Abstract
Non-dopamine (putative GABAergic) neurons in the ventral tegmental area are in a position to influence mesolimbic functions by their inhibitory terminals that impinge locally on dopamine neurons and via their GABAergic efferents that innervate mesolimbic structures. In the present study we investigated responses of non-dopamine and dopamine neurons, recorded intracellularly in the rat midbrain slice, to orphanin FQ/nociceptin, the endogenous ligand for opioid receptor-like orphan receptors.
When recording in either non-dopamine or dopamine neurons, orphanin FQ/nociceptin reduced the frequency of spike firing and caused membrane hyperpolarization under current-clamp, or produced outward current under voltage-clamp. Such responses were concentration-dependent and reversed at −108 mV and −102 mV in non-dopamine and dopamine neurons, respectively.
Hyperpolarizations to orphanin FQ/nociceptin were not altered by tetrodotoxin or the opioid receptor antagonist naloxone, but were reduced by the opioid receptor-like orphan receptor antagonist [Phe11φCH2-NH)Gly2]NC(1–13)NH2 (1 μM).
In dopamine neurons, orphanin FQ/nociceptin reduced the frequency of bicuculline- and tetrodotoxin-sensitive spontaneous inhibitory postsynaptic potentials, and reduced the amplitude of stimulus-evoked inhibitory postsynaptic potentials.
Taken together, the above data provide evidence that both non-dopamine and dopamine neurons are important substrates for orphanin FQ/nociceptin within the ventral tegmental area. Simultaneous inhibition of both non-dopamine and dopamine pathways by orphanin FQ/nociceptin may account for its influences on various ventral tegmental area-related functions.
Keywords: Orphanin FQ/nociceptin, GABAergic neurons, inhibition and disinhibition, inhibitory postsynaptic potential, ventral tegmental area
Introduction
The ventral tegmental area (VTA) is the origin of the brain dopamine-rewarding pathway that has been implicated in the reinforcing properties of abused drugs including opiates (Wise, 1996). Besides the principal dopamine neurons, the secondary (non-dopamine) neurons in this area are getting more attention due to their strategic position to influence mesolimbic functions. VTA non-dopamine neurons are suggested to contain γ-aminobutyric acid (GABA), with axons distributed locally to impinge on dopamine neurons or sent outside to innervate mesolimbic structures (Carr & Sesack, 2000; Laviolette & van der kooy, 2001; O'brien & White, 1987; Steffensen et al., 1998; van bockstaele & Pickel, 1995). Indeed, an opioid enhancement of VTA dopamine activity clearly results from inhibition of local GABAergic neurons (Johnson & North, 1992a).
The opioid receptor-like orphan receptor has been identified on the basis of its close structural homology with classical opioid receptors (Mollereau et al., 1994). The receptor and the endogenous agonist orphanin FQ/nociceptin (OFQ/N) (Meunler et al., 1995; Reinscheid et al., 1995) are found in a number of brain regions including the VTA (Anton et al., 1996; Darland et al., 1998; Henderson & Mcknight, 1997; Norton et al., 2002). At the cellular level, OFQ/N shares actions similar to those of classical opioids (Grudt & Williams, 1995; Henderson & Mcknight, 1997). Thus, a potent inhibitory action of this agent, via opening of potassium channels, has been detected in widespread brain regions, such as hypothalamic nuclei (Emmerson & Miller, 1999), hippocampus (Amano et al., 2000), amygdala (Meis & Pape, 1998), locus coerulerus (Connor et al., 1996), dorsal raphe (Vaughan & Christie, 1996) and periaqueductal grey (Vaughan et al., 1997).
Possible link of OFQ/N with the mesolimbic dopamine system comes from several lines of in vivo evidence that OFQ/N (1) decreases the outflow of dopamine in nucleus accumbens after intracerebral injections (Murphy et al., 1996; Murphy & Maidment, 1999), (2) reduces the morphine-induced dopamine release in nucleus accumbens (di giannuario et al., 1999), and (3) blocks the acquisition of morphine-dependent place preference (Murphy et al., 1999). Given that non-dopamine neurons play an important role in modulating VTA dopamine neuronal activity, in the present study we are interested to evaluate whether or not OFQ/N, like opiates, can influence dopaminergic functions via a GABA-mediated disinhibitory mechanism. To achieve this, we performed experiments to investigate the actions of OFQ/N on non-dopamine and dopamine neurons, and on inhibitory synaptic transmissions to dopamine neurons in the VTA slice preparation. Part of these results has been reported in abstract form (Zheng et al., 2000).
