system (for review, see Safieh-Garabedian et al., 1999). For example, thymulin injections, in high concentrations, reduced the ET-induced mechanical and thermal hyperalgesia and the elevation of cytokine levels (Safieh-Garabedian et al., 1996; 1998). On the other hand, thymulin injections, in low concentrations, resulted in hyperalgesia, an effect partly mediated via PGE2 and cytokines (Safieh-Garabedian et al., 2000). It has been also shown that these actions were abolished by sub-diaphragmatic vagotomy, indicating supraspinal mechanisms and the involvement of capsaicin sensitive primary afferent fibers in mediating the hyperalgesic actions of thymulin (Saadé et al., 1998).
In this study, we report on the analgesic and anti-inflammatory actions of a synthetic peptide analogue of thymulin (PAT), which was initially synthesized with the potential for clinical applications as an immunomodulating agent (Pleau et al., 1979). To characterize the analgesic and anti-inflammatory actions of PAT, we utilized two animal models of inflammation and hyperalgesia, using intraplantar (i.pl.) and intraperitoneal (i.p.) endotoxin (ET) injections. It has been previously shown that pro-inflammatory cytokines and NGF are important mediators of ET-induced hyperalgesia (Safieh-Garabedian et al., 1997). The aim of this study was to investigate whether PAT could affect the inflammatory hyperalgesia and to compare the efficacy of this molecule to that of steroidal, NSAID and peptides with known anti-inflammatory and anti-hyperalgesic actions.
Methods
Animals
Adult male Sprague-Dawley rats (200–250 g) were used in all the experiments. The animals were housed under optimum conditions of light and temperature (12 h light–dark cycle and 22±3°C), with food and water provided ad libitum. All experiments were carried out with strict adherence to National Institutes of Health guide for the care and use of laboratory animals for pain experimentation, and were approved by the Institutional Animal Care Committee.
Behavioural measurements
Thermal and mechanical pain tests were performed for 3 consecutive days prior to any injections to establish baseline values. The paw pressure test was used to assess the threshold of mechanical nociception and the hot plate (HP), paw immersion (PI) and tail immersion (TF) tests were performed for the assessment of thermal nociception. For the HP test, animals were placed individually on a hot plate (52.8°–53.2°C) and the latency of the first sign of paw licking or jumping was taken as an index of the pain threshold. In the TF test, the tail of each animal was immersed into a beaker of distilled water (T=50.5°C) and the withdrawal latency for tail flicking was recorded; scores were based on three trials with a 5 min interval between consecutive tests. For the PI test, the hind paws were dipped alternately into a beaker of distilled water (T=48°C) and the latency to onset of paw removal was recorded. The paw pressure (PP) test was performed by applying a constant pressure of 0.20 kg cm2, alternately to the left and right hind paws, with a 3 min interval between consecutive applications. The pressure was discontinued when the animals displayed a typical reaction characterized by a vigorous flexion reflex (for more details, see Kanaan et al., 1996).
For both the PP and PI tests, the threshold of each test was based on the average of two measurements performed alternately on each hind paw of each animal with a minimum of 3 min between two consecutive trials.
Induction of inflammatory hyperalgesia
Two animal models were used to establish inflammatory hyperalgesia, one for localized and the other for systemic inflammation. In the localized model, rats received i.pl. injection of a solution (1.25 μg in 50 μl saline) of ET (lipopolysaccharide from Salmonella typhosa, Sigma) in one hind paw which resulted in both thermal and mechanical hyperalgesia, restricted to the injected leg, as described previously (Kanaan et al., 1996). In the second model, rats received i.p. injection of ET (50 μg in 100 μl saline).
Recordings of the body temperature, blood pressure and respiratory rate were performed on moderately restrained rats. This control procedure was performed, at least, on one group of rats from each model and aimed to determine whether PAT injections (5 μg or 50 μg) can alter the above-mentioned physiological parameters.
Protocol 1
Different groups (n=5 each) of rats received i.pl. injections of either sterile saline (50 μl) or ET (1.25 μg in 50 μl saline) alone or preceded by pretreatment with various drugs. These groups were used either in behavioural observations or for leg tissue sampling for the determination of the levels of various pro-inflammatory mediators.
Two groups injected with saline were used either for behavioural observation of nociceptive thresholds (one group) or for tissue sampling (the other group).
Four groups received either i.p. injection of PAT alone (25 μg in 100 μl saline; 114 pmol) or PAT followed after 30 min by ET (1.25 μg, i.pl.) as one group per each of the following dosages of PAT: 1, 5 and 25 μg/rat (4.5 to 114 pmol), and were subjected to the various behavioural tests for nociception over a period of 24 h. The reported results on nociception were based on measurements made at 9 h post injection, the time that corresponds to the peak of hyperalgesia as shown previously by Kanaan et al. (1996).
