Abstract
The effect of histamine on the rate of lymphatic vessel constrictions and lymphatic smooth muscle membrane potential was examined in the guinea-pig mesentery.
Histamine (0.01–5 μM) increased the frequency and decreased the amplitude of constrictions in lymphatic vessels under intraluminal perfusion. This response was accompanied by a depolarization of the smooth muscle membrane potential, an increase in the activity of spontaneous transient depolarizations (STDs), the proposed pacemaker for constrictions in these vessels, and an increase in the occurrence of action potentials.
Responses to histamine were inhibited by the H1 receptor antagonist pyrilamine (0.2 μM), but unaffected by NO synthase inhibition with NG-nitro L-arginine (L-NOARG, 100 μM) and lysis of the endothelium.
In about 50% of the vessels, a decrease in constriction frequency, STD activity and a smooth muscle hyperpolarization were observed in response to dimaprit (10 μM), suggesting the presence of H2 receptors. These vessels had also a significantly lower basal contractile rate. Lymphatic vessel pumping was not affected by R-α-methylhistamine (10–50 μM), ruling out a role for H3 receptor stimulation in the histamine response.
The present results suggest a direct action of histamine on the lymphatic smooth muscle via stimulation of H1 (and in some vessels H2) receptors. H1 receptors enhance and H2 receptors slow down lymphatic pumping, the dominant effect being an increased contractile activity. Correlation of these effects with histamine-induced changes in membrane potential and STD activity suggests the involvement of these electrical changes in the initiation of the contractile response.
Keywords: Lymphatic pumping, smooth muscle, histamine, spontaneous transient depolarization, membrane potential, inflammatory mediators, rhythmic constriction
Introduction
The lymphatic circulation maintains tissue fluid homeostasis through removing fluid and proteins that accumulate into the interstitium and return them to the systemic circulation. Lymph transport depends mainly upon the active constriction of the successive chambers that comprise the collecting lymphatic vessels. The contractile mechanism is intrinsic to the smooth muscle present in the vessel wall and consequent to L-type Ca2+ channel-mediated action potential (McHale & Allen, 1983). In lymphatic vessels found in the mesentery of the guinea-pig, the pacemaker mechanism underlying the generation of action potential has been proposed to be due to the summation of spontaneous electrical events termed, spontaneous transient depolarizations (STDs, Van Helden, 1993; Van Helden et al., 1996).
Lymphatic vessels adapt their pumping activity to changes in fluid load. This function is particularly important during inflammatory reactions where the increase in vascular permeability and resultant elevation in interstitial fluid and protein concentration give rise to oedema that must be offset by an appropriate rise in lymph flow. Numerous mediators, released during inflammation have been involved in the increase in vascular fluid leakage and resultant oedema. Some of them exhibit vasoactive properties and have been shown to also affect lymphatic vessel contractile activity (for review, see von der Weid, 2001). Due to the multi-target action of these mediators, it is difficult to determine whether change in lymphatic contractile activity is driven by the oedema-mediated filling of the vessel and subsequent increase in constrictions or by the inflammatory mediator(s) stimulating the vessel directly.
Histamine is a well-known inflammatory mediator present in mast cell that can upon release cause vasodilatation, increased vascular permeability, gastric secretion and contraction of bronchiolar and gastrointestinal smooth muscle (see Babe & Serafin, 1996). Histamine has also been suggested as early as thirty years ago to play a role in the transport of lymph (Haddy et al., 1972). More recently, histamine has been shown to modulate lymphatic smooth muscle contractility in several isolated preparation of lymphatic vessels (Ohhashi et al., 1978; Ferguson et al., 1988; Watanabe et al., 1988; Dobbins et al., 1990; Takahashi et al., 1990; Hashimoto et al., 1994; Reeder et al., 1996). These studies consistently reported that histamine increase lymphatic smooth muscle contractile activity through activation of H1 receptors. Histamine was also shown to induce a decrease in lymphatic vessel contractility. However, mechanisms proposed to be involved in this action may vary according to the preparation. In porcine tracheobronchial lymph vessels, Reeder et al. (1996) reported that, in addition to being located on the smooth muscle, H1 receptors were also present on the endothelium, where their stimulation caused the production of nitric oxide. Endothelial production of nitric oxide was suggested by the observation that histamine induced an increase in vessel tone either in the absence of endothelium or in the presence of a nitric oxide synthase inhibitor (Reeder et al., 1996). In isolated bovine mesenteric lymphatics, low concentrations of histamine (0.01–1 μM) caused a dose-dependent decrease in the rhythm of constrictions that was shown to be mediated by H2 receptor activation (Watanabe et al., 1988).
Despite the importance of membrane potential changes in the modulation of lymphatic smooth muscle contractility, investigations on the effect of histamine on the lymphatic smooth muscle membrane potential have not been assessed. Thus, the purpose of the present study was 2 fold. (1) To examine the effects of the inflammatory mediator, histamine, on the contractile activity of perfused lymphatic vessels from the guinea-pig mesentery and on the lymphatic smooth muscle membrane potential and (2) To investigate the role of STD, the proposed electrical pacemaker for lymphatic rhythmical activity, in the responses to histamine.
Methods
Tissue preparation
Guinea-pigs (4–15 days of age) of either sex were killed by decapitation during deep anaesthesia consequent to inhalation of halothane (5–10%). This procedure has been approved by the University of Calgary Animal Care and Ethics Committee and is conform to the guidelines established by the Canadian Council on Animal Care. The small intestine and attached mesentery were rapidly removed and placed in a physiological saline solution (PSS), of the following composition (mM): CaCl2, 2.5; KCL, 5; MgCl2, 2; NaCl, 120; NaHCO3, 25; NaH2PO4, 1; glucose, 11. The pH was maintained at 7.4 by constant bubbling with 95% O2/5% CO2.
