Abstract
In cultured porcine coronary artery endothelial cells, we have recently shown that substance P and bradykinin stimulated different types of Ca2+-dependent K+ (KCa) current. A large part of this current was insensitive to iberiotoxin and apamin. The aim of the present study was to characterize the KCa channel responsible for this current.
In cell-attached configuration and asymmetrical K+ concentration, 100 nM bradykinin or substance P activated a 10 pS K+ channel. In inside-out configuration, the channel was half-maximally activated by 795 nM free Ca2+.
Apamin (1 μM) added to the pipette solution failed to inhibit the channel activity while charybdotoxin (50 nM), completely blocked it. Perfusion at the intracellular face of the cell, of an opener of intermediate conductance KCa channel, 500 μM 1-ethyl-benzimidazolinone (1-EBIO) increased the channel activity by about 4.5 fold.
In whole-cell mode, bradykinin and substance P stimulated an outward K+ current of similar amplitude. Charybdotoxin inhibited by 75% the bradykinin-induced current and by 80% the substance P-induced current. Charybdotoxin plus iberiotoxin (50 nM each) inhibited by 97% the bradykinin-response. Charybdotoxin plus apamin did not increase the inhibition of the substance P-response obtained in the presence of charybdotoxin alone.
1-EBIO activated a transient outward K+ current and hyperpolarized the membrane potential by about 13 mV. Charybdotoxin reduced the hyperpolarization to about 3 mV.
Taken together these results show that bradykinin and substance P activate a 10 pS KCa channel, which largely contributes to the total K+ current activated by these agonists. Despite its small conductance, this channel shares pharmacological characteristics with intermediate conductance KCa channels.
Keywords: Endothelial cells, KCa channel, substance P, bradykinin, charybdotoxin, apamin
Introduction
Regulation of vascular tone depends on a balance between vasodilator and vasoconstrictor signals. Physical stimuli, circulating hormones, neurotransmitters, endothelium-derived factors, such as nitric oxide (NO), endothelium-derived hyperpolarizing factor (EDHF), and endothelin contribute to the establishment of the appropriate vascular tone.
In porcine coronary artery, bradykinin and substance P are both endothelium-dependent vasodilators (Pacicca et al., 1992). Their interaction with the receptors on the endothelial cells activates the phospholipase C pathway, which leads to an increase in inositol 1, 4, 5-trisphosphate (IP3) and diacylglycerol (DAG) levels (Graier et al., 1991; Farmer & Burch, 1992). IP3 causes a release of Ca2+ from the internal stores accompanied by a transient hyperpolarization due to the gating of KCa channels (Himmel et al., 1993). Moreover, this change in membrane potential increases the electrical driving force for Ca2+ ions in favour of a sustained Ca2+ influx from the extracellular space (Luckhoff & Busse, 1990).
Three types of KCa channels have been described: large conductance K+ (BKCa) channels (100–250 pS) that are blocked by iberiotoxin (Galvez et al., 1990), noxiustoxin and charybdotoxin (Miller et al., 1985), intermediate conductance K+ (IKCa) channels (18–60 pS) that are blocked by charybdotoxin, and small conductance K+ (SKCa) channels (6–14 pS) that are blocked by apamin (Blatz & Magleby, 1986). All three types were shown to be present in endothelial cells (for review see Nilius et al., 1997).
In rat mesenteric artery, stimulation of endothelial cells with acetylcholine leads to a K+ efflux through KCa channels. The increase of the K+ concentration into the myoendothelial space was supposed to be responsible for the hyperpolarization of smooth muscle cells through the gating of inward rectifying K+ (Kir) channels and the activation of the Na+/K+-ATPase pump (Edwards et al., 1998). At present, peptide toxins are largely used in order to inhibit KCa channels on endothelial cells and to block the EDHF response in several systems (Andersson et al., 2000; Bény & Schaad, 2000; Quignard et al., 2000). Indeed, it has been demonstrated that a mixture of apamin and charybdotoxin inhibits the EDHF-induced response, by specifically acting in endothelial cells (Doughty et al., 1999). In this context, the knowledge of the different KCa channels present in endothelial cells is crucial in order to determine definitively their possible involvement in the endothelium-dependent hyperpolarization.
We previously showed that in endothelial cells of porcine coronary artery, bradykinin and substance P trigger a Ca2+ elevation which leads to a membrane hyperpolarization of similar amplitude (Brunet & Bény, 1989; Frieden et al., 1999). Interestingly, whole cell experiments show that the two peptides do not activate the same type of KCa current (Frieden et al., 1999). We have shown that in both cases a large part of the current stimulated by bradykinin and substance P is insensitive to apamin and iberiotoxin. Thus the remaining outward K+ current activated by both peptides is likely due to unidentified K+ channel/s.
In order to identify this/these channel/s in primary cultures of porcine coronary artery endothelial cells, we performed patch clamp experiments in single channel and whole-cell modes, as well as membrane potential measurements using peptide toxins as pharmacological tool.
Methods
Endothelial cell primary cultures
Left anterior and right posterior descending branches of coronary artery from domestic pigs, Sus Scrofa, were obtained after electrocution at the slaughterhouse.
Endothelial cells were isolated by gentle rubbing of the internal face of the vessel with a scalpel. They were then centrifuged at 800×g for 8 min in the culture medium M199, (Gibco) supplemented with 10% foetal calf serum, 2 mM glutamine, non-essential amino acids (13 ml added to 1000 ml of M199; Gibco), MEM vitamin solution (13 ml added to 1000 ml of M199; Gibco) and gentamicin (50 mg l−1). The cell pellet was resuspended in 500 μl of culture medium M199. Ten μl of the cell suspension were mixed with 10 μl of Tripan blue and the number of living cells was counted in a Neubauer chamber. The volume of culture medium was adjusted to obtain a density of about 90,000 cells ml−1. Cells were plated on collagen-coated glass coverslips in culture dishes. Cells were cultured at 37°C under 5% CO2. The culture medium was changed three times a week. Cells were used after 2–5 days of primary culture. Endothelial cells were identified by their fusiform morphology in islets during 4 to 5 days, and a monolayer of polygonal cells (cobblestone-like) after 5–6 days of culture, as previously described (Bény & Pacicca, 1994).