Methods
Tissue preparation
Horizontal midbrain slices containing the VTA were prepared from adult male Sprague–Dawley rats (150–250 g) as described previously (Johnson & North, 1992a,b). Use of animals followed procedures approved by the Oregon Health & Sciences University Institutional Animal Care and Use Committee. Briefly, each rat was anaesthetized with halothane prior to severing major thoracic blood vessels. The brain was rapidly removed and a tissue block containing the midbrain was mounted on a plexiglass platform and immersed in artificial cerebrospinal fluid at 4°C in a vibratome. Horizontal slices (300 μM) were cut, starting from the ventral surface. The slice was placed on a nylon mesh in a 0.5 ml recording chamber, immersed and perfused with artificial cerebrospinal fluid (35±1°C; at a rate of 2 ml min−1) composed of (in mM) NaCl 126, KCl 2.5, NaH2PO4 1.2, MgCl2 1.2, CaCl2 2.4, NaHCO3 18 and glucose 11, which was saturated with 95% O2 and 5% CO2 (pH 7.4).
Electrophysiological recordings
Intracellular recordings were performed in the VTA with the aid of a dissecting microscope (Johnson & North, 1992a,b). Recordings were made using glass microelectrodes filled with 2 M potassium chloride, with tip resistance of 40–100 MΩ. Bridge current clamp and single electrode discontinuous voltage clamp were used for recording the membrane potentials and currents, respectively. Single electrode voltage clamp recordings were made at a gain of 0.8–2.5 nA mV−1 with a switching frequency of 2–4 KHz. The headstage signal was monitored continuously on an oscilloscope. Potentials and currents were amplified with an Axoclamp 2A amplifier and recorded using Axotape or pClamp software (IBM compatible computer) together with MacLab chart software (Macintosh computer).
Spontaneous inhibitory postsynaptic potentials (IPSPs) were recorded continuously and analysed off-line using Axograph software. For evoked synaptic potentials, bipolar stimulation electrodes (sharpened tungsten wire; tip separation 300–500 μm) were placed in the slice within 500 μm of the recording electrode. Synaptic events were evoked by single square-wave electrical pulses (0.1 ms, 0.1 Hz) of constant voltage and were isolated pharmacologically. GABAA receptor-mediated IPSPs were induced by a single focal stimulus in the presence of antagonists for ionotropic glutamate receptors (6-cyano-7-nitroquinoxalone, 10 μM, and D-2-amino-5-phosphono-pentanoic acid, 50 μM) to block excitatory synaptic events.
Neuronal identification
Neurons recorded in the VTA were classified into two categories: dopamine and non-dopamine (putative GABAergic) neurons, according to their characteristic electrophysiological and pharmacological properties (Johnson & North, 1992b; Lacey et al., 1989). Non-dopamine neurons were identified by (i) their ability to fire narrow action potentials (half-amplitude duration, 0.2–0.6 ms) at high rate, (ii) a more negative resting membrane potential than that of dopamine neurons, (iii) a small or absent time-dependent membrane rectification to hyperpolarizing voltage commands (known as Ih), and (iv) membrane hyperpolarization or outward current in response to opioid l receptor agonist [met5]enkephalin but not to dopamine. Dopamine neurons were identified by (i) a wider action potential (half-amplitude duration>1 ms), (ii) pronounced Ih, and (iii) hyperpolarization to dopamine but not to [met5]enkephalin.
Drugs
All drugs were added to the superfusate at known concentration. OFQ/N and [Phe1φ(CH2-NH)Gly2]NC(1–13)NH2 were purchased from Tocris Cookson Ltd. (Bristol, U.K.). Bicuculline methiodide, dopamine hydrochloride, [met5]enkephalin and tetrodotoxin (TTX) were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Naloxone hydrochloride, 6-cyano-7-nitroquinoxalone and D-2-amino-5-phosphonopentanoic acid were obtained from Research Biochemicals Int. (Natick, MA, U.S.A.). Antagonist drugs were superfused 8–10 min before testing effect of antagonists.
