Abstract
Endothelium-dependent responses were assessed in myometrial small arteries isolated from pregnant women, using pressure myography. Responses to bradykinin were unaffected by the combined presence of the nitric oxide synthase (NOS) inhibitor, N-nitro-L-arginine methyl ester (L-NAME, 100 μM) and the cyclo-oxygenase inhibitor, indomethacin (10 μM). The additional presence of clotrimazole (50 μM) attenuated, but did not abolish, vasodilator responses to bradykinin. Raising extracellular [K+] (by between 1 and 15 mM) did not evoke a vasodilator response, nor did the cannabinoids, anandamide and methanandamide. Responses to bradykinin were attenuated in the presence of the gap junction inhibitors 18-α-glycyrrhetinic acid (18-α GA, 100 μM), carbenoxolone (100 μM) and palmitoleic acid (50 μM). SR141716A, the CB1 receptor antagonist attenuated responses to bradykinin, but only at high concentrations (10 μM). These results suggest that gap junctional communication is involved in the nitric oxide (NO)- and prostanoid-independent vasodilator responses to bradykinin in myometrial small arteries in normal pregnancy.
Keywords: Gap junctions, EDHF, pregnancy, myometrial arteries
Introduction
Endothelium-dependent vasorelaxation responses in several human blood vessels, including subcutaneous (Buus et al., 2000; Coats et al., 2001; Coleman et al., 2001), omental (Pascoal et al., 1998) and myometrial small arteries (Kenny et al., 2002) have been reported to be insensitive to combined inhibition of the cyclo-oxygenase and nitric oxide (NO) synthase enzymes. In some of these preparations the non-NO, non-prostanoid response was associated with vascular smooth muscle hyperpolarization consistent with the release of an endothelium-derived hyperpolarizing factor (EDHF) (Buus et al., 2000; Coleman et al., 2001).
A number of candidates have been proposed to be EDHFs including epoxyeicosatrienoic acids (EETs) (Campbell et al., 1996), endocannabinoids (Randall et al., 1996) and K+ (Edwards et al., 1998). More recently, it has been suggested that EDHF responses may involve the transfer of a substance, or electrical current, directly between the endothelium and underlying vascular smooth muscle via heterocellular gap junctions (Chaytor et al., 1998; Taylor et al., 1998; Harris et al., 2000; Coleman et al., 2001).
The aim of the present study was to investigate the mechanisms involved in the vasodilator action of EDHF-type responses in human myometrial small arteries isolated from pregnant women.
Methods
This investigation conformed to the principles outlined in the Declaration of Helsinki (1989). Ethical permission for the study was obtained from the Nottingham City Hospital Trust Ethical Committee and all subjects had given informed written consent to participation. Forty-nine healthy women had normotensive pregnancies with singleton foetuses subsequently shown to be above the tenth centile of birth weight for gestational age. They were undergoing elective Caesarean section at term for reasons such as previous cephalopelvic disproportion or malpresentation. Following delivery of the baby and placenta at Caesarean section, a full thickness biopsy of myometrium measuring approximately 2 cm by 1 cm was obtained from the upper margin of the transverse lower uterine incision. Specimens were placed in oxygenated physiological salt solution (PSS) at 4°C. With the use of a stereomicroscope, arteries between 100–400 μm in diameter were identified, promptly dissected from the samples and utilized immediately.
Each artery was mounted on a pressure myograph as described in detail elsewhere (Wallis et al., 1996). In brief, vessels were secured between two glass cannulae. One cannula was closed and the other connected to a system containing PSS, which in turn was linked to a pressure-servo unit. This allowed the intraluminal pressure to be precisely controlled. Vessels were circulated with PSS that was bubbled continuously with a 5% CO2–95% O2 gas mixture and circulated with a Masterflex pump. The vessel was imaged using a video camera and analysed with an appropriate dimension analyser (Living Systems Instrumentation, Burlington, VT, U.S.A.), which was linked to a MacLab data acquisition system. The intraluminal pressure was set at 60 mmHg and the temperature of the organ bath was maintained at 37°C. The vessels were allowed to equilibrate under these conditions for 90 min. Over this time, vessels developed a variable degree of myogenic tone, which was supplemented by the addition of the thromboxane mimetic U46619 to reduce diameter by approximately 50% of the original diameter. Vessels were then exposed to increasing concentrations of the endothelium-dependent vasodilator, bradykinin in 3 fold increments. Following washing, and inducing tone, the concentration response curves to bradykinin were repeated in the presence and absence of various inhibitors. Initial experiments determined the effect of the cyclo-oxygenase inhibitor, indomethacin (10 μM) and the NOS inhibitor, N-nitro-L-arginine methyl ester (L-NAME, 100 μM) on responses to bradykinin. In subsequent experiments indomethacin (10 μM) and L-NAME (100 μM) were present throughout.