Measurement of vessel contractile activity
Small collecting lymphatic vessels (diameter <230 μm) supplying the jejunum and ileum were dissected together with their associated artery and vein and left intact within the surrounding mesentery. The mesentery was used to pin out the tissues on the Sylgard-coated base of a 2 ml organ bath, mounted on the stage of an inverted microscope (Olympus, CK40) and continuously superfused at a flow rate of 3 ml min−1 with PSS heated to 36°C. The lumen of the vessel was perfused to induce a consistent rate of vessel constrictions, as constriction frequency has been found to be enhanced by vessel filling (Florey, 1927; Smith, 1949). Perfusion was performed by inserting a fine glass micropipette cannula into the lumen, after the vessel has been cut. The cannula was connected to an infusion pump via a Teflon tubing, allowing the vessel lumen to be perfused in the direction of the valves at a flow rate of 2.5 μl min−1. This flow rate was selected from preliminary experiments as the most reliable in inducing a regular rhythmical contractile activity in lymphatic vessel in the range of diameter used and for the duration of the experiment (typically 3–4 h). As normal Ca2+–PSS tended to block the cannula, a low-calcium solution, where 0.3 mM CaCl2 was substituted for 2.5 mM, was used. Perfusion with this solution did not affect vessel contractile activity and did not affect endothelial responsiveness (see von der Weid et al., 1996). The lymphatic vessels were observed using a video camera attached to the microscope and connected to a TV monitor. Changes in vessel diameter and constriction frequency of the lymphatic chambers were continuously measured in real time (or off-line from images recorded on videotape) using a video-dimension analyser (Model V94, Living Systems Instrumentation, Burlington, VT, U.S.A.). This device, designed to sense the optically denser wall of the vessel, at a chosen scan line seen on the monitor, followed any change in vessel diameter with a rapid (<20 ms) time resolution. Data were then recorded on a computer via an analogue-to-digital converter (MacLab/8s, ADInstruments, Mountain View, CA, U.S.A.).
Preparations were allowed a 30 min equilibration period prior to the first agonist application. Concentration–response relationships to histamine were obtained by applying increasing concentrations of histamine, with a washout period of at least 30 min between successive applications. In experiments where the effects of inhibitors were investigated, successive concentrations of histamine were applied to the superfusion solution after the inhibitor was present for at least 15 min. Histamine (or other agonists) was applied for 4 min into the superfusion solution and effects on vessel constriction frequency and amplitude were assessed during the 4 min of maximum effect. Data were averaged and expressed in constriction min−1 (±s.e.m.) and μm (±s.e.m.), respectively. When expressed as percentage of control rate or amplitude, values were compared to the mean of the constriction min−1 or amplitude values obtained during a 5 min period immediately preceding the application of histamine. The pEC50 values were determined from individual concentration–response relationships by manual graph interpolation.
Electrophysiology
Lymphatic vessels and attached mesentery were pinned into a small organ bath (volume 100 μl), mounted on the stage of an inverted microscope (TMS, Nikon) and superfused (flow rate of 3 ml min−1) with PSS heated to 36°C. Resting membrane potential was measured using conventional glass intracellular microelectrodes with resistances of 150–250 MΩ when filled with 0.5 M KCl. Electrodes were connected to an amplifier (Intra 767, World Precision Instruments, Sarasota, FL, U.S.A.) through an Ag-AgCl half-cell. Resting membrane potential was monitored on a digital oscilloscope (VC6525, Hitachi) and simultaneously recorded on a computer via a MacLab/8s. Impalements of smooth muscle cells were obtained from the adventitial side of lymphatic vessels, cut into short segments (length 125–350 μm) with fine dissecting scissors. Short segments were used in order to ensure simplified electrical properties of the smooth muscle such that electrical activity, even if generated at localized foci within the smooth muscle, produced similar potential changes in all the smooth muscle cells of the segment (Van Helden, 1993).
Lymphatic smooth muscle impalements were characterized by a sharp drop in potential that settled after 10–15 s to a value typically more negative than −45 mV. Impalements were maintained for more than 5 min in >90% of the cases and up to 3 h in the best of them. In experiments where the effect of histamine was studied in the presence of inhibitors, histamine was applied first as a control and then, at least 20 min later in the presence of the inhibitor that had been superfused for at least 10 min. This protocol was usually performed during the same impalement. However in some instances, successive impalements were obtained from neighbouring cells in the same segment. No significant difference in the response induced by histamine applied at the same concentration 20 min apart in the absence of an inhibitor was observed.
STD activity was assessed by measuring the frequency and amplitude of events greater than 1 mV. STD frequency and amplitude occurring during an interval of 15–60 s (depending on the stability of the recording, but typically 30 s) before the application of the histamine were compared with that occurring during a period of the same duration while the maximum response to histamine was observed.
Lysis of the endothelium
The lymphatic endothelium was damaged in vitro by repeatedly passing brief streams of air through the lumen of the vessel (5–6 times for 5–10 s) at a rate of about 3 μl min−1. The success of the endothelial destruction was confirmed by applying acetylcholine (ACh, 10 μM) followed by sodium nitroprusside (SNP, 100 μM) in the superfusion solution, while the vessel lumen was perfused. A negative response to ACh and a positive response to SNP were used as confirmation of the success of the procedure. Endothelial destruction based on this testing procedure proved successful in about 50% of treated vessels. The use of SNP was necessary, as it has been shown that 40% of guinea-pig mesenteric lymphatic vessels that had an intact endothelium did not respond in any way to either ACh or SNP. The main reason for that was shown to be due to a high basal production of nitric oxide (von der Weid et al., 1996).
Chemicals and drugs
Histamine and dimaprit were obtained from ICN, cimetidine, acetylcholine (ACh), sodium nitroprusside (SNP), R-α-methylhistamine, NG-nitro L-arginine (L-NOARG) and pyrilamine were purchased from Sigma/Aldrich. All drugs were dissolved in distilled water (with the exception of L-NOARG that was dissolved in 0.1 M HCl) to give 10 mM stock solutions.