Data analysis
Numerical data were expressed as mean±s.e.m. Linear regression was used to estimate a reversal potential of OFQ/N for each neuron; results were pooled to calculate an average value. Concentration-response curves were fitted to the equation y=ax/(x+b) using the KaleidaGraph curve-fitting program (Synergy Software, Reading, PA, U.S.A.) on a Macintosh computer; ‘y' is the magnitude of drug effect, ‘a' is the maximal drug effect, ‘x' is the concentration of the drug, and ‘b' is the IC50 (concentration producing 50% maximal hyperpolarization). An IC50 value was calculated for each neuron; results were pooled to calculate an average value. One-way ANOVA with repeated measures and t-test were used to test statistical significance. Differences were considered statistically significant when P<0.05.
Results
Effect of Orphanin FQ/Nociceptin on membrane properties of VTA neurons
We recorded 31 non-dopamine VTA neurons that were either firing spontaneously (n=9) or were silent (n=23) at zero holding current. OFQ/N (300 nM) reduced the spike discharge rate in non-dopamine neurons firing spontaneously from 14±3 to 3±2 Hz (n=9; P<0.05, paired t-test). As illustrated in Figure 1, those silent non-dopamine neurons displayed a resting membrane potential of −64±1 mV (n=23), a small or absent Ih, and membrane hyperpolarization or outward current in response to [met5]enkephalin (30 μM) but not to dopamine (30 μM). Their membrane input resistance was 151±22 MΩ (n=23). Under current clamp, OFQ/N (300 nM) produced a membrane hyperpolarization in most of these neurons (n=21; Figure 1A), reaching an average of 7.5±0.9 mV (range from 2.1 to 16.4 mV). The remaining two neurons displayed no detectable response to OFQ/N (300 nM; data not shown). Under voltage clamp, OFQ/N (300 nM) produced an outward current of 63±26 pA in non-dopamine VTA neurons (n=5). Both current and voltage clamp experiments were done with zero holding current, with a membrane potential of −61 to −68 mV.
Figure 1.
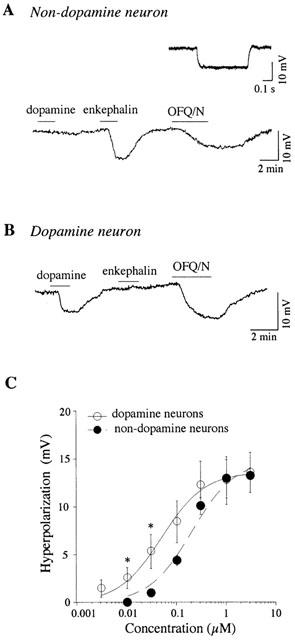
OFQ/N inhibits VTA neurons. (A) Continuous voltage trace recorded from a non-dopamine neuron under current clamp mode illustrates membrane hyperpolarization in response to OFQ/N (300 nM) at a resting membrane potential of −67 mV. These neurons are also hyperpolarized by [met5]enkephalin (30 μM) but not by dopamine (30 μM). Non-dopamine neurons exhibit no obvious sag in voltage in response to a 300 pA hyperpolarizing pulse (inset). (B) OFQ/N (300 nM) also evokes membrane hyperpolarization in a VTA dopamine neuron (potential initially held at −60 mV). Dopamine neurons are hyperpolarized by dopamine (30 μM) but not by [met5]enkephalin (30 μM). (C) Concentration-response curves for OFQ/N show that it is equally efficacious for inducing membrane hyperpolarization in non-dopamine (n=5) and dopamine (n=6) neurons in the VTA. However, OFQ/N, at submaximal concentrations, is more potent for hyperpolarizing dopamine neurons compared to non-dopamine neurons. Significant difference (P<0.05): * vs the respective value of non-dopamine neurons.
