Abstract
Since all 5-HT1 receptors couple to Gi–type G proteins and inhibit adenylyl cyclase, the functional significance of five distinct subtypes of 5-HT1 receptors has been unclear.
In previous studies we have used transfected cells to demonstrate that 5-HT1B receptors can couple more efficiently than 5-HT1A receptors to activation of extracellular signal-regulated kinase (ERK) and to inhibition of adenylyl cyclase. These findings suggested the possibility that individual 5-HT1 receptors differentially couple to isoforms of Giα.
In the present study we utilized a model system in which pertussis toxin resistant forms of human Giα1, Giα2, and Giα3 were used to directly compare the coupling of human 5-HT1A, 5-HT1B, and 5-HT1D receptors to each Giα in transfected human HeLa cells.
5-HT1A receptors displayed a preference for Giα1 and Giα2, relative to Giα3. Pertussis toxin resistant forms of Giα1, Giα2, and Giα3 rescued 73%, 76%, and 44%, respectively, of the ERK activation stimulated by 5-HT in the absence of pertussis toxin.
In contrast, pertussis toxin resistant forms of Giα1, Giα2, and Giα3 rescued 32%, 118%, and 35% of 5-HT1B receptor-stimulated activity, respectively, indicating that 5-HT1B receptors coupled primarily through Giα2. A similar preference for Giα2 was found in studies of the 5-HT1D receptor, where toxin resistant Giα1, Giα2, and Giα3 rescued 30%, 70%, and 40% of activity, respectively.
In conclusion, the observed differential coupling of 5-HT1 receptors to isoforms of Giα, provides additional evidence for our previous findings that the subtypes of 5-HT1 receptors exhibit similar, but distinct, functions.
Keywords: 5-HT, serotonin, 5-HT1 receptors, ERK, MAP kinase, G proteins, Gi, pertussis toxin
Introduction
At least 16 types of mammalian receptors for serotonin (5-HT) have been identified and classified within seven families (Hoyer et al., 1994; Scalzitti & Hensler, 1996). The physiological significance of such a large number of receptors is currently unclear. This is especially true regarding those receptors classified as 5-HT1 receptors, which have been postulated to play a role in the treatment and pathophysiology of a number of disorders including depression, anxiety, and migraine headaches. All of the 5-HT1 receptors, designated 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F, couple to G proteins of the Gi class and inhibit adenylyl cyclase. Although these receptors have clear distinctions in pharmacology and structure, there is little currently known about differences in coupling to cellular signals. However, we have previously demonstrated that 5-HT1B receptors couple more effectively than 5-HT1A receptors to activation of the mitogen-activated protein (MAP) kinase ERK and to inhibition of adenylyl cyclase in Chinese Hamster Ovary (CHO) cells (Mendez et al., 1999). In those studies we directly compared the intrinsic activity of each receptor subtype in stably transfected cells expressing receptors at the same densities. The observed differences in receptor function suggested the possibility that the receptors differentially couple to isoforms of Giα.
However, binding studies utilizing membranes from infected Spodoptera frugiperda (Sf9) insect cells over-expressing receptors and G proteins, suggest that differential receptor/G protein coupling, in fact, might not occur. All three isoforms of Giα have been reported to reconstitute high-affinity binding by 5-HT1A, 5-HT1B, and 5-HT1D receptors (Butkerait et al., 1995; Clawges et al., 1997; Brys et al., 2000). Nevertheless, it cannot be assumed that these findings translate into similar non-preferential utilization of G proteins in the coupling of receptors to cellular signals in mammalian cells. In fact, Garnovskaya et al. (1997) found that pertussis toxin resistant forms of Giα1 were ineffective in rescuing coupling of 5-HT1A receptors to Na+/H+ exchange in transfected CHO cells.
In the present studies we examined the coupling of human 5-HT1A, 5-HT1B, and 5-HT1D receptors to the MAP kinase ERK. The ERK pathway is known to enhance cell survival, and is required for normal neuronal functioning (Encinas et al., 1999; Erhardt et al., 1999). In particular, our studies were aimed at uncovering differences in the coupling of 5-HT1 receptors to Giα. Since Giα is selectively ADP-ribosylated by pertussis toxin, we utilized toxin resistant mutants of human Giα to ‘rescue' receptor/G protein-coupling from pertussis toxin-catalyzed inhibition of 5-HT1 receptor/G protein coupling. In this way, the efficacy of receptor coupling to each subtype of Giα could be studied in isolation. An advantage to this approach, over some other methods, is that such studies directly address functional specificity in a cell type-independent manner. Since pertussis toxin prevents the coupling of receptors to endogenous Giα, the relative endogenous expression of Giα isoforms in the particular cell type used does not alter the observed results.
In order to simulate the G protein-coupling of endogenous human 5-HT1 receptors, as closely as possible, we utilized a model system in which the receptors, G proteins, and cell line were all human. Studies presented here demonstrate that 5-HT1B and 5-HT1D receptors couple more selectively than 5-HT1A receptors to subtypes of Giα. The expression of multiple receptors that display similar, but distinct, coupling to G proteins and cellular signals provides insight into the large number of receptors (at least 16) required to mediate the diverse and highly complex actions of 5-HT in the central nervous system.
