Abstract
The consequences of the reduced production of nitric oxide (NO) by cells from regenerated endothelium were investigated by measuring membrane potential of smooth muscle cells (SMCs), isometric tension and cyclic nucleotides content in porcine coronary arteries with intimal thickening, four weeks following angioplasty.
Under basal conditions, SMCs of coronary arteries with regenerated endothelium were depolarized by 10 mV. This depolarization was associated with 82% decreased level of cGMP without alteration in cAMP.
Sodium nitroprusside (SNP, 1 μM) repolarized SMCs of the previously denuded coronary arteries. This repolarization was abolished by 1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, 10 μM) and not suppressed by glibenclamide (10 μM), iberiotoxin (IbTX, 100 nM) and the combination of charybdotoxin (ChTX, 40 nM) plus apamin (100 nM).
Four-aminopyridine (4-AP, 1-5 mM) generated spontaneous rhythmic activities only in coronary arteries with regenerated endothelium which were abolished by SNP. Nevertheless, 4-AP did not suppress the repolarization induced by SNP.
In vascular segments with regenerated endothelium, contracted with prostaglandin F2α (PGF2α), relaxation to bradykinin (BK, 30 nM) was unaltered despite a reduced production of cGMP (−70%). Indomethacin (10 μM) plus Nω-nitro-L-arginine (L-NA, 30 μM) reduced relaxation (−12% and −35% for native and regenerated endothelium, respectively) but did not abolish it.
The hyperpolarizations induced by BK were not altered by the presence of indomethacin and L-NA and were unchanged in segments with regenerated endothelium.
These data are consistent with a contribution of impairment in NO production to the depolarization of SMCs. Nevertheless, EDHF responses to BK are sufficient to maintain a normal relaxation after angioplasty.
Keywords: Regenerated endothelium, endothelium-derived hyperpolarizing factor, nitric oxide, cyclic nucleotides, bradykinin
Introduction
Endothelial dysfunction is an early marker of coronary disease as increased vasoconstrictions and impaired endothelium-dependent relaxations precede the formation of atherosclerotic lesions. A reduced bioavailability of endothelial factors, such as nitric oxide (NO), contributes to these processes (Shimokawa et al., 1987; Yamamoto et al., 1987; Cohen et al., 1988; Lüscher, 2000). Four weeks after angioplasty porcine coronary arteries with regenerated endothelium present selective impairment of endothelium-dependent relaxations similar to those described in injured human coronary arteries (Shimokawa et al., 1987; 1989; Borg-Capra et al., 1997). Cultured cells from regenerated endothelium have a reduced capacity to produce NO under basal conditions or during stimulation with serotonin, bradykinin and calcium ionophore (Fournet-Bourguignon et al., 2000). At rest, smooth muscle cells are depolarized in coronary artery segments covered with regenerated endothelium (Thollon et al., 1999a). The first aim of the present study was to investigate a possible relation between the altered production of NO under resting conditions and the abnormal electrical activity of smooth muscle cells in the previously injured blood vessels. The second aim was to understand why the relaxations to bradykinin were maintained while the production of NO was reduced in cells from regenerated endothelium. In the porcine coronary artery, the kinin causes relaxation not solely by activation of the NO-pathway, suggesting a contribution of endothelium-derived hyperpolarizing factor (EDHF) in the response (Richard et al., 1990; Cowan & Cohen, 1991; Nagao & Vanhoutte, 1992). Previous studies suggested a greater contribution of EDHF, as the hyperpolarizations in response to bradykinin were larger in the most depolarized cells of unstretched previously injured coronary arteries (Thollon et al., 1999a). However, the experimental conditions for evaluation of endothelium-dependent hyperpolarizations in open coronary artery segments, pinned to the bottom of the chamber, are different from those used for organ chambers experiments where coronary artery rings are stretched to their optimal tension, and also markedly different from coronary arteries subjected to a physiological transmural pressure. Thus, the present study evaluated the contribution of prostacyclin, NO and EDHF during the relaxations caused by bradykinin in segments from porcine coronary arteries with regenerated endothelium.
