Abstract
We have examined the relationship between neutrophil accumulation, NO• production and nitrated protein levels in zymosan-mediated inflammation in rat skin in vivo.
Rats were anaesthetized and cutaneous inflammation was induced by zymosan (injected intradermally, i.d.). Experiments were carried out up to 48 h, in recovery procedures as appropriate. Assays for neutrophil accumulation (measurement of myeloperoxidase), nitric oxide (assessment of NO2−/NO3−) and nitrated proteins (detected by ELISA and Western blot) were performed in skin extracts.
The results demonstrate a close temporal relationship between these parameters. Samples were assayed at 1, 4, 8, 24 and 48 h after i.d. injection of zymosan. The highest levels measured of each parameter (P<0.001 compared with vehicle) were found at 4–8 h, with a reduction towards basal levels by 24 h.
Selective depletion of circulating neutrophils with anti-neutrophil antibody abolished neutrophil accumulation and protein nitration. In addition substantially decreased NO levels were found.
A selective inducible nitric oxide synthase (iNOS) inhibitor, N-3-aminomethyl-benzyl-acetamidine-dihydrochloride (1400W) also significantly reduced neutrophil levels and NO production and substantially inhibited protein nitration.
We conclude that the neutrophil leukocyte plays an essential role in the formation of iNOS-derived NO and nitrated proteins in inflammation, in a time-dependent and reversible manner. The NO-derived iNOS also has a role in stimulating further neutrophil accumulation into skin. This suggests a close mechanistic coupling between neutrophils, NO production and protein nitration.
Keywords: inducible nitric oxide synthase, nitric oxide, nitrotyrosine, rodent, zymosan
Introduction
Many inflammatory processes are associated with increased nitric oxide (NO) production and an upregulation of inducible nitric oxide synthase (iNOS), also known as NOS II. The polymorphonuclear leukocyte has a primary role in inflammation and the rapid accumulation of neutrophils in inflamed tissue is an essential defensive component in the response of the body to injury (Becker, 1990). The pro-inflammatory potential of the neutrophil in disease is also well established (Becker, 1990; Scapini et al., 2000). Interestingly, the direct contribution of the neutrophil leukocyte to events associated with NO production and protein nitration is not established in vivo, despite extensive knowledge of the pro-inflammatory potential of neutrophil-derived reactive species, such as superoxide (O2−) and the highly reactive oxidant peroxynitrite (ONOO−) in tissue injury (Beckman et al., 1990; Halliwell, 1982; Salvemini et al., 1996a, 1999; Szabo et al., 1995). ONOO− is formed in inflamed tissue where the increased production of NO and O2− favours their reaction (Ischiropoulos et al., 1992; Beckman, 1994). The cytotoxic mechanisms of ONOO− include protein nitration, lipid peroxidation, inhibition of cellular metabolic pathways and signal transduction processes, and DNA strand breakages (Beckman, 1996).
It is generally assumed that NO, O2− and ONOO− are primarily generated at sites of inflammation by monocytes and macrophages (Halliwell, 1982; Ischiropoulos et al., 1992; Babior et al., 1973; Hazen et al., 1999). Indeed, it has been suggested that activated neutrophils produce little ONOO− as the O2− production far exceeds that of NO (Grisham et al., 1998). Early studies in animal models of inflammation (e.g. rat air pouch (Salvemini et al., 1995) and experimental peritonitis in the rat (Boughton-Smith & Ghelani, 1995)) suggest that removal of neutrophils by colchicine does not affect NO production. In addition, studies by Cuzzocrea (1997b) in the rat have highlighted the ability of zymosan-activated plasma, in the absence of white blood cells, to mediate increased NO production and ONOO− formation, possibly related to multi-organ dysfunction in shock. More recently, the use of iNOS-deficient mice has enabled the role of iNOS in inflammatory responses to be elucidated and the lack of iNOS was correlated with reduced neutrophil accumulation, in response to either zymosan (Ajuebor et al., 1998) or carrageenan (Cuzzocrea et al., 2000). By comparison, no change was observed in neutrophils accumulating in response to zymosan in the joints of iNOS deficient mice, where an involvement of NO derived from iNOS in cartilage destruction was implicated (van de loo et al., 1998). We have previously demonstrated that zymosan induces inflammatory oedema formation, increased blood flow and neutrophil accumulation in rat skin and that iNOS-derived NO is associated with the increased blood flow and the potentiation of inflammatory oedema by 4 h after zymosan administration (Ridger et al., 1997b), although others have suggested that NO derived from iNOS can act in a functionally significant manner as early as 2.5 h after inflammatory insult (Omote et al., 2001). The upregulation of iNOS in response to zymosan has also been reported in a range of other rat models (Cuzzocrea et al., 1997a; Paya et al., 1997; Setoguchi et al., 1996); and in particular Cuzzocrea and colleagues (Cuzzocrea et al., 1997a,b) has demonstrated that iNOS inhibitors attenuate zymosan-induced inflammation and shock.
Neutrophils and the reactive species that they generate have been implicated in tyrosine nitration in vitro. (Evans et al., 1996) and lung lavage samples (Lamb et al., 1999a, b). However, the contradictory literature outlined above highlights the need for a comprehensive study of the ability of the neutrophil to influence NO production and nitration of tissue proteins in a well-defined inflammatory model in vivo. Zymosan, a preparation of yeast cell walls, stimulates a non-allergic, non-septic inflammation (Williams & Jose, 1981) that has been traditionally related to activation of the alternative complement pathway (Keystone et al., 1977), and generation of C5a, a potent mediator of mast cell activation and neutrophil accumulation (Becker, 1972; Johnson et al., 1975). Zymosan is used to induce inflammation in models of inflammatory diseases that include athritis. In addition, other inflammatory mediators are known to contribute to this response including cytokines, chemokines and eicosanoids (Ajuebor et al., 1998; van de loo et al., 1998; Beaubien et al., 1990; Coates & McColl, 2001). We now provide in vivo evidence that implicates the neutrophil leukocyte in the formation of NO derived from iNOS and formation of nitrated proteins in a temporal manner. We also show the substantial reduction of NO production and nitrated protein levels when circulating neutrophils are depleted with a selective anti-rat neutrophil antiserum. In addition, the use of 1400W, an established, selective iNOS inhibitor (Garvey et al., 1997) reveals that the pro-inflammatory effects of NO derived from iNOS plays a significant role in the nitration of proteins during zymosan-induced inflammation.