Single channel recordings
We performed single channel recordings in cell-attached and inside-out configurations. Coverslips containing endothelial cells were placed in a custom-made perfusion chamber and observed with an inverted microscope (Nikon Diaphot 200, Tokyo, Japan). Quartz capillaries were pulled with a programmable puller (P-2000 Sutter Instrument CO, Novato, California U.S.A.). Borosilicate capillaries were pulled with a BB-CH-PC puller (Mecanex SA, Nyon, Switzerland) and coated with Sylgard 184 (Dow Corning, Seneffe, Belgium) to improve the recording quality. Patch pipettes had a resistance of 5–10 MΩ. Patch-clamp recordings were performed using an EPC-9 amplifier (EPC-9; HEKA Electronics, List Medical, Darmstadt, Germany). Currents were filtered at 1 kHz, sampled at 16 kHz and acquired with Pulse software (HEKA Electronics, List Medical, Darmstadt, Germany) on a Macintosh Quadra 650 computer. Currents were monitored on a digital oscilloscope (Gould, DSO 1604). Recordings were performed on islets of cells at room temperature (20–25°C).
Standard pipette solution contained (mM): KCl 5.6, NaCl 130 and HEPES 10 (pH=7.45 adjusted with NaOH 1N) for experiments in asymmetrical K+ concentration, and KCl 130, HEPES 10 (pH=7.45 adjusted with NaOH 1N) for experiments in symmetrical K+ concentration. Standard bathing solution contained (mM): KCl 130, MgCl2 1, CaCl2 2, HEPES 8, Glucose 10 (pH=7.5 adjusted with KOH 10 N). The Ca2+-free bathing solution was obtained by omission of MgCl2 and CaCl2 and addition of 2 mM EGTA. Calcium-dependence of the channel was determined using solutions containing between 100 nM to 100 μM of CaCl2 buffered with 5 mM EGTA. Solutions were exchanged using a custom-made solution exchanger. We selected the channel we were interested in according to its unitary conductance and K+ selectivity. Patches containing large conductances (>40 pS) and/or too many channels were discarded.
Recording of current amplitude was performed maintaining the holding potential during 10 sweeps of 1 s each in a range of values between −80 mV and +40 mV. Channel events detection and open time duration histograms were performed using the MacTAC software (Instrutech Corp, and SKALAR Instr Inc, Seattle, WA, U.S.A.). The open probability (Po) of the channel was calculated as the time spent in the open state (to) divided by the total time of recording (t): Po=to/t. Open probability was usually calculated every 20 s on 10 sweeps of 1 s each. When several identical channels (N) were simultaneously opened on the same patch, the open probability of one channel was calculated as:
Where toN is the time spent by a channel at the open level N. The mean open time (τ) was calculated according to Fenwick et al. (1982).
Whole-cell recordings
Borosilicate glass capillaries were used for experiments in whole-cell configuration. Patch pipettes were pulled with a BB-CH-PC puller and had a resistance of 3–5 MΩ. Patch clamp recordings were made using the same set-up as for single channel recordings.
To determine the current-potential relationship, repetitive 300 ms voltage steps (−20, 20, 40 mV) were applied throughout the recording (10 sweeps of 40 s each), from a holding potential varying between −50 and −30 mV. To normalize the results, we expressed the current conductance as density (pS/pF), and thus membrane capacitance was measured before each experiment by applying a 10 mV voltage step. The capacitive current transients were fitted with a single exponential (Pulsefit; HEKA Electronik) and the membrane capacitance (Cm) was calculated according to the following equations (de roos et al., 1996):
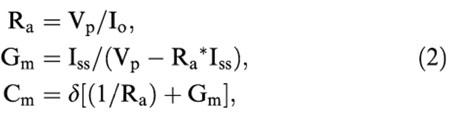 |
Where Ra is the access resistance, Vp the applied voltage step (10 mV), Io the peak current, Iss the steady-state current, Gm the membrane conductance and δ the decay constant of the transient. The slope conductance of the activated currents was expressed as a function of the cell capacitance. The membrane capacitance was 28.6±3.0 pF (n=10) for one cell and 49.0±7.1 pF (n=11) for two coupled cells, in agreement with the values reported by Frieden et al. (1999). The absence of any ramification of the cell surface insures a proper space clamp of the holding potential of the cells. Furthermore, when we patched two coupled cells, we could not detect any difference compared to one single cell in terms of the kinetics/amplitude of the response or of reversal potential (after normalization to cell capacitance). It was shown that clusters of endothelial cells until eight cells have adequate space clamp (Mendelowitz et al., 1992). In our study, we performed recording on single cells or two coupled cells.
Experiments were performed at room temperature (20–25°C). Cells were superfused with a solution containing (mM): NaCl 130, KCl 5.6, MgCl2 1, CaCl2 2, Glucose 10, HEPES 8 (pH 7.5 with NaOH). Standard pipette solution contained (mM): KCl 130, MgCl2 1, MgATP 5, HEPES 10 (pH 7.3 with NaOH).
Perfusion of patch-pipette
Outside-out experiments were most of the time unsuccessful, and thus we used a 2PK+ pipette perfusion system (Ala Scientific Instruments, Inc., Westbury, New York, U.S.A.) in inside-out experiments, in order to test toxin sensitivity of the channel. The volume of solution inside the patch pipette was about 15 μl. Pipette was first filled with the standard pipette solution until about one third of the final volume, and after seal formation a suction was applied from a small tube near the pipette tip, connected to the 2PK system. This aided the toxin-enriched solution to easily reach the patch membrane.