Statistical analysis
Experimental data have been expressed as the mean±one standard error of the mean (s.e.m.). Statistical significance was assessed using paired or unpaired Student's t-test (as specified in the text) with P<0.05 being considered significant.
Results
Studies were performed on lymphatic vessels that were intraluminally perfused. Under control conditions, lymphatic vessel chambers displayed a regular contractile activity ranging between 2 and 17 constrictions min−1 (mean rate 8.6±0.5 constrictions min−1, n=51). Constrictions were typically ample, leading, in 1.1 to 1.5 s, to nearly closure of the chamber lumen. The chambers then regained their resting diameter, with the whole constriction–relaxation process lasting for 3–6 s. Constrictions were sometimes preceded by a small dilation consequent to constriction of the upstream chamber.
Effect of histamine on the constriction rate of perfused lymphatic vessels
Histamine increased the constriction rate of lymphatic vessels in a concentration-dependent fashion (Figures 1 & 2). A maximum rate of 26.5±2.4 constrictions min−1 was achieved at a histamine concentration of 5 μM (n=5). At 1 μM histamine, a concentration close to the pEC50 (6.1±0.1, n=7), a rate of 18.3±1.8 constrictions min−1 was reached (n=7). Concentrations higher than 5 μM caused a prolonged constriction, or ‘fibrillation' of the vessel, characterized by a sustained constriction with small amplitude oscillations.
Figure 1.
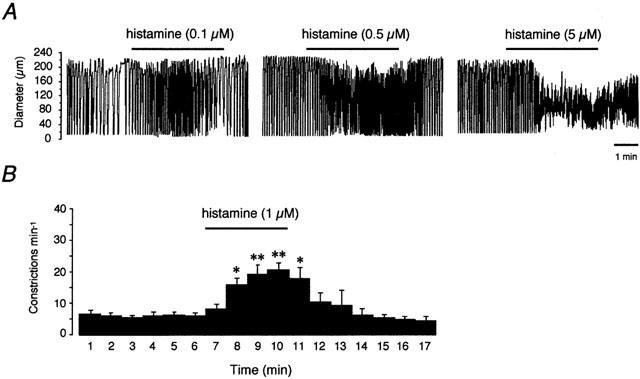
Effect of histamine on the contractile activity of lymphatic vessel of the guinea-pig mesentery. (A) Original traces of vessel diameter changes in an actively constricting lymphatic chamber where downward deflections represent constrictions. Histamine increased the frequency and decreased the amplitude of the constrictions in a concentration-dependent manner. (B) Time-course histogram showing the mean response (±s.e.m.) of seven vessels to 1 μM histamine applied for 4 min. *P<0.05; **P<0.01 vs mean of 5 min of control (paired Student's t-test).
Figure 2.
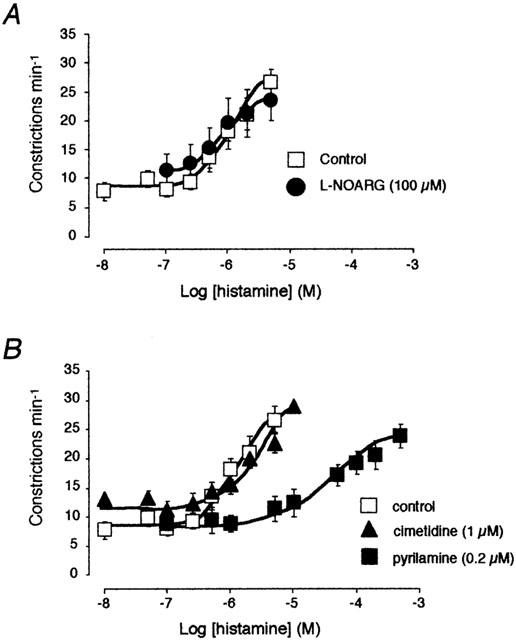
Effect of the NO-synthase inhibitor L-NOARG (100 μM, (A)) and the histamine receptor antagonists pyrilamine (0.2 μM) and cimetidine (1 μM, (B)) on the concentration–response curve to histamine. The same concentration–response curve to histamine (control) was used in both graphs.
Effect of histamine on the amplitude of constrictions
In the presence of histamine, lymphatic vessel constrictions were decreased in a concentration-dependent manner (Figure 1A). At the concentration of 5 μM, the mean constriction amplitude was reduced to 39±14% of control. Approximately half of that effect, 68±15%, was reached at a concentration of 0.75 μM (n=7).
Role of the endothelium and the endothelial release of nitric oxide in the response to histamine
Histamine has been suggested to induce a decrease in lymphatic vessel tone in porcine tracheobronchial lymph vessels through the endothelial release of nitric oxide (Reeder et al., 1996). To test this possibility, histamine was applied in the presence of L-NOARG. In six lymphatic vessel preparations, treatment with 100 μM L-NOARG caused an increase in basal constriction rate from 5.8±0.9 to 9.6±1.1 constrictions min−1 (174±22% of control). This effect might be due to the inhibition of a constitutive production of NO and/or flow-induced production of NO consequent to the intraluminal perfusion (see von der Weid et al., 1996). As illustrated in Figure 2A, the histamine-induced increase in constriction frequency was not significantly affected in the presence of L-NOARG.
To examine the role of the endothelium further, the contractile activity of lymphatic vessels was recorded after the endothelium had been lysed. The lysis procedure did not affect either the basal constriction rate (P>0.1), nor the amplitude of constrictions (218±29 μm before lysis vs 199±32 μm after lysis, P>0.1). The response to histamine (1 and 5 μM) was compared with that obtained in the same preparations while the endothelium was still intact. No significant difference was observed, with 1 μM histamine causing the constriction rate to increase from 9.3±1.0 to 15.6±3.8 constrictions min−1 before lysis and from 7.3±1.1 to 11.2±1.7 constriction min−1 after lysis (increase from control rate: 163±27% vs 155±10%, respectively, n=4). Similarly, 5 μM histamine increased the constriction frequency from 9.3±1.6 to 20.6±4.7 constriction min−1 before lysis and from 6.7±1.4 to 18.6±2.7 constrictions min−1 after lysis (increase from control rate: 227±32% vs 365±144%, respectively, n=4).