The efficacy of PAT was compared with that of steroidal, NSAID and peptides known to antagonize IL-1β and PGE2 induced hyperalgesia in another four groups of rats. One group was pretreated with injections of Lys-D-Pro-Thr (10 mg kg−1 in 100 μl saline; 1.1 μmol, i.p.) 30 min before ET injection. This tripeptide is related to IL-1β-(193-195) and is known to antagonize IL-1β evoked hyperalgesia (Ferreira et al., 1988). In the second group, rats received injections of α-MSH related tripeptide, Lys-D-Pro-Val (10 mg kg−1 in 100 μl saline; 1.1 μmol, i.p.) 30 min before ET injection. Lys-D-Pro-Val has been shown to antagonize both IL-1β and PGE2 induced hyperalgesia (Poole et al., 1992). The dose utilized for both tripeptides was determined in a previous study (Safieh-Garabedian et al., 1997). The third and fourth groups were each treated by either dexamethasone or indomethacin in doses established as appropriate in previous studies (Benedetti & Butler, 1990; Borchard et al., 1992). Dexamethasone (200 μg kg−1; 1.01 μmol) and indomethacin (4 mg kg−1; 11.2 μmol) were injected 30 min before and 5 h after ET injection (for details, see Kanaan et al., 1997; Safieh-Garabedian et al., 1997).
Finally, six groups of rats injected with saline (one group), PAT, 25 μg alone (one group), ET alone or ET preceded by PAT, 25 μg (two groups for each treatment) were sacrificed at 1 or 3 h post ET injection for the determination of the levels of TNF-α or IL-1β, IL-6 and NGF, respectively.
Protocol 2
This protocol is based on systemic injection of high doses of ET (50 μg in 100 μl saline, i.p.). Such injections have been shown to produce sickness behaviour, fever and hyperalgesia (Maier et al., 1993).
One group of rats received i.p. injection of ET (50 μg) whereas another group was pretreated with PAT (25 μg, i.p.) 30 min before ET, and the TF and PP tests were carried out at 1, 3 and 6 h after the ET injection. Saline (100 μl, i.p.) or PAT (50 μg, in 100 μl, i.p.) injections only were performed on two control groups (as one group per treatment). Body temperature was monitored rectally in all rats from the various groups over a period of 6 h following ET injections. The effects of pretreatment with PAT at the doses of 25, 100 and 200 μg were tested in another three groups of rats treated with ET.
For the determination of the levels of cytokines and PGE2, liver tissues were sampled from four groups of ratssacrificed 1 h following injections of saline, PAT (25 μg), ET (50 μg) or PAT followed after 30 min by ET, as one group per each treatment.
Cytokine, PGE2 and NGF determination
In the experiments, which involved tissue removal for cytokine and NGF assay, the animals were terminally anaesthetized (sodium pentobarbital, 50 mg kg−1) and the entire hind paw skin (from left and right feet, protocol 1d) or liver biopsies (protocol 2b) were removed. The tissue samples were weighed; snap frozen on dry ice and stored at −70°C to be processed for the determination of the levels of IL-1β, TNF-α, IL-6, PGE2 and NGF.
Skin tissue was homogenized in phosphate buffered saline (PBS; pH=7.4) containing 0.4 M NaCl, 0.05% Tween-20, 0.5% bovine serum albumin, 0.1 mM phenylmethylsulfonyl fluoride, 0.1 mM benzethonium chloride, 10 mM EDTA and 20 KI ml−1 aprotinin. The homogenates were centrifuged at 12,000 g for 60 min at 4°C. Two-site enzyme-linked immunosorbent assay (ELISA) was used to measure cytokines and NGF content in the supernatants.
PGE2 was determined by a competitive ELISA using a commercial kit (Biosource), as recommended by the manufacturer. Briefly, both standards and samples (100 μl of each) were added to goat anti-mouse IgG coated microtiter plates followed by the addition of alkaline phosphatase conjugated PGE2 (50 μl) and an anti-PGE2 monoclonal antibody (50 μl). After incubation for 2 h with shaking, at room temperature, the plates were washed and the colour was developed using p-nitrophenyl phosphate solution. The optical density was measured at 405 nm.
NGF was measured using an immunoassay kit (Promega) as described by the manufacturer. Standards and samples (100 μl of each) were added to microtiter plates, in duplicate, coated with polyclonal anti-NGF antiserum. After overnight incubation (4°C) and washing, monoclonal anti-NGF antiserum (100 μl) was added to all the wells and the plates incubated overnight (4°C). Following washing of the plates, 100 μl of IgG HRP conjugate was added to all the wells and the plates incubated for 2.5 h at room temperature. After washing, the colour was developed using tetramethylbenzidine and the optical density was measured at 450 nm.
The cytokines IL-1β, TNF-α and IL-6 were assayed as above, with reagents provided by the National Institute for Biological Standards and Control, Blanche Lane, U.K. and detailed previously (Safieh-Garabedian et al., 2000). Plates were coated with affinity purified polyclonal sheep anti-cytokine antisera and the capturing antibody used was biotinylated affinity purified polyclonal sheep antisera.
Injected Drugs
PAT (Glu-Ala-Lys-Ser-Gln-Gly-Gly-Ser-Asp) custom synthesized by Quantum Biotechnologies Inc. (Canada) was dissolved in sterile saline and injected at concentrations varying from 1 to 200 μg per rat. ET (lipopolysaccharide from Salmonella typhosa, Sigma) was dissolved in saline. The tripeptides Lys-D-Pro-Thr and Lys-D-Pro-Val were custom synthesized by Cambridge Research Biochemicals, Cambridge, U.K. and provided by Dr Stephen Poole, National Institute of Biological Standards and Control. Injectable dexamethasone phosphate (Laboratory Renaudin, France) was diluted in saline to the appropriate dosage and injected immediately. Indomethacin was prepared by dissolving indomethacin-lactose (gift from Algorithm, Lebanon) in phosphate buffered saline (pH 7.4).