Methods
General methods
Most agents were purchased from either the Sigma Chemical Company (Dorset, U.K.) or Amersham Pharmacia Biotech (Bucks, U.K.). Other agents were acquired as follows: rabbit anti-neutrophil antiserum from Accurate Chemicals (Westbury, NY, U.S.A.); 1400W and SIN-1 from Alexis Biochemicals (Nottingham, U.K.); avidin-biotinylated HRP complex, diaminobenzidine, swine anti-rabbit immunoglobulin biotinylated antibody rabbit anti-goat biotinylated antibody from DAKO (Cambridgeshire, U.K.); rabbit polyclonal anti-rat iNOS antibody, goat polyclonal anti-neutrophil elastase antibody from Santa Cruz Biotechnology (Santa Cruz, U.S.A.); 125I-BSA from ICN (Belgium); K-Blue substrate from Neogen (Lexington, KY, U.S.A.); sheep polyclonal anti-rat albumin antibody from The Binding Site (U.K.); rabbit polyclonal anti-3-nitrotyrosine (3-NT) antibody from Upstate Biotechnology Incorporated (Lake Placid, NY, U.S.A.).
In vivo procedures
Male Wistar rats (180–250 g) were purchased from Tucks Ltd. (Essex, U.K.). Experiments were either performed according to the Animals (Scientific Procedures) Act 1986. Sodium pentobarbitone (Sagatal, 50 mg kg−1 i.p.) was used to induce and maintain anaesthesia as assessed using the pedal reflex. For time course studies, the dorsal skin was shaved and zymosan (0.1 mg site−1), made up in Tyrode's solution (0.1 ml) was injected i.d. in duplicate, according to a randomized balanced site pattern. Animals were injected −48, −24, −8, −4, −2, −1 h before being killed by cervical dislocation. At zero hours the rats were killed and skin sites were removed (16 mm diameter) and stored at −80°C until analysis. The iNOS inhibitor 1400W (5 mg kg−1, s.c. in saline) or saline for control were given 5 min before and 3 h after i.d. treatments. In a separate series of experiments the rabbit anti-rabbit neutrophil anti-serum (2 ml kg −1, i.p.) as used by this group (Ridger et al., 1997b), or rabbit serum for control were given 17 h before the start of the experiment. A 2 ml blood sample was taken by cardiac puncture to determine cell counts, just before the termination of the experiment.
Frozen skin samples were pulverized and then homogenized (Polytron, Brinkman, NY, U.S.A.) in either 10 mM PBS (pH 7.4, 4°C) containing 0.05% Tween 20, for measure of nitrated proteins and NO2−/NO3−, or 50 mM phosphate buffer (pH 6.0) containing 0.5% hexadecyl trimethylammonium bromide (HTAB), for MPO assay. Homogenates were centrifuged at 25 000×g for 25 min at 5°C. The supernatant was stored at −80°C.
Detection of protein nitration by competitive enzyme-linked immunoabsorbent assay (ELISA)
The assay was modified from that of Khan et al. (1998). Briefly, BSA nitrated with tetranitromethane and containing 4–5 nitrotyrosine residues per protein was made up in 50 mM carbonate buffer (pH 9.0) and coated onto 96-well plates. Samples (0.02–0.5 mg protein 0.1 ml−1) or standard nitrated BSA (0.01–150 pmol 0.1 ml−1) were added in duplicate, and incubated for 2 h at 37°C with 0.1 ml primary rabbit polyclonal anti-nitrotyrosine antibody (1 : 30,000) to allow competition between immobilized nitrated BSA and nitrated protein in sample or standard. After washing, sequential 1 h incubations were performed with 0.1 ml biotinylated donkey anti-rabbit IgG (1 : 5000) and an avidin-biotinylated horseradish peroxidase complex. Colour development was with 0.1 ml substrate (o-phenylene-diamine in 0.03% sodium perborate) for up to 10 min. Antibody binding was determined from the absorbance at 492 nm.
Detection of protein nitration by Western blot
The procedure (Greenacre et al., 1999) was as follows: Skin protein (20 μg) was applied to the SDS-polyacrylamide gels (Protean II, Bio-Rad Laboratories Ltd., Hemel Hempstead, U.K.). The resolved bands were transferred to Immobilon-P, 0.45 μm pore size polyvinylidene difluoride membranes which were blocked for 3 h in low fat milk (2% in Tris Buffer Saline Tween containing 50 mM Tris-HCl, 150 mM NaCl, 0.02% (v v−1) Tween 20, pH 7.4) and probed with a rabbit primary anti-nitrotyrosine polyclonal antibody (diluted 1 : 5000 in 2% milk, for 2 h). Membranes were washed and incubated for 1 h in 0.2% milk containing HRP-conjugated goat anti-rabbit immunoglobulin antibody. Nitrated protein bands were detected using enhanced chemiluminescence. To evaluate the success of the transfer, some of the blots were stained with Coomassie blue. To confirm that protein bands were not due to non-specific binding, blots were also incubated with the blocked primary antibody, pre-incubated for 12 h with 10 mM free 3-NT. Molecular weight standards and nitrated RSA, made from the addition of 10 μl of 200 mM peroxynitrite to 5 mg RSA (made up in 1 ml 0.5 M phosphate buffer at pH 7.4), were applied to each gel.
Determination of neutrophil accumulation
MPO activity was used as an index of neutrophil accumulation as determined by measuring the H2O2-dependent oxidation of tetramethylbenzidine (TMB). Values were calculated from a purified leukocyte MPO standard (Sigma). Duplicate samples were diluted 1 : 2 with 50 mM phosphate buffer (pH 6.0) containing 0.5% HTAB and incubated with 0.1 ml of ‘K-Blue' substrate (stabilized H2O2 and TMB) for 10 min. The plate was shaken for 5 s and absorbance measured at 620 nm. MPO activity was expressed as MPO U site−1.
Determination of NO production
NO production was determined by the formation of NO2−/NO3− by the Griess reaction. NO2−/NO3− levels were measured after the NO3− was converted to NO2− by nitrate reductase from Aspergillus species (1 unit ml−1) and NADPH (1 mM) for 30 min at 37°C. NO2− levels were assessed spectrophotometrically at 540 nm by comparing the absorbance after adding Griess reagent (Sulfanilic aicd (1% w v−1) and N-(1-Naphythyl)ethylenediamine (0.1 w v−1) in 5% phosphoric acid) to that of a NaNO2 standard.
Immunohistochemistry using streptavidin biotin immunoperoxidase detection
Tissue was embedded in paraffin and sliced (5 μm). The sections were deparaffinized and treated with 0.3% H2O2 for 15 min at 23°C to block endogenous peroxidase before rehydration in H2O for 5 min. Sections were microwaved (880W at maximum temperature and pressure for 2 min) and then cooled. They were washed with TRIS buffered saline containing 0.05% Tween 20 (TBS) for 5 min and then treated with normal serum followed by incubation with either rabbit anti-rat iNOS (1 100−1) or goat anti-rat neutrophil elastase (1 100−1) for 2 h at 21±2°C. The slides were then washed twice with TBS for 5 min and incubated with the respective secondary antibody (either swine anti-rabbit (biotinylated F(ab′)2 fragment of swine anti-rabbit immunoglobulin (1 400−1 dilution) from Dako or rabbit anti-goat Ig biotin (1 300−1 dilution) from Dako) for 2 h at 21±2°C followed by wash, before incubation with streptavidin-biotin complex/horseradish peroxidase (1 100−1 in TBS for 30 min). Sections, after washing, were developed with a diaminobenzidine (DAB)-substrate chromogen kit. Sections were then stained with haematoxylin, dehydrated, cleansed, covered and viewed. Stained regions of skin were quantified by an image analyser (software by Foster-Findlay Associates, Newcastle on Tyne, U.K.). The brown peroxidase reaction product was assessed by measurement of pixels in intact tissue and results given as per cent area stained.