Effect of pyrilamine on the histamine-induced increase in constriction rate
Pyrilamine did not alter the basal rate of lymphatic pumping, but strongly inhibited the contractile response to histamine. This inhibitory effect is illustrated in Figure 2B, which shows that pyrilamine (0.2 μM) totally abolished the increase in constriction rate induced by histamine up to a concentration of 1 μM, leading to a rightward shift of the concentration–response curve (pEC50=4.7±0.2). Calculation of the pKB gave a value of 8.1.
Role of H2 receptors on the histamine-induced change in lymphatic vessels contractile activity
The presence of H2 receptors has been demonstrated in lymphatic vessels of the bovine mesentery (Watanabe et al., 1988). In the present study, involvement of H2 receptors in the response to histamine was tested first using the antagonist cimetidine. As illustrated in Figure 2B, the concentration–response curve of histamine in the presence of 1 μM cimetidine was not significantly different from the control one, with a similar pEC50 value (6.2±0.1, n=12). Similarly, cimetidine did not affect the concentration–response curve to histamine in the presence of pyrilamine (not shown, n=4). The possibility that H2 receptors might be involved in the response to histamine was suggested by the observation that the H2-receptor agonist, dimaprit reduced or arrested spontaneous constriction in some vessels, however, it had little or no effect in others (Figure 3A). In 11 preparations, dimaprit (10 μM) caused a decrease in constrictions from 6.5±1.4 constrictions min−1 in control to 3.0±1.0 constrictions min−1 in the presence of dimaprit. In 11 other vessels, dimaprit up to a concentration of 50 μM did not significantly affect the constriction rate (11.7±1.0 constrictions min−1 in control and 12.2±1.3 constrictions min−1 in the presence of dimaprit). According to their response to dimaprit, the effect of histamine was re-evaluated in those vessels where both histamine and dimaprit had been tested. Vessels that responded to dimaprit had a basal constriction rate lower than that of vessels that did not respond to dimaprit. Both populations reaching the same maximal effect at concentrations of 1–5 μM (n=5; Figure 3B).
Figure 3.
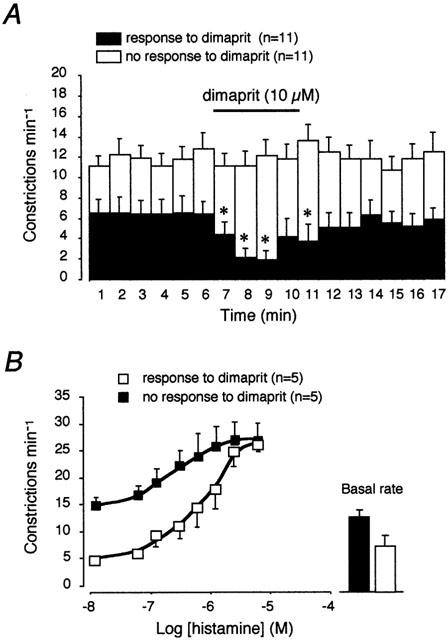
Effect of H2 receptor activation in lymphatic vessels. (A) Time course histogram of the effect of dimaprit (10 μM) on the lymphatic vessel contractile activity. Half of the vessel tested responded to dimaprit with a decrease in constriction rate (closed columns), whereas the other half did not respond at all (open columns). *P<0.05 vs mean of 5 min of control (paired Student's t-test). (B) Concentration–response curves to histamine in vessels that responded to dimaprit and in vessels that did not respond to dimaprit. These latter showed a higher basal rate (closed column in right inset panel) and a flatter slope to reach a maximal response similar to that of the vessels responding to dimaprit.
Effects of R-α-methylhistamine
To test a possible involvement of H3 receptors in the response to histamine, R-α-methylhistamine was used. At concentration as high as 50 μM, R-α-methylhistamine did not cause a change in the amplitude or the rate of constrictions in the mesenteric lymphatic vessels (not shown, n=5).
Effects of histamine on the lymphatic smooth muscle membrane potential and STD activity
Microelectrode recordings of smooth muscle membrane potential were obtained from short lymphatic vessel segments (length 125–350 μm) with the endothelium left intact. The mean resting membrane potential obtained from 26 recordings was −50.0±1.1 mV. Spontaneous transient depolarizations (STDs) occurring in these smooth muscles (Van Helden, 1993) were recorded in >90% of the segments.
Superfusion of vessel segments with histamine caused changes in the smooth muscle membrane potential characterized by a depolarization and an increase in STD activity (Figure 4). These effects often led to action potential firing, subsequent constriction and most of the time loss of the impalement (see example in Figure 4Ab). To minimize vessel contractility in response to histamine and to allow a better observation of STDs underlying action potentials, experiments were often performed in the presence of nifedipine (2–10 μM). None of the parameters of interest (membrane potential, STD frequency and amplitude changes) being significantly affected by this procedure (P>0.1, unpaired Student's t-tests), the two sets of data have been pooled and the mean values are presented below. Histamine (0.1 and 0.5 μM) induced a depolarization that culminate (time to peak 44±3 s) at 4.4±1.0 mV (n=8) and 10.0±2.0 mV, respectively (n=12, see Figure 4a,b). The membrane potential then returned to its control value. The histamine-induced depolarization was associated with an increase in STD activity. As illustrated in Figure 4C, STD frequency and amplitude were significantly enhanced during application of 0.1 and 0.5 μM histamine. The establishment of a complete concentration–response curve was precluded by the difficulty to maintain recording during application of high concentrations of histamine. However, in four segments, where impalements could be maintained, depolarizations as large as 20 mV were observed in response to histamine concentrations of 1–5 μM.
Figure 4.