We also recorded 111 VTA dopamine neurons. OFQ/N (300 nM) reduced the spike discharge rate in dopamine neurons firing spontaneously from 2.5±0.2 Hz to 0.1±0.06 Hz (n=29; P<0.05, paired t-test). Among 82 dopamine neurons that were held at −60 mV under current clamp, OFQ/N (300 nM) hyperpolarized most of these neurons (Figure 1B), reaching an average of 10.2±0.5 mV (n=77). The remaining five neurons displayed no detectable response to OFQ/N (300 nM; data not shown).
The OFQ/N-induced hyperpolarization was concentration-dependent in both non-dopamine and dopamine neurons of the VTA. As seen in Figure 1C, 30 nM OFQ/N induced a hyperpolarization of 5.4±1.8 mV in dopamine neurons (n=6), but it only produced 1.0±0.4 mV of hyperpolarization in non-dopamine neurons (n=5; P<0.05). However, at the nearly maximal concentration of 1 μM, the magnitude of hyperpolarization was the same for both types of neuron (12.8±2.4 mV for 6 dopamine neurons and 13.0±2.0 mV for 5 non-dopamine neurons). The IC50 for non-dopamine neurons (238±68 nM, n=5) was significantly higher than that of dopamine neurons (61±16 nM, n=6; P<0.05). Thus, OFQ/N was more potent in dopamine neurons for inducing membrane hyperpolarization. However, at higher concentrations, OFQ/N was equally efficacious in each type of neuron.
The OFQ/N-induced hyperpolarization (n=2) and outward current (n=5) in VTA non-dopamine neurons reversed at −108±5 mV (Figure 2A,B), close to the value of EK predicted by the Nernst equation (−107 mV). In dopamine neurons, the reversal potential of OFQ/N-induced hyperpolarization was −102±5 mV (n=5). The reversal potential was shifted to −80±4 mV (n=4) by raising the extracellular potassium concentration to 7.5 mM (Figure 2C); this value is also close to the value of EK predicted by the Nernst equation (−78 mV).
Figure 2.
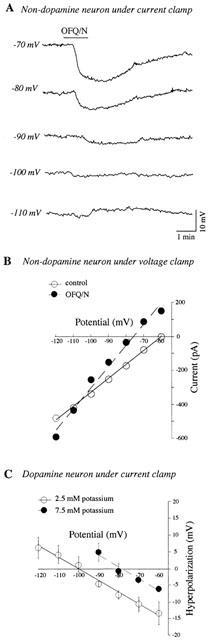
Reversal potential for OFQ/N-induced changes in membrane potential or current in VTA neurons. (A) Voltage-dependent effects of OFQ/N (300 nM) on membrane potential in current clamp in a non-dopamine neuron. The estimated Erev was −106 mV. (B) Current-voltage plot showing effects of OFQ/N (300 nM) on membrane currents under voltage clamp in a non-dopamine neuron. Erev is estimated at −106 mV. (C) Plot showing effects of various test potentials on magnitude of membrane hyperpolarization produced by OFQ/N (300 nM) in dopamine neurons recorded in normal (2.5 mM) and in a raised concentration (7.5 mM) of extracellular potassium. The average Erev for OFQ/N-induced hyperpolarization was −108±5 mV in 2.5 mM potassium (n=5), and was −80±4 mV in 7.5 mM potassium (n=4). In (A) and (C), OFQ/N is applied sequentially for each test voltage. In (B), OFQ/N is applied continuously during sequential voltage steps.
Neither TTX (0.5–1.0 μM) nor the opioid receptor antagonist naloxone (3 μM) affected OFQ/N-induced hyperpolarization in non-dopamine (n=3, Figure 3A) and dopamine neurons (n=4; Figure 3B). [Phe1φ(CH2-NH)Gly2]NC(1–13) NH2 (1 μM), the putative antagonist for opioid receptor-like orphan receptors (Guerrini et al., 1998), partially and reversibly reduced the effect of OFQ/N in both non-dopamine (51±4% of control, n=4; Figure 3A) and dopamine (50±3% of control, n=4; Figure 3B) neurons. This agent also caused a hyperpolarization in five of eight VTA neurons (6.8±0.5 mV), which is consistent with a partial agonist action as was described previously (Emmerson & Miller, 1999).
Figure 3.