In a series of experiments, responses to bradykinin were obtained in the absence and presence of a variety of agents which have been reported to interfere with endothelium-dependent vasodilator responses, including: (1) clotrimazole (50 μM), (McCulloch et al., 1997), (2) 18-α-glycyrrhetinic acid (18α-GA, 100 μM) (Taylor et al., 1998), (3) carbenoxolone (100 μM) (Edwards et al., 1999), (4) palmitoleic acid (50 μM) (Harris et al., 2000), or (5) SR141716A (1 and 10 μM) (Harris et al., 2000; 2002). At the end of each experiment the vessels were washed for approximately 60 min in calcium free PSS in order to obtain the maximum passive diameter of each artery. In a separate series of experiments vessels were constricted (50% reduction in diameter) by myogenic tone supplemented with U46619, and then exposed to either (1) ananadamide (10 nM–3 μM in 3 fold increments), or (2) methanadamide (10 nM–3 μM) or (3) raised extracellular [K+] (with additional increments of 2, 5, 10, 15 and 20 mM), to determine whether these agents caused vasodilatation.
Drugs and solutions
9,11-Dideoxy-11α,9α-epoxy-methanoprostglandin F2α (U46619), bradykinin acetate, indomethacin, L-NAME, clotrimazole, Ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), Ethylenediaminetetraacetic acid (EDTA), 18-α-glycyrrhetinic acid (18α-GA), 3β-Hydroxy-11-oxoolean-12-en-30-oic acid 3-hemisuccinate (carbenoxolone), cis-9-Hexadecenoic acid (palmitoleic acid) and R(+)-arachidonyl-1′-hydroxy-2′-propylamide (methanandamide) (Sigma, U.K.), and N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride (SR141716A), supplied by Sanofi-Winthrop. All drugs were made up fresh from stock solutions on the day of the experiment and dissolved in physiological salt solution with the exception of SR141716A and clotrimazole which were dissolved in ethanol and palmitoleic acid and 18α-GA, which were dissolved in dimethyl sulphoxide (DMSO). Ananadamide was synthesized by Dr P. DeBank (University of Nottingham) from arachidonoyl chloride and ethanolamine and dissolved in an inert oil/water emulsion. Extracellular [K+] was increased by equimolar exchange of NaCl with KCl. All drugs were added to the extraluminal side of the vessel. Control experiments confirmed that the vehicles had no significant effect on constrictor tone or bradykinin-induced relaxations at the final concentration employed.
The composition of the PSS was as follows (in mM): CaCl2.2H2O 1.6, NaCl 119, NaHCO3 25, KCl 4.7, KH2PO4 1.18, MgSO4.7H2O 1.17, Na2EDTA 0.023, Glucose 5.5 (pH 7.4 when gassed with 5% CO2-95% O2). Calcium-free PSS was prepared by omitting calcium and adding 0.5 mM EGTA.
Drug analysis
Vasodilator responses to bradykinin were calculated as percentages of the maximum possible vasodilator range, i.e. the diameter in calcium-free PSS minus the diameter in the presence of myogenic and U46619-induced tone. Results are shown graphically as means (±s.e.mean), with n=the number of patients. Rma x refers to the maximum relaxation achieved in the presence of the highest concentration of bradykinin used (1 μM). Statistical evaluation of concentration response curves within each group was performed using repeated measures analysis of variance (ANOVA) (with significance determined at P<0.05) with post-hoc testing (Bonferroni/Dunn).
Results
Cumulative concentration response curves to bradykinin were reproducible over two successive runs (results not shown). Our preliminary experiments showed that responses to bradykinin were unaffected by combined inhibition of cyclo-oxygenase and NOS (see also Kenny et al., 2002). Thereafter, all experiments were performed in the presence of indomethacin (10 μM) and L-NAME (100 μM).
In the presence of Clotrimazole (50 μM), higher concentrations of U46619 were required to reduce diameter by 50%. Clotrimazole significantly attenuated but did not abolish response to bradykinin (Rma x control 78.3±5.7% vs Rmax clotrimazole 46.0±7.9%, n=5, P<0.05) (Figure 1).
Figure 1.