Methods
Materials
5-HT, tranylcypromine, and pertussis toxin were purchased from Sigma (St. Louis, MO, U.S.A.).
Cell culture
HeLa cells were obtained from American Type Culture Collection (Rockville, MD, U.S.A.), and were routinely cultured in Eagle's minimum essential medium supplemented with non-essential amino acids and 10% dialyzed foetal bovine serum (dialyzed in membranes with 1000 Dalton molecular weight cut-offs against a 100-fold greater volume of 150 mM NaCl to remove endogenous 5-HT), 100 units penicillin-100 μg streptomycin/ml (95% air, 5% CO2).
Transient transfections of cells
cDNAs for the human 5-HT1B and 5-HT1D receptors were obtained from the American Type Culture Collection (Rockville, MD, U.S.A.). cDNA for the human 5-HT1A receptor has been previously described (Fargin et al., 1989; Cowen et al., 1996). cDNAs for pertussis toxin resistant mutants of human Giα1 (C351I), Giα2 (C352I) and Giα3 (C351I) were obtained from the Guthrie cDNA Resource Center (Sayre, PA, U.S.A.). Expression of all sequences was under the control of the CMV promoter. Transient transfections were performed 48 hours prior to cellular studies using the Profectin calcium phosphate procedure (Promega, Madison, WI, U.S.A.). For studies of receptor coupling, cells were cultured in 60 mm plates and co-transfected with 6 μg of receptor plasmid DNA plus 6 μg of G protein plasmid DNA or empty vector. For studies of Giα expression, cells were cultured in 100 mm plates and transfected with 20 μg of receptor plasmid DNA.
Immunoblots
Monoclonal anti-phospho-ERK1/ERK2 (Thr202/Tyr204) was obtained from Cell Signalling (Beverly, MA, U.S.A.). Goat polyclonal anti-Giα recognizing all Giα subtypes, rabbit polyclonal total ERK1/ERK2, and horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). The day prior to use, cells were washed with phosphate-buffered saline and cultured overnight under serum- and geneticin free conditions. Cells were stimulated with the specified concentrations of agonists, and routinely lysed with a 26-gauge needle in (mM) HEPES 25 (pH 7.4), NaF 50, EDTA 5, sodium orthovanadate 1, 250 μM 4-(2-aminoethyl)-benzene-sulfonylfluoride hydrochloride, 0.1% aprotinin, and 10 μg ml−1 leupeptin. In studies of Giα expression, NaCl 150 mM, 1% Triton X-100, and β-glycerolphosphate 1 mM were included in the lysis buffer. Proteins were separated on 12% resolving gels (Bio-Rad Laboratories, Hercules, CA, U.S.A.) and transferred to 0.45 μM Immobolin-P polyvinylidene difluoride membranes (Millipore Corporation, Bedford, MA, U.S.A.). Membranes were blocked overnight with 3% powdered milk before incubation with primary and secondary antibodies. Bound antibodies were visualized using Enhanced Luminol Chemiluminescence Reagent (NEN Life Sciences, Boston, MA, U.S.A.) and exposure to a Kodak Image Station 440CF with a cooled, full-frame-capture CCD camera (Kodak). Net intensity of bands was calculated directly from stored images using Kodak Digital Science 1D Image Analysis Software (version 3.5) on defined regions of interest.
Binding assays
Cells were transfected, as described above, with 6 μg of receptor plasmid DNA plus 6 μg of empty vector. The day prior to use, cells were washed with phosphate-buffered saline and cultured overnight under serum-free conditions. Receptor binding assays were performed using the radioligand [3H]-5-HT (Veldman & Bienkowski, 1992), obtained from Perkin-Elmer Life Sciences (Boston, MA, U.S.A.). Assays contained 10–20 μg of membrane protein, 15 nM [3H]-5-HT, and 1 μM tranylcypromine in a total volume of 100 μl. Displaceable binding of [3H]-5-HT was determined in the presence of 10 μM 5-HT.
Results
Activation of MAP kinase was assayed by measuring MAP kinase kinase (MEK)-dependent phosphorylation of ERK1 and ERK2 at threonine 202 and tyrosine 204 (Cobb & Goldsmith, 1995). When nontransfected HeLa cells were treated with 5-HT, no increase in the level of activated, phosphorylated ERK was detected (Figure 1A). Therefore, although HeLa cells were found in binding studies to apparently express endogenous receptors for 5-HT (Table 1), they did not express subtypes of 5-HT receptors that couple to activation of ERK. In contrast, 5-HT did stimulate phosphorylation of ERK in cells transfected with cDNA for 5-HT1 receptors. When transfected cells expressing human 5-HT1A receptors were treated with 5-HT, a 3.8-fold activation of ERK was observed. This activation represented primarily ERK2, as the observed band (Figure 1B) migrated at a relative weight equal to the lower band of a band doublet of p44 ERK1/p42 ERK2 seen from PC12 cell lysate run on the same gel (not shown). Activation of ERK2 by 5-HT was mediated by Gi, as pretreatment with pertussis toxin caused almost complete inhibition.