Statistics
Results are presented as mean±s.e.mean (unless stated) and analysed statistically by ANOVA followed by Bonferroni's or by Dunnett's modified t-test.
Results
Zymosan induced protein nitration, neutrophil accumulation and NO production in skin
Zymosan induced an inflammatory response in skin, in keeping with our previous results (Ridger et al., 1997b). The time-dependent effects of zymosan on protein nitration, neutrophil accumulation and NO production in rat skin are shown in Figure 1. Significant neutrophil accumulation was observed by 4 h that had increased by 8 h, but then returned to basal levels after 24 h. Surprisingly, there was a close correlation between neutrophil accumulation and increases in protein nitration, that had also returned to basal levels by 24 h. In addition an increase in NO production in skin was detected within 2 h (P<0.01) reaching a peak after 4 h (P<0.001) and remaining elevated after 8 and 24 h, returning to basal levels after 48 h.
Figure 1.
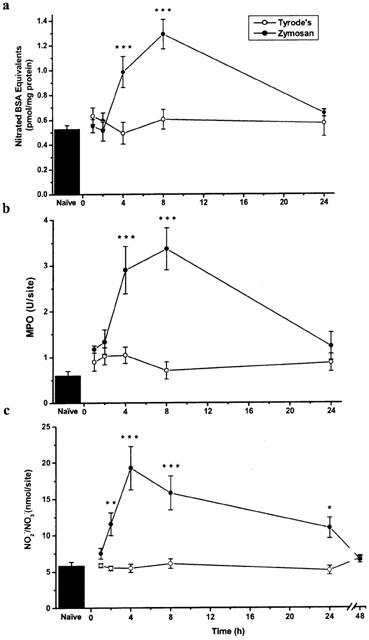
The effects of i.d. zymosan (0.1 mg site−1) over 1–48 h on (a) protein nitration, (b) neutrophil accumulation, and (c) NO• production in rat dorsal skin. Results are expressed as mean±s.e.mean, n=5. *P<0.05, **P<0.01, ***P<0.001 compared to Tyrode's vehicle at corresponding time point.
Immunohistochemical detection of iNOS in skin
Minimal iNOS staining was detected in naïve skin, as shown in Figure 2a. At 4 h after i.d. zymosan there was enhanced staining in Figure 2b, indicating increased accumulation of neutrophils and iNOS. By 24 h, Figure 2c is indicative of a lack of neutrophils in skin, in keeping with results shown in Figure 1. Interestingly, Figure 2c indicates the presence of iNOS protein, despite evidence that NO production had declined by 24 h. An image analyser was used to determine the mean area (±s.d.) of stained tissue in three slides (10 readings taken from each) from the same skin site with results as follows: iNOS 13±2 μm2 in naïve, 25±2 at 4 h after zymosan (P<0.01) and 26±7 at 24 h after zymosan (P<0.05).
Figure 2.
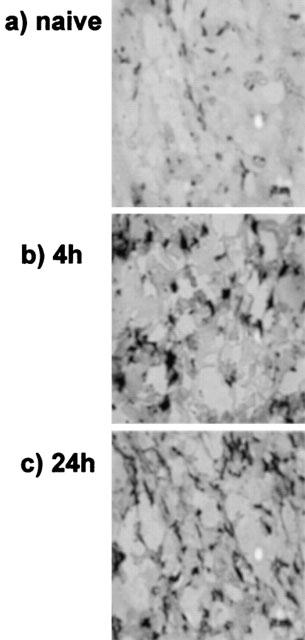
Detection of neutrophils and iNOS as determined by using horse radish peroxide-mediated observation of skin slices. Immunocytochemical analysis of (a) naïve skin and at (b) 4 h and (c) 24 h after intradermal zymosan (0.1 mg site−1).
The effect of selective neutrophil depletion
The rabbit anti-rat neutrophil anti-serum produced a complete and selective depletion of circulating neutrophils in the blood of rats as previously observed (see Table 1). The effects of depletion of circulating neutrophils on zymosan-induced responses is shown in Figure 3. After 4 h, zymosan induced significant protein nitration, neutrophil accumulation and NO production compared to treatment with vehicle, in rats pre-treated with irrelevant anti-serum. Depletion of circulating neutrophils abolished the increased levels of neutrophils and protein nitration with by comparison a significant, but not total, reduction, in NO levels.
Table 1.
The effect of an anti-rat neutrophil anti-serum and rabbit irrelevant anti-serum on rat peripheral blood counts
Figure 3.
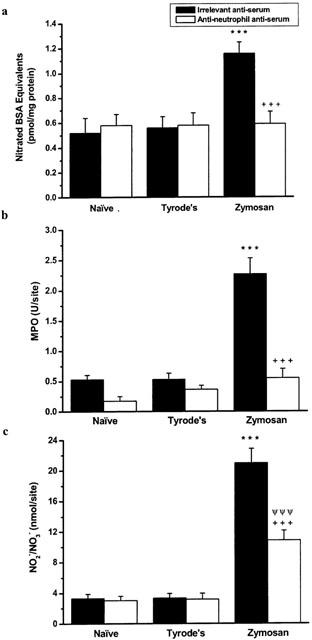
The effects of neutrophil depletion, with an anti-rat neutrophil antiserum, on (a) protein nitration, (b) neutrophil accumulation and (c) NO• production in rat dorsal skin. Skin samples were removed 4 h after i.d. zymosan (0.1 mg site−1). Data expressed as mean±s.e.mean, n=5 animal. ***P<0.001, zymosan compared to Tyrode's vehicle in animals with neutrophils, ΨΨΨP<0.001, zymosan compared to Tyrode's vehicle in neutrophil depleted animals, +++P<0.001, zymosan in animals with neutrophils compared to zymosan in neutrophil depleted animals.
The effect of a selective iNOS inhibitor
Figure 4 shows the effects of the selective iNOS inhibitor 1400W on zymosan induced responses. Pre-treatment with 1400W significantly attenuated protein nitration, NO2−/NO3− levels and neutrophil accumulation induced by zymosan. However, while 1400W pre-treatment reduced protein nitration in zymosan treated skin to levels similar to basal, there was still significant neutrophil accumulation and NO2−/NO3−.