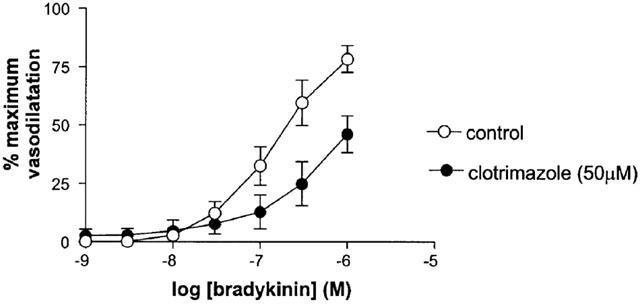
The effect of clotrimazole on responses to bradykinin (in the presence of L-NAME and indomethacin) in human isolated myometrial small arteries. Each point represents the mean±s.e.mean (n=5).
The inhibitors of gap junction communication, 18α-GA (100 μM), carbenxolone (100 μM) and palmitoleic acid (50 μM), virtually abolished responses to bradykinin (Figure 2).
Figure 2.
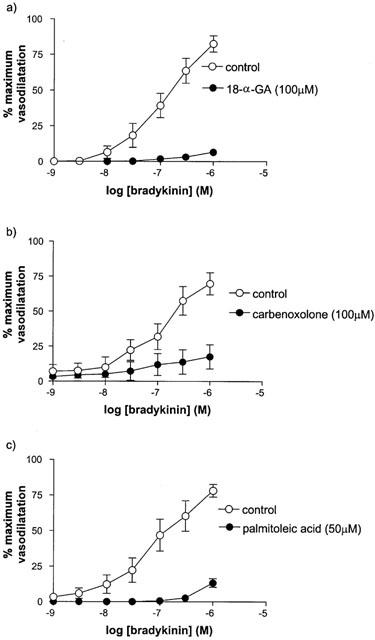
The effect of (a) 18-α-GA, (b) carbenoxelone and (c) palmitoleic acid on responses to bradykinin (in the presence of L-NAME and indomethacin) in human isolated myometrial small arteries. Each point represents the mean±s.e.mean (n=6).
SR141716A (1 μM) had no effect on responses to bradykinin (Rmax control 75.0±9.9% vs Rmax SR141716A 72.4±10.6%, n=6, P>0.05). By contrast a higher concentration of SR141716A (10 μM) significantly attenuated responses to bradykinin (Figure 3).
Figure 3.
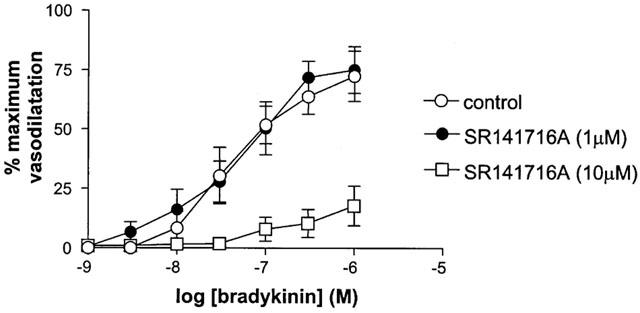
The effect of 1 μM and 10 μM SR 141716A on responses to bradykinin (in the presence of L-NAME and indomethacin) in human isolated myometrial small arteries. Each point represents the mean±s.e.mean (n=6).
Anandamide and methanandamide did not evoke a relaxation response at concentrations up to 30 μM (Rmax anandamide 3.0±1.1%, n=4, Rmax methanandamide 2.2±0.8%, n=5). Similarly incremental concentrations of K+ (additional to the 5.9 mM K+ already present in the PSS) did not evoke a relaxation and at concentrations of 15 mM and above caused a further increase in tone. In both sets of experiments, vasodilator responses to bradykinin were apparent.
Discussion
A number of recent reports have indicated that endothelium-dependent responses are independent of NO and prostanoid production in small arteries isolated from humans (Pascoal et al., 1998; Buus et al., 2000; Coats et al., 2001; Coleman et al., 2001; Kenny et al., 2002). The non-NO, non-prostanoid responses in human subcutaneous arteries have been shown to be sensitive to depolarization, by raising extracellular KCl (Coats et al., 2001; Kenny et al., 2002) and to be associated with vascular smooth muscle hyperpolarization (Buus et al., 2000; Coleman et al., 2001). These observations are consistent with the release of an EDHF in these vessels. In the present study we investigated a number of potential mediators of the EDHF-type response in human myometrial small arteries.