Figure 1.

5-HT1A receptors couple to activation of ERK through pertussis toxin sensitive G proteins. (A) Nontransfected HeLa cells were treated for 5 min with 10 μM 5-HT (lane 2), and then lysed. (B) HeLa cells transfected with cDNA for the human 5-HT1A receptor were treated overnight in the presence (lanes 3 and 4) or absence (lanes 1 and 2) of 20 ng ml−1 pertussis toxin before treatment with 10 μM 5-HT (lanes 2 and 4) for 5 min, and subsequent lyses. Total lysate was analysed by immunoblotting with antibody to phospho-ERK1/ERK2 (p-ERK). Membranes were then stripped and analysed with antibody to total ERK1/ERK2 (Total). Net intensities of bands were calculated from three separate experiments, performed in duplicate, and expressed as the means±s.e.mean (×103). *P<0.05; ***P<0.001; n.s., statistically not significant vs absence of 5-HT, two-sided paired Student t-test calculated separately for both the presence and absence of pertussis toxin. Representative immunoblots from one of the three experiments are shown to demonstrate that treatment with pertussis toxin does not alter the levels of total ERK.
Table 1.
Transfected 5-HT1 receptors are all expressed at the same density
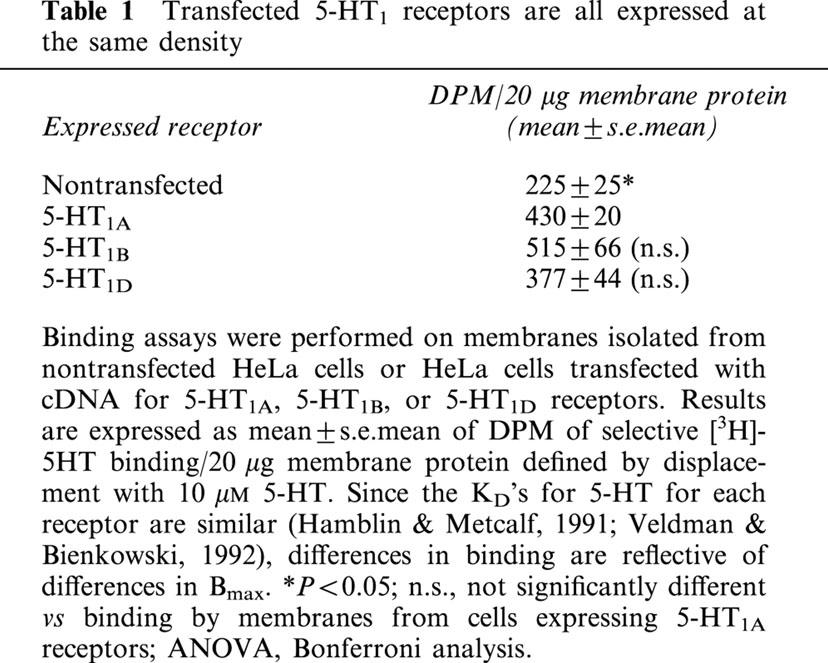
In contrast, when cells were co-transfected with cDNA for the 5-HT1A receptor and pertussis toxin resistant forms of human Giα1, Giα2, or Giα3, all three toxin-resistant subtypes were found to ‘rescue' receptor-mediated activation of ERK from inhibition by pertussis toxin (Figure 2). However, the receptor demonstrated a preference for Giα1 and Giα2, as transfection with toxin resistant Giα3 caused the smallest activation of ERK. Pertussis toxin resistant forms of Giα1, Giα2, and Giα3 rescued 73, 76 and 44%, respectively, of the increase in levels of activated ERK stimulated by 5-HT in the absence of pertussis toxin. While both Giα1 and Giα2 effectively coupled 5-HT1A receptors to activation of ERK, concentration–response curves revealed somewhat more efficient coupling by Giα2. The EC50 (calculated by nonlinear regression analysis of the net intensities of bands) for 5-HT-stimulated activation was 12 nM for cells expressing toxin resistant Giα2, but 40 nM for cells expressing Giα1 (Figure 3A). The EC50 for cells transfected with Giα3 was similar to that for Giα1, 32 nM, though the maximal effect was significantly reduced. Interestingly, the basal levels of activated ERK were higher in cells transfected with toxin resistant Giα2 than in cells transfected with toxin resistant Giα1 and Giα3.
Figure 2.

5-HT1A receptors couple to activation of ERK through multiple subtypes of Giα. HeLa cells co-transfected with cDNA for the human 5-HT1A receptor and pertussis toxin resistant forms of either (A) Giα1, (B) Giα2, or (C) Giα3 were treated overnight in the presence (lanes 3 and 4) or absence (lanes 1 and 2) of 20 ng ml−1 pertussis toxin before treatment with 10 μM 5-HT (lanes 2 and 4) for 5 min, and subsequent lyses. Total lysate was analysed by immunoblotting with antibody to phospho-ERK1/ERK2 (p-ERK). Membranes were then stripped and analysed with antibody to total ERK1/ERK2 (Total). Net intensities of bands were calculated from three separate experiments, performed in duplicate, and expressed as the means±s.e.mean (×103). **P<0.01; ***P<0.001 vs absence of 5-HT, two-sided paired Student t-test calculated separately for both the presence and absence of pertussis toxin. Representative immunoblots from one of the three experiments are shown to demonstrate that treatment with pertussis toxin does not alter the levels of total ERK.