Data analysis
The baseline threshold of each behavioural nociceptive test was established by averaging the measurements made on each experimental group during 2 or 3 days before any treatment. These thresholds were measured, following the same method, at different time intervals after drug treatment. The variations of thresholds of each test in each experimental group were determined, unless otherwise indicated, with reference to the baseline value using ANOVA followed by Bonferroni post-hoc test.
The determination of the levels of cytokines and NGF, was made by averaging the measurements performed in each experimental group. Their variations were assessed by comparisons with the levels absorbed in control group injected with sterile saline, using the same statistical method. The Instat 3 and Prism softwares (GraphPad software, CA, U.S.A.) were used to perform all statistical analysis and graphic illustrations.
Results
Effect of PAT on ET-induced localized inflammatory hyperalgesia
Injection of ET (1.25 μg in 50 μl saline, i.pl.) in the hind paws of rats caused a significant decrease in the nociceptive thresholds which peaked at 9 h (peak of hyperalgesia) as determined by the PP test for mechanical hyperalgesia and the PI and TF tests for thermal hyperalgesia (P<0.001 for all tests). Treatment with PAT (1, 5 and 25 μg) reduced, in a dose dependent manner, the ET-induced hyperalgesia (Figure 1). With the dose of 25 μg PAT, the latencies to onset of the various responses returned to their control levels before the ET injection (P>0.05 for all values compared with the baseline or saline values). Injection of PAT alone (25 μg in 100 μl saline, i.p.) did not produce significant alterations in the latencies of the various pain tests (Figure 1).
Figure 1.
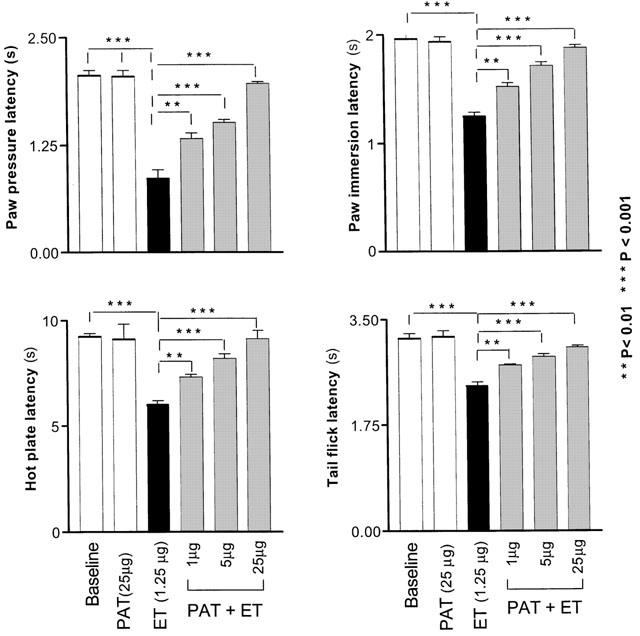
Dose-dependent reduction of the endotoxin-induced hyperalgesia by PAT. Each bar represents the mean±s.e.mean of measurements made on a separate group of rats (n=5) for each treatment. All measurements were performed at 9 h following the injections, the time that corresponds to the peak of hyperalgesia induced by ET. The significance of differences was measured for each experimental group in reference to the control group (injected with saline).
Comparison of efficacy of PAT with other drugs and antagonists
When comparing the effects of PAT on ET-induced hyperalgesia (1.25 μg, i.pl.) with the effects obtained with the peptides Lys-D-Pro-Val (10 mg kg−1) and Lys-D-Pro-Thr (10 mg kg−1), the results obtained demonstrated that PAT was much more effective than both peptides (Figure 2). Compared to indomethacin (4 mg kg−1, i.p., two injections) or dexamethasone (200 μg kg−1, i.p., two injections), PAT exerted comparable or even stronger analgesic effects and at much lower concentration (Figure 2).
Figure 2.
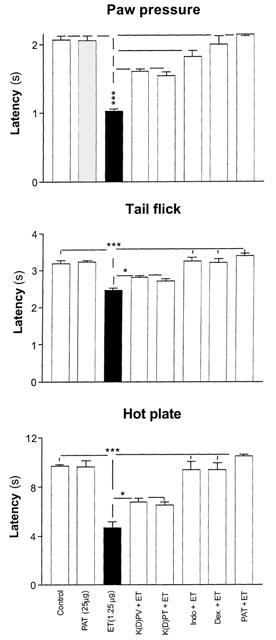
The effect of PAT pretreatment on ET-induced inflammatory hyperalgesia is compared with pretreatment with steroidal, NSAID and other analgesic tripeptides. Each bar represents the mean±s.e.mean of measurements performed on a separate group of rats (n=5) for each treatment. All measurements were performed at 9 h following the injections. The significance of differences was measured in reference to the value of saline control for each test. Abbreviations: K(D)PV, Lys. D. Pro. Val, (10 mg kg−1; 1.1 μmol); K(D)PT, Lys. D. Pro. Thr, (10 mg kg−1; 1.1 μmol); Indo; Indomethacin (4 mg kg−1; 11.2 μmol); Dex., Dexamethasone (200 μg kg−1; 1.01 μmol); PAT, peptide analogue of thymulin (114 pmol).
