Abstract
Oestrogen and tamoxifen activate large conductance Ca2+-activated K+ (BKCa) channels in smooth muscle through a non-genomic mechanism that depends on the regulatory β1 subunit and an extracellular binding site. It is unknown whether a ‘pure' anti-oestrogen such as ICI 182,780 (Faslodex™), that has no known oestrogenic properties, would have any effect on BKCa channels. Using single channel patch clamp techniques on canine colonic myocytes, the hypothesis that ICI 182,780 would activate BKCa channels was tested. ICI 182,780 increased the open probability of BKCa channels in inside-out patches with an EC50 of 1 μM. These data suggest that molecules with the ability to bind nuclear oestrogen receptors, regardless of oestrogenic or anti-oestrogenic nature, activate BKCa channels through this nongenomic, membrane-delimited mechanism. The identity and characteristics of this putative binding site remain unclear; however, it has pharmacological similarity to oestrogen receptors α and β, as ICI 182,780 interacts with it.
Keywords: 17-β oestradiol, tamoxifen, Slo, MaxiK channel, β1 subunit
Introduction
Oestrogen (17-β oestradiol) has nongenomic, or alternative, effects unrelated to the nuclear events (Falkenstein et al., 2000) and some anti-oestrogens share this property. These compounds exert effects outside the classical signalling pathway of altering gene transcription. For example, oestrogen and tamoxifen activate BKCa channels in smooth muscle (Valverde et al., 1999; Dick et al., 2001). BKCa channels have a large unitary conductance and are Ca2+/voltage-sensitive. In smooth muscle cells, BKCa channels contain a regulatory β1 subunit that alters channel function in many ways (McManus et al., 1995). Appreciation for the physiological importance of the β1 subunit has recently increased, as genetically engineered mice lacking this regulatory subunit are hypertensive and demonstrate altered smooth muscle reactivity (Brenner et al., 2000). An extracellular binding site and the β1 subunit are essential for sensitivity of BKCa channels to oestrogen and tamoxifen (Valverde et al., 1999; Dick et al., 2001; 2002; Dick & Sanders, 2001). The identity of the extracellular binding site is not known (Nadal et al., 2001).
Tamoxifen is used clinically as an oestrogen receptor antagonist for the treatment and prevention of breast cancer. However, tamoxifen has mixed oestrogenic and anti-oestrogenic properties, ranging from full agonist to full antagonist depending on the tissue and endpoint examined. Tamoxifen inhibits the binding of oestrogen to nuclear receptors preventing cell proliferation. In contrast, tamoxifen has oestrogenic properties in liver, bone, and uterus, where it alters blood lipids, bone density, and endometrial thickness (Jordan, 2000). Because of oestrogenic properties, tamoxifen is associated with adverse chemotherapeutic reactions including tumour flare, hot flashes, and the stimulation of liver and myometrial carcinomas (Jordan, 2000; Howell et al., 2000). Thus, the search for oestrogen receptor antagonists without oestrogenic properties has been an area of intense interest. ICI 182,780 is a highly specific oestrogen receptor antagonist marketed under the trade name Faslodex (Howell et al., 2000). ICI 182,780 is used for treating oestrogen receptor-dependent tumours, and, in contrast to tamoxifen, has no known oestrogenic properties.
The question remains as to whether the ability of tamoxifen to activate BKCa channels is due to its partial oestrogenic properties. This was determined by assessing the effect of a pure anti-oestrogen, ICI 182,780. The results delineate a novel mechanism of action and indicate that the putative extracellular binding site has pharmacology in common with oestrogen receptors α or β, as a pure anti-oestrogen interacts with it. This interaction is translated, by an unknown mechanism, into changes in BKCa channel activity.
Methods
Canine colonic smooth muscle cells were isolated by enzymatic dispersion described previously (Dick et al., 2001; 2002). The methods were approved by an Animal Care and Use Committee and were consistent with NIH guidelines. Myocytes were suffused at 3 ml min−1 with (mM) KCl 140, HEPES 10, Tris 5; pH 7.1. Additionally, this solution contained either 1 mM EGTA or 1 mM HEDTA (N-[2-hydroxy ethyl]-ethylenediamine-triacetic acid) and between 0.01–10 μM free Ca2+ (Maxchelator 2.05; Pacific Grove, CA, U.S.A.).