Membrane potential recordings
Coverslips containing endothelial cells were placed on a custom-made chamber and observed on an inverted microscope (Diaphot TMD, Nikon). Cells were continuously perfused with a Krebs-buffered solution containing (mM): NaCl 118.7, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 24.8, glucose 10.1, pH 7.4, and gassed with a mixture of 95% O2 and 5% CO2. The membrane potential was measured with a conventional glass microelectrode containing 3 M KCl (90–150 MΩ) and connected to an amplifier (WPI Intra707) with an Ag-AgCl half-cell. The membrane potential was monitored on a digital oscilloscope (Gould, type 1425) and recorded on a potentiometric recorder (W+W Electronics, U.S.A. 312). Criteria for accepting a record were a stable membrane potential below −20 mV, and a sharp penetration and withdrawal. Membrane potential was also recorded in current-clamp mode. In that case, amphotericin B-perforated patch was performed, and the pipette was filled with a solution containing 200–300 μg ml−1 of amphotericin B.
Chemical and drugs
Bradykinin and Substance P were purchased from Bachem Feinchemikalien AG (Bubendorf, Switzerland). Apamin, charybdotoxin and iberiotoxin were purchased from Alomone labs (Jerusalem, Israel). 1-ethyl-2-benzimidazolinone (1-EBIO) was purchased from Tocris (Bristol, U.K.), and amphotericin B was purchased from Sigma (Switzerland).
Statistics
Data were expressed as mean±standard error of the mean (s.e.mean), n referring to number of experiments. Student's t-test was used to compare results with a P value <0.05 as level of significance. Half-maximal activation of the channel (EC50) was obtained by fitting the data with a Boltzmann equation.
Results
Small conductance K+ channel stimulated by bradykinin and substance P
In order to recognize the current due to K+ channels at a holding potential of 0 mV, an asymmetrical K+ gradient was chosen during experiments, the pipette solution containing 5.6 mM KCl and the bath solution 130 mM KCl. In cell-attached configuration, no channel activity was observed, but the application of bradykinin (100 nM) to the bath solution transiently activated a channel of small conductance 10.7±0.2 pS (n=16). The reversal potential of this current was −83.1±2.1 mV (n=16), close to the estimated K+ equilibrium potential (EK≅−80 mV). Likewise application of substance P (100 nM) activated a channel of 10.7±0.3 pS (n=12), this conductance not being different from the one activated by bradykinin. The reversal potential of the current was −85.0±2.4 mV (n=12), close to EK. Bradykinin activated the channel with a maximal open probability (Po) of 0.4±0.1 (n=16) and substance P activated the channel to 0.3±0.1 (n=12; P=0.19; bradykinin vs substance P; Figure 1A,B). During the kinin-induced activation only one open level of the channel was observed. An increase in number of openings (N) occurred whereas the mean open time (τ) remained constant τ=3.9±0.4 ms and τ=4.1±0.7 ms for bradykinin and substance P, respectively (Figure 1C,D).
Figure 1.
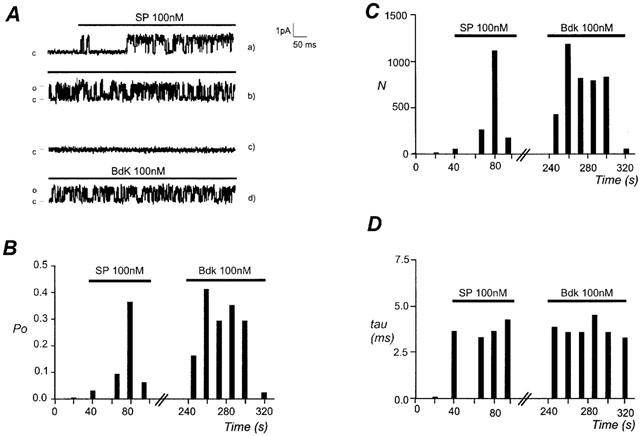
Effects of bradykinin and substance P on open probability, number of openings and mean open time of SKap-ins channel. Results were obtained on the same cell during successive applications of substance P (100 nM) and bradykinin (100 nM), perfused in the bath during the time indicated by the bar. During recording the holding potential was maintained at 0 mV. (A) Original current traces in cell-attached configuration at a holding potential of 0 mV: (a), (b), shows the activation induced by substance P (horizontal bar); (c), (d) during the perfusion of bradykinin (horizontal bar). (B,C,D) Open state probability (Po), number of openings (N) and mean open time (τ) are expressed versus the time of peptide application.
Excision of the patch in the inside-out configuration, and exposure of the intracellular face to a standard bath solution containing 2 mM Ca2+ restored the channel activity. The current-voltage (I-V) relationship for the channel is shown in Figure 2A. The conductance of the channel (ginside-out=10.2±1.1 pS) was not significantly different from the conductance activated by bradykinin and substance P. The reversal potential of the current was −79.6±1.0 mV, close to the EK, and confirmed a high K+ selectivity of this channel. In high symmetrical K+ concentration the conductance of the channel was increased to 30.9±3.9 pS (n=4) and the reversal potential was 1.0±2.2 mV.
Figure 2.
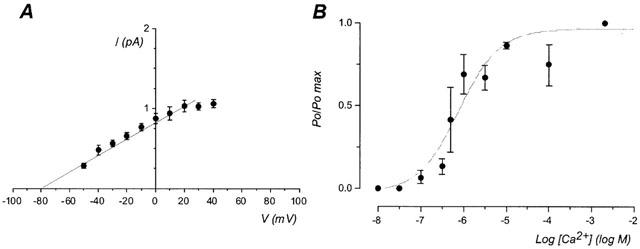
Characterization in inside-out configuration of the SKap-ins channel. (A) Current-voltage relationship obtained in inside-out configuration in asymmetrical K+ concentration, pipette solution containing 5.6 mM K+ and bath solution containing 130 mM K+. Each point is the mean±s.e.mean of 13 experiments. (B) Effect of various intracellular [Ca2+] on the normalized open state probability (Po/Pomax), at holding potential of 0 mV. Values are given as mean±s.e.mean for 3–7 experiments. Data were fitted with a Boltzmann equation and EC50 was 795 nM [Ca2+]i.