Effect of PAT on cytokine up-regulation by ET
Injection of ET in the hind paw of rats resulted in a significant (P<0.001) increase in the concentrations of pro-inflammatory cytokines and NGF when compared to their levels in saline injected animals or the non-injected legs of the same animals. This increase reached a peak at 1 h for TNF-α and at 3 h for IL-1β, IL-6 and NGF after ET injection (Figure 3). Pretreatment with PAT (25 μg, i.p.) abolished the increased concentration of TNF-α and significantly reduced the levels of IL-1β (from 2850.6±255.4 to 1686.0±266.0 pg/hind paw, P<0.05), IL-6 (from 2831.0±285.0 to 1158.0±197.0 pg/hind paw, P<0.01) and NGF (from 23.0±1.73 to 16.73±2.70 ng/hind paw, P<0.01) (Figure 3). Injections of PAT (25 μg) in control animals did not produce significant alterations in the concentration of the cytokines and NGF as shown in Figure 3.
Figure 3.
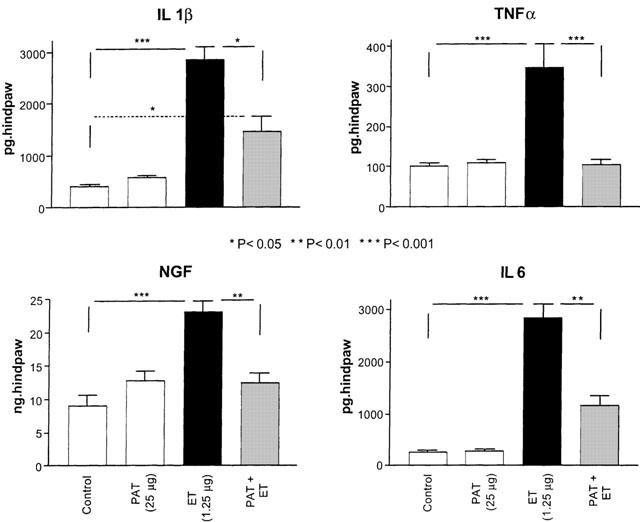
Pretreatment with PAT prevented the increase in cytokines and NGF concentration due to ET injection. Each bar represents the determination of the level of each cytokine and NGF in the skin tissues of the injected paws of a separate group of rats (n=5) for the indicated treatment. The levels of cytokines and NGF were measured at the time of peak of their upregulation by ET, which corresponds to 1 h for TNF-α and 3 h for the remaining factors. The significance of differences was measured by comparing their levels with saline injected controls.
Effect of PAT on ET-induced systemic inflammatory hyperalgesia
Injections of ET (50 μg, i.p.) resulted in a significant decrease in the nociceptive thresholds over a period of 6–9 h as assessed by the PP and TF tests. Pretreatment with PAT (50 μg in 100 μl saline, i.p.), 30 min before systemic ET, abolished the ET-induced mechanical (Figure 4A) and thermal (Figure 4B) hyperalgesia.
Figure 4.
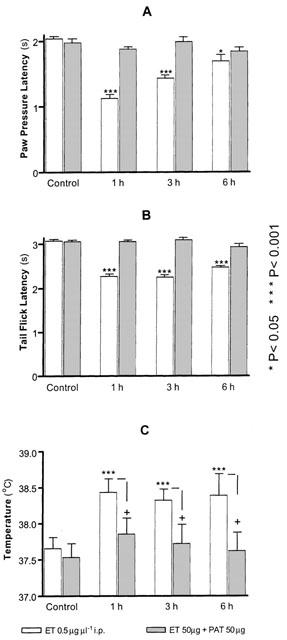
Pretreatment with PAT prevented the hyperalgesia and fever induced by systemic injection of ET. Two groups (n=5) of rats were used, one injected with ET the other received ET preceded by PAT injection. The pairs of bars in each panel represent the measurements made before (control) or at the indicated time interval following treatments. The significance of difference was established by the two-tailed student t-test with reference to the control (*) before injection or between the two groups of each time interval (+).
ET is a known pyrogen, and its injection resulted in a significant increase in body temperature by 1 h (38.43±0.28°C vs control 37.65±0.16°C), which remained significantly elevated after 6 h (Figure 4C). Pretreatment with PAT reversed the elevated body temperature due to ET injection (Figure 4C).
Pretreatment of different groups of rats with different doses of PAT (25, 50, 100 and 200 μg) showed that PAT reduced significantly the effect of ET at the dose of 25 μg and its effect peaked with the dose of 50 μg (Figure 5).
Figure 5.

Dose-dependent effect of PAT pretreatment on the hyperalgesia induced by systemic injection of ET. Each bar, in each panel, represents the mean±s.e.mean of measurements made on one group of rats for the indicated treatment. The significance of difference was established with reference either to control (*) measured before any treatment or to the peak of hyperalgesia induced by ET (+).