Methods
Endothelial denudation
The experiments were performed in accordance with the guidelines of the French Ministry of Agriculture for the use and care of animals. Large-White pigs, (8 weeks of age; 18–24 kg) were anaesthetized with an intramuscular injection of a mixture composed of Tiletamine plus Zolazepam (20 mg kg−1) and Atropine Sulphate (50 μg kg−1). One coronary artery (right or one branch of left) was denuded along the full length, by inflating a balloon catheter three times (Thollon et al., 1999a).
Membrane potential and isometric tension
Four weeks after denudation, the animals were anaesthetized again with Tiletamine plus Zolazepam. The heart was removed and placed in ice-cold oxygenated modified Krebs solution of the following composition (mM): NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 2.5, NaHCO3 25, EDTA 0.026 and glucose 11. The coronary arteries were dissected free, cleaned of adherent fat and connective tissue and maintained in oxygenated Krebs solution. Each coronary artery with regenerated endothelium was compared with one coronary artery with native endothelium from the same heart. Rings of coronary arteries (4 mm long) were cut open along the longitudinal axis and pinned down to the bottom of an experimental chamber, the endothelial side upward. For simultaneous measurement of membrane potential and tension one end of the segment was pinned down to the experimental chamber and the other was connected to a force transducer using a wire passed through three ties performed with surgical thread (Prolene 8/0 Ethicon). For these experiments, the preparations were progressively stretched to the predetermined optimal tension (45–50 mN) during the equilibration period (30–45 min). All the strips were superfused continuously at 5 ml min−1 with oxygenated, modified Krebs solution. The membrane potential was measured in subintimal vascular smooth muscle cells with glass microelectrodes (30–40 MΩ), filled with 3 M KCl after crossing both the endothelial cells and the internal elastic lamina (Thollon et al., 1999a).
Experimental design
In the first set of experiments, with no applied tension, coronary segments were exposed to a NO donor (sodium nitroprusside, SNP) in order to evaluate the possible link between depolarization of SMC and reduced production of NO by endothelial cells after regeneration of the endothelium. After equilibration in Krebs solution for 30 min, the vascular segments were initially treated for 30 min with ODQ (1 μM) or potassium channels blockers (40 nM ChTx plus 100 nM Apamin; 100 nM IbTX; 10 μM glibenclamide or 1–5 mM 4-AP) and then were exposed to SNP (1 μM, 13 min) in the presence of the same inhibitors. For each experiment, two segments from coronary arteries with regenerated endothelium were exposed to SNP (with or without inhibitors) and were compared to two coronary segments with native endothelium from the same heart. For the experiments with ODQ (six pigs), the vascular preparations were frozen in liquid nitrogen at the end of the exposure to SNP for determination of cGMP level. For this group, two additional coronary segments from each coronary arteries (maintained at room temperature in Krebs solution) were used for the evaluation of the basal content of cGMP. They were frozen in liquid nitrogen at the same time as the corresponding vascular segment exposed to SNP and were maintained at −80°C for further determination of cGMP content.
In the second set of experiments, the coronary segments were stretched to the optimal tension and contracted with PGF2α (2–6 μM) in order to evaluate the contribution of the three endothelial factors (nitric oxide, prostacyclin and EDHF) to the relaxation evoked by bradykinin (30 nM). For each animal, three coronary segments from each coronary artery (with native and regenerated endothelium) were used: (1) one segment was tested for measurement of membrane potential hyperpolarization and determination of cyclic nucleotides content at the maximal relaxation induced by bradykinin; (2) one was maintained in Krebs solution at room temperature, for the evaluation of basal levels of cyclic nucleotides in the same coronary artery (‘Basal'); and (3) the third placed in the experimental chamber closed to the tested segment in order to determine the influence of the incubation period (‘PGF2α') on cGMP and cAMP contents. After equilibration in Krebs solution (30–40 min), each coronary segment tested was initially contracted with PGF2α (2–6 μM) alone or in combination with inhibitors of cyclo-oxygenase (indomethacin, 10 μM) and/or nitric oxide synthase (Nω-nitro-L-arginine, 30 μM). Seven pigs were used for each group. Once a steady tone was established in the tested segment (40 min with inhibitors, more than 1 h without inhibitors), the corresponding vascular preparations under incubation (‘PGF2α') and under basal conditions (‘Basal') were frozen and stored at −80°C. Then, bradykinin was applied to the coronary segment which was frozen in liquid nitrogen at maximal relaxation (‘+BK'). The membrane potential was measured at the end of the resting period, when the contraction had reached a stable level and during the exposure to bradykinin (2–2.5 min).