In contrast to the observations of Edwards et al. (1998), who provided evidence to suggest that K+ was an EDHF in rat arteries, no vasodilator response was seen in human myometrial, pressurized small arteries, in response to raising the concentration of extracellular K+ over the range of 1–15 mM. Thus K+ is unlikely to be an EDHF in these vessels. Coleman et al. (2001) also precluded a role for K+ in mediating EDHF responses in human small arteries isolated from subcutaneous fat.
Endogenous cannabinoids have been suggested as possible candidates as EDHF. Evidence for this has been derived from studies which have demonstrated that anandamide evokes relaxant responses and that EDHF-type responses to a variety of endothelium-dependent agonists are inhibited by the cannabinoid receptor antagonist, SR141716A (Randall et al., 1996). In the present study neither anandamide, nor its more metabolically stable analogue, methanandamide, evoked a vasodilator response in human small myometrial arteries. Similarly, SR141716A, at a concentration selective for CB1 receptors (1 μM), did not attenuate the response to bradykinin. These findings suggest that an endogenous cannabinoid is unlikely to be an EDHF in the myometrial vascular bed.
A number of reports have suggested that EDHF is an epoxide of arachidonic acid produced from the expoxygenase pathway by a cytochrome P-450 mono-oxygenase system (Campbell et al., 1996; Coats et al., 2001). This contention is largely based on the observation that inhibitors of cytochrome P450 can block EDHF-type responses in vessels isolated from several vascular beds. In the present study, we have shown that the response to bradykinin was attenuated, although not abolished, by clotrimazole (50 μM). This could be taken as evidence in support of a role for EETs in this preparation. However, this contention should be treated with caution, since it has been reported that clotrimazole can act as a K+ channel blocker, and thus interfere with EDHF at its site of action, rather than preventing its synthesis (Zygmunt et al., 1996). In addition, clotrimazole has been reported to block gap junction communication (Harris et al., 2000).
Overall, our observations suggest the involvement of gap junction communication in mediating EDHF-type responses in human myometrial small arteries since 18α-GA, carbenoxolone and palmitoleic acid virtually abolished non-NO, non-prostanoid responses to bradykinin. Furthermore, SR141716A at a high concentration (10 μM), also abolished the response to bradykinin. Each of these agents have been shown to inhibit cell–cell coupling, mediated via functional gap junctions, in dye transfer studies (Chaytor et al., 1999; Harris et al., 2000). It should be noted that some of these agents have other actions. For example, it has recently been reported that some glycyrrhetinic derivatives (carbenoxelone and 18-β-glycyrrhetinic acid (18-β-GA)) inhibit hyperpolarization in endothelial cells of guinea-pig and rat arteries (Tare et al., 2002). It should be noted that the observation that carbenoxelone inhibited endothelial-dependent responses in the guinea-pig coronary artery (Tare et al., 2002), contrast with observations in the guinea-pig carotid artery in which carbenoxelone was reported to have no effect on endothelial hyperpolarization (Edwards et al., 1999). Interestingly, Tare et al. (2002) reported that 18-α-GA had only small, variable effects against endothelial and vascular smooth muscle hyperpolarizations in the guinea-pig coronary artery. This could be taken as evidence that endothelium-dependent responses in this preparation do not involve functional gap junctions and that 18-α-GA does not act as a non-selective inhibitor of endothelium-independent hyperpolarization. The observation that 18-α-GA abolished responses to bradykinin in the present study thus, implicates the involvement of functional gap junctions. More importantly, the balance of evidence demonstrating that several chemically distinct agents that have been shown to interfere with gap junction communication, inhibited non-NO, non-prostanoid-mediated responses to bradykinin, suggest this is the mechanism of EDHF-type responses in human myometrial small arteries.
In conclusion, we have demonstrated that endothelium-dependent vasodilatation in a human small artery involves functional gap junctions.
Acknowledgments
Louise Kenny was an MRC/RCOG Clinical Training Fellow. This work was supported in part by an MRC Development Grant no. G9826907 (PN Baker, WR Dunn). SR141716A was a generous gift from Sanofi-Winthrop.