Figure 4.
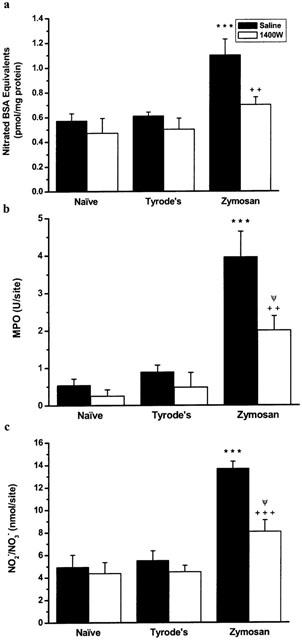
The effects of the iNOS inhibitor 1400W (5 mg kg−1, s.c.), administered 5 min pre- and 3 h post-zymosan (0.1 mg site−1, i.d.) on (a) protein nitration, (b) neutrophil accumulation and (c) NO• production in rat dorsal skin. Skin samples were removed 4 h after i.d. zymosan. Data expressed as mean±s.e.mean, n=5 animals. ***P<0.001, zymosan compared to Tyrode's vehicle in absence of 1400W, ΨP<0.05, zymosan compared to Tyrode's vehicle in presence of 1400W, ++P<0.01 +++P<0.001, zymosan in the presence of 1400W compared to zymosan in the absence of 1400W.
Zymosan induced nitration of a 66 kDa protein
The representative Western blot in Figure 5 shows the detection of nitrated proteins. The samples of naïve skin, zymosan and Tyrode's treated skin used in the blots were also used in the ELISA, Figure 1, allowing direct comparison. A single strong immunopositive protein was identified with a molecular weight of 66 kDa in skin 4 h and 8 h after zymosan treatment (Figure 5a). The nitrated 66 kDa protein has the same molecular weight as nitrated RSA suggesting that albumin may be nitrated. The nitration of this protein also occurs basally in rat skin as the immunopositive band was found in all skin samples, albeit to a lesser extent. The specificity of the rabbit polyclonal anti-3-NT antibody was confirmed by incubation with 10 mM free 3-NT which resulted in a loss of the immunopositive band (Figure 5b). Even loading of protein onto the gel was confirmed by Coomassie blue staining (Figure 5c).
Figure 5.
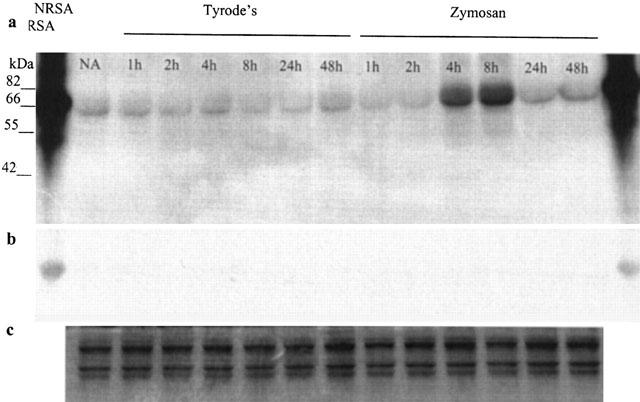
Detection of nitrated proteins in zymosan-treated rat dorsal skin by Western blot. A representative blot with resolved and transferred protein incubated with rabbit polyclonal anti-3-NT IgG is shown in (a). Results for naïve skin (NA) and for Tyrode and zymosan (0.1 mg site−1) injected sites are shown. A comparison is made with results from nitrated RSA (NRSA). The effect of 10 mM free 3-NT is shown in (b). Even protein loading onto the gel is shown in (c) by visualization of the loading lanes using Coomassie blue stain.
Discussion
Surprisingly, little is known about the direct relationship of the neutrophil to the generation of NO and protein nitration in inflammation. This led us to investigate a neutrophil-dependent model of inflammation in rat skin. Experiments in skin allows substantial information to be obtained through use of multiple sites, of relevance to inflammatory disease. This study provides important evidence to extend previous findings that have implicated neutrophils in tyrosine nitration (e.g. Salvemini et al., 1996a,b). The results suggest a close coupling between these phenomena in that levels are concomitantly raised in the first hours after inflammatory insult, but then the decrease of neutrophils with time is associated with a reduction in NO generation and in the detection of nitrated proteins. However, NO2−/NO3− levels remain significantly raised at 24 h, whereas nitrated protein and neutrophil levels do not, indicative that NO is formed from a non-neutrophil source at this later timepoint. The detection of nitrated proteins is not related to inflammatory oedema that we have previously shown to occur at an earlier stage than neutrophil accumulation (Ridger et al., 1997b). The depletion of circulating neutrophils leads to a reduction in protein nitration in inflamed tissues, thus providing evidence for neutrophil-mediated nitration in vivo during a non-allergic, non-septic inflammatory response. Interestingly, NO2−/NO3− levels remain high. This provides further evidence that NO is formed from a non-neutrophil source and that neutrophils play an important role in protein nitration. In addition, the iNOS inhibitor, 1400W, significantly inhibited neutrophil accumulation and nitrated proteins levels, thus revealing the importance of iNOS. Therefore the increase in protein nitration during acute inflammatory processes within these tissues appears secondary to neutrophil accumulation and the generation of iNOS-derived NO.
The specific consequences of tissue protein nitration are unclear. Western blotting revealed that only one major protein was found to be nitrated at 4–8 h after zymosan; although it is highly likely that the other less abundant particulate/membrane bound or insoluble proteins are also nitrated. This nitrated protein possessed a molecular weight of around 66 kDa and was considered to be albumin, based on molecular weight and the abundance of albumin in skin. Previous studies show nitration in proteinaceous exudates in inflamed tissues after stimuli that include zymosan (Setoguchi et al., 1996) and that immunohistochemical staining for nitrotyrosine may often appear diffuse (Ischiropoulos et al., 1995; Viera et al., 1999; Greenacre & Ischiropoulos, 2001), in keeping with a major nitration of albumin. Furthermore, a single 66 kDa protein was nitrated in inflammatory models in skin that included ischemic rat skin grafts (Um et al., 1998) and naïve and inflamed UVB-irradiated mouse skin (Hattori et al., 1996). We have previously identified albumin as a major nitrated protein after peroxynitrite administration to skin (Greenacre et al., 1999). In addition, in support of the occurrence of albumin nitration under basal and inflammatory conditions, of particular interest is the very recent preliminary work of Cullen et al. (2001) who have identified nitrated albumin (by Western blot followed by proteomic sequence analysis) as the major nitrated protein in the healthy and inflamed (nitration increased after systemic LPS) opossum gall bladder. We have previously shown that ONOO− has potent oedema-inducing effects in skin when compared with degraded ONOO− and induces protein nitration in this tissue (Greenacre et al., 1999; Ridger et al., 1997a). The inflammatory oedema formation may serve as a protective anti-oxidant mechanism by providing plasma albumin. Certainly, the abundance of albumin has led to the proposal that it is an important extra-cellular anti-oxidant (Halliwell et al., 1988) acting in a sacrificial manner to protect the oxidative and nitrative damage of more ‘critical' proteins.