Systemic injection of ET, on the other hand, produced significant up-regulation of IL-1β (P<0.001), IL-6 (P<0.01), TNF-α (P<0.01) and PGE2 (P<0.01) levels in the liver when compared to rats injected with either sterile saline or PAT (25 μg in 100 μl saline, i.p.) alone (Figure 6). Pretreatment with PAT exerted strong reduction of IL-6 and PGE2 to the control level and significant attenuation of the increased levels of TNF-α (P<0.05) and IL-1β (P<0.01) by ET (Figure 6).
Figure 6.
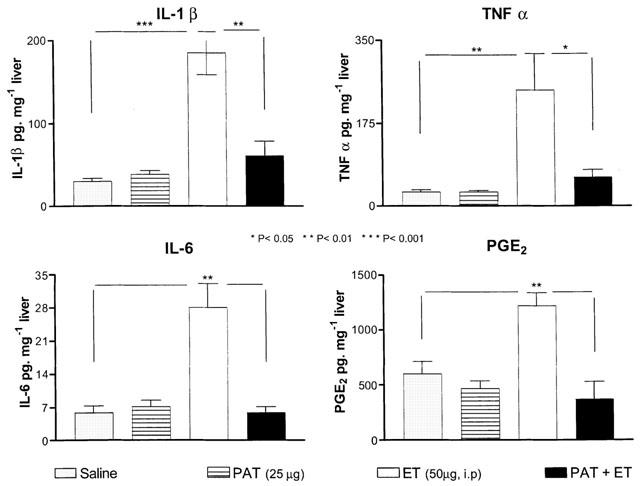
Pretreatment with PAT reduced the up-regulation of cytokine and PGE2 levels by systemic injection of ET. Each bar represents the mean±s.e.mean of measurements performed on one group of rats for the indicated treatment, 1 h following the injection.
Discussion
Two models of ET-induced inflammatory hyperalgesia have been characterized during the last decade. The first was based on systemic inflammation induced by i.p. injection of high doses of ET (Maier et al., 1993) and the observed hyperalgesia, fever and illness were attributed to the up-regulation of cytokines levels, specially IL-1β and PGE2 (for a review, see Konsman et al., 2002). The second described inflammatory The second described inflammatory hyperalgesia that was induced by local (i.pl.) injection of a small dose of ET in rats and mice (Kanaan et al., 1996). Further investigations showed that this localized inflammatory hyperalgesia was preceded by significant up-regulation of pro-inflammatory cytokines and NGF (Kanaan et al., 1998; Safieh-Garabedian et al., 1997) and pretreatment with either anti-inflammatory drugs (Safieh-Garabedian et al., 1997) or anti-inflammatory cytokines (IL-10) prevented the ET-induced hyperalgesia (Kanaan et al., 1998). The present study was based on the use of both models, and showed significant inhibitory effects exerted by PAT on the ET-induced hyperalgesia and up-regulation of cytokine levels. It is interesting to note that, PAT injection to naïve rats, at the dosage used, did not produce significant alteration of physiological parameters such as blood pressure, respiratory rate, temperature and normal motor behaviour. On the other hand, PAT treatment of rats subjected to systemic injection of ET, appeared to alleviate the illness behaviour induced by systemic inflammation. This observation will be considered in more detail later in this discussion.
Since the demonstration of the role of pro-inflammatory cytokines (Cunha et al., 1992; Ferreira et al., 1998; and for review see Poole et al., 2000) and NGF (for review, see Lewin & Mendell, 1993; McMahon, 1996) in mediating inflammatory response and hyperalgesia, the targeting of these molecules has become a major focus for the treatment of inflammatory diseases (Dinarello et al., 1993). On the other hand, it has been shown that PGE2 plays a major role as a key regulator of cytokine production and the inflammatory cascade (Portanova et al., 1996; Vane et al., 1994). A new therapeutic approach for the treatment of pain and inflammatory diseases has been initiated, for three decades based on the inhibition of prostaglandins by NSAID (Vane, 1971). Despite extensive medical use of several generations of NSAID, the outcome of selective targeting of COX-2 mechanisms is still debated (Wallace et al., 1999, and for review, see Fröhlich, 1997; Furst, 1998; Vane, 1998). Three major issues constituted the main topic of this debate: (1) the variation of toxicity of NSAID depending on selective inhibition of either COX-1 or COX-2 (for review, see Furst, 1998; Vane, 1998); (2) the other therapeutic aspects of NSAID considered independent from COX inhibition (Furst, 1998; Osnes et al., 1996); (3) the limited anti-inflammatory effects of COX-2 inhibition in some inflammatory situations and its possible detrimental side effects (Wallace et al., 1999; Fitzgerald & Patrono, 2001). This debate, in addition to other clinical limitations for the use of NSAID, provided the basis for a new trend considering the pro-inflammatory mediators as potential targets for therapeutic intervention in inflammatory diseases (Ferreira et al., 1988; Hamilton et al., 1999; Huber et al., 2000; Poole et al., 1992).