Figure 3.
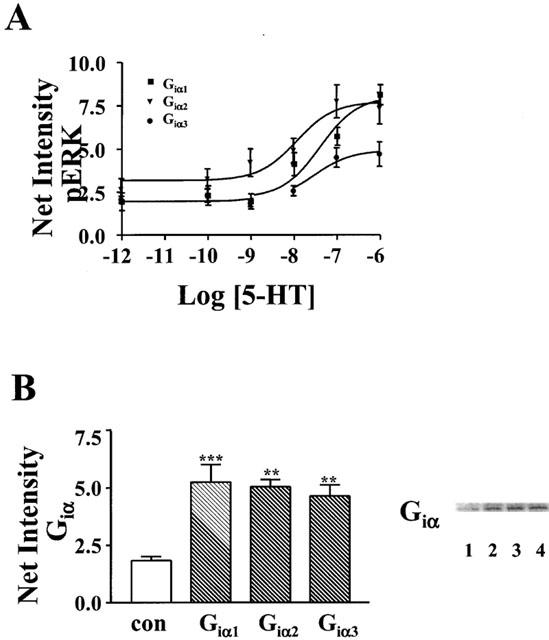
5-HT1A receptors couple most efficiently to activation of ERK through Giα2 despite equal expression of all transfected Giα subtypes. (A) HeLa cells co-transfected with cDNA for the human 5-HT1A receptor and pertussis toxin resistant forms of either Giα1, Giα2, or Giα3 were treated overnight with 20 ng/ml pertussis toxin before treatment with the indicated concentrations of 5-HT for 5 min, and subsequent lyses. (B) HeLa cells were transfected with cDNA for pertussis toxin resistant forms of either Giα1 (lane 2), Giα2 (lane 3), or Giα3 (lane 4) and the density of expression of Giα subunits were compared to nontransfected (con) cells (lane 1). (A) Total lysate was analysed by immunoblotting with antibody to phospho-ERK1/ERK2 (p-ERK) or (B) 20 μg of total lysate was analysed by immunoblotting with antibody recognizing all forms of Giα. Net intensities of bands were calculated from three separate experiments, performed in duplicate, and expressed as the means±s.e.mean (×103) **P<0.01; ***P<0.001 vs nontransfected (con) cells, ANOVA, Bonferroni analysis. A representative immunoblot from one of the three experiments is shown.
The observed preferential coupling of 5-HT1A receptors to Giα1 and Giα2, relative to Giα3, was not the result of differences in the levels of transfected Giα subunits. Each subunit was expressed at an approximately 2.5-fold greater density than endogenously expressed Giα subunits (Figure 3B).
As was seen with 5-HT1A receptors, coupling of 5-HT1B receptors to activation of ERK was almost completely inhibited by pertussis toxin (Figure 4A). However, the relative preference for coupling to subtypes of Giα was different than that found for 5-HT1A receptors. Giα2 much more effectively rescued receptor-mediated activation of ERK than Giα1 and Giα3. In contrast to the complete (118%) rescue by Giα2, pertussis toxin resistant forms of Giα1 and Giα3 rescued only 32 and 35%, respectively, of the activation of ERK stimulated by 5-HT in the absence of toxin (Figure 5). Significantly, the more selective G protein-coupling by 5-HT1B receptors, relative to 5-HT1A receptors, was not the result of expression of a lower density of transfected 5-HT1B receptors. The level of displaceable binding of [3H]5-HT to membranes prepared from cells transfected with cDNA for the 5-HT1B receptor was similar to that from membranes prepared from cells transfected with cDNA for the 5-HT1A receptor (Table 1).
Figure 4.
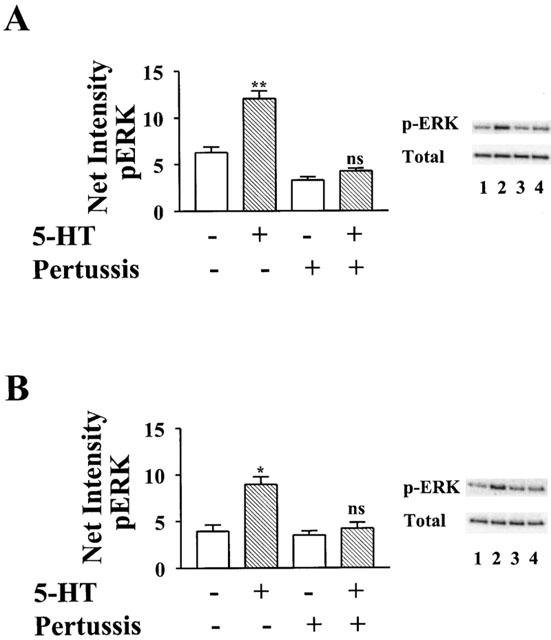
5-HT1B and 5-HT1D receptors couple to activation of ERK through pertussis toxin sensitive G proteins. (A) HeLa cells transfected with cDNA for the human 5-HT1B receptor or (B) human 5-HT1D receptor were treated overnight in the presence (lanes 3 and 4) or absence (lanes 1 and 2) of 20 ng ml−1 pertussis toxin before treatment with 10 μM 5-HT (lanes 2 and 4) for 5 min, and subsequent lyses. Total lysate was analysed by immunoblotting with antibody to phospho-ERK1/ERK2 (p-ERK). Membranes were then stripped and analysed with antibody to total ERK1/ERK2 (Total). Net intensities of bands were calculated from three separate experiments, performed in duplicate, and expressed as the means±s.e.mean (×103). *P<0.05; **P<0.01; n.s., statistically not significant vs absence of 5-HT, two-sided paired Student t-test calculated separately for both the presence and absence of pertussis toxin. Representative immunoblots from one of the three experiments are shown to demonstrate that treatment with pertussis toxin does not alter the levels of total ERK.