In inside-out configuration the calcium-dependence of the channel was studied. As shown in Figure 2B, the open probability was determined for various intracellular Ca2+ concentrations. In the absence of intracellular Ca2+, there was no activity of the channel, whereas an increase of the cytosolic calcium concentration increased the open probability of the channel with an EC50=795 nM.
Effect of apamin on channel activity in single channel recordings
The small unitary conductance, the high K+ selectivity, and the calcium-dependence suggested that this channel belongs to the family of SKCa channel, a class of KCa channels sensitive to the bee venom apamin (Blatz & Magleby, 1986). In order to investigate the pharmacological profile of this SKCa channel, 1 μM apamin was added to the standard pipette solution. In cell-attached configuration the channel was activated by bradykinin or substance P with a maximal open probability of 0.2±0.1 (n=5). The unitary conductance was not significantly different, gbradykinin=12.0±0.8 pS (n=4), and gsubstance P=12.0±0.9 pS (n=5) upon stimulation with bradykinin and substance P, respectively. Excision of the patch in inside-out configuration recovered a single channel activity with characteristics comparable with those obtained in the absence of apamin (data not shown). Thus, no differences in the channel characteristics were observed in the absence or presence of the inhibitor inside the patch pipette.
Effect of charybdotoxin on channel activity in single channel recordings
We filled the patch pipette with a toxin-free pipette solution, until one third of the final volume. After establishing the seal an additional aspiration was applied in order to back fill the patch pipette as described in Methods. In this manner, the charybdotoxin was progressively applied to the external face of the patch. No channel activity was observed in cell-attached configuration. Excision of the patch, in a bath solution containing 2 mM Ca2+, showed the presence of a channel with the same conductance as in control condition. Indeed at a holding potential of 0 mV the amplitude of the currents was 1.0±0.1 pA (n=4). The open probability of the channels was estimated at 0.4±0.1 (n=4). Within 5–6 min after the seal formation the channel activity was completely blocked (n=4; Figure 3A) by the application of charybdotoxin.
Figure 3.
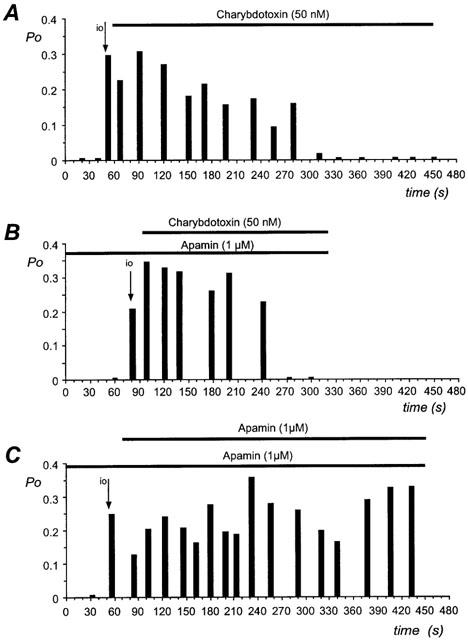
Effect of toxins on open probability of SKap-ins channel. The open probability of the channel is plotted against the recording time during the application of the toxin at a holding potential of 0 mV. Each panel represents the recording obtained on a different cell. The horizontal bar indicates the presence of the toxin. (A) The perfusion of the patch pipette with charybdotoxin (50 nM), decreased the channel activity. (B) The pipette solution enriched with apamin (1 μM) is perfused with charybdotoxin (50 nM). (C) The pipette solution enriched with apamin (1 μM) is perfused with apamin (1 μM). The arrow indicated the transition between the cell-attached and the inside-out (io) configuration. Data are representative recordings from 2–4 experiments.
With a similar procedure as the one described above, a patch pipette containing 1 μM apamin was perfused in combination with 50 nM charybdotoxin. The channel activity was blocked with the same delay as before (Figure 3B, n=3), showing that the channel was apamin-insensitive but blocked by the external presence of charybdotoxin.
In order to verify that the decrease in channel activity after 5–6 min was genuinely due to a charybdotoxin inhibition and not to a channel run-down, similar time-course experiments were performed using a pipette solution containing 1 μM apamin, perfused with 1 μM apamin. The channel activity was recorded in inside-out configuration during 7–8 min after the establishment of the seal. During this period the open probability of the channel did not significantly change (Figure 3C, n=2). Thus a run-down phenomenon cannot account for the decrease of the channel activity following application of charybdotoxin.
Effect of charybdotoxin on current stimulation in whole-cell recordings
In whole-cell configuration bradykinin and substance P stimulated an outward current with a maximal conductance of 202.0±23.5 pS/pF (n=24) and 216.3±30.2 pS/pF (n=17; P=0.71) respectively. In K+ asymmetrical concentration, and bath and pipette solutions containing 5.6 mM and 130 mM KCl respectively, the extrapolated reversal potentials of the peptide-induced currents were −77.4±2.4 mV and −78.3±2.5 mV for bradykinin and substance P, respectively. This indicated that the current was mostly carried by K+ ions, which is in agreement with our previous findings (Frieden et al., 1999).
We tested whether the application of charybdotoxin induces an inhibition of the current stimulated by bradykinin or substance P. Figure 4C shows that charybdotoxin alone reduced the conductance of the bradykinin-stimulated current to 50.8±15.9 pS/pF (n=9, P<0.05 vs control of 202.0±23.5 pS/pF) corresponding to an inhibition of 75%. A combination of charybdotoxin and iberiotoxin, a specific inhibitor of BKCa channels, reduced the conductance of the bradykinin-induced current to 6.4±6.4 pS/pF (n=4; P<0.05 vs control; P=0.1 vs a conductance of 50.8±15.9 pS/pF in the presence of charybdotoxin), corresponding to an inhibition of 97%.