Despite the demonstration of promising results in the laboratory, most anti-cytokine drugs have resulted in poor efficacy in clinical trials (Huber et al., 2000). This has been thought to be due to targeting of one mediator only, which may not be sufficient to block complex inflammatory responses (Camussi & Lupia, 1998). PAT in a single dose, however, was effective in decreasing the level of several of these inflammatory mediators, which may prove to be an important effect not shown by other anti-inflammatory molecules. As illustration, PAT exerted stronger inhibition of ET-induced hyperalgesia than K(D)PV or K(D)PT and at a dosage, at least 10 times lower than that used for both peptides. Furthermore, these tripeptides were reported to antagonize either IL-1β (KDPT) or IL-1β and PGE2 (KDPV) mechanisms (Ferreira et al., 1988; Poole et al., 1992), while PAT showed an almost equal reversal of the upregulations of interleukins, TNF-α and NGF by i.pl. injection of ET.
Another interesting aspect in the reported effects of PAT treatment was the amelioration of the symptoms of illness behaviour induced by systemic injection of ET. This amelioration took the form of reversal of the hyperalgesia, improvement of the motor behaviour and prevention of febrile reactions to ET. Accumulating evidence has shown that ET does not cross the blood brain barrier (Dascombe & Milton, 1979) and can induce the illness behaviour and fever through PGE2-dependent mechanisms (Cao et al., 1997; van dam et al., 1993, for a review, see Konsman et al., 2002). Therefore, the anti-febrile effect of PAT can be attributed to the inhibition of COX-2 mechanisms leading to PGE2 formation. This assumption can be correlated with the observed down-regulation of the levels of cytokine and PGE2, which were shown to be up-regulated in the liver following systemic injection of ET. Preliminary results from our laboratory have demonstrated that PAT inhibits the ET-induced nuclear activation of NF-κB (unpublished data), the transcription factor required for the expression of pro-inflammatory cytokine genes (Osnes et al., 1996). Thus, the inhibition of COX-2 and the deactivation of NF-κB can constitute a solid substrate for the explanation of the anti-hyperalgesic and anti-inflammatory effects of PAT. This assumption, however, does not exclude possible action of PAT, like thymulin, on the afferent nerve fibers involved in nociception and neuroimmune regulations (Saadé et al., 1998). Recent evidence from our groups demonstrates that PAT exerts strong inhibition of neuropathic manifestations, which are not necessarily the end-product of inflammatory mechanisms (Saadé et al., unpublished data).
In conclusion, the observed antihyperalgesic action of PAT can be attributed to its inhibitory effects on the inflammatory cascade through the down-regulation of the levels of pro-inflammatory cytokines and NGF. This inhibition may be attributed, at least in part, to the inhibition of COX-2 mechanisms targeted by the NSAID drugs. Thus, PAT may exert its antihyperalgesic effects, at least partially, like the NSAID drugs by reducing the inflammatory reactions.
Acknowledgments
The authors wish to thank Messrs Sawsan Sharrouf, Cynthia Massaad and Riad Maalouf for their technical assistance. This study received partial support from the Franco–Lebanese CEDRE Project and from the Lebanese National Research Council for Scientific Research.
Abbreviations
- COX
cyclo-oxygenase
- ELISA
enzyme-linked immunosorbent assay
- ET
endotoxin
- HP
hot plate
- IL
interleukin
- i.p.
intraperitoneal
- i.pl.
intraplantar
- K(D)PT
Lys-D-Pro-Thr
- K(D)PV
Lys-D-Pro-Val
- NGF
nerve growth factor
- NSAID
non-steroidal anti-inflammatory drugs
- PAT
peptide analogue of thymulin
- PG
prostaglandin
- PI
paw immersion
- PP
paw pressure
- TF
tail flick
- TNF
tumour necrosis factor
References
- BARNES P.J., ADCOCK I. Antiinflammatory actions of steroids: molecular mechanisms. Trends Pharmacol. Sci. 1993;14:436–441. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- BENEDETTI C., BUTLER S.H.Systemic analgesics Management of Pain 1990Philadelphia: Lea and Febiger; 1640–1675.ed. Bonica, J.J [Google Scholar]
- BORCHARD R.E., BARNES C.D., ELTHERINGTON L.G. Drug dosage in laboratory animals. 1992Florida: Boca Raton; A handbook, 3rd edn [Google Scholar]