Measurement of cyclic nucleotides
At the time of assay, frozen tissue (stored at −80°C) was placed in medium containing acetate buffer (0.05 M, pH=5.8) and a phosphodiesterase inhibitor, 3-isobutyl-1-1-methylxanthine (IBMX 0.1 mM). The coronary segment was then homogenized and sonicated. The total protein content was determined in each homogenate using the Biorad method. The homogenate was centrifuged at 2000 g for 20 min at 4°C. The levels of cyclic GMP (cGMP) and cyclic AMP (cAMP) were determined in the supernatant by radioimmunoassay using the Amerlex method (Amersham, RPA525). The results are expressed in pmol per mg of total protein content.
Drugs
The following drugs were used: 4-aminopyridine, apamin, bradykinin, charybdotoxin, glibenclamide, iberiotoxin, indomethacin, Nω-nitro-L-arginine, prostaglandin F2α, 1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and sodium nitroprusside. All drugs were from Sigma Chemical Co. (St Louis, MO, U.S.A.), except the three toxins that were from Latoxan (Valence, France).
Statistical analysis
Data are expressed as the means±s.e.mean from n experiments. The number n corresponds to the number of animals used per group. To compare the responses between the coronary arteries with regenerated endothelium to those with native endothelium from the same animals, Student's t-test for paired observations was used. Differences were considered to be statistically significant at P<0.05.
Results
Reduced basal cGMP content associated with a depolarization of SMC
Four weeks after surgery, the smooth muscle cells from coronary artery segments with regenerated endothelium were polarized significantly less than those from corresponding control arteries (−45.8±1.1 mV vs −56.1±1.4 mV, respectively, n=14 pigs). In the coronary arteries with regenerated endothelium, the basal level of cGMP was decreased (0.72±0.17 vs 4.10±0.68 pmol mg−1 protein, n=14) while the content of cAMP was not changed significantly.
cGMP-dependent repolarization induced by a NO donor
In order to evaluate the possible link between both alterations, coronary segments from six animals were exposed to exogenous NO, using sodium nitroprusside (SNP). Under basal conditions, the addition of sodium nitroprusside (1 μM, 13 min), repolarized the smooth muscle cells of coronary arteries with regenerated endothelium without affecting the membrane potential in the control arteries (Figure 1). Concomitantly, after repolarization by SNP, the levels of cGMP in these vascular segments were not significantly different (2.48±1.07 and 2.03±0.60 pmol mg−1 protein, for coronary segments with native and regenerated endothelium, respectively) while in the same blood vessels, the basal values of cGMP were lower in the presence of regenerated endothelium (1.06±0.33 pmol mg−1 protein) in comparison with the corresponding basal coronary segments with native endothelium (4.07±0.71 pmol mg−1 protein). Thus, after 13 min-exposure to SNP, in the absence of inhibitors of phosphodiesterases, an increase in cGMP was persistent in coronary segments with regenerated endothelium when compared to basal segments from the same coronary arteries (P=0.056, n=6) while the cGMP content tend to decrease in coronary segments with native endothelium (P=0.067, n=6).
Figure 1.
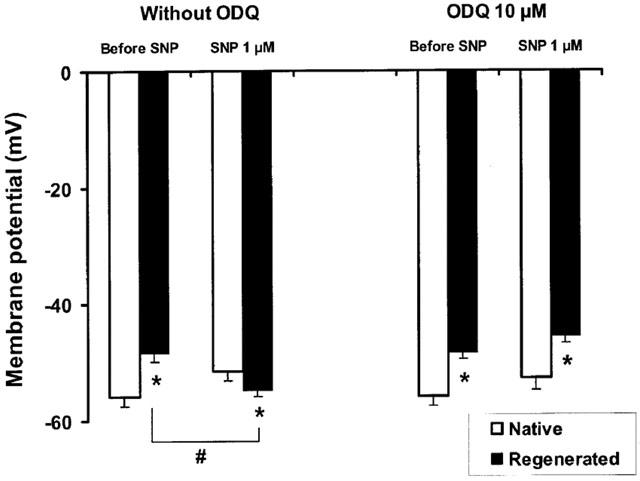
Membrane potential of smooth muscle cells in porcine coronary arteries with native or regenerated endothelium after exposure to a NO donor, sodium nitroprusside (SNP, 1 μM) in the presence or absence of a soluble guanylate cyclase inhibitor, ODQ (10 μM). Data are means±s.e.mean from six experiments for each group. *P<0.05: statistically significant differences from the corresponding control coronary arteries with native endothelium. #P<0.05: statistically significant differences from values before the addition of SNP.