Figure 4.
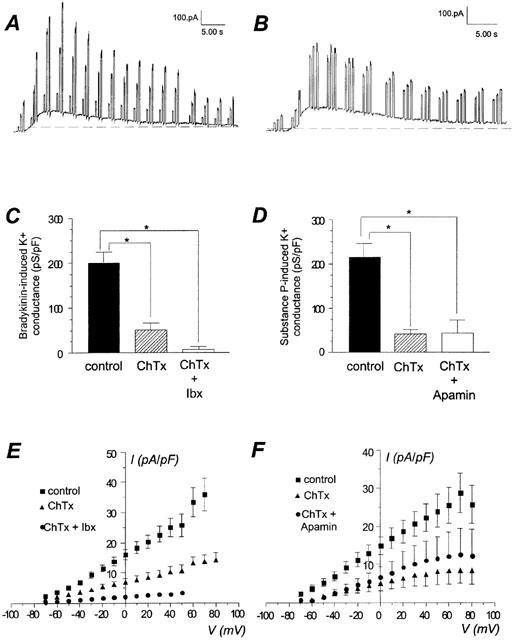
Effect of peptide toxins on maximal conductance stimulated by bradykinin and substance P in whole-cell configuration. (A, B) Original recordings of a response to bradykinin and substance P, respectively. Holding potential was −50 mV, voltage steps (−20, 20, 40 mV) were repetitively applied. (C) Comparison of the whole-cell bradykinin-induced maximal K+ conductance in control condition (100 nM), in the presence of charybdotoxin alone (50 nM, ChTx), or a mixture of charybdotoxin and iberiotoxin (50 nM each inhibitor, ChTx+Ibx). E, current-voltage relationship of the bradykinin-stimulated current in control condition, in presence of charybdotoxin alone (ChTx), or a mixture of charybdotoxin and iberiotoxin (ChTx+Ibx). Data are the mean±s.e.mean of 4–24 experiments. (D) Comparison of the whole-cell substance P-induced maximal K+ conductance in control condition (100 nM), in the presence of charybdotoxin alone (50 nM, ChTx), or a mixture of charybdotoxin (50 nM) and apamin (1 μM, ChTx+Apamin). (F) Current-voltage relationship of the substance P-stimulated current in control condition, in presence of charybdotoxin (ChTx) alone, or a mixture of charybdotoxin and apamin (ChTx+Apamin). Data are the mean±s.e.mean of 8–17 experiments. *For P<0.05 vs control condition.
Figure 4D shows that charybdotoxin also reduced the conductance of the substance P-induced current to 41.5±9.9 pS/pF (n=5; P<0.05 vs control 216.3±30.1 pS/pF) corresponding to an inhibition of 81% of the response. The mixture of charybdotoxin and apamin, a specific inhibitor of SKCa channels, did not further reduce the conductance of the outward K+ current.
Effect of 1-EBIO
1-ethyl-2-benzimidazolinone (1-EBIO) is known as a selective opener of intermediate conductance K+ channel (IKCa) in cultured bovine aortic endothelial cells (Edwards et al., 1999). In inside-out configuration, addition of 500 μM 1-EBIO to a bath solution containing 500 nM Ca2+ increased the channel activity in a transient manner. On average 1-EBIO increases the open probability of the channel 4.5±1.5 fold (n=3, Figure 5A). In one case out of four, 1-EBIO failed to affect the channel activity. 1-EBIO did not change the unitary conductance of the channel (g=10.3±1.1 pS).
Figure 5.
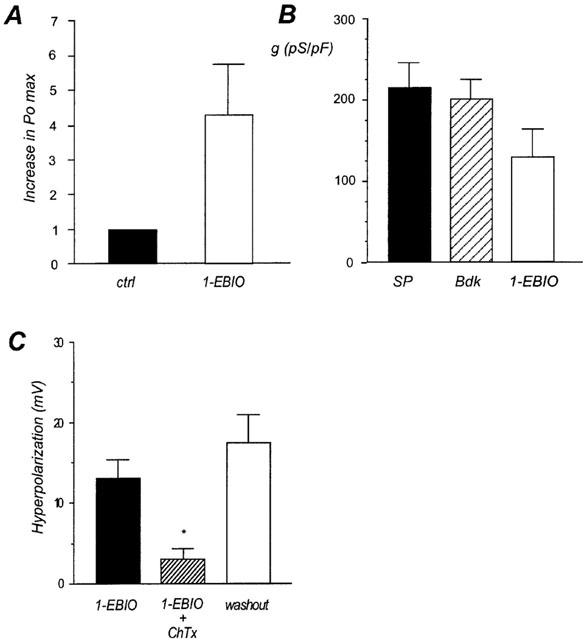
Effect of 1-EBIO on endothelial cells. (A) Increase of the Po max of the SKap-ins channel induced by application of 500 μM 1-EBIO at the intracellular side of the membrane in inside-out configuration. Columns are the mean±s.e.mean of three experiments on different cells. Po max in control condition was normalized to 1. (B) Comparison of the maximal K+ current conductance induced by substance P (100 nM, black column), bradykinin (100 nM, hatching column) and 1-EBIO (500 μM, white column) in whole-cell configuration. (C) Comparison of 1-EBIO (500 μM) induced hyperpolarization in control condition (black column), in the presence of charybdotoxin (ChTx, 50 nM, hatching column) and after washout of charybdotoxin (white column). *For P<0.05 vs control condition.
We then determined the effect of 1-EBIO on whole-cell current. The opener of IKCa channels provoked a transient outward current of 133.2±38.1 pS/pF with a reversal potential of −82.3±4.4 mV (n=3). The current induced by 1-EBIO corresponded to about 60% of the bradykinin- and substance P-induced currents (Figure 5B). Single channel and whole-cell results suggest that in endothelial cells, K+ channels sensitive to 1-EBIO are present.
The effect of 1-EBIO was also tested on the membrane potential. Under resting conditions, endothelial cells had a membrane potential of −29.7±7.3 mV (n=7).