- CAMUSSI G., LUPIA E. The future of anti-tumour necrosis factor (TNF) products in the treatment of rheumatoid arthritis. Drugs. 1998;55:613–620. doi: 10.2165/00003495-199855050-00001. [DOI] [PubMed] [Google Scholar]
- CAO C., MATSUMURA K., YAMAGATA K., WATANABE Y. Involvement of cyclooxygenase-2 in LPS-induced fever and regulation of its mRNA by LPS in the rat brain. Am. J. Physiol. 1997;272:R1712–R1725. doi: 10.1152/ajpregu.1997.272.6.R1712. [DOI] [PubMed] [Google Scholar]
- CUNHA F.Q., POOLE S., LORENZETTI B.B., FERREIRA S.H. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DASCOMBE M.J., MILTON A.S. Study on the possible entry of bacterial endotoxin and prostaglandin E2 into the central nervous system from the blood. Br. J. Pharmacol. 1979;66:565–572. doi: 10.1111/j.1476-5381.1979.tb13695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINARELLO C.A., GELFAND J.A., WOLFF S.M. Anticytokine strategies in the treatment of the systemic inflammatory response syndrome. J. Am. Med. Assoc. 1993;269:1829–1835. [PubMed] [Google Scholar]
- FERREIRA S.H., LORENZETTI B.B., BRISTOW A.F., POOLE S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- FITZGERALD G.A., PATRONO C. The coxibs, selective inhibitors of cyclooxygenase-2. N. Engl. J. Med. 2001;345:433–442. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- FRÖHLICH J.C. A classification of NSAIDs according to the relative inhibition of cyclooxygenase isoenzymes. TIPS. 1997;18:30–34. doi: 10.1016/s0165-6147(96)01017-6. [DOI] [PubMed] [Google Scholar]
- FURST D.E. Perspectives on the cyclooxygenase-2/cyclooxygenase-1 hypothesis. J. Clin. Rheumatol. 1998;5:S40–S48. doi: 10.1097/00124743-199810001-00007. [DOI] [PubMed] [Google Scholar]
- GOLD R., BUTTGEREIT F., TOYOTA K.V. Mechanism of action of glucocorticosteroid hormones: possible implications for therapy of neuroimmunological disorders. J. Neuro. 2001;117:1–8. doi: 10.1016/s0165-5728(01)00330-7. [DOI] [PubMed] [Google Scholar]
- HAMILTON K., CLAIR E.W., O'DELL J.R. Anticytokine therapy: a new era in the treatment of rheumatoid arthritis. N. Engl. J. Med. 1999;340:310–312. doi: 10.1056/NEJM199901283400411. [DOI] [PubMed] [Google Scholar]
- HUBER T.S., GAINES G.C., WELBORN M.B., III, ROSENBURG J.J., SEEGER J.M., MOLDAWER L.L. Anticytokine therapies for acute inflammation and the systemic inflammatory response syndrome: Il-10 and ischemia/reperfusion injury as new paradigm. Shock. 2000;13:425–434. doi: 10.1097/00024382-200006000-00002. [DOI] [PubMed] [Google Scholar]
- KANAAN S.A., POOLE S., SAADÉ N.E., JABBUR S.J., SAFIEH-GARABEDIAN B. Interleukin-10 reduces the endotoxin induced hyperalgesia in mice. J. Neuroimmunol. 1998;86:142–150. doi: 10.1016/s0165-5728(98)00027-7. [DOI] [PubMed] [Google Scholar]
- KANAAN S.A., SAADÉ N.E., HADDAD J.J., ABDELNOOR A.M., JABBUR S.J., SAFIEH-GARABEDIAN B. Endotoxin-induced local inflammation and hyperalgesia in rats and mice: a new model of inflammatory pain. Pain. 1996;66:373–379. doi: 10.1016/0304-3959(96)03068-0. [DOI] [PubMed] [Google Scholar]
- KANAAN S.A., SAFIEH-GARABEDIAN B., HADDAD J.J., ATWEH S.F., ABDELNOOR A.M., JABBUR S.J., SAADÉ N.E. Effects of various analgesic and anti-inflammatory drugs on endotoxin-induced hyperalgesia in rats and mice. Pharmacology. 1997;54:285–297. doi: 10.1159/000139498. [DOI] [PubMed] [Google Scholar]
- KONSMAN J.P., PARNET P., DANTZER P. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- LEWIN G.R., MENDELL L.M. Nerve growth factor and nociception. Trends Neurosci. 1993;16:353–359. doi: 10.1016/0166-2236(93)90092-z. [DOI] [PubMed] [Google Scholar]
- MAIER S.F., WIERTELAK E.P., GOEHLER L., MARTIN D., WATKINS L.R. Interleukin-1 mediates the behavioral hyperalgesia produced by lithium chloride and endotoxin. Brain Res. 1993;623:321–325. doi: 10.1016/0006-8993(93)91446-y. [DOI] [PubMed] [Google Scholar]
- MCMAHON S.B. NGF as a mediator of inflammatory pain. Philos. Trans. R. Soc. Lond. 1996;351:431–440. doi: 10.1098/rstb.1996.0039. [DOI] [PubMed] [Google Scholar]
- MITCHELL J.A., WARNER T.D. Cyclo-oxygenase-2: pharmacology, physiology, biochemistry and relevance to NSAID therapy. Br. J. Pharmacol. 1999;128:1121–1132. doi: 10.1038/sj.bjp.0702897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSNES L.T.N., FOSS K.B., JOØ G.B., OKKENHAUG C., WESTVIK A.B., ØVSTEBØ R., KIERULF P. Acetylsalicylic acid and sodium salicylate inhibit LPS-induced NF-κB/c-Rel nuclear translocation, and synthesis of tissue factor (TF) and tumor necrosis factor alfa (TNF-α) in human monocytes. Thromb. Haemost. 1996;76:970–976. [PubMed] [Google Scholar]