Tyrosine nitration was proposed as a marker of endogenous formation of ONOO− (Beckman, 1996). It is now realised that other reactions can also induce nitration of tyrosine and tyrosine containing proteins (e.g. the reaction of nitrite with hypochlorous acid and the reaction of myeloperoxidase with hydrogen peroxide; van der vliet et al., 1997; Eiserich et al., 1998). Furthermore, Baldus et al. (2001) have recently shown that myeloperoxidase released by activated neutrophils can become localized in the vascular wall and then act to nitrate fibronectin. Nitrotyrosine is not observed in myeloperoxidase knockout mice, thus establishing the importance of myeloperoxidase. This paper is directly relevant to our findings. It highlights the link between neutrophils and protein nitration. It is possible that fibronection could have been nitrated in our studies. However, if fibronectin or its fragments were major nitrated proteins one would expect to see a series of different molecular weight bands on the Western blot.
There is evidence, mainly from cellular studies, that a number of less abundant proteins and peptides have altered functions after protein nitration and they may have more critical roles than albumin in the inflammatory process. These include α1-proteinase inhibitor (Halliwell, 1988), cytochrome P450 (Roberts et al., 1998), chemokines (Sato et al., 2000a,b, manganese superoxide dismutase (Macmillan-Crow & Thompson, 1999) and cyclo-oxygenase (Boulos et al., 2000). The early increase in nitration within 4 h of zymosan treatment and rapid return to basal levels of nitration by 24 h indicates that protein nitration may have an important role in the acute inflammatory process, being a tightly controlled process with specific mechanisms existing for the removal, repair, or degradation of nitrated proteins. Mechanisms for the removal of nitrated proteins have been suggested (Kamisaki et al., 1998) and this laboratory has evidence for such activity in rat skin (Greenacre et al., 2000). Furthermore, the finding of the temporal correlation of tissue nitration with neutrophil accumulation is intriguing, especially when considering the neutrophil chemoattractants involved in this response. The chemokines interleukin-8 (IL-8) and macrophage inflammatory protein-1alpha (MIP-1α) are potent neutrophil chemoattractants and play a key functional role in neutrophil accumulation observed in response to zymosan (Beaubien et al., 1990; Mahesh et al., 1999). Interestingly, the tyrosine at position 13 of IL-8 is important for chemotactic activity (Schraufstatter et al., 1995) and nitration of IL-8 leads to an inhibition of its neutrophil chemotactic activity (Sato et al., 2000a); as does nitration of MIP-1α (Sato et al., 2000b). This could be directly related to the transient nature of the neutrophil accumulation, such that inactivation of the chemotactic factors, due to nitration, may be associated with the return to basal levels of neutrophils as observed in the skin by 24 h. Thus the process may work as a negative feedback, limiting the inflammatory potential of the neutrophil in tissues.
The compound 1400W is one of the most selective iNOS inhibitors available to date (Garvey et al., 1997). The results show that the increased protein nitration observed in skin 4 h after zymosan treatment was totally dependent on iNOS activity, whilst increases in NO production and neutrophil infiltration were partially dependent on iNOS. The data suggest that basal protein nitration in skin is most likely due to cNOS activity, since iNOS inhibition did not reduce the basal levels of nitrated protein detected. The doses of 1400W were sufficient for inhibition of iNOS as they were between 1–5 times higher than those used to protect against lipopolysaccharide and iNOS-dependent vascular leakage in the rat (Garvey et al., 1997) and have been reported to inhibit iNOS selectivity without non-specific toxicity (Thomsen et al., 1997). The partial reduction of zymosan-induced neutrophil infiltration by 1400W is indicative of a role for iNOS derived NO• in mediating neutrophil accumulation. The role of NO in modulating neutrophil infiltration is not clear and may vary from tissue and inflammatory model, but generally it is thought that low levels of NO reduce inflammatory cell adhesion to the endothelium (Kubes et al., 1991; Lefer et al., 1999). It has been demonstrated that neutrophil-derived oxidants promote PMN adherence to microvessels in vivo (Suzuki et al., 1991) and it has been suggested that the effects of NO that prevent polymorphonuclear leukocyte accumulation are related to its ability to sequester O2− (Kubes et al., 1993). Our findings suggest that high levels of NO induced by iNOS stimulate neutrophil accumulation and in keeping with results from Koarai et al. (2000) who demonstrated reduced eosinophil infiltration in mouse airway inflammation after 1400W treatment. That the anti-infiltrative action of 1400W on neutrophils is due to iNOS inhibition is supported by studies in iNOS deficient mice which showed reduced neutrophil accumulation after i.p. zymosan (Ajuebor et al., 1998) or pleural carrageenan treatment (Cuzzocrea et al., 2000). The former study also provided evidence for the concept of iNOS derived NO in stimulating increased levels of cytokines and chemokines such as the macrophage inflammatory protein-2, and this may be relevant to the role of iNOS-derived NO in neutrophil accumulation. In addition, it is possible that NO is associated with the enhanced production of other mediators of neutrophil accumulation such as LTB4 (Grisham et al., 1998). Alternatively, since iNOS derived NO has been shown to increase microvascular blood flow during zymosan-induced skin inflammation (Ridger et al., 1997b), reduced neutrophil infiltration following iNOS inhibition may be secondary to a reduction in blood flow. The presence of iNOS as determined by immunohistochemistry at 24 h, but lack of evidence for iNOS-derived NO production at this time point is of interest, but the reasons are unclear. It is suggestive that iNOS is not functional at the later timepoint.
A recent study has used a similar experimental approach to our own to identify an essential role of eosinophils in protein nitration in asthma; an anti-IL-5 antibody was used to selectively deplete circulating eosinophils and to demonstrate in turn an absence of protein nitration in allergen-challenged mice that lacked functioning eosinophils (Iijima et al., 2001). The results are supported by evidence that the human eosinophil is a major source of oxidants during asthma (Macpherson et al., 2001).
In conclusion, these results identify the neutrophil as playing an essential role in providing oxidants during the acute stages of zymosan-induced inflammation in rat skin. They highlight NO derived from iNOS as a key intermediate between the neutrophil and the resulting nitrative stress. The finding that tissue nitration is closely coupled with the time-dependent increase and then decrease of neutrophil accumulation in the inflamed tissue is indicative of specific roles for nitrated proteins in the ongoing inflammatory process, although precise in vivo consequences of nitrated proteins are unclear and can only be hypothesized at the moment. However, the evidence presented in this study is indicative of an intimate coupling phenomenon where tissue nitration levels are in direct proportion to the infiltrating neutrophils. The evidence supports the concept that therapeutic interventions that block inappropriate neutrophil accumulation, NO generation via iNOS and the resultant nitrated protein may play vital roles in the treatment of non-allergic, non-septic skin inflammation.