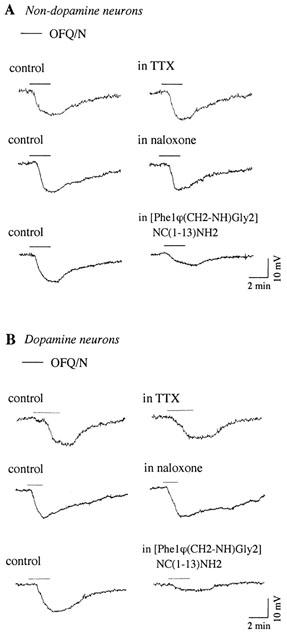
Pharmacology of OFQ/N-induced hyperpolarization in non-dopamine (A) and dopamine (B) neurons. Hyperpolarization to OFQ/N (300 nM) Ms not affected by TTX (0.5 μM) or by the opioid receptor antagonist naloxone (3 μM), but it is reduced by the antagonist [Phe1φ(CH2-NH)Gly2]NC(1–13)NH2 (1 μM). Each illustration is derived from a different neuron.
Effect of orphanin FQ/nociceptin on spontaneous and evoked inhibitory postsynaptic potentials
Spontaneous IPSPs with variable frequency and amplitude were observed in some dopamine neurons with membrane potential initially held at −70 mV (n=9). As described previously (Johnson & North, 1992b; Sugita et al., 1992), these spontaneous IPSPs were TTX- and bicuculline-sensitive (Figure 4A), and driven by spontaneous action potentials generated in local GABA-containing interneurons that make synaptic connections with dopamine neurons. As seen in Figure 4A,B, OFQ/N (300 nM) reversibly reduced the frequency, but not the amplitude, of spontaneous IPSPs from 6.2±1.2 Hz to 0.9±0.3 Hz (n=9; P<0.05, paired t-test). OFQ/N (300 nM) also caused 7.4±1.4 mV hyperpolarization in these neurons (n=9; Figure 4A).
Figure 4.
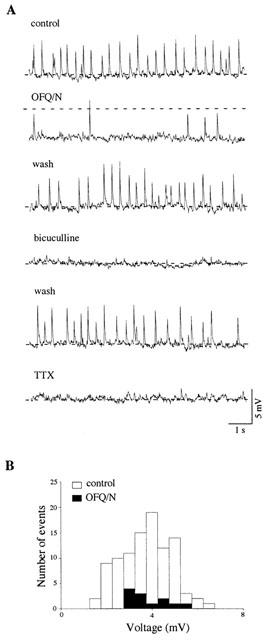
OFQ/N reduces spontaneous IPSPs in VTA dopamine neurons. (A) OFQ/N (300 nM) attenuates the frequency of bicuculline (30 μM)- and TTX (0.5 μM)-sensitive spontaneous IPSPs in a dopamine neuron. Dashed line indicates −70 mV. Note hyperpolarization induced by OFQ/N. (B) Histogram shows the distribution of IPSP amplitude of this neuron during control and OFQ/N application. In each condition, data are collected for 30 s and events are binned in 0.5 mV intervals.
As seen in Figure 5, OFQ/N reduced the amplitude of IPSPs evoked by focal electrical stimulation of the midbrain slice. When recorded in dopamine neurons with membrane potential held initially at −60 mV, OFQ/N (300 nM) reduced the amplitude of evoked IPSPs by 21±4% (n=10). Moreover, when pairs of electrical stimuli were used to evoke IPSPs in dopamine neurons, OFQ/N (300 nM) significantly increased the paired-pulse ratio from 0.59±0.05 to 0.70±0.04 (n=10; P<0.05, paired t-test). This suggests that OFQ/N inhibits GABA release onto dopamine neurons via a presynaptic site of action.
Figure 5.