In segments from the same coronary arteries, the inhibitor of soluble guanylate cyclase, ODQ (10 μM), inhibited the repolarization of the smooth muscle cells (Figure 1, n=6) and maintained reduced levels of cGMP despite the presence of SNP (0.20±0.06 and 0.13±0.04 pmol mg−1 protein, for coronary segments with native and regenerated endothelium, respectively).
No involvement of Ca2+-dependent and ATP-sensitive K+ channels in the repolarization induced by SNP
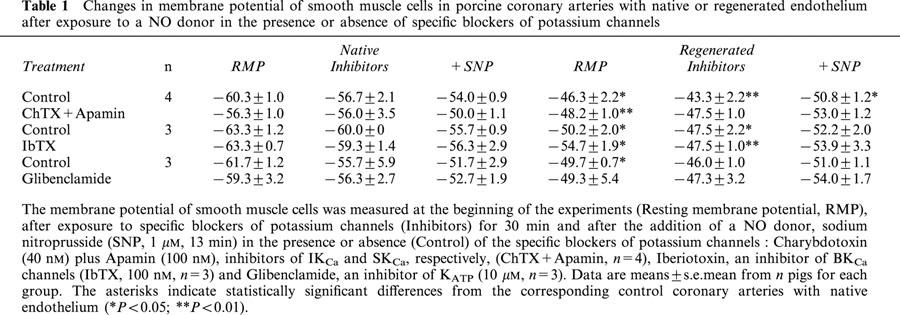
In order to investigate the possible involvement of potassium channels in this repolarization induced by sodium nitroprusside, coronary segments were treated initially with different specific blockers (for 30 min) before the addition of SNP (1 μM) for 13 min, in the presence of the blockers. Under basal conditions, apamin (100 nM) plus charybdotoxin (40 nM), selective blockers of SKCa and IKCa, respectively, had no significant effect on the resting membrane potential (RMP) in both coronary arteries and did not affect the repolarization induced by SNP in the previously denuded vessels (Table 1, four pigs). Likewise iberiotoxin (100 nM), a blocker of BKCa channel, did not influence RMP of the blood vessels with native and regenerated endothelium and the repolarizing effect of SNP in the arteries with regenerated endothelium (Table 1, three pigs). Glibenclamide (10 μM), an inhibitor of KATP channel, had no significant effect on the RMP of blood vessels with native and regenerated endothelium and did not change the effect of SNP (Table 1, three pigs).
Table 1.
Changes in membrane potential of smooth muscle cells in porcine coronary arteries with native or regenerated endothelium after exposure to a NO donor in the presence or absence of specific blockers of potassium channels
Effects of 4-AP on RMP and repolarization induced by SNP
Four-aminopyridine (4-AP, 5 mM), a blocker of Kv channels, did not depolarize significantly the coronary segments (−51±4.7 vs −56.6±2.0 mV, P=0.094 and −38.4±5.1 vs −48.2±2.7 mV, P=0.069, for blood vessels with native and regenerated endothelium, respectively, n=5 animals) and generated spontaneous rhythmic activity only in coronary arteries with regenerated endothelium (in all five treated segments). In the presence of 4-aminopyridine at lower concentration (1 mM), leading to partial block of Kv channels, this rhythmic electrical activity followed different patterns: small oscillations, short or long bursts of spikes which were observed at resting membrane potential of about −40 mV or sustained frequency of spikes when the smooth muscle cells were highly depolarized (Figure 2). The effect of sodium nitroprusside on these rhythmic electrical activities was dependent on their initial profiles. A rapid (less than 3 min) suppression of the abnormal electrical phenomenon was observed in the presence of small oscillations or burst of spikes (not shown). Sustained frequency of spikes, generally observed at higher concentration of 4-AP, needed a more prolonged exposure to sodium nitroprusside to disappear: first the amplitude of spikes increased and the frequency decreased with partial repolarization of cells, then burst of spikes appeared and finally the cells became quiescent (Figure 3). The amplitude of repolarization observed in the presence of sodium nitroprusside was not modified by 4-AP, but the levels of membrane potential reached during exposure to SNP in the presence of 4-AP were significantly less negative compared to control conditions (Figure 4).