Acknowledgments
This work was supported by the U.K. Arthritis Research Campaign (S.A.B. Greenacre), U.K. Medical Research Council (A. Rawlingson), Fundação Cearense de Amparo à Pesquisa (FUNCAP-CE-Brazil) and CNPq-Brazil. We thank Ms Esther Seeds for help with blood counts and Dr Khalid Shendi for help with immunohistochemistry.
Abbreviations
- ANOVA
analysis of variance
- HTAB
hexadecyl trimethylammonium bromide
- MPO
myeloperoxidase
- cNOS
constitutive nitric oxide synthase
- eNOS
endothelial nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- iNOS
NOSII inducible nitric oxide synthase
- NO2−
nitrite
- NO3−
nitrate
- O2−
superoxide
- ONOO−
peroxynitrite
- RNS
reactive nitrogen species
- RSA
rat serum albumin
- TBS
Tris buffered saline
References
- AJUEBOR M.N., VIRAG F., FLOWER R.J., PERRETTI M., SZABO C. Role of inducible nitric oxide synthase in the regulation of neutrophil migration in zymosan-induced inflammation. Immunology. 1998;95:625–630. doi: 10.1046/j.1365-2567.1998.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BABIOR B.M., KIPNES R.S., CURNUTTE J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALDUS S., EISERICH J.P., MANI A., CASTRO L., FIGUEROA M., CHUMLEY P., MA W., TOUSSON A., WHITE C.R., BULLARD D.C., BRENNAN M.L., LUSIS A.J., MOORE K.P., FREEMAN B.A. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J. Clin. Invest. 2001;108:1759–1770. doi: 10.1172/JCI12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAUBIEN B.C., COLLINS P.D., JOSE P.J., TOTTY N.F., HSUAN J., WATERFIELD M.D., WILLIAMS T.J. A novel neutrophil chemoattractant generated during an inflammatory reaction in the rabbit peritoneal cavity in vivo. Purification, partial amino acid sequence and structural relationship to interleukin 8. Biochem. J. 1990;271:797–801. doi: 10.1042/bj2710797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER E.L. The relationship of the chemotactic behavior of the complement-derived factors, C3a, C5a, and C567, and a bacterial chemotactic factor to their ability to activate the proesterase 1 of rabbit polymorphonuclear leukocytes. J. Exp. Med. 1972;135:376–387. doi: 10.1084/jem.135.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER E.L. The short and happy life of neutrophil activation. J. Leukoc. Biol. 1990;47:378–389. doi: 10.1002/jlb.47.4.378. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S. Peroxynitrite versus hydroxyl radical: the role of nitric oxide in superoxide-dependent cerebral injury. Ann. N.Y. Acad. Sci. 1994;738:69–75. doi: 10.1111/j.1749-6632.1994.tb21791.x. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., BECKMAN T.W., CHEN J., MARSHALL P.A., FREEMAN B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUGHTON-SMITH N.K., GHELANI A. Role of induced nitric oxide synthase and increased NO levels in zymosan peritonitis in the rat. Inflamm. Res. 1995;44:S149–150. doi: 10.1007/BF01778306. [DOI] [PubMed] [Google Scholar]
- BOULOS C., JIANG B.A., BALAZY M. Diffusion of peroxynitrite into the human platelet inhibits cyclooxygenase via nitration of tyrosine residues. J. Pharmacol. Exp. Ther. 2000;293:222–229. [PubMed] [Google Scholar]
- COATES N.J., MCCOLL S.R. Production of chemokines in vivo in response to microbial stimulation. J. Immunol. 2001;166:5176–5182. doi: 10.4049/jimmunol.166.8.5176. [DOI] [PubMed] [Google Scholar]
- CULLEN J., LINDAMAN B.A., MAES E.B., EPHGRAVE K.S., BATES J.N., CONKLIN J.L. Detection of peroxynitrite during acalculous cholecystitis. Proceedings of the 3rd International Conference on Peroxynitrite and Reactive Nitrogen Species in Biology and Medicine. A. 2001. p. 49.
- CUZZOCREA S., MAZZON E., CALABRO G., DUGO L., DE SARRO A., VAN DE LOO F.A., CAPUTI A.P. Inducible nitric oxide synthase-knockout mice exhibit resistance to pleurisy and lung injury caused by carrageenan. Am. J. Respir. Crit. Care Med. 2000;162:1859–1866. doi: 10.1164/ajrccm.162.5.9912125. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., O'CONNOR M., SALZMAN A.L., CAPUTI A.P., SZABO C. Role of peroxynitrite and activation of poly (ADP-ribose) synthase in the vascular failure induced by zymosan-activated plasma. Br. J. Pharmacol. 1997b;122:493–503. doi: 10.1038/sj.bjp.0701387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., SAUTEBIN L., RIZZO A., CRISAFULLI C., CAMPO G.M., COSTANTINO G., CALAPAI G., NAVA F., DIROSA M., CAPUTI A.P. Multiple organ failure following zymosan-induced peritonitis is mediated by nitric oxide. Shock. 1997a;8:268–275. doi: 10.1097/00024382-199710000-00006. [DOI] [PubMed] [Google Scholar]
- EISERICH J.P., HRISTOVA M., CROSS C.E., JONES A.D., FREEMAN B.A., HALLIWELL B., VAN DER VLIET A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- EVANS T.J., BUTTERY L.D., CARPENTER A., SPRINGALL D.R., POLAK J.M., COHEN J. Cytokine-treated human neutrophils contain inducible nitric oxide synthase that produces nitration of ingested bacteria. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9553–9558. doi: 10.1073/pnas.93.18.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARVEY E.P., OPLINGER J.A., FURFINE E.S., KIFF R.J., LASZLO F., WHITTLE B.J., KNOWLES R.G. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J. Biol. Chem. 1997;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- GREENACRE S.A., EVANS P., HALLIWELL B., BRAIN S.D. Formation and loss of nitrated proteins in peroxynitrite-treated rat skin in vivo. Biochem. Biophys. Res. Commun. 1999;262:781–786. doi: 10.1006/bbrc.1999.1309. [DOI] [PubMed] [Google Scholar]
- GREENACRE S.A., ISCHIROPOULOS H. Tyrosine nitration: localisation, quantification, consequences for protein function and signal transduction. Free Radic. Res. 2001;34:541–581. doi: 10.1080/10715760100300471. [DOI] [PubMed] [Google Scholar]