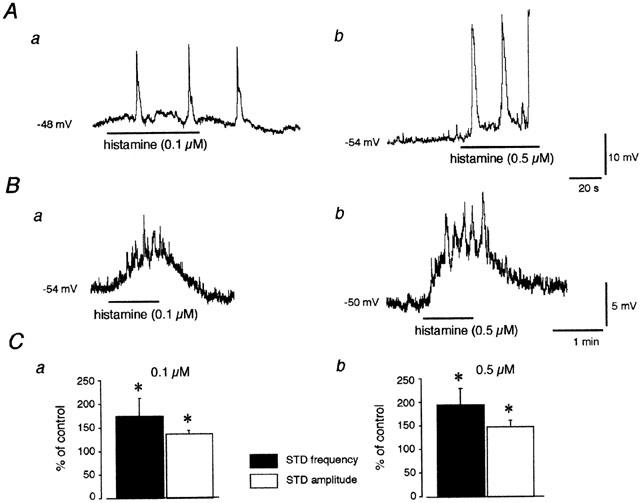
Effects of histamine on membrane potential and action potential and STD activity in guinea-pig mesenteric lymphatic smooth muscle. (A) Intracellular microelectrode recordings from two different preparations in response to 0.1 μM (a) and 0.5 μM histamine (b) applied for 1 min (horizontal bars). Histamine caused a depolarization and an increase in the occurrence of action potentials. These events were observed to be coupled to vessel constrictions that led in one case (b) in the lost of the impalement. (B) In the presence of nifedipine to block action potentials, application of the same concentrations of histamine to two other vessels caused a depolarization and an increase in the frequency and amplitude of STDs (upward deflections). (C) STD frequency and amplitude measured during the maximum response to histamine (0.1 μM, a) and (0.5 μM, b) expressed as a percentage of the values obtained during the same impalement, before histamine application. Column values are means±s.e.m. of eight (a) and 13 experiments (b), some of which were carried out in the presence of nifedipine. Resting membrane potential values in this and subsequent figures are indicated on the left side of the traces.
The effects of histamine on the membrane potential were inhibited in the presence of 0.2 μM pyrilamine. Lymphatic smooth muscle that was depolarized from −55.2±1.6 to −38.0±4.7 mV by histamine (0.5–5 μM, n=5) in control conditions, was not affected when histamine was applied in the presence of pyrilamine, and stayed at −54.5±1.7 mV. The increase in STD activity was also abolished (Figure 5).
Figure 5.
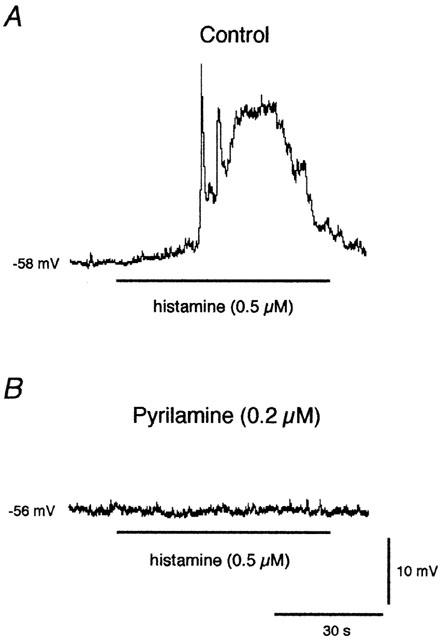
Effect of pyrilamine on the membrane potential response to histamine. The marked depolarization caused by a 1-min application of 0.5 μM histamine (horizontal bar, (A)) was completely abolished while the preparation was superfused with 0.2 μM pyrilamine. (B) Scale bars apply to both recordings, obtained from the same impalement. Nifedipine (2 μM) was present throughout the experiment to avoid histamine-induced action potential-associated constrictions.
Effect of dimaprit on the lymphatic smooth muscle membrane potential and STD activity
Dimaprit (10 μM) induced a change in the smooth muscle membrane potential in four out of the nine preparations investigated. In these vessels, dimaprit caused a hyperpolarization of 5.3±0.7 mV, which was associated with a small, but significant decrease in STD frequency and amplitude (P=0.01 and 0.02, respectively; Figure 6B). In the five other preparations, no change in membrane potential or STD activity was observed in response to dimaprit up to a concentration of 50 μM.
Figure 6.
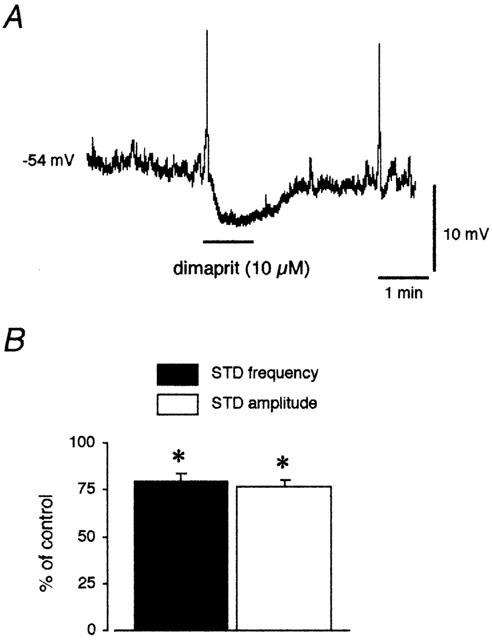
Effect of dimaprit on the membrane potential of lymphatic smooth muscle. (A) Dimaprit (10 μM) applied for 1 min (horizontal bar) induced a hyperpolarization and a decrease in STD activity in about 50% of the vessel tested. (B) Histograms of STD frequency and amplitude measured during the maximum response to dimaprit in the responding preparations, expressed as a percentage of the values obtained during the same impalement, before dimaprit application. Column values are means±s.e.mean of four experiments. *P<0.02 vs control (paired Student's t-test).