Perfusion of 500 μM 1-EBIO in the bath solution resulted in a transient hyperpolarization of the membrane potential of 13.1±2.3 mV (n=7). According to previous data (Frieden et al., 1999), 100 nM bradykinin and substance P induced a change of the membrane potential of 27.9±2.3 mV (n=6) and 36.8±7.8 (n=3), respectively. Application of 50 nM charybdotoxin during 12–15 min strongly reduced the hyperpolarization of 1-EBIO (3.1±1.3 mV; n=5). Following washout of charybdotoxin, 1-EBIO-induced response was completely restored (17.4±3.5; n=3; Figure 5C).
Discussion
In primary cultured endothelial cells, bradykinin and substance P activate an outward K+ current of the same amplitude (Frieden et al., 1999). Despite the similarity between the responses induced by bradykinin and substance P, we have shown that the two peptides do not involve the same type of KCa current (Frieden et al., 1999). Bradykinin-induced current is partially sensitive to iberiotoxin but insensitive to apamin, which reflects an activation of BKCa channels, previously characterized at the single channel level (Baron et al., 1996). On the contrary, a large part of the substance P-induced current is sensitive to apamin but insensitive to iberiotoxin. In both cases an important residual K+ current is present and is insensitive to iberiotoxin and apamin. In the present study we show that this residual current is sensitive to charybdotoxin. Moreover, at single channel level we characterize a SKCa channel insensitive to apamin, blocked by charybdotoxin, and gated by 1-EBIO, that likely participates to the charybdotoxin-sensitive current. In order to avoid confusion about the nomenclature, in the discussion below we name this channel SKap-ins (apamin-insensitive).
Characterization of SKap-ins at single channel level
Bradykinin and substance P activate a K+ channel of the same conductance of 10 pS. This channel shows a rather high sensitivity to Ca2+ (EC50=795 nM) which fits with the values shown for a 10 pS KCa channel in cultured bovine aortic endothelial cells (Vaca et al., 1992) and for the hSK1 type channels re-expressed in Xenopus oocytes (Kohler et al., 1996). Moreover, maximal value of intracellular Ca2+ concentrations reached during the activation with bradykinin and substance P, i.e. about 1 μM, is compatible with the Ca2+-sensitivity of the channel. These data suggest that SKap-ins channels could likely participate in the hyperpolarization induced by these peptides (Frieden et al., 1999; Sharma & Davis, 1994). SKCa channels have already been described in pig coronary artery and aortic endothelial cells (Sharma & Davis, 1994; Groschner et al., 1992) but their precise pharmacological profile was not investigated.
The channel characterized in our study belongs to the small conductance class, according to the classification based on the conductance, while it belongs to the intermediate conductance class regarding its sensitivity to toxins. Indeed apamin added to the pipette solution failed to inhibit the channel activity, and did not change the conductance of the current. On the contrary, addition to the pipette solution of 50 nM charybdotoxin blocked the channel activity. These results show that in cultured porcine coronary artery endothelial cells an apamin-insensitive, charybdotoxin-sensitive SKCa channel is present. A similar pharmacological profile of a SKCa channel has already been shown in rat aortic endothelial cells in intact tissue (Marchenko & Sage, 1996).
SKCa channel with such properties was shown to be activated by 1-EBIO (Warth et al., 1999). Furthermore, 1-EBIO is described as an opener of IKCa channels (Edwards et al., 1999; Devor et al., 1996), even if its specificity is not definitely assessed (Pedarzani et al., 2001). Therefore, we tested this compound as a pharmacological tool to characterize our channel. Single channel experiments have shown that 1-EBIO activates in a transient manner the SKap-ins channel. In whole-cell configuration, 1-EBIO activates a transient outward K+ current with a maximal conductance that corresponds to 60% of the conductance stimulated by bradykinin and substance P. In addition, 1-EBIO provokes a membrane hyperpolarization of about half amplitude compared to the hyperpolarization induced by bradykinin and substance P. In agreement with the effect of 1-EBIO on the SKap-ins channel, the hyperpolarization was strongly inhibited by charybdotoxin. A similar effect on the membrane potential has been shown in the rat mesenteric vessel (Adeagbo, 1999) and rat hepatic artery (Edwards et al., 1999). Hence, the stimulatory effect of 1-EBIO and its charybdotoxin sensitivity, would fit with the presence of a residual current (insensitive to apamin and iberiotoxin), which results from the activation of SKap-ins channel.
To date four types of SKCa channels have been cloned in human and rat. They are named hSK1 (human)/rSK1 (rat), rSK2, rSK3 and hSK4 and differ in their unitary conductance, and sensitivity for Ca2+ and toxins (Kohler et al., 1996). Because of the unitary conductance, the Ca2+-sensitivity and the pharmacological profile, the channel described above is similar to the SK4 type, recently identified as homologue to hIK1, stimulated by 1-EBIO and inhibited by charybdotoxin (Warth et al., 1999).
Characterization of SKap-ins current in whole-cell recordings
Whole-cell experiments show that bradykinin and substance P provoke an outward K+ current reaching similar maximal conductance. Charybdotoxin alone inhibits about 75% of the bradykinin-induced current. The combination of charybdotoxin and iberiotoxin does not significantly potentiate the inhibition of the bradykinin-stimulated current. Charybdotoxin is largely used to inhibit the BKCa channel type (Miller et al., 1985), but it also inhibits IKCa channels (Cai et al., 1998) and apamin-insensitive K+ channels (KChtx) of small conductance (Marchenko & Sage, 1996). In porcine coronary artery endothelial cells, a BKCa channel of 285 pS is present (Baron et al., 1996). This channel is activated by bradykinin and is responsible for 40% of the outward K+ current induced by the peptide, and specifically inhibited by iberiotoxin (Frieden et al., 1999). We suggest that the large effect provoked by charybdotoxin is due to an inhibition of BKCa and SKap-ins channels, both recruited by bradykinin during the response.