- GREENACRE S.A., ROBERTS C., HALLIWELL B., BRAIN S.D. “Denitration” of albumin by skin homogenates assessed by modified ELISA. Nitric Oxide. 2000;4:A295. [Google Scholar]
- GRISHAM M.B., GRANGER D.N., LEFER D.J. Modulation of leukocyte-endothelial interactions by reactive metabolites of oxygen and nitrogen: relevance to ischemic heart disease. Free Radic. Biol. Med. 1998;25:404–433. doi: 10.1016/s0891-5849(98)00094-x. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. Production of superoxide, hydrogen peroxide and hydroxyl radicals by phagocytic cells: a cause of chronic inflammatory disease. Cell Biol. Int. Rep. 1982;6:529–542. doi: 10.1016/0309-1651(82)90175-8. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. Albumin – an important extracellular antioxidant. Biochem. Pharmacol. 1988;37:569–571. doi: 10.1016/0006-2952(88)90126-8. [DOI] [PubMed] [Google Scholar]
- HATTORI Y., NISHIGORI C., TANAKA T., UCHIDA K., NIKAIDO O., OSAWA T., HIAI H., IMAMURA S., TOYOKUNI S. 8-hydroxy-2′-deoxyguanosine is increased in epidermal cells of hairless mice after chronic ultraviolet. J. Invest. Dermatol. 1996;107:733–737. doi: 10.1111/1523-1747.ep12365625. [DOI] [PubMed] [Google Scholar]
- HAZEN S.L., ZHANG R., SHEN Z., WU W., PODREZ E.A., MACPHERSON J.C., SCHMITT D., MITRA S.N., MUKHOPADHYAY C., CHEN Y., COHEN P.A., HOFF H.F., ABU-SOUD H.M. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation in vivo. Circ. Res. 1999;85:950–958. doi: 10.1161/01.res.85.10.950. [DOI] [PubMed] [Google Scholar]
- IIJIMA H., DUGUET A., EUM S.Y., HAMID Q., EIDELMAN D.H. Nitric oxide and protein nitration are eosinophil dependent in allergen-challenged mice. Am. J. Respir. Crit. Care Med. 2001;163:1233–1240. doi: 10.1164/ajrccm.163.5.2003145. [DOI] [PubMed] [Google Scholar]
- ISCHIROPOULOS H., AL-MEHDI D.H., FISHER A.B. Reactive species in ischemic rat lung injury: contribution of peroxynitrite. Am. J. Physiol. 1995;269:L158–L164. doi: 10.1152/ajplung.1995.269.2.L158. [DOI] [PubMed] [Google Scholar]
- ISCHIROPOULOS H., ZHU L., CHEN J., TSAI M., MARTIN J.C., SMITH C.D., BECKMAN J.S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- JOHNSON A.R., HUGLI T.E., MULLER-EBERHARD J.H. Release of histamine from rat mast cells by the complement peptides C3a and C5a. Immunology. 1975;28:1067. [PMC free article] [PubMed] [Google Scholar]
- KAMISAKI Y., WADA K., BIAN K., BALABANLI B., DAVIS K., MARTIN E., BEHBOD F., LEE Y.C., MURAD F. An activity in rat tissues that modifies nitrotyrosine-containing proteins. Proc. Natl. Acad. Sci. U.S.A. 1998;95:11584–11589. doi: 10.1073/pnas.95.20.11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYSTONE E.C., SCHORLEMMER H.U., POPE C., ALLISON A.C. Zymosan-induced arthritis: a model of chronic proliferative arthritis following activation of the alternative pathway of complement. Arthritis Rheum. 1977;20:1396–1401. doi: 10.1002/art.1780200714. [DOI] [PubMed] [Google Scholar]
- KHAN J., BRENNAND D.M., BRADLEY N., GAO B., BRUCKDORFER R., JACOBS M., BRENNAN D.M. 3-NT in the proteins of human plasma determined by an ELISA. Biochem. J. 1998;330:795–801. doi: 10.1042/bj3300795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOARAI A., ICHINOSE M., SUGIURA H., YAMAGATA S., HATTORI T., SHIRATO K. Allergic airway hyperresponsiveness and eosinophil infiltration is reduced by a selective iNOS inhibitor, 1400W, in mice. Pulm. Pharmacol. Ther. 2000;13:267–275. doi: 10.1006/pupt.2000.0254. [DOI] [PubMed] [Google Scholar]
- KUBES P., KANWAR S., NIU X.F., GABOURY J.P. Nitric oxide synthesis inhibition induces leukocyte adhesion via superoxide and mast cells. FASEB J. 1993;7:1293–1299. doi: 10.1096/fasebj.7.13.8405815. [DOI] [PubMed] [Google Scholar]
- KUBES P., SUZUKI M., GRANGER D.N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMB N.J., GUTTERIDGE J.M., BAKER C., EVANS T.W., QUINLAN C.J. Oxidative damage to proteins of bronchoalveolar lavage fluid in patients with acute respiratory distress syndrome: evidence neutrophil-mediated hydroxylation, nitration, and chlorination. Crit. Care Med. 1999a;27:1738–1744. doi: 10.1097/00003246-199909000-00007. [DOI] [PubMed] [Google Scholar]
- LAMB N.J., QUINLAN G.J., WESTERMAN S.T., GUTTERIDGE J.M., EVANS T.W. Nitration of proteins in bronchoalveolar lavage fluid from patients with acute respiratory distress syndrome receiving inhaled nitric oxide. Am. J. Respir. Crit. Care Med. 1999b;160:1031–1034. doi: 10.1164/ajrccm.160.3.9810048. [DOI] [PubMed] [Google Scholar]
- LEFER D.J., JONES S.P., GIROD W.G., BAINES A., GRISHAM M.B., COCKRELL A.S., HUANG P.L., SCALIA R. Leukocyte-endothelial cell interactions in nitric oxide synthase-deficient mice. Am. J. Physiol. 1999;276:H1943–H1950. doi: 10.1152/ajpheart.1999.276.6.H1943. [DOI] [PubMed] [Google Scholar]
- MACMILLAN-CROW L.A., THOMPSON J.A. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch. Biochem. Biophys. 1999;366:82–88. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- MACPHERSON J.C., COMHAIR S.A., ERZURUM S.C., KLEIN D.F., LIPSCOMB M.F., KAVURU M.S., SAMOSZUK M.K., HAZEN S.L. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J. Immunol. 2001;166:5763–5772. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- MAHESH J., DALY J., CHEADLE W.G., KOTWAL C.J. Elucidation of the early events contributing to zymosan-induced multiple organ dysfunction syndrome using MIP-1alpha, C3 knockout, and C5-deficient mice. Shock. 1999;12:340–349. doi: 10.1097/00024382-199911000-00003. [DOI] [PubMed] [Google Scholar]
- OMOTE K., HAZAMA K., KAWAMATA T., KAWAMATA M., NAKAYAKA Y., TORIYABE M., NAMIKI A. Peripheral nitric oxide in carrageenan-induced inflammation. Brain Res. 2001;912:171–175. doi: 10.1016/s0006-8993(01)02733-0. [DOI] [PubMed] [Google Scholar]