Data were analysed with the Analysis of Single Channel Data program (Guy Droogmans; University of Leuven, Belgium). NPo (number of channels multiplied by the open probability) was measured before, during, and after exposing the patches to ICI 182,780 (Tocris Cookson Inc., Ballwin, MO, U.S.A.). ICI 182,780 was made as a 10 mM stock in DMSO and diluted to the final concentration in bathing solutions. DMSO, at concentrations of 0.1% and less, has no effect on BKCa channels (Dick et al., 2001). NPo and unitary conductance were determined from all-points amplitude histograms. Data are presented as the mean±s.e. from n cells. Statistical analyses were made with Student's paired t-test or one-way analysis of variance (with repeated measures and Bonferroni post-hoc analysis as necessary). The threshold for statistical significance was P<0.05. Statistical tests were run in SigmaStat (version 2; Jandel Scientific Software; San Rafael, CA, U.S.A.).
Results
ICI 182,780 increases the NPo of BKCa channels by a nongenomic mechanism
BKCa channels were studied in inside-out patches of membrane taken from canine colonic myocytes as described previously (Dick & Sanders, 2001; Dick et al., 2001, 2002). ICI 182,780 (10 μM) increased NPo when applied to the bath (Figure 1A–D). In contrast to the effect of tamoxifen, ICI 182,780 increased NPo without affecting the unitary conductance of BKCa channels (Figure 1E).
Figure 1.
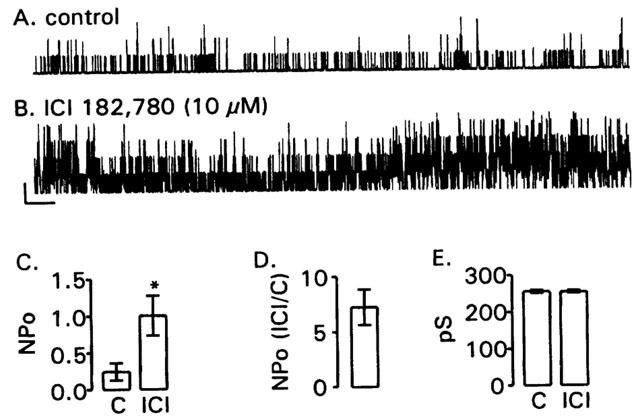
ICI 182,780 increases BKCa channel NPo in excised membrane patches. Representative 1 min recordings of BKCa channel activity in an inside-out patch before (panel A; control; C) and after a 5 min exposure to 10 μM ICI 182,780 (ICI; panel B). Patch potential was +80 mV; 100 nM free Ca2+. ICI 182,780 increased NPo without effect on the unitary conductance. Scale bar represents 20 pA and 3 s. Panel C group data (n=13) demonstrating the effect of 10 μM ICI 182,780 on BKCa channel NPo. The asterisk indicates a significant effect of ICI 182,780 on NPo (P=0.001 by Student's paired t-test). Panel D NPo in the presence of 10 μM ICI 182,780 normalized to control. Panel E ICI 182,780 had no effect on the unitary conductance of BKCa channels (P=0.60 by Student's paired t-test).
Effects of ICI 182,780 on BKCa channels are rapid, but not readily reversible
The increase of BKCa channel NPo by ICI 182,780 was observed in cell-free patches of membrane, indicating it is a nongenomic effect. Additionally, a hallmark of nongenomic steroid effects is the speed of the effect. Figure 2 shows that ICI 182,780 (10 μM) rapidly increased BKCa channel NPo. The time from ICI 182,780 (10 μM) application to reach a steady-state NPo was 4.8±0.7 min (n=13). The effect of ICI 182,780, however, was not readily reversible. As can be seen in Figure 2A, brief exposure to ICI 182,780 caused an increase in NPo that persisted for approximately 30 min. In six cells, NPo was 306±38% higher than control 21±2 min after 10 μM ICI 182,780 was washed out of the bath.