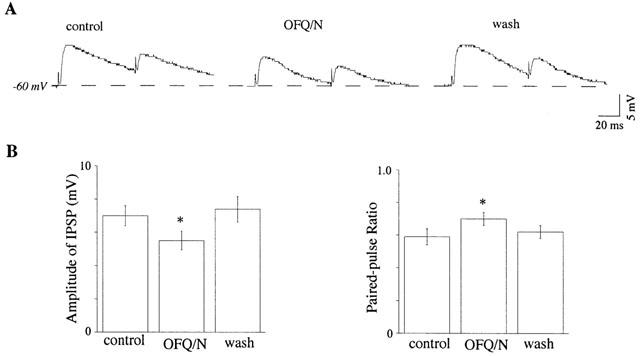
OFQ/N reduces the evoked GABAA IPSPs in VTA dopamine neurons. (A) OFQ/N (300 nM) decreases the amplitude of IPSPs evoked by paired stimuli (interpulse interval of 70 ms) recorded in a dopamine neuron. The membrane potential is clamped manually at −60 mV by injecting current. (B) Histograms summarize the group data (n=10) for the effects of OFQ/N on the amplitude of the first IPSP and the paired-pulse ratio (the ratio of the second IPSP divided by the first). Significant difference (P<0.05): * vs the value of control.
Discussion
Data from the present study demonstrate direct inhibitory effects of OFQ/N on non-dopamine (putative GABAergic) and dopamine neurons in the VTA. Opening of potassium channels is consistent with OFQ/N's effect on both neuronal type in the VTA, as well as in many other neurons in various brain regions (see Introduction). Our findings that OFQ/N-induced hyperpolarization is antagonized by [Phe1φ(CH2-NH)Gly2]NC(1–13)NH2 but not by naloxone suggest that OFQ/N acts on VTA neurons via opioid receptor-like orphan receptors rather than opioid receptors, despite structural homology. Furthermore, both non-dopamine and dopamine neurons in the VTA are found to be substrates for OFQ/N, which contrasts with the report that functional μ opioid receptors are expressed only by GABAergic VTA neurons (Dilts & Kalivas, 1988), and their activity is inhibited by a direct action of opioid agonists (Johnson & North, 1992a).
Like opioids (Johnson & North, 1992a), OFQ/N reduces the frequency of GABAA receptor-mediated spontaneous IPSPs and the amplitude of evoked IPSPs in dopamine neurons. Local GABAergic neurons in the VTA produce the spontaneous IPSPs recorded in dopamine neurons (Sugita et al., 1992), but they also contribute to IPSPs evoked by electrical stimulation. Thus, similar to opioids, OFQ/N could also disinhibit dopamine neurons by reducing the tonic inhibition from local interneurons, and/or by reducing the stimulus-induced release of GABA from inhibitory terminals. The net effect of OFQ/N on mesolimbic dopamine output would theoretically result from a balance of direct inhibition and disinhibition. However, the possibility that OFQ/N disinhibits dopamine neurons is somehow undermined by the findings that (1) OFQ/N at relatively low concentration tends to be more potent in hyperpolarizing dopamine neurons in the slice preparation, and (2) only an inhibitory effect of OFQ/N has been reported on dopamine outflow in nucleus accumbens in anaesthetized rats (Murphy & Maidment, 1999), even though a facilitatory action of OFQ/N on dopamine outflow in the striatum has also been reported in conscious animals (Konya et al., 1998).
Although most VTA GABAergic neurons recorded in the present study are presumed to be interneurons, some of these may actually be efferent neurons that project GABA-containing axons to mesolimbic structures (Steffensen et al., 1998; van bockstaele & Pickel, 1995). Interestingly, this GABA-containing pathway arising from the VTA has been implicated in dopamine-independent mechanisms for reward (Laviolette & van der kooy, 2001). At first glance, the reduction of GABAergic neuronal activity in the present in vitro study does not seem compatible with the local increase in GABA release that was reportedly produced by an intra-VTA injection of OFQ/N in anaesthetized rats (Murphy & Maidment, 1999). However, our data may in fact be compatible if we consider a possible role of VTA-nucleus accumbens neuronal circuits, which are cut in the slice preparation. These neuronal circuits are composed of reciprocal projections between GABAergic neurons in the nucleus accumbens and VTA dopamine and non-dopamine neurons (Kalivas et al., 1993; Oades & Hilliday, 1987; van bockstaele & Pickel, 1995). OFQ/N-induced reductions in VTA dopamine and GABAergic neuronal activities could disinhibit the GABAergic projection neurons in the nucleus accumbens, which may be responsible for the increased GABA content and further suppression of dopamine neuronal activity in the VTA.