Figure 5.
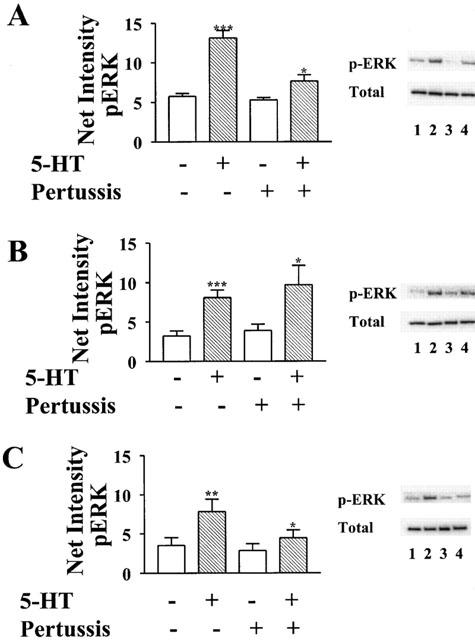
5-HT1B receptors couple preferentially to Giα2. HeLa cells co-transfected with cDNA for the human 5-HT1B receptor and pertussis toxin resistant forms of either (A) Giα1, (B) Giα2, or (C) Giα3 were treated overnight in the presence (lanes 3 and 4) or absence (lanes 1 and 2) of 20 ng ml−1 pertussis toxin before treatment with 10 μM 5-HT (lanes 2 and 4) for 5 min, and subsequent lyses. Total lysate was analysed by immunoblotting with antibody to phospho-ERK1/ERK2 (p-ERK). Membranes were then stripped and analysed with antibody to total ERK1/ERK2 (Total). Net intensities of bands were calculated from three separate experiments, performed in duplicate, and expressed as the means±s.e.mean (×103). *P<0.05; **P<0.01; ***P<0.001 vs absence of 5-HT, two-sided paired Student t-test calculated separately for both the presence and absence of pertussis toxin. Representative immunoblots from one of the three experiments are shown to demonstrate that treatment with pertussis toxin does not alter the levels of total ERK.
5-HT1D receptors were found to be similar to 5-HT1B receptors in their coupling to Giα. As was found with 5-HT1B receptors, treatment with pertussis toxin almost completely uncoupled endogenous Giα from 5-HT1D receptors (Figure 4B). Pertussis toxin resistant Giα2 rescued 70% of receptor-mediated activation of ERK. In contrast, toxin resistant forms of Giα1 and Giα3 rescued only 30 and 40%, respectively, of the activity stimulated by 5-HT in the absence of pertussis toxin (Figure 6).
Figure 6.
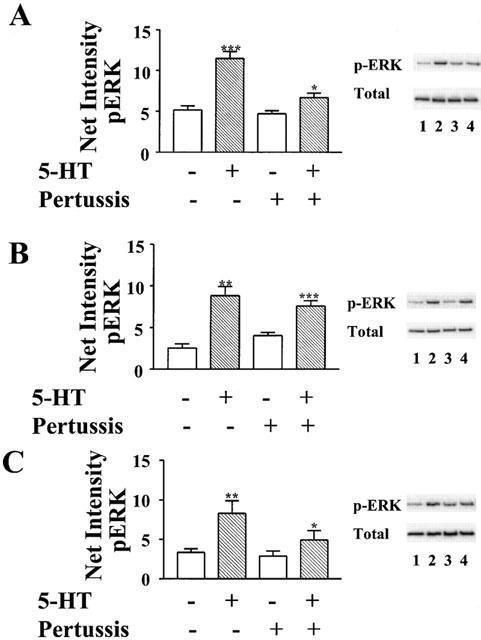
5-HT1D receptors are similar to 5-HT1B receptors in preferentially coupling to Giα2. HeLa cells co-transfected with cDNA for the human 5-HT1D receptor and pertussis toxin resistant forms of either (A) Giα1, (B) Giα2, or (C) Giα3 were treated overnight in the presence (lanes 3 and 4) or absence (lanes 1 and 2) of 20 ng ml−1 pertussis toxin before treatment with 10 μM 5-HT (lanes 2 and 4) for 5 min, and subsequent lyses. Total lysate was analysed by immunoblotting with antibody to phospho-ERK1/ERK2 (p-ERK). Membranes were then stripped and analysed with antibody to total ERK1/ERK2 (Total). Net intensities of bands were calculated from three separate experiments, performed in duplicate, and expressed as the means±s.e.mean (×103). *P<0.05; **P<0.01; ***P<0.001 vs absence of 5-HT, two-sided paired Student t-test calculated separately for both the presence and absence of pertussis toxin. Representative immunoblots from one of the three experiments are shown to demonstrate that treatment with pertussis toxin does not alter the levels of total ERK.