Figure 2.
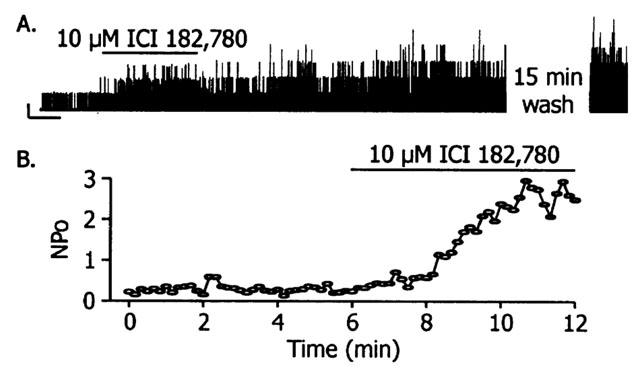
Rapid, but not readily reversible, effect of ICI 182,780 on BKCa NPo. Panel A shows a representative recording of BKCa channel activity in an inside-out patch. Patch potential was +100 mV; 100 nM free Ca2+. Current was recorded for 2 min prior to exposing the patch to 10 μM ICI 182,780 for 3 min, which increased NPo. NPo remained elevated after ICI 182,780 was washed out; an effect that persisted after a 15 min wash. Scale bar represents 20 pA and 1 min Panel B is a plot of NPo vs time from another patch under the same recording conditions. NPo was steady after patch excision and increased with the application of 10 μM ICI 182,780.
The increase in NPo by ICI 182,780 is concentration-dependent and saturable
Increasing concentrations of ICI 182,780 (0.1–10 μM) were added to solutions bathing the cytoplasmic face of inside-out patches. ICI 182,780 increased NPo in a concentration-dependent manner. The EC50 was 1.0±0.2 μM (n=10; Figure 3). The unitary conductance under control conditions was 260±4 pS (n=10) and did not change with exposure to ICI 182,780 (P=0.69 by one-way repeated measures analysis of variance).
Figure 3.
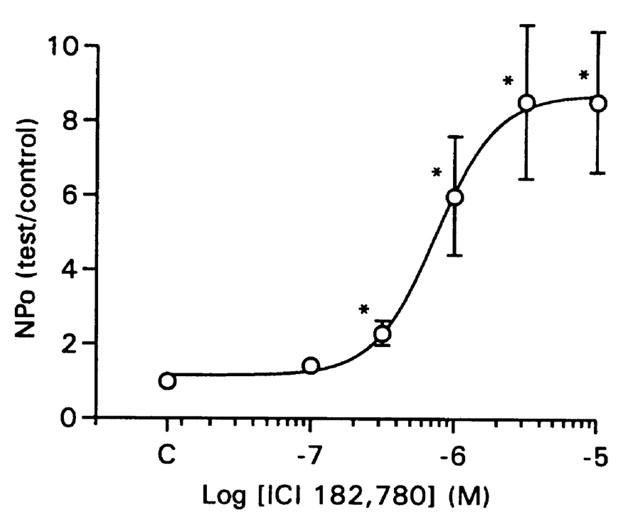
Concentration-dependent effect of ICI 182,780 on BKCa channel NPo. Group data (n=10) showing the concentration-dependent effect of ICI 182,780 on BKCa channel NPo at +80 mV and 100 nM free Ca2+. NPo was measured at least 5 min after each concentration of ICI 182,780 was applied. The EC50 was 1 μM. Asterisks indicate a difference from control (C) by one-way repeated measures analysis of variance.
ICI 182,780 shifts the voltage-dependence of BKCa gating to more negative potentials
In all the experiments described thus far, the bath free Ca2+ concentration was 100 nM, near the lower limit where the influence of the β1 subunit on BKCa channel gating is thought to decline. To determine the effect of ICI 182,780 on channel gating over a wider range of free Ca2+ concentrations, and thus NPo, two different solutions were used. In the first, the bath contained 1 mM EGTA and no added Ca2+, setting the free Ca2+ concentration near 10 nM. Under these low Ca2+ conditions, ICI 182,780 increased NPo 420±95% (n=8). The second solution contained 1 mM HEDTA and 10 μM free Ca2+. Under these high Ca2+ conditions, ICI 182,780 increased NPo 431±107% (n=7). Additionally, an activation curve was made in 100 nM free Ca2+. ICI 182,780 (10 μM) shifted the voltage of half activation from 106±6 to 86±5 mV (n=7; P<0.0001 by Student's paired t-test; Figure 4).