- PLEAU J.M., DARDENNE M., BLANOT D., BRICAS E., BACH J.F. Antagonistic analogue of serum thymic factor (FTS) interacting with FTS cellular receptor. Immunol. Lett. 1979;1:179–182. [Google Scholar]
- PLEAU J.M., FUENTES V., MORGAT J.L., BACH J.F. Specific receptors for serum thymic factor (FTS) in lymphoblastoid cultured cell lines. Proc. Natl. Acad. Sci. U.S.A. 1980;77:2861–2865. doi: 10.1073/pnas.77.5.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POOLE S., BRISTOW A.F., LORENZETTI B.B., GAINES DAS R.E., SMITH T.W., FERREIRA S.H. Peripheral analgesic activities of peptides related to α-melanocyte stimulating hormone and interleukin-1β193-195. Br. J. Pharmacol. 1992;106:489–492. doi: 10.1111/j.1476-5381.1992.tb14361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POOLE S., CUNHA F.Q., FERREIRA S.H.Bradykinin, cytokines and inflammatory hyperalgesia Pain and neuroimmune interactions 2000New York: Kluwer Academic/Plenum Publishers; 31–45.eds. Saadé, N.E., Apkarian, A.V., Jabbur, S.J [Google Scholar]
- PORTANOVA J.P., ZHANG Y., ANDERSON G.D., HAUSER S.D., MASFERRER J.L., SEIBERT K., GREGORY S.A., ISAKSON P.C. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J. Exp. Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAADÉ N.E., MAJOR S.C., ATWEH S.F., JABBUR S.J., KANAAN S.A., SAFIEH-GARABEDIAN B. Involvement of capsaicin sensitive primary afferents in thymulin-induced hyperalgesia. J. Neuroimmunol. 1998;91:171–179. doi: 10.1016/s0165-5728(98)00176-3. [DOI] [PubMed] [Google Scholar]
- SAFIEH-GARABEDIAN B., DARDENNE M., KANAAN S.A., ATWEH S.F., JABBUR S.J., SAADÉ N.E. The role of cytokines and prostaglandin-E2 in thymulin induced hyperalgesia. Neuropharmacology. 2000;39:1653–1661. doi: 10.1016/s0028-3908(99)00247-6. [DOI] [PubMed] [Google Scholar]
- SAFIEH-GARABEDIAN B., JALAKHIAN R.H., JABBUR S.J., SAADÉ N.E., KANAAN S.A.Thymulin in high doses reduces endotoxin-induced hyperalgesia by reducing interleukin-1β and nerve growth factor levels in the hind paw of rats Pain mechanisms and Management 1998Amsterdam: IOS Press; 131–138.eds. Apkarian, A.V. & Ayrapetian, S [Google Scholar]
- SAFIEH-GARABEDIAN B., JALAKHIAN R.H., SAADÉ N.E., HADDAD J.J., JABBUR S.J., KANAAN S.A. Thymulin reduces hyperalgesia induced by peripheral endotoxin injection in rats and mice. Brain Res. 1996;717:179–183. doi: 10.1016/0006-8993(95)01532-9. [DOI] [PubMed] [Google Scholar]
- SAFIEH-GARABEDIAN B., KANAAN S.A., HADDAD J.J., ABOU JAOUDE P., JABBUR S.J., SAADÉ N.E. Involvement of interleukin-1β, nerve growth factor and prostaglandin-E2 in endotoxin induced localized inflammatory hyperalgesia. Br. J. Pharmacol. 1997;121:1619–1626. doi: 10.1038/sj.bjp.0701313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAFIEH-GARABEDIAN B., KANAAN S.A., JABBUR S.J., SAADÉ N.E. Cytokine mediated or direct effects of thymulin on the nervous system. Neuroimmunomodulation. 1999;6:39–44. doi: 10.1159/000026362. [DOI] [PubMed] [Google Scholar]
- SAFIEH-GARABEDIAN B., KENDALL M.D., KHAMASHTA M.A., HUGHES G.R.V. Thymulin and its role in immunomodulation. J. Autoimmunity. 1992;5:547–555. doi: 10.1016/0896-8411(92)90152-g. [DOI] [PubMed] [Google Scholar]
- VAN DAM A.M., BROUNS M., MAN-A-HING W., BERKENBOSCH F. Immunocytochemical detection of prostaglandin E2 in microvasculature and in neurons of rat brain after administration of bacterial endotoxin. Br. Res. 1993;613:331–336. doi: 10.1016/0006-8993(93)90922-a. [DOI] [PubMed] [Google Scholar]
- VANE J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- VANE J.R. Differential inhibition of cyclooxygenase isoforms: An explanation of the action of NSAIDs. J. Clin. Rheumatol. 1998;4:S3–S10. doi: 10.1097/00124743-199810001-00002. [DOI] [PubMed] [Google Scholar]
- VANE J.R., MITCHELL J.A., APPLETON I., BISHOP-BAILEY D., CROXTALL J., WILLOUGHBY D.A. Inducible isoforms of cyclooxygenase and nitric oxide synthase in inflammation. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACE J.L., CHAPMAN K., MCKNIGHT W. Limited anti-inflammatory efficacy of cyclo-oxygenase-2 inhibition in carrageenan-airpouch inflammation. Br. J. Pharmacol. 1999;126:1200–1204. doi: 10.1038/sj.bjp.0702420. [DOI] [PMC free article] [PubMed] [Google Scholar]