- PAYA M., GARCIA PASTOR P., COLOMA J., ALCARAZ M.J. Nitric oxide synthase and cyclo-oxygenase pathways in the inflammatory response induced by zymosan in the rat air pouch. Br. J. Pharmacol. 1997;120:1445–1452. doi: 10.1038/sj.bjp.0701073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIDGER V.C., GREENACRE S.A., HANDY R.L., HALLIWELL B., MOORE P.K., WHITEMAN M., BRAIN S.D. Effect of peroxynitrite on plasma extravasation, microvascular blood flow and nociception in the rat. Br. J. Pharmacol. 1997a;122:1083–1088. doi: 10.1038/sj.bjp.0701498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIDGER V.C., PETTIPHER E.R., BRYANT C.E., BRAIN S.D. Effect of the inducible nitric oxide synthase inhibitors aminoguanidine and L-N6-(1-iminoethyl)lysine on zymosan-induced plasmaextravasation in rat skin. J. Immunol. 1997b;159:383–390. [PubMed] [Google Scholar]
- ROBERTS E.S., LIN H.I., CROWLEY J.R., VULETICH J.L., OSAWA Y., HOLLENBERG P.F. Peroxynitrite-mediated nitration of tyrosine and inactivation of the catalytic activity of cytochrome P450. Chem. Res. Toxicol. 1998;11:1067–1074. doi: 10.1021/tx980099b. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., RILEY D.P., LENNON P.J., WANG Z.Q., CURRIE M.G., MACARTHUR H., MISKO T.P. Protective effects of a superoxide dismutase mimetic and peroxynitrite decomposition catalysts in endotoxin-induced intestinal damage. Br. J. Pharmacol. 1999;127:685–692. doi: 10.1038/sj.bjp.0702604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., MANNING P.T., ZWEIFEL B.S., SEIBERT K., CONNOR J., CURRIE M.G., NEEDLEMAN P., MASFERRER J.L. Dual inhibition of nitric oxide and prostaglandin production contributes to the antiinflammatory properties of nitric oxide synthase inhibitors. J. Clin. Invest. 1995;96:301–308. doi: 10.1172/JCI118035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., BOUDRON D.M., STERN M.K., CURRIE M.G., MANNING P.T. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur. J. Pharmacol. 1996a;303:217–320. doi: 10.1016/0014-2999(96)00140-9. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., WYATT P.S., BOURDON D.M., MARINO M.H., MANNING P.T., CURRIE M.G. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996b;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO E., SIMPSON K.L., GRISHAM M.B., KOYAMA S., ROBBINS R.A. Reactive nitrogen and oxygen species attenuate interleukin-8-induced neutrophil chemotactic activity in vitro. J. Biol. Chem. 2000a;275:10826–10830. doi: 10.1074/jbc.275.15.10826. [DOI] [PubMed] [Google Scholar]
- SATO E., SIMPSON K.L., GRISHAM M.B., KOYAMA S., ROBBINS R.A. Inhibition of MIP-1alpha-induced human neutrophil and monocyte chemotactic activity by reactive oxygen and nitrogen metabolites. J. Lab. Clin. Med. 2000b;135:161–169. doi: 10.1067/mlc.2000.104307. [DOI] [PubMed] [Google Scholar]
- SCAPINI P., LAPINET-VERA J.A., GASPERINI S., CALZETTI F., BAZZONI F., CASSATELLA M.A. The neutrophil as a cellular source of chemokines. Immunol. Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- SCHRAUFSTATTER I.U., MA M., OADES Z.G., BARRITT D.S., COCHRANE C.G. The role of Tyr13 and Lys15 of interleukin-8 in the high affinity interaction with the interleukin-8 receptor type A. J. Biol. Chem. 1995;270:10428–10431. doi: 10.1074/jbc.270.18.10428. [DOI] [PubMed] [Google Scholar]
- SETOGUCHI K., TAKEYA M., AKAIKE T., SUGA M., HATTORI R., MAEDA H., ANDO M., TAKAHASHI K. Expression of inducible nitric oxide synthase and its involvement in pulmonary granulomatous inflammation in rats. Am. J. Pathol. 1996;149:2005–2022. [PMC free article] [PubMed] [Google Scholar]
- SUZUKI M., GRISHAM M.B., GRANGER D.N. Leukocyte-endothelial cell adhesive interactions: role of xanthine oxidase-derived oxidants. J. Leukoc. Biol. 1991;50:488–494. doi: 10.1002/jlb.50.5.488. [DOI] [PubMed] [Google Scholar]
- SZABO C., SALZMAN A.L., ISCHIROPOULOS H. Peroxynitrite-mediated oxidation of dihydrorhodamine 123 occurs in early stages of endotoxic and hemorrhagic shock and ischemia-reperfusion injury. FEBS Lett. 1995;372:229–232. doi: 10.1016/0014-5793(95)00984-h. [DOI] [PubMed] [Google Scholar]
- THOMSEN L.L., SCOTT J.M., TOPLEY P., KNOWLES R.G., KEERIE A.J., FREND A.J. Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: studies with 1400W, a novel inhibitor. Cancer Res. 1997;57:3300–3304. [PubMed] [Google Scholar]
- UM S.C., SUZUKI S., TOYOKUNI S., KIM B.M., TANAKA T., HIAI H., NISHIMURA Y. Involvement of nitric oxide in survival of random pattern skin flap. Plast. Reconstr. Surg. 1998;101:785–792. doi: 10.1097/00006534-199803000-00030. [DOI] [PubMed] [Google Scholar]
- VAN DE LOO F.A., ARNTZ O.J., VAN ENCKEVORT F.H., VAN LENT P.L., VAN DEN BERG W.B. Reduced cartilage proteoglycan loss during zymosan-induced arthritis in NOS2-deficient mice and in anti-interleukin-1-treated wild-type mice with unabated joint inflammation. Arthritis Rheum. 1998;41:634–646. doi: 10.1002/1529-0131(199804)41:4<634::AID-ART10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- VAN DER VLIET A., EISERICH J.P., HALLIWELL B., CROSS C.E. Formation of reactive nitrogen species during peroxidase-catalysed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J. Biol. Chem. 1997;272:7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- VIERA L., YE Y.Z., ESTEVEZ A.G., BECKMAN J.S. Immunohistochemical methods to detect nitrotyrosine. Methods Enzymol. 1999;301:373–381. doi: 10.1016/s0076-6879(99)01101-5. [DOI] [PubMed] [Google Scholar]
- WILLIAMS T.J., JOSE P.J. Mediation of increased vascular permeability after complement activation. Histamine-independent action of rabbit C5a. J. Exp. Med. 1981;153:136–153. doi: 10.1084/jem.153.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]