Discussion
In lymphatic vessels of the guinea-pig mesentery that rhythmically constricted in response to intraluminal perfusion, the predominant effect of histamine was to increase the frequency and decrease the amplitude of constrictions. In non-perfused lymphatic vessel segments that are mechanically quiescent, intracellular microelectrode recordings from smooth muscle showed that histamine induced a depolarization of the membrane potential and the occurrence of action potentials leading to constrictions of the vessel segments. Importantly, in the presence of histamine, the smooth muscle membrane potential also exhibited an enhanced STD activity, further validating the proposal that STDs play an important role in the pacemaker mechanism generating action potentials and lymphatic constrictions (see Van Helden, 1993; Van Helden et al., 1996).
Our observations demonstrate that histamine directly stimulates lymphatic vessel pumping in this isolated preparation and suggest the involvement of the smooth muscle membrane potential via depolarization and increase in STD occurrence in this process. Perfusion or filling of lymphatic vessels has been shown to be a key modulator of lymphatic pumping activity (Florey, 1927a,b; Smith, 1949; Misslin, 1961; McHale & Roddie, 1976; Van Helden et al., 1995). In the present study, lymphatic vessels were not subjected to perfusion during electrophysiological measurements, as microelectrode measurements are impaired due to perfusion-induced constrictions. Thus, the causal relationship between electrical and mechanical events cannot be unequivocally established under the present experimental conditions.
The effects of histamine on blood vessels as well as on other smooth muscle preparations has been shown to involve H1 and H3 histamine receptors. Activation of H2 receptors usually leads to relaxations (Leurs et al., 1995). The present observation that histamine-induced increase in contractile and electical activities in lymphatic vessels were inhibited by pyrilamine suggests the involvement of H1 receptors. This observation is further supported by the calculated pKB value for pyrilamine (8.1), which is similar to the pKB value of 8.4 reported for the same antagonist in human bronchial arteries (Liu et al., 1990). Persistence of the histamine effects after lysis of the endothelium indicates a localization of the receptors on the smooth muscle. In addition, the absence of effect of R-α-methylhistamine seems to rule out activation of H3 receptors. Our results are in agreement with those obtained in other lymphatic vessel preparations. Histamine has been observed to produce a positive chronotropic effect on spontaneously contracting bovine mesenteric lymphatic vessels, through stimulation of smooth muscle H1 receptors (Watanabe et al., 1988). In porcine tracheobronchial lymph vessels that are characterized by a relative absence of spontaneous constrictions, histamine exerts a powerful contractile effect (Ferguson et al., 1993) via stimulation of H1 receptors located on the smooth muscle (Reeder et al., 1996). In this latter study, histamine was shown also to act on endothelial H1 receptors, causing NO-dependent relaxation (Reeder et al., 1996). This finding contrasts with the present results where no endothelium-dependent or NO-mediated action was detected in response to histamine. This discrepancy with the previous observations may be explained by the fact that administration of histamine to the vessel only via the superfusion solution may account for limited access of the lumen and thus the endothelium. However previous results showing that acetylcholine (von der Weid et al., 1996; 2001b) and 5-HT (von der Weid et al., 2001a), administrated to the preparation in the same way caused an endothelium-dependent release of NO, seems to rule out this possibility.
About 50% of the guinea-pig mesenteric lymphatic vessels were observed to respond by a decrease in the perfusion-induced constriction rate when exposed to the H2 receptor agonist, dimaprit. This observation suggests the presence of H2 receptors in some vessels. H2 receptor stimulation by dimaprit was observed in the same proportion of vessels to cause a hyperpolarization and a decrease in STD frequency and amplitude. These observations further demonstrate the good correlation that exists between frequency of constrictions and activity of STDs. Watanabe et al. (1988) also reported slowing of the contractile activity in bovine mesenteric lymphatic vessels in response to H2 receptor stimulation by dimaprit. They showed that histamine, in addition to the H1-mediated positive chronotropic effect observed in all vessels, produced in about two-thirds of them a negative chronotropic effects through stimulation of smooth muscle H2 receptors. The authors attributed the variation in the histamine response to regional difference in the distribution of H2 receptors. Although, this explanation is plausible in their preparation, it would not appear to apply to the present investigation, as all the vessels have been isolated from the same mesenteric location. Importantly in the present study, vessels that responded to dimaprit were observed to have a significantly lower basal constriction rate. This intriguing observation was not investigated further, but could account for H2 receptors being spontaneously stimulated, independently of the presence of histamine. This phenomenon has been recently demonstrated in transfected CHO cells (Smit et al., 1996).
Both histamine H1 and H2 receptors belong to the large family of heptahelical G-protein-coupled receptors. H1 receptors have been described to be coupled to Gq/11 proteins and the phospholipase C-catalysed formation of InsP3 and 1,2-diacylglycerol. Histamine induces the production of InsP3 in a variety of tissues, including airway, intestinal and vascular smooth muscle (see review by Leurs et al., 1995). This InsP3 production leads to the subsequent release of Ca2+ from intracellular Ca2+ stores and an increase in cytosolic Ca2+. In lymphatic vessels of the guinea-pig mesentery, an increase in InsP3 production has been proposed to mediate the generation of STD (Van Helden, 1993; Van Helden et al., 1996), as illustrated by the increase in STD activity and vessel constriction rate observed in the presence of agonists known to stimulate InsP3 synthesis (Van Helden, 1993; Van Helden & Bull, 1995; von der Weid et al., 2001b). Although the intracellular pathway(s) activated by histamine in lymphatic smooth muscle was not addressed specifically in the present study, the demonstration that histamine increased both STD activity and vessel constriction rate via activation of H1 receptors, supports the concept that Ca2+ released from InsP3-sensitive Ca2+ stores plays a pivotal role in the generation of STDs and increase in contractile activity (Van Helden et al., 1996).