Figure 4.
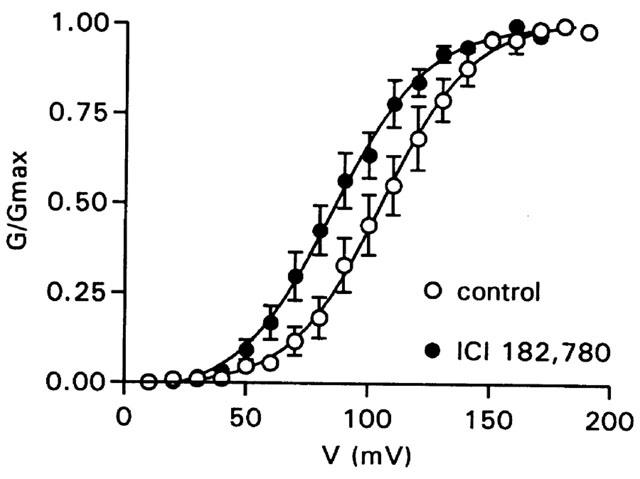
ICI 182,780 shifts the voltage-dependence of BKCa gating. Group data (n=7) demonstrating a negative shift in the voltage of half activation by 10 μM ICI 182,780. The patch potential was held at 0 mV and stepped to potentials from +10 to +200 mV; 100 nM free Ca2+. Conductance was calculated from current, normalized to the maximum, and fit with a Boltzmann sigmoidal function.
Discussion
ICI 182,780 activates BKCa channels in smooth muscle, a previously unrecognized action that is rapid, concentration-dependent, and similar to that of oestrogen and tamoxifen (Valverde et al., 1999; Dick & Sanders, 2001; Dick et al., 2001). This is a nongenomic, or alternative, effect as it does not involve changes in gene transcription (Falkenstein et al., 2000). The activation of BKCa channels by [xeno]oestrogens is not strictly due to oestrogenic properties, as a pure anti-oestrogen, ICI 182,780, has similar effects. The extracellular binding site has pharmacology in common with oestrogen receptor α and β, as oestradiol, tamoxifen, and ICI 182,780 interact with it. This binding is translated, by an unknown mechanism, into changes in BKCa channel activity.
ICI 182,780 (AstraZeneca, Cheshire, U.K.) is a steroidal oestrogen receptor antagonist (Howell et al., 2000). ICI 182,780 is thought to be a pure anti-oestrogen (i.e., it does not have any known oestrogen agonist activity). There are few data regarding nongenomic mechanisms of action for ICI 182,780. One example is inhibition of L-type Ca2+ channels (i.e., Ba2+ current) by ICI 182,780 in A7r5 cells (Ruehlmann et al., 1998). ICI 182,780 also increased K+ current in three of four cells tested; however, the nature of the current, and the concentration-dependence and magnitude of the ICI 182,780 effect were not determined. ICI 182,780 also reduces coronary vascular tone in isolated perfused rat hearts (Ruehlmann et al., 1998). This could be due, in part, to inhibition of L-type Ca2+ channels or activation of K+ channels. Increased outward current could be due to diminished inward current, and not the activation per se, of an outward current. These results suggest that previous observations could be explained by activation of BKCa channels.