Figure 2.
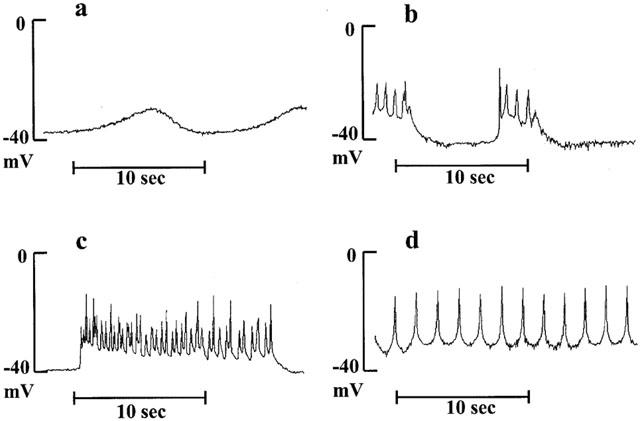
Representative traces of changes in membrane potential of smooth muscle cells in porcine coronary arteries with regenerated endothelium after exposure to a low concentration of 4-aminopyridine (4-AP, 1 mM). Abnormal electrical activities, small membrane potential oscillations (a), short (b) or long (c) burst of spikes or sustained spiking (d) were only observed in previously denuded blood vessels.
Figure 3.
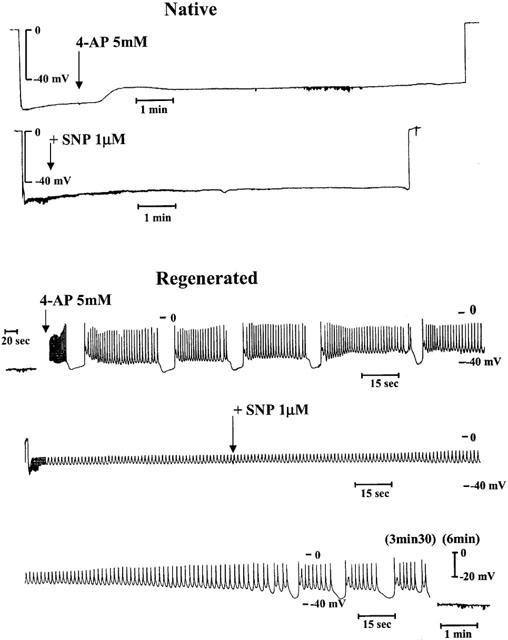
Representative traces of changes in membrane potential of smooth muscle cells in porcine coronary arteries with native (top panel) or regenerated endothelium (bottom panel) after exposure to a NO donor, sodium nitroprusside (SNP, 1 μM) in the presence of 4-aminopyridine (4-AP, 5 mM). Note that for the bottom panel the traces constitute an uninterrupted recording (the scales were expanded in order to show the spiking activity).
Figure 4.