Abbreviations
- EDHF
endothelium-derived hyperpolarizing factor
- NO
nitric oxide
- NOS
nitric oxide synthase
References
- BUUS N.H., SIMONSEN U., PILEGAARD H.K., MULVANY M.J. Nitric oxide, prostanoid and non-NO, non-prostanoid involvement in acetylcholine relaxation of isolated human small arteries. Br. J. Pharmacol. 2000;129:184–192. doi: 10.1038/sj.bjp.0703041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL W.B., GEBREMEDHIN D., PRATT P.F., HARDER D.R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Central role of heterocellular gap junctional communication in endothelium-independent relaxations of rabbit arteries. J. Physiol. (Lond.) 1998;508:561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T., MARTIN P.E., EVANS W.H., RANDALL M.D., GRIFFITH T.M. The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J. Physiol. (Lond.) 1999;520:539–550. doi: 10.1111/j.1469-7793.1999.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COATS P., JOHNSTON F., MACDONALD J., MCMURRAY J.J., HILLIER C. Endothelium-derived hyperpolarizing factor: identification and mechanisms of action in human subcutaneous resistance arteries. Circulation. 2001;103:1702–1708. doi: 10.1161/01.cir.103.12.1702. [DOI] [PubMed] [Google Scholar]
- COLEMAN H.A., TARE M., PARKINGTON H.C. K+ currents underlying the action of endothelium-derived hyperpolarising factor in guinea-pig, rat and human blood vessels. J. Physiol. 2001;531:359–373. doi: 10.1111/j.1469-7793.2001.0359i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., FELETOU M., GARDENER M.J., THOLLON C., VANHOUTTE P.M., WESTON A.H. Role of gap junction in the responses to EDHF in rat and guinea-pig small arteries. Br. J. Pharmacol. 1999;128:1788–1794. doi: 10.1038/sj.bjp.0703009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS D., MARTIN P.E.M., EVANS H.W., KENDALL D.A., GRIFFITH T.M., RANDALL M.D. Role of gap junctions in endothelium-derived hyperpolarising factor responses and mechanisms of K+-relaxation. Eur. J. Pharmacol. 2000;402:119–128. doi: 10.1016/s0014-2999(00)00512-4. [DOI] [PubMed] [Google Scholar]
- HARRIS D., MCCULLOCH A.I., KENDALL D.A., RANDALL M.D. Characterization of vasorelaxant responses to anandamide in the rat mesenteric arterial bed. J. Physiol. 2002;593:893–902. doi: 10.1113/jphysiol.2001.013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNY L.C., BAKER P.N., RANDALL M.D., KENDALL D.A., DUNN W.R. Differential mechanisms of endothelial-dependent vasodilator responses in myometrial small arteries in normal pregnancy and preeclampsia. Clin. Sci. 2002;103:67–73. doi: 10.1042/cs1030067. [DOI] [PubMed] [Google Scholar]
- MCCULLOCH A.I., BOTTRILL F.E., RANDALL M.D., HILEY C.R. Characterization and modulation of EDHF-mediated relaxations in the rat isolated superior mesenteric arterial bed. Br. J. Pharmacol. 1997;120:1431–1438. doi: 10.1038/sj.bjp.0701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASCOAL I.F., LINDHEIMER M.D., NALBANTIAN-BRANDT C., UMANS J.G. Preeclampsia selectively impairs endothelium-dependent relaxation and leads to oscillatory activity in small omental arteries. J. Clin. Invest. 1998;101:464–470. doi: 10.1172/JCI557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL M.D., ALEXANDER S.P.H., BENNETT T., BOYD E.A., FRY J.R., GARDINER S.M., KEMP P.A., MCCULLOCH A.I., KENDALL D.A. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem. Biophys. Res. Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- TARE M., COLEMAN H.A., PARKINGTON H.C. Glycyrrhetinic derivatives inhibit hyperpolarization in endothelial cells of guinea pig and rat arteris. Am. J. Physiol. 2002;282:H335–H341. doi: 10.1152/ajpheart.2002.282.1.H335. [DOI] [PubMed] [Google Scholar]
- TAYLOR H.J., CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Inhibition of the gap junctional component of endothelium-independent relaxations in rabbit iliac artery by 18-alpha glycyrrhetinic acid. Br. J. Pharmacol. 1998;125:1–3. doi: 10.1038/sj.bjp.0702078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLIS S.J., FIRTH J., DUNN W.R. Pressure-induced myogenic responses in human isolated cerebral resistance arteries. Stroke. 1996;27:2287–2291. doi: 10.1161/01.str.27.12.2287. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., EDWARDS G., WESTON A.H., DAVIS S.C., HOGESTATT E.D. Effects of cytochrome P450 inhibitors on EDHF-mediated relaxation in the rat hepatic artery. Br. J. Pharmacol. 1996;118:1147–1152. doi: 10.1111/j.1476-5381.1996.tb15517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