Discussion
Our studies demonstrate that human 5-HT1A, 5-HT1B, and 5-HT1D receptors exhibit clear differences in coupling to subtypes of Giα. 5-HT1B and 5-HT1D receptors were found to much more efficiently utilize Giα2 relative to Giα1 and Giα3. In contrast, 5-HT1A receptors demonstrated less selectivity, particularly with regards to Giα1 vs Giα2. These findings represent a progression of our earlier studies in which we used transfected CHO cells to demonstrate more efficient regulation of ERK and adenylyl cyclase by 5-HT1B receptors relative to 5-HT1A receptors (Mendez et al., 1999). Significantly, in each study the coupling by receptor subtypes was compared in an individual cell line, under identical conditions. Therefore, the difficulties inherent in comparing receptors expressed in different cell types was avoided. Together, these studies provide evidence that although all 5-HT1 receptors, to some degree, negatively regulate adenylyl cyclase, they differentially couple to G proteins and consequently exhibit differences in coupling to cellular signals.
CHO cells were not utilized in the present study because they express, at low density, endogenous 5-HT1B receptors that couple to activation of ERK (Mendez et al., 1999). That low level of expression was useful in our earlier studies in which the coupling of receptors to cellular signals was studied at various receptor densities. However, interpretation of results from studies of receptor coupling to G proteins is facilitated when all receptors are activated with the physiological agonist (i.e. 5-HT, in the present studies). In this manner, problems resulting from the use of different receptor-selective agonists are avoided. For example, it has been reported that the relative preference of 5-HT1A receptors for subtypes of Giα is modulated by the particular receptor agonist studied (Gettys et al., 1994). It is quite possible that the same also occurs with 5-HT1B and 5-HT1D receptors.
Significantly, our finding that 5-HT1A receptors more effectively utilized Giα1 than did 5-HT1B and 5-HT1D receptors, cannot be attributed to differences in expression of receptors or G proteins. Binding studies demonstrated that the density of 5-HT1B and 5-HT1D receptors was similar to the density of 5-HT1A receptors. Similarly, the poor coupling by all 5-HT1 receptor subtypes to Giα3, was not the result of lower levels of expression of the Gα subunit relative to Giα1 and Giα2. Immunoblot analysis demonstrated similar levels of expression of all three Giα subunits.
Our finding that all three subtypes of 5-HT1 receptors effectively utilize Giα2 to activate ERK is consistent with findings from studies of other Gi-coupled receptors. Winitz et al. (1994) demonstrated that a dominant-negative form of Giα2 inhibited thrombin and ATP receptor-coupling to activation of ERK. Conversely, pertussis toxin resistant forms of Giα2 have been reported to rescue activation of ERK by A1-adenosine receptors (Pace et al., 1995), and constitutively active forms independently stimulate activation of the MAP kinase (Edmastsu et al., 1998). However, our results additionally demonstrate that some Gi-coupled receptors can also, to varying degrees, utilize Giα1 and Giα3 to stimulate activation of ERK. Interestingly, in our studies of G protein coupling to 5-HT1A receptors, we found that transfection with Giα2 resulted in increased basal activity relative to that seen with transfection of Giα1 and Giα3. This may represent an enhancement of receptor constitutive activity by Giα2.
The observed differences in 5-HT1 receptor-coupling to isoforms of Giα provide an explanation for our previous demonstration of differential coupling of receptors to cellular signals (Mendez et al., 1999). While all subtypes of Giα, by definition, inhibit the activity of adenylyl cyclase in in vitro studies, there is increasing evidence that the particular isoforms differentially regulate cellular pathways in intact cells. This may result, in part, from their expression in different cellular locations. For example, in LLC–PK1 renal epithelial cells, Giα2 is localized to the basolateral membrane where it negatively couples to adenylyl cyclase, while Giα3 is expressed both in the apical membrane where it stimulates Na+ channel activity and in the Golgi (Ercolani et al., 1990). Interestingly, Garnovskaya et al. (1997) found that pertussis toxin resistant forms of Giα2 and Giα3, but not Giα1, rescued coupling of 5-HT1A receptors to activation of Na+/H+ exchange in CHO cells. Our findings suggest that this likely reflected a lack of coupling of Giα1 to Na+/H+ exchange rather than a lack of coupling of Giα1 to 5-HT1A receptors. Perhaps, in CHO cells, the expression of Giα1 is localized such that it cannot modulate Na+/H+ exchange. Our demonstration that 5-HT1A, but not 5-HT1B and 5-HT1D, receptors efficiently utilize Giα1 relative to Giα2, is significant in that the differential coupling was observed in studies of the same cellular signal (ERK), in the same cells, under identical conditions.