Coupling of the H2 receptor to adenylate cyclase and the production of cAMP is well accepted and has been demonstrated in several smooth muscle preparations (Leurs et al., 1995). In the population of lymphatic vessels that appeared to express H2 receptors, dimaprit depressed constriction rate, decreased STD frequency and amplitude and hyperpolarized the smooth muscle. These effects are similar to the responses observed in guinea-pig mesenteric lymphatic vessels exposed to isoproterenol, forskolin, IBMX or membrane permeant analogues of cAMP (von der Weid & Van Helden, 1996). The data therefore are consistent with H2 activation inducing an increase in the concentration of cAMP, thereby decreasing STD and constriction frequencies.
Histamine is an important mediator in inflammation. It is known to be present in the lymphatic vessel environment during inflammatory challenges and has been detected in lymph draining injured tissues (Edery & Lewis, 1963) and in cat mesenteric lymph after brief ischemia-reperfusion (Fu et al., 1997). Systemic administration of histamine has been observed to produce significant increases in lymph flow (Lewis & Winsey, 1970; Haddy et al., 1972; Amelang et al., 1981; Svensjo et al., 1982; McNamee, 1983). These effects have been thought to be due to the histamine-induced increase in the transvascular efflux of fluid and protein at the level of the transcapillary venules (Haddy et al., 1972; Svensjo et al., 1982). The present finding that histamine directly stimulates lymphatic vessel contractile activity might be of considerable importance, as it supports the idea that the augmented rate of lymph flow during inflammation may be due not only to the histamine-induced increase in lymph formation, but also to a direct action of histamine on the lymphatic vessel that enhanced the active lymph transport mechanism (see Johnston, 1985) and help resolving the inflammation-associated edema. This beneficial effect might be altered at high histamine concentrations, as the induced vessel ‘fibrillation' would lead to a decreased lymph flow. Other mediators released during inflammation have been shown to decrease or increase lymphatic contractile activity (see review by von der Weid, 2001). They could amplify and/or counteract the effect of histamine. Whether the direct action of histamine and/or other inflammatory mediators on lymphatic pumping plays an important role in the resolution of edema during inflammation is difficult to assess, but this aspect certainly needs to be taken into account.
Acknowledgments
This study was supported by grants from the Alberta Heritage Foundation for Medical Research (AHFMR) and the Heart and Stroke Foundation of Canada. J.L.R. Fox was the recipient of an AHFMR Summer Studentship and P.-Y. von der Weid is an AHFMR Scholar. The authors wish to thank S. Roizes for excellent technical assistance and Dr M.D. Hollenberg for his valuable comments on the manuscript.
Abbreviations
- ACh
acetylcholine
- L-NOARG
NG-nitro L-arginine
- SNP
sodium nitroprusside
- STD
spontaneous transient depolarization
References
- AMELANG E., PRASAD C.M., RAYMOND R.M., GREGA G.J. Interactions among inflammatory mediators on edema formation in the canine forelimb. Circ. Res. 1981;49:298–306. doi: 10.1161/01.res.49.2.298. [DOI] [PubMed] [Google Scholar]
- BABE K.S., SERAFIN W.E.Histamine, bradykinin and their antagonists Goodman and Gilman's The Pharmacological Basis of Therapeutics 1996New York: McGraw-Hill; 581–600.ed. Hardman, J., Limbird, L. pp [Google Scholar]
- DOBBINS D.E., BUEHN M.J., DABNEY J.M. Constriction of perfused lymphatics by acetylcholine, bradykinin and histamine. Microcirc. Endothelium Lymphatics. 1990;6:409–425. [PubMed] [Google Scholar]
- EDERY H., LEWIS G.P. Kinin-forming activity and histamine in lymph after tissue injury. J. Physiol. 1963;169:568–583. doi: 10.1113/jphysiol.1963.sp007280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON M.K., SHAHINIAN H.K., MICHELASSI F. Lymphatic smooth muscle responses to leukotrienes, histamine and platelet activating factor. J. Surg. Res. 1988;44:172–177. doi: 10.1016/0022-4804(88)90046-7. [DOI] [PubMed] [Google Scholar]
- FERGUSON M.K., WILLIAMS U.E., LEFF A.R., MITCHELL R.W. Heterogeneity of tracheobronchial lymphatic smooth muscle responses to histamine and 5-hydroxytryptamine. Lymphology. 1993;26:113–119. [PubMed] [Google Scholar]
- FLOREY H.W. Observations on the contractility of lacteals. Part I. J. Physiol. 1927a;62:267–272. doi: 10.1113/jphysiol.1927.sp002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOREY H.W. Observations on the contractility of lacteals. Part II. J. Physiol. 1927b;63:1–18. doi: 10.1113/jphysiol.1927.sp002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FU L.W., O'NEILL C.A., LONGHURST J.C. Increased histamine and 5-HT in portal vein plasma and mesenteric lymph during brief ischemia and reperfusion. Am. J. Physiol. 1997;273:H1135–H1141. doi: 10.1152/ajpheart.1997.273.3.H1135. [DOI] [PubMed] [Google Scholar]
- HADDY F.J., SCOTT J.B., GREGA G.J. Effects of histamine on lymph protein concentration and flow in the dog forelimb. Am. J. Physiol. 1972;223:1172–1177. doi: 10.1152/ajplegacy.1972.223.5.1172. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO S., KAWAI Y., OHHASHI T. Effects of vasoactive substances on the pig isolated hepatic lymph vessels. J. Pharmacol. Exp. Ther. 1994;269:482–488. [PubMed] [Google Scholar]