Charybdotoxin alone also inhibits 80% of the substance P-induced current, showing that a charybdotoxin-sensitive current is largely responsible for the substance P-induced response. This is in agreement with the single channel experiments presented above, where substance P activates the SKap-ins channel that is sensitive to charybdotoxin. However, such a large inhibition was unexpected given the fact that apamin alone inhibits about 70% of the current (data not shown), which is in agreement with previous data (Frieden et al., 1999). This contradiction arises from the assumption that the contribution of the different channels to the global current is additive, which might not be the case. Another explanation would be that charybdotoxin, being not a highly selective compound, would also inhibit the apamin-sensitive channel. Since we did not find an apamin-sensitive channel, we cannot further speculate about its pharmacological profile. Several different hypotheses can be proposed to explain the apparent absence of an apamin-sensitive channel in single channel recordings. First, it may be that the unitary conductance of this channel is too small to be properly detected. Second, the channel could be located on region(s) of the cell membrane not accessible to the patch pipette (i.e. basal side of the cell). Finally, we cannot rule out that the apamin-sensitive SKCa channel has a similar conductance to the SKap-ins channel, making them difficult to discriminate. The use of other approaches than the patch-clamp will be necessary to clarify this point.
The present study combined with the previous one (Frieden et al., 1999) give a more precise view of the KCa channels present in cultured endothelial cells and their different involvement during the stimulation with bradykinin and substance P. While bradykinin activates SPap-ins and BKCa channels, substance P stimulates SKap-ins and SKCa (apamin sensitive) channels. As both agonists trigger the IP3 pathway leading to a Ca2+ release from the reticulum as well as a Ca2+ influx, it remains to be elucidated how bradykinin and substance P can gate different types of KCa channels.
In conclusion, in porcine coronary artery endothelial cells, SKap-ins channels are partially involved in the release of K+. Different hypotheses concerning the nature of EDHF, it has been proposed that K+ could be the EDHF in this vessel (Bény & Schaad, 2000) and in other tissues (Edwards et al., 1998). Additionally we suggest that the inhibition of the EDHF using charybdotoxin is not necessarily a consequence of the inhibition of IKCa channels. Other KCa channels, such as the SKap-ins channels, can be responsible for this phenomenon in endothelial cells.
Acknowledgments
This work was supported by the Swiss National Science Foundation, grant 31-62502.00. We thank D. Solomos for preparing the cell cultures, F. Gribi for technical assistance, R. Henauer for his excellent technical competency and C. Bouvier. We thank N. Demaurex, C. Luscher, C. Blanchet and A. Maturana for helpful discussion during manuscript revision.
Abbreviations
- BdK
bradykinin
- BKCa
large conductance K+ channel
- ChTx
charybdotoxin
- DAG
diacylglycerol
- 1-EBIO
1-ethyl-benzimidazolinone
- EDHF
endothelium-derived hyperpolarizing factor
- Ibx
iberiotoxin
- IKCa
intermediate conductance K+ channel
- IP3
inositol 1, 4, 5-trisphosphate
- KCa
Ca2+-dependent K+ channel
- NO
nitric oxide
- SKap-ins
apamin-insensitive small conductance K+ channel
- SKCa
small conductance K+ channel
- SP
substance P
References
- ADEAGBO A.S. 1-Ethyl-2-benzimidazolinone stimulates endothelial K(Ca) channels and nitric oxide formation in rat mesenteric vessels. Eur. J. Pharmacol. 1999;379:151–159. doi: 10.1016/s0014-2999(99)00489-6. [DOI] [PubMed] [Google Scholar]
- ANDERSSON D.A., ZYGMUNT P.M., MOVAHED P., ANDERSSON T.L., HOGESTATT E.D. Effects of inhibitors of small- and intermediate-conductance calcium-activated potassium channels, inwardly-rectifying potassium channels and Na(+)/K(+) ATPase on EDHF relaxations in the rat hepatic artery. Br. J. Pharmacol. 2000;129:1490–1496. doi: 10.1038/sj.bjp.0703226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARON A., FRIEDEN M., CHABAUD F., BENY J.L. Ca2+-dependent non-selective cation and potassium channels activated by bradykinin in pig coronary artery endothelial cells. J. Physiol. 1996;493:691–706. doi: 10.1113/jphysiol.1996.sp021415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÉNY J.L., PACICCA C. Bidirectional electrical communication between smooth muscle and endothelial cells in the porcine coronary artery. Am. J. Physiol. 1994;266:H1465–1472. doi: 10.1152/ajpheart.1994.266.4.H1465. [DOI] [PubMed] [Google Scholar]
- BÉNY J.L., SCHAAD O. An evaluation of potassium ions as endothelium-derived hyperpolarizing factor in porcine coronary arteries. Br. J. Pharmacol. 2000;131:965–973. doi: 10.1038/sj.bjp.0703658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLATZ A.L., MAGLEBY K.L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986;323:718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- BRUNET P.C., BÉNY J.L. Substance P and bradykinin hyperpolarize pig coronary artery endothelial cells in primary culture. Blood Vessels. 1989;26:228–234. doi: 10.1159/000158770. [DOI] [PubMed] [Google Scholar]
- CAI S., GARNEAU L., SAUVE R. Single-channel characterization of the pharmacological properties of the K(Ca2+) channel of intermediate conductance in bovine aortic endothelial cells. J. Memb. Biol. 1998;163:147–158. doi: 10.1007/s002329900379. [DOI] [PubMed] [Google Scholar]