The activation of smooth muscle BKCa channels by [xeno]oestrogens depends on the presence of the regulatory β1 subunit (Dick & Sanders, 2001; Dick et al., 2001; Valverde et al., 1999). There are four known regulatory β subunits, but only β1 and β4 (Behrens et al., 2000) confer oestrogen sensitivity upon BKCa channels. Studies using membrane impermeant [xeno]oestrogens suggest an extracellular binding site exists, perhaps within the extracellular loop of the BKCa channel β1 subunit (Valverde et al., 1999; Dick et al., 2002). However, preliminary data indicate that a chimeric β subunit containing the β1 extracellular loop and the β2 intracellular and transmembrane portions does not respond to oestrogen or tamoxifen (Orio et al., 2002). It remains to be determined whether this chimeric β1/2 subunit binds [xeno]oestrogens or whether additional portions of the β1 subunit are necessary for transducing the response to BKCa channels. It is possible that a yet unidentified mediator, such as oestrogen receptor α or β, may be a necessary molecular component.
Gene transcripts for both the α and β isoforms of the oestrogen receptor are present in smooth muscle (Hodges et al., 2000); however, the hypothesis that one or both of these receptor isoforms is involved in this signalling pathway to BKCa channels has not been tested. As attractive as the possibility seems, there are difficulties with suggesting that either oestrogen receptor α or β is a transducing element in the membrane (Nadal et al., 2001). Neither oestrogen receptor α nor β has a predicted transmembrane spanning domain. This limitation might be overcome by post-translational modification; however, neither isoform has an obvious site. Additionally, while Xenopus oocytes (Valverde et al., 1999) and human embryonic kidney cells (Dick et al., 2001) serve as competent expression systems for studying the β1-dependent effects of [xeno]oestrogens on BKCa channels, neither is thought to possess endogenous oestrogen receptors, and are commonly used to express them (Kahlert et al., 2000; Watson, 1991). Whether the plasma membrane receptor for [xeno]oestrogens is identical to, related to, or completely distinct from oestrogen receptor α or β remains a matter of debate.
Regardless of the molecular machinery involved, ICI 182,780 activates smooth muscle BKCa channels in a non-genomic fashion with an EC50 near 1 μM. This effect on BKCa channels in smooth muscle would not be expected to have clinical significance, as the therapeutic serum concentration in humans is approximately 12.6 ng ml−1 (≈21 nM; (Howell et al., 1996)). Thus, the effects of ICI 182,780 on BKCa channel NPo, and L-type Ca2+ channels (Ruehlmann et al., 1998), in vitro are achieved only at much higher concentrations. However, the data suggest that molecules with the ability to bind nuclear oestrogen receptors, regardless of oestrogenic or anti-oestrogenic nature, activate BKCa channels through this membrane-delimited mechanism. Further research is required to identify the putative extracellular binding site for [xeno]oestrogens.
Acknowledgments
I thank Nancy Horowitz for preparing the cells used in this study. I appreciate the comments of Drs. Kenton M. Sanders and James L. Kenyon regarding this manuscript. Dr. Dick is the recipient of a postdoctoral National Research Service Award (F32 DK09947). NIH DK41315 supported this work.
Abbreviations
- BKCa
large conductance Ca2+-activated K+ channel
- tamoxifen
[Z]-1-[p-dimethylaminoethoxy-phenyl]-1,2-diphenyl-1-butene
- ICI 182,780
(7α-[9-[(4,4,5,5,5-pentafluoropentyl)sulphinyl]nonyl]-estra-1,3,5(10)-triene-3,17β-diol)
- HEDTA
N-[2-hydroxyethyl]-ethylenediamine-triacetic acid
References
- BEHRENS R., NOLTING A., REIMANN F., SCHWARZ M., WALDSCHUTZ R., PONGS O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel β subunit family. FEBS Lett. 2000;474:99–106. doi: 10.1016/s0014-5793(00)01584-2. [DOI] [PubMed] [Google Scholar]