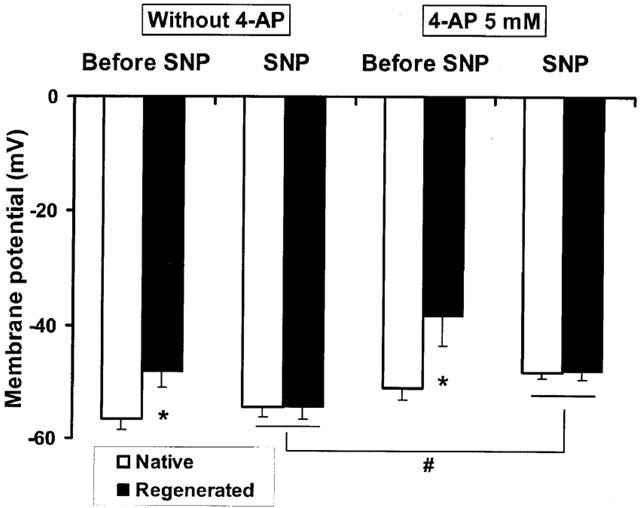
Membrane potential of smooth muscle cells in porcine coronary arteries with native or regenerated endothelium after exposure to a NO donor, sodium nitroprusside (SNP, 1 μM) in the presence or absence of 4-aminopyridine (4-AP, 5 mM). Data are means±s.e.mean from five experiments for each group. *P<0.05: statistically significant differences from the corresponding control coronary arteries with native endothelium. #P<0.05: statistically significant differences from the control conditions.
Involvement of different endothelial factors to the relaxation evoked by BK
In this second set of experiments, the contribution of the three relaxing factors (nitric oxide, prostacyclin and EDHF) was analysed simultaneously during the relaxation of coronary artery segments with native and regenerated endothelium to bradykinin. The contribution of EDHF was quantified by direct measurement of membrane potential hyperpolarization and those of prostacyclin and NO by determination of their second messenger level, cAMP and cGMP, respectively. Three groups (with seven animals per group) were constituted: (a) control solution (‘LNA (−)/Indo (−)', three factors involved); (b) in the presence of cyclo-oxygenase inhibitor (indomethacin, 10 μM) to block the production of prostacyclin (‘LNA (−)/Indo (+)') and (c) in the presence of combined inhibition of nitric oxide synthase (with Nω-nitro-L-arginine, 30 μM) and cyclo-oxygenase (‘LNA (+)/Indo (+)'), whereby the only remaining factor must be EDHF. For each experiment, two additional segments from the same coronary artery were used for the determination of cyclic nucleotides content under resting conditions (‘Basal') and after the same incubation period as that of the tested segment (‘PGF2α').
Effects of stretch to optimal tension and contraction with PGF2α on membrane potential
Under the applied tension (45–50 mN), the smooth muscle cells of the coronary arteries with regenerated endothelium were polarized significantly less than the corresponding control arteries in the three groups (Figure 5, ‘RMP'). As for unstretched blood vessels, this depolarization was associated with a decrease in cyclic GMP level (−73%) while the cyclic AMP contents were not changed significantly (Figure 6, ‘Basal').
Figure 5.
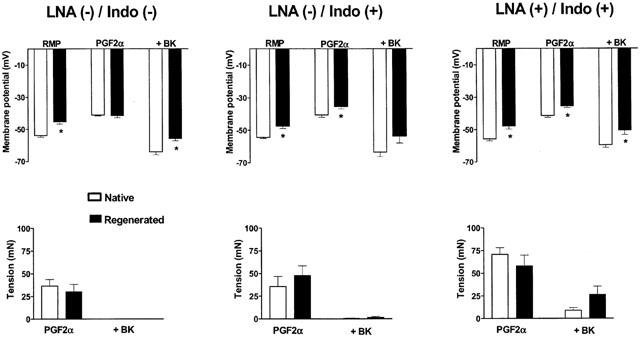
Top panels: membrane potential of smooth muscle cells after contractions with prostaglandin F2α (2–6 μM) (PGF2α) and during relaxations to bradykinin (30 nM) (+BK) in porcine coronary arteries with native or regenerated endothelium. RMP represents the resting membrane potential under resting tension, before addition of the drugs. Bottom panels: corresponding values of tension after contraction with PGF2α (PGF2α) and relaxation with bradykinin (+BK). Data are means±s.e.mean from seven experiments for each group. The experiments were performed in the absence (−) or in the presence (+) of indomethacin (10 μM) (Indo) alone or in addition to Nω-nitro-L-arginine (30 μM) (LNA). The asterisks indicate statistically significant differences from the corresponding control coronary arteries with native endothelium (P<0.05).
Figure 6.