Interestingly, our findings differ from those obtained in binding studies utilizing membranes from infected Sf9 cells. In those studies Giα1, Giα2, and Giα3, each were found to reconstitute high-affinity binding by all three 5-HT1 receptors (Butkerait et al., 1995; Clawges et al., 1997; Brys et al., 2000). However, our studies were different in that we attempted to more closely duplicate the coupling of endogenous receptor to endogenous G protein that would occur in human cells. While Sf9 cells are non-mammalian cells, our studies utilized human HeLa cells. Similarly, the previous studies utilized rat and mouse G proteins, while our studies examined coupling to human G proteins. These differences, as well as the much higher levels of protein expression achieved in Sf9 cells, relative to mammalian cells, could account for our different results.
Significantly, since our study utilized toxin resistant mutants of human Giα to ‘rescue' receptor/G protein-coupling, we were able to study the efficacy of receptor coupling to each subtype of Giα in isolation. In that pertussis toxin prevents the coupling of receptors to endogenous Giα, the relative endogenous expression of Giα isoforms in the particular cell type studied does not alter the observed results. Although HeLa cells express endogenous Giα3 and Giα1 in a 10 : 1 ratio, with little Giα2 (Raymond et al., 1993), our results are relevant to any type of cell expressing 5-HT1 receptors, regardless of the composition of expressed Giα.
Our studies, in that they model the coupling of 5-HT1 receptors to G proteins in human cells, may therefore be relevant to human central nervous system (CNS) neurons, and consequently to understanding the etiology of mood disorders. There is increasing evidence that patients with depression and bipolar disorder exhibit alterations in the expression of G proteins (Young et al., 1994; Avissar et al., 1997). Our findings suggest that changes in the expression of Giα2 could have an effect on the activation of ERK stimulated by each of the three 5-HT1 receptors. In contrast, alterations in the level of Giα1 would impact primarily the activity elicited by 5-HT1A receptors. Such changes in G protein expression could be postulated to contribute to the pathophysiology of mood disorders since the ERK pathway is required for normal neuronal functioning, and is known to enhance cell survival (Encinas et al., 1999; Erhardt et al., 1999).
Our findings that subtypes of 5-HT1 receptors display similar, but distinct, patterns of coupling to G proteins and cellular signals (Mendez et al., 1999) is consistent with the hypothesis that a large number of receptors, with subtle differences, are required to mediate the actions of 5-HT. Serotonergic medications are known to have diverse and complex actions on the central nervous system. They are used to treat such complicated disorders as depression, anxiety, eating disorders, obsessive–compulsive disorder, and schizophrenia. As the functions of individual 5-HT receptors continue to be elucidated, it may become possible to design medications that act more selectively at the specific receptor/receptors relevant to particular disorders.
Acknowledgments
These studies were supported by NIMH grant MH60100 to D.S. Cowen. S.L. Lin is a Senior Research Fellow of Vion Pharmaceuticals, Inc.
Abbreviations
- 5-HT
5-hydroxytryptamine or serotonin
- ERK
extracellular signal-regulated kinase
- MAP kinase
mitogen-activated protein kinase
References
- AVISSAR S., NECHAMKIN Y., ROITMAN G., SCHREIBER G. Reduced G protein functions and immunoreactive levels in mononuclear leukocytes of patients with depression. Am. J. Psychiatry. 1997;154:211–217. doi: 10.1176/ajp.154.2.211. [DOI] [PubMed] [Google Scholar]
- BRYS R., JOSSON K., CASTELLI M.P., JURZAK M., LIJNEN P., GOMMEREN W., LEYSEN J.E. Reconstitution of the human 5-HT1D receptor-G-protein coupling: evidence for constitutive activity and multiple receptor conformations. Mol. Pharmacol. 2000;57:1132–1141. [PubMed] [Google Scholar]
- BUTKERAIT P., ZHENG Y., HALLAK H., GRAHAM T.E., MILLER H.A., BURRIS K.D., MOLINOFF P.B., MANNING D.R. Expression of the human 5-hydroxytryptamine1A receptor in Sf9 cells. J. Biol. Chem. 1995;270:18691–18699. doi: 10.1074/jbc.270.31.18691. [DOI] [PubMed] [Google Scholar]
- CLAWGES H.M., DEPREE K.M., PARKER E.M., GRABER S.G. Human 5-HT1 receptor subtypes exhibit distinct G protein coupling behaviors in membranes from Sf9 cells. Biochem. 1997;36:12930–12938. doi: 10.1021/bi970112b. [DOI] [PubMed] [Google Scholar]