- DE ROOS A.D., VAN ZOELEN E.J., THEUVENET A.P. Determination of gap junctional intercellular communication by capacitance measurements. Pfluegers Arch. 1996;431:556–563. doi: 10.1007/BF02191903. [DOI] [PubMed] [Google Scholar]
- DEVOR D.C., SINGH A.K., FRIZZELL R.A., BRIDGES R.J. Modulation of Cl− secretion by benzimidazolones. I. Direct activation of a Ca2+-dependent K+ channel. Am. J. Physiol. 1996;271:L775–L784. doi: 10.1152/ajplung.1996.271.5.L775. [DOI] [PubMed] [Google Scholar]
- DOUGHTY J.M., PLANE F., LANGTON P.D. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Am. J. Physiol. 1999;276:H1107–H1112. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., GARDENER M.J., FELETOU M., BRADY G., VANHOUTTE P.M., WESTON A.H. Further investigation of endothelium-derived hyperpolarizing factor (EDHF) in rat hepatic artery: studies using 1-EBIO and ouabain. Br. J. Pharmacol. 1999;128:1064–1070. doi: 10.1038/sj.bjp.0702916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARMER S.G., BURCH R.M. Biochemical and molecular pharmacology of kinin receptors. Annu. Rev. Pharmacol. Toxicol. 1992;32:511–536. doi: 10.1146/annurev.pa.32.040192.002455. [DOI] [PubMed] [Google Scholar]
- FENWICK E.M., MARTY A., NEHER E. Sodium and calcium channels in bovine chromaffin cells. J. Physiol. 1982;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDEN M., SOLLINI M., BENY J. Substance P and bradykinin activate different types of K(Ca) currents to hyperpolarize cultured porcine coronary artery endothelial cells. J. Physiol. 1999;519:361–371. doi: 10.1111/j.1469-7793.1999.0361m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALVEZ A., GIMENEZ-GALLEGO G., REUBEN J.P., ROY-CONTANCIN L., FEIGENBAUM P., KACZOROWSKI G.J., GARCIA M.L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J. Biol. Chem. 1990;265:11083–11090. [PubMed] [Google Scholar]
- GRAIER W.F., SCHMIDT K., KUKOVETZ W.R. Bradykinin-induced Ca2+-influx into cultured aortic endothelial cells is not regulated by 1, 4, 5-trisphosphate or inositol 1, 3, 4, 5-tetrakisphosphates. Second Messengers Phosphoproteins. 1991;13:187–197. [PubMed] [Google Scholar]
- GROSCHNER K., GRAIER W.F., KUKOVETZ W.R. Activation of a small-conductance Ca2+-dependent K+ channel contributes to bradykinin-induced stimulation of nitric oxide synthesis in pig aortic endothelial cells. Biochem. Biophys. Acta. 1992;1137:162–170. doi: 10.1016/0167-4889(92)90198-k. [DOI] [PubMed] [Google Scholar]
- HIMMEL H.M., WHORTON A.R., STRAUSS H.C. Intracellular calcium, currents, and stimulus-response coupling in endothelial cells. Hypertension. 1993;21:112–127. doi: 10.1161/01.hyp.21.1.112. [DOI] [PubMed] [Google Scholar]
- KOHLER M., HIRSCHBERG B., BOND C.T., KINZIE J.M., MARRION N.V., MAYLIE J., ADELMAN J.P. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- LUCKHOFF A., BUSSE R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pfluegers Arch. 1990;416:305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- MARCHENKO S.M., SAGE S.O. Calcium-activated potassium channels in the endothelium of intact rat aorta. J. Physiol. 1996;492:53–60. doi: 10.1113/jphysiol.1996.sp021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDELOWITZ D., BACAL K., KUNZE D.L. Bradykinin-activated calcium influx pathway in bovine aortic endothelial cells. Am. J. Physiol. 1992;262:H942–H948. doi: 10.1152/ajpheart.1992.262.4.H942. [DOI] [PubMed] [Google Scholar]
- MILLER C., MOCZYDLOWSKI E., LATORRE R., PHILLIPS M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985;313:316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- NILIUS B., VIANA F., DROOGMANS G. Ion channels in vascular endothelium. Annu. Rev. Physiol. 1997;59:145–170. doi: 10.1146/annurev.physiol.59.1.145. [DOI] [PubMed] [Google Scholar]
- PACICCA C., VON DER WEID P.Y., BENY J.L. Effect of nitro-L-arginine on endothelium-dependent hyperpolarizations and relaxations of pig coronary arteries. J. Physiol. 1992;457:247–256. doi: 10.1113/jphysiol.1992.sp019376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEDARZANI P., MOSBACHER J., RIVARD A., CINGOLANI L.A., OLIVER D., STOCKER M., ADELMAN J.P., FAKLER B. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J. Biol. Chem. 2001;276:9762–9769. doi: 10.1074/jbc.M010001200. [DOI] [PubMed] [Google Scholar]
- QUIGNARD J.F., FELETOU M., EDWARDS G., DUHAULT J., WESTON A.H., VANHOUTTE P.M. Role of endothelial cell hyperpolarization in EDHF-mediated responses in the guinea-pig carotid artery. Br. J. Pharmacol. 2000;129:1103–1112. doi: 10.1038/sj.bjp.0703175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARMA N.R., DAVIS M.J. Mechanism of substance P-induced hyperpolarization of porcine coronary artery endothelial cells. Am. J. Physiol. 1994;266:H156–H164. doi: 10.1152/ajpheart.1994.266.1.H156. [DOI] [PubMed] [Google Scholar]
- VACA L., SCHILLING W.P., KUNZE D.L. G-protein-mediated regulation of a Ca2+-dependent K+ channel in cultured vascular endothelial cells. Pfluegers Arch. 1992;422:66–74. doi: 10.1007/BF00381515. [DOI] [PubMed] [Google Scholar]
- WARTH R., HAMM K., BLEICH M., KUNZELMANN K., VON HAHN T., SCHREIBER R., ULLRICH E., MENGEL M., TRAUTMANN N., KINDLE P., SCHWAB A., GREGER R. Molecular and functional characterization of the small Ca2+-regulated K+ channel (rSK4) of colonic crypts. Pfluegers Arch. 1999;438:437–444. doi: 10.1007/s004249900059. [DOI] [PubMed] [Google Scholar]