In summary, the present findings provide evidence that both non-dopamine and dopamine neurons represent substrates for OFQ/N action within the VTA. Simultaneous inhibition of both non-dopamine and dopamine pathways by OFQ/N may work in concert to account for its influences on various VTA-related functions (Devine et al., 1996; di giannuario et al., 1999; Murphy et al., 1999).
Acknowledgments
This work is supported by USPHS grant MH40416 to SW Johnson and DA10703 to DK Grandy.
Abbreviations
- GABA
γ-aminobutyric acid
- IPSP
inhibitory postsynaptic potential
- OFQ/N
orphanin FQ/nociceptin
- TTX
tetrodotoxin
- VTA
ventral tegmental area
References
- AMANO T., MATSUBAYASHI H., TAMURA Y., TAKAHASHI T. Orphanin FQ-induced outward current in rat hippocampus. Brain Res. 2000;853:269–274. doi: 10.1016/s0006-8993(99)02245-3. [DOI] [PubMed] [Google Scholar]
- ANTON B.A., FEIN J., TO T., LI X., SILBERSTEIN L., EVANS C.J. Immunohistochemical location of ORL-1 in the central nervous system of the rat. J. Comp. Neurol. 1996;368:229–251. doi: 10.1002/(SICI)1096-9861(19960429)368:2<229::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- CARR D.B., SESACK S.R. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000;38:114–123. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- CONNOR M., VAUGHAN C.W., CHIENG B., CHRISTIE M.J. Nociceptin receptor coupling to a potassium conductance in rat locus coerleus neurons in vitro. Br. J. Pharmacol. 1996;119:1614–1618. doi: 10.1111/j.1476-5381.1996.tb16080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARLAND T., HEINRICHER M.M., GRANDY D.K. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–221. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- DEVINE D.P., TAYLOR L., REINSCHEID R.P., MONSMA F.J., JR, CIVELLI O., AKIL H. Rats rapidly develop tolerance to the locomotor-inhibiting effects of the novel neuropeptide orphanin FQ. J. Neurochem. Res. 1996;21:1387–1396. doi: 10.1007/BF02532380. [DOI] [PubMed] [Google Scholar]
- DI GIANNUARIO A., PIERETTI S., CATALANI A., LOIZZO A. Orphanin FQ reduces morphine-induced dopamine release in the nucleus accumbens: a microdialysis study in rats. Neurosci. Lett. 1999;272:183–186. doi: 10.1016/s0304-3940(99)00579-0. [DOI] [PubMed] [Google Scholar]
- DILTS R.P., KALIVAS P.W. Localization of mu opioid and neurotesin receptors within the A10 region of the rat. Ann. N.Y. Acad. Sci. 1988;537:472–474. [Google Scholar]
- EMMERSON P.J., MILLER R.J. Pre- and post-synaptic actions of opioid and orphan opioid agonists in the rat arcuate nucleus and ventromedial hypothalamus in vitro. J. Physiol. 1999;517:431–445. doi: 10.1111/j.1469-7793.1999.0431t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUDT T.J., WILLIAMS J.T. Opioid receptors and the regulation of ion conductances. Rev. Neurosci. 1995;6:279–286. doi: 10.1515/revneuro.1995.6.3.279. [DOI] [PubMed] [Google Scholar]
- GUERRINI R., CALO G., RIZZI A., BIGONI R., BIANCHI C., SALVADORI S., REGOLI D. A new selective antagonist of the nociceptin receptor. Br. J. Pharmacol. 1998;123:163–165. doi: 10.1038/sj.bjp.0701640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDERSON G., MCKNIGHT A.T. The orphan opioid receptor and its endogenous ligand–nociceptin/orphanin FQ. Trends Pharmacol. 1997;18:293–300. [PubMed] [Google Scholar]
- JOHNSON S.W., NORTH R.A. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 1992a;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON S.W., NORTH R.A. Two types of neuron in the rat ventral tegmental area and their synaptic inputs. J. Physiol. 1992b;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALIVAS P.W., CHURCHILL L., KLITENICK M.A. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57:1047–1060. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- KONYA H., MASUDA H., ITOH K., NAGAI K., KAKISHITA E., MATSUOKA A. Modification of dopamine release by nociceptin in conscious rat striatum. Brain Res. 1998;788:341–344. doi: 10.1016/s0006-8993(98)00075-4. [DOI] [PubMed] [Google Scholar]