- BRENNER R., PEREZ G.J., BONEV A.D., ECKMAN D.M., KOSEK J.C., WILER S.W., PATTERSON A.J., NELSON M.T., ALDRICH R.W. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- DICK G.M., HUNTER A.C., SANDERS K.M. Ethylbromide tamoxifen, a membrane-impermeant antiestrogen, activates smooth muscle calcium-activated large conductance potassium channels from the extracellular side. Mol. Pharmacol. 2002;61:1105–1113. doi: 10.1124/mol.61.5.1105. [DOI] [PubMed] [Google Scholar]
- DICK G.M., ROSSOW C.F., SMIRNOV S., HOROWITZ B., SANDERS K.M. Tamoxifen activates smooth muscle BK channels through the regulatory β1 subunit. J. Biol. Chem. 2001;267:34594–34599. doi: 10.1074/jbc.M104689200. [DOI] [PubMed] [Google Scholar]
- DICK G.M., SANDERS K.M. (Xeno)estrogen-sensitivity of smooth muscle BK channels conferred by the regulatory β1 subunit: A study of β1 knockout mice. J. Biol. Chem. 2001;276:44835–44840. doi: 10.1074/jbc.M106851200. [DOI] [PubMed] [Google Scholar]
- FALKENSTEIN E., TILLMANN H.C., CHRIST M., FEURING M., WEHLING M. Multiple actions of steroid hormones – a focus on rapid, nongenomic effects. Pharmacol. Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- HODGES Y.K., TUNG L., YAN X.D., GRAHAM J.D., HORWITZ K.B., HORWITZ L.D. Estrogen receptors α and β: prevalence of estrogen receptor β mRNA in human vascular smooth muscle and transcriptional effects. Circulation. 2000;101:1792–1798. doi: 10.1161/01.cir.101.15.1792. [DOI] [PubMed] [Google Scholar]
- HOWELL A., DEFRIEND D.J., ROBERTSON J.F., BLAMEY R.W., ANDERSON L., ANDERSON E., SUTCLIFFE F.A., WALTON P. Pharmacokinetics, pharmacological and anti-tumour effects of the specific anti-oestrogen ICI 182780 in women with advanced breast cancer. Br. J. Cancer. 1996;74:300–308. doi: 10.1038/bjc.1996.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWELL A., OSBORNE C.K., MORRIS C., WAKELING A.E. ICI 182,780 (Faslodex): development of a novel, ‘pure' antiestrogen. Cancer. 2000;89:817–825. doi: 10.1002/1097-0142(20000815)89:4<817::aid-cncr14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- JORDAN V.C. Progress in the prevention of breast cancer: concept to reality. J. Steroid Biochem. Mol. Biol. 2000;74:269–277. doi: 10.1016/s0960-0760(00)00103-5. [DOI] [PubMed] [Google Scholar]
- KAHLERT S., NUEDLING S., VAN EICKELS M., VETTER H., MEYER R., GROHE C. Estrogen receptor α rapidly activates the IGF-1 receptor pathway. J. Biol. Chem. 2000;275:18447–18453. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- MCMANUS O.B., HELMS L.M., PALLANCK L., GANETZKY B., SWANSON R., LEONARD R.J. Functional role of the β subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14:645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- NADAL A., ROPERO A.B., FUENTES E., SORIA B. The plasma membrane estrogen receptor: nuclear or unclear. Trends Pharmacol. Sci. 2001;22:597–599. doi: 10.1016/s0165-6147(00)01846-0. [DOI] [PubMed] [Google Scholar]
- ORIO P., ROJAS P., TORO L., LATORRE R.MaxiK channel activation by estradiol and tamoxifen: role of the extracellular domain of the β1 subunit Biophys. J. 200282205A(abstract) [Google Scholar]
- RUEHLMANN D.O., STEINERT J.R., VALVERDE M.A., JACOB R., MANN G.E. Environmental estrogenic pollutants induce acute vascular relaxation by inhibiting L-type Ca2+ channels in smooth muscle cells. FASEB J. 1998;12:613–619. doi: 10.1096/fasebj.12.7.613. [DOI] [PubMed] [Google Scholar]
- VALVERDE M.A., ROJAS P., AMIGO J., COSMELLI D., ORIO P., BAHAMONDE M.I., MANN G.E., VERGARA C., LATORRE R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the β subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- WATSON C.S. Human estrogen receptor introduced into the Xenopus oocyte represses expression from an artificial frog estrogen response element. J. Steroid Biochem. Mol. Biol. 1991;38:423–431. doi: 10.1016/0960-0760(91)90330-8. [DOI] [PubMed] [Google Scholar]