- JOHNSTON M.G.Involvement of lymphatic collecting ducts in the physiology and pathophysiology of lymph flow Experimental Biology of the Lymphatic Circulation 1985Amsterdam: Elsevier; 81–120.Ed. Johnston M.G. pp [Google Scholar]
- LEURS R., SMIT M.J., TIMMERMAN H. Molecular pharmacological aspects of histamine receptors. Pharmacol. Ther. 1995;66:413–463. doi: 10.1016/0163-7258(95)00006-3. [DOI] [PubMed] [Google Scholar]
- LEWIS G.P., WINSEY N.J. The action of pharmacologically active substances on the flow and composition of cat hind limb lymph. Br. J. Pharmacol. 1970;40:446–460. [PMC free article] [PubMed] [Google Scholar]
- LIU S.F., YACOUB M., BARNES P.J. Effect of histamine on human bronchial arteries in vitro. Naunyn Schmied. Arch. Pharmacol. 1990;342:90–93. doi: 10.1007/BF00178978. [DOI] [PubMed] [Google Scholar]
- MCHALE N.G., ALLEN J.M. The effect of external Ca2+ concentration on the contractility of bovine mesenteric lymphatics. Microvasc. Res. 1983;26:182–192. doi: 10.1016/0026-2862(83)90069-9. [DOI] [PubMed] [Google Scholar]
- MCHALE N.G., RODDIE I.C. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J. Physiol. 1976;261:255–269. doi: 10.1113/jphysiol.1976.sp011557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNAMEE J.E. Histamine decreases selectivity of sheep lung blood-lymph barrier. J. Appl. Physiol. 1983;54:914–918. doi: 10.1152/jappl.1983.54.4.914. [DOI] [PubMed] [Google Scholar]
- MISSLIN H. Experimenteller Nachweis der autochtonen Automatie der Lymphgefäße. Experientia. 1961;17:29–30. [Google Scholar]
- OHHASHI T., KAWAI Y., AZUMA T. The response of lymphatic smooth muscles to vasoactive substances. Pflugers Arch. 1978;375:183–188. doi: 10.1007/BF00584242. [DOI] [PubMed] [Google Scholar]
- REEDER L.B., DEFILIPPI V.J., FERGUSON M.K. Characterization of the effects of histamine in porcine tracheobronchial lymph vessels. Am. J. Physiol. 1996;271:H2501–H2507. doi: 10.1152/ajpheart.1996.271.6.H2501. [DOI] [PubMed] [Google Scholar]
- SMITH R.O. Lymphatic contractility: A possible mechanism of lymphatic vessels for the transport of lymph. J. Exp. Med. 1949;90:497–509. doi: 10.1084/jem.90.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMIT M.J., LEURS R., ALEWIJNSE A.E., BLAUW J., VAN NIEUW AMERONGEN G., VAN DE VREDE Y., ROOVERS E., TIMMERMAN H. Inverse agonism of histamine H2 antagonist accounts for upregulation of spontaneously active histamine H2 receptors. Proc. Natl. Acad. Sci. U.S.A. 1996;93:6802–6807. doi: 10.1073/pnas.93.13.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENSJO E., ADAMSKI S.W., SU K., GREGA G.J. Quantitative physiological and morphological aspects of microvascular permeability changes induced by histamine and inhibited by terbutaline. Acta Physiol. Scand. 1982;116:265–273. doi: 10.1111/j.1748-1716.1982.tb07140.x. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI N., KAWAI Y., OHHASHI T. Effects of vasoconstrictive and vasodilative agents on lymphatic smooth muscles in isolated canine thoracic ducts. J. Pharmacol. Exp. Ther. 1990;254:165–170. [PubMed] [Google Scholar]
- VAN HELDEN D.F. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J. Physiol. 1993;471:465–479. doi: 10.1113/jphysiol.1993.sp019910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN HELDEN D.F., BULL N. Neuropeptide Y enhances pacemaking in smooth muscle of guinea-pig mesenteric lymphatics. Proc. Austral. Physiol. Pharmacol. Soc. 1995;26:130P. [Google Scholar]
- VAN HELDEN D.F., VON DER WEID P.-Y., CROWE M.J.Electrophysiology of lymphatic smooth muscle Interstitium, Connective Tissue, and Lymphatics 1995London: Portland Press; 221–236.Eds. Bert, J., Laine, G.A., McHale, N.G., Reed, R., Winlove, P. pp [Google Scholar]
- VAN HELDEN D.F., VON DER WEID P.-Y., CROWE M.J.Intracellular Ca2+ release: a basis for electrical pacemaking in lymphatic smooth muscle Smooth Muscle Excitation 1996London: Academic Press; 355–373.Ed. Bolton, T.B., Tomita, T. pp [Google Scholar]
- VON DER WEID P.-Y. Lymphatic vessel pumping and inflammation-the role of spontaneous constrictions and underlying electrical pacemaker potentials. Aliment. Pharmacol. Ther. 2001;15:1115–1129. doi: 10.1046/j.1365-2036.2001.01037.x. [DOI] [PubMed] [Google Scholar]
- VON DER WEID P.-Y., CHAN A.K., FOX J.L.R. Effects of 5-HT and histamine on the contractile and electrical activity of lymphatic vessels 2001a591–596.Proceedings of the 7th World Congress for Microcirculation
- VON DER WEID P.-Y., CROWE M.J., VAN HELDEN D.F. Endothelium-dependent modulation of pacemaking in lymphatic vessels of the guinea-pig mesentery. J. Physiol. 1996;493:563–575. doi: 10.1113/jphysiol.1996.sp021404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON DER WEID P.-Y., VAN HELDEN D.F. β-Adrenoceptor-mediated hyperpolarization in lymphatic smooth muscle of guinea pig mesentery. Am. J. Physiol. 1996;270:H1687–H1695. doi: 10.1152/ajpheart.1996.270.5.H1687. [DOI] [PubMed] [Google Scholar]
- VON DER WEID P.-Y., ZHAO J., VAN HELDEN D.F. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am. J. Physiol. 2001b;280:H2707–H2716. doi: 10.1152/ajpheart.2001.280.6.H2707. [DOI] [PubMed] [Google Scholar]
- WATANABE N., KAWAI Y., OHHASHI T. Dual effects of histamine on spontaneous activity in isolated bovine mesenteric lymphatics. Microvasc. Res. 1988;36:239–249. doi: 10.1016/0026-2862(88)90025-8. [DOI] [PubMed] [Google Scholar]