- LACEY M.G., MERCURI N.B., NORTH R.A. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J. Neurosci. 1989;9:1233–1241. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVIOLETTE S.R., VAN DER KOOY D. GABAA receptors in the ventral tegmental area control bidrectional reward signaling between dopaminergic and non-dopaminergic neural motivational system. Eur. J. Neurosci. 2001;13:1009–1015. doi: 10.1046/j.1460-9568.2001.01458.x. [DOI] [PubMed] [Google Scholar]
- MEIS S., PAPE H.C. Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to nociceptin/orphanin FQ. J. Neurosci. 1998;18:8133–8144. doi: 10.1523/JNEUROSCI.18-20-08133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEUNLER J.C., MOLLEREAU C., TOLL L., SUAUDEAU C., MOISAND C., ALVINERIE P., BUTOUR J.L., GUILLEMOT J.C., FERRARA P., MONSARRAT B., MAZARGUIL H., VASSART G., PARMENTIER M., COSTENEIN J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- MOLLEREAU C., PARMENTIER M., MAILLEUX P., BUTOUR J.L., MOISAND C., CHALON P., CAPUT D., VASSART G., MENUMIER J.C. ORL1, a novel member of the opioid receptor family. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- MURPHY N.P., LEE Y., MAIDMENT N.T. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- MURPHY N.P., LY H.T., MAIDMENT N.T. Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience. 1996;75:1–4. doi: 10.1016/0306-4522(96)00322-3. [DOI] [PubMed] [Google Scholar]
- MURPHY N.P., MAIDMENT N.T. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J. Neurochem. 1999;73:179–186. doi: 10.1046/j.1471-4159.1999.0730179.x. [DOI] [PubMed] [Google Scholar]
- NORTON C.S., NEAL C.R., KUMAR S., AKIL H., WATSON S.J. Nociceptin/Orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J. Comp. Neurol. 2002;444:358–368. doi: 10.1002/cne.10154. [DOI] [PubMed] [Google Scholar]
- OADES R.D., HILLIDAY G.M. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. Rev. 1987;12:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- O'BRIEN D.P., WHITE F.J. Inhibition of nondopamine cells in the ventral tegmental area by benzodiazepine: relationship to A10 dopamine cell activity. Eur. J. Pharmacol. 1987;142:343–354. doi: 10.1016/0014-2999(87)90072-0. [DOI] [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MONSMA F.J.JR., CIVELLI O. Orphanin FQ: a neuropeptide that activates an opioid like G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- STEFFENSEN S.C., SVINGOS A.L., PICKEL V.M., HENRIKSEN S.J. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J. Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGITA S., JOHNSON S.W., NORTH R.A. Synaptic inputs to GABAA and GABAB receptors originate from discrete afferent neurons. Neurosci. Lett. 1992;134:207–211. doi: 10.1016/0304-3940(92)90518-c. [DOI] [PubMed] [Google Scholar]
- VAN BOCKSTAELE E.J., PICKEL V.M. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res. 1995;682:215–221. doi: 10.1016/0006-8993(95)00334-m. [DOI] [PubMed] [Google Scholar]
- VAUGHAN C.W., CHRISTIE M.J. Increase by the ORL1 receptor (opioid receptor-like1) ligand, nociceptin, of inwardly rectifying K+ conductance in dorsal raphe nucleus neurons. Br. J. Pharmacol. 1996;117:1609–1611. doi: 10.1111/j.1476-5381.1996.tb15329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAUGHAN C.W., INGRAM S.L., CHRISTIE M.J. Actions of the ORL1 receptor ligand nociceptin on membrane properties of rat periaqueductal gray neurons in vitro. J. Neurosci. 1997;17:996–1003. doi: 10.1523/JNEUROSCI.17-03-00996.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISE R.A. Addictive drugs and brain stimulation reward. Annu. Rev. Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- ZHENG F., GRANDY D.K., JOHNSON S.W. Effect of Orphanin FQ/Nociceptin on rat ventral tegmental area neurons. Soc. Neurosci. Abstr. 2000;26:1159. [Google Scholar]