- COBB M., GOLDSMITH E. How MAP kinases are regulated. J. Biol. Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- COWEN D.S., SOWERS R.S., MANNING D.R. Activation of a mitogen-activated protein kinase (ERK2) by the 5-hydroxytryptamine1A receptor is sensitive not only to inhibitors of phosphatidylinositol 3-kinase, but to an inhibitor of phosphatidylcholine hydrolysis. J. Biol. Chem. 1996;271:22297–22300. doi: 10.1074/jbc.271.37.22297. [DOI] [PubMed] [Google Scholar]
- EDMASTSU H., KAZIRO Y., ITOH H. Expression of an oncogenic mutant G alpha i2 activates Ras in Rat-1 fibroblast cells. FEBS Lett. 1998;440:231–234. doi: 10.1016/s0014-5793(98)01457-4. [DOI] [PubMed] [Google Scholar]
- ENCINAS M., IGLESIAS M., LLECHA N., COMELLA J.X. Extracellular-regulated kinases and phosphatidylinositol 3-kinase are involved in brain-derived neurotrophic factor-mediated survival and neurogenesis of the neuroblastoma cell line SH-SY5Y. J. Neurochem. 1999;73:1409–1421. doi: 10.1046/j.1471-4159.1999.0731409.x. [DOI] [PubMed] [Google Scholar]
- ERCOLANI L., STOW J.L., BOYLE J.F., HOLTZMAN E.J., LIN H., GROVE J.R., AUSIELLO D.A. Membrane localization of the pertussis toxin-sensitive G-protein subunits alpha I-2 and alpha I-3 and expression of a metallothionein-alpha I-2 fusion gene in LLC-PK 1 cells. Proc. Natl. Acad. Sci. U.S.A. 1990;87:4635–4639. doi: 10.1073/pnas.87.12.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERHARDT P., SCHREMSER E.J., COOPER G.M. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol. Cell. Biol. 1999;19:5308–5315. doi: 10.1128/mcb.19.8.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARGIN A., RAYMOND J.R., REGAN J.W., COTECCHIA S., LEFKOWITZ R., CARON M.G. Effector coupling mechanisms of the cloned 5-HT1A receptor. J. Biol. Chem. 1989;264:14848–14852. [PubMed] [Google Scholar]
- GARNOVSKAYA M.N., GETTYS T.W., VAN BIESEN T., PRPIC V., CHUPRIN J.K., RAYMOND J.R. 5-HT1A receptor activates Na+/H+ exchange in CHO-K1 cells through Giα2 and Giα3. J. Biol. Chem. 1997;272:7770–7776. doi: 10.1074/jbc.272.12.7770. [DOI] [PubMed] [Google Scholar]
- GETTYS T.W., FIELDS T.A., RAYMOND J.R. Selective activation of inhibitory G-protein α-subunits by partial agonists of the human 5-HT1A receptor. Biochem. 1994;33:4283–4290. doi: 10.1021/bi00180a024. [DOI] [PubMed] [Google Scholar]
- HAMBLIN M.W., METCALF M.A. Primary structure and functional characterization of a human 5-HT1D-type serotonin receptor. Mol. Pharmacol. 1991;40:143–148. [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P.A. International Union of Pharmacology classification of receptors for 5-Hydroxytryptamine (Serotonin) Pharmacol. Reviews. 1994;46:157–203. [PubMed] [Google Scholar]
- MENDEZ J., KADIA T.M., SOMAYAZULA R.K., EL-BADAWI K.I., COWEN D.S. Differential coupling of 5-HT1A and 5-HT1B receptors to activation of ERK2 and inhibition of adenylyl cyclase. J. Neurochem. 1999;73:162–168. doi: 10.1046/j.1471-4159.1999.0730162.x. [DOI] [PubMed] [Google Scholar]
- PACE A.M., FAURE M., BOURNE H.R. Gi2-mediated activation of the MAP kinase cascade. Mol. Biol. Cell. 1995;6:1685–1695. doi: 10.1091/mbc.6.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAYMOND J.R., OLSEN C.L., GETTYS T.W. Cell-specific physical and functional coupling of human 5-HT1A receptors to inhibitory G protein α-subunits and lack of coupling to GSα. Biochem. 1993;32:11064–11073. doi: 10.1021/bi00092a016. [DOI] [PubMed] [Google Scholar]
- SCALZITTI J.M., HENSLER J.G.Serotonin receptors: role in psychiatry Handbook of Psychiatric Genetics 1996Boca Raton: CRC Press; 113–145.eds. Blum, K., Noble, E.P. pp [Google Scholar]
- VELDMAN S.A., BIENKOWSKI M.J. Cloning and pharmacological characterization of a novel human 5-hydroxytryptamine1D receptor subtype. Mol. Pharmacol. 1992;42:439–444. [PubMed] [Google Scholar]
- WINITZ S., GUPTA S.H., QIAN N., HEASLEY L.E., NEMENOFF R.A., JOHNSON G.L. Expression of mutant Gi2 α subunit inhibits ATP and thrombin stimulation of cytoplasmic phospholipase A2-mediated arachidonic acid release independent of Ca2+ and mitogen-activated protein kinase regulation. J. Biol. Chem. 1994;269:1889–1895. [PubMed] [Google Scholar]
- YOUNG T.Y., LI P.P., KAMBLE A., SIU K.P., WARSH J.J. Mononuclear leukocyte levels of G proteins in depressed patients with bipolar disorder or major depression. Am. J. Psychiatry. 1994;151:594–596. doi: 10.1176/ajp.151.4.594. [DOI] [PubMed] [Google Scholar]