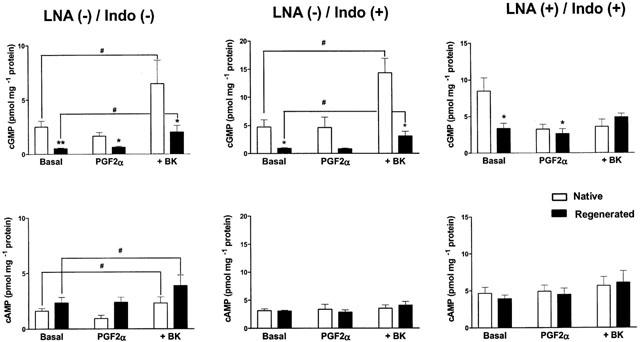
Levels of cyclic GMP (top panels) and cyclic AMP (bottom panels) in porcine coronary arteries with native or regenerated endothelium: under basal conditions (Basal), after incubation with prostaglandin F2α (2 to 6 μM) (PGF2α) and at the maximal level of relaxation with bradykinin (30 nM) (+BK, same segments as those tested in the experiments of Figure 5). Both vascular segments are frozen at the same time and correspond to the same vascular tissue. Data are means±s.e.mean from seven experiments for each group. The experiments were performed in the absence (−) or in the presence (+) of indomethacin (10 μM) (Indo) alone or in addition to Nω-nitro-L-arginine (30 μM) (LNA). *P<0.05; **P<0.01: statistically significant differences from the corresponding control coronary arteries with native endothelium. #P<0.05: statistically significant differences from the basal conditions.
Prostaglandin F2α induced similar contractions which were associated with a depolarization of vascular smooth muscle cells in both types of coronary arteries (Figure 5, ‘PGF2α').
Relaxation evoked by BK
In both types of preparations, bradykinin (30 nM) induced complete endothelium-dependent relaxations under control conditions and in the presence of indomethacin only (Figure 5). With no production of nitric oxide and prostacyclin (Figure 5, ‘LNA(+)/Indo (+)'), the relaxations induced by bradykinin were reduced to 88.0±4.7% and 64.7±9.9% for the blood vessels with native and regenerated endothelium, respectively.
Alteration in NO production during the relaxation evoked by BK
In the absence of inhibitors of cyclo-oxygenase and NO synthase (‘LNA (−)/Indo (−)'), bradykinin induced an increase in cGMP which was greater than for cAMP in both preparations. The levels of cGMP at maximal relaxation were significantly lower in coronary arteries with regenerated endothelium while the levels of cAMP were not modified significantly (Figure 6, ‘+BK'). As expected, the production of cAMP in response to bradykinin was inhibited by indomethacin (10 μM) (Figure 6, ‘LNA (−)/Indo (+)') and that of cGMP was inhibited by the addition of Nω-nitro-L-arginine (30 μM) (Figure 6, ‘LNA (+)/Indo (+)').
Normal EDHF response during the relaxation evoked by BK
The contribution of EDHF was measured by recording of membrane potential during the two first minutes of relaxation evoked by bradykinin (30 nM), just before freezing the tissue. In both types of blood vessels, the addition of bradykinin produced a total change in cell membrane potential (repolarization plus hyperpolarization) which was not modified by the presence of LNA and indomethacin. However, the membrane potential reached in the presence of bradykinin was more positive in the arteries with regenerated endothelium than in those with native endothelium (Figure 5, ‘+BK').
Discussion
Consequences of altered production of NO at rest
As previously demonstrated, the smooth muscle cells from porcine coronary arteries with regenerated endothelium are depolarized in comparison with those from corresponding control arteries (Thollon et al., 1999a, b). This depolarization favours the appearance of spontaneous spikes that generate phasic contractions in arteries with regenerated endothelium, while such membrane potential instability and associated contractile oscillations are not observed in arteries with native endothelium under the present experimental conditions. Such rhythmic spontaneous contractions occur in diseased human coronary arteries (Ross et al., 1980; Kalsner, 1985), precede formation of atherosclerotic lesions (Davies et al., 1996) and are associated with proliferation of vascular smooth muscle cells in human vasospasm (Golenhofen et al., 1981). These oscillations in tension are also described in other relevant models of human vascular diseases such as diabetic pigs fed with high fat and high cholesterol diets (Dixon et al., 1999). Thus, a depolarization of smooth muscle cells under resting conditions is probably a key phenomenon favouring spastic activities of pathological blood vessels. A reduction in resting membrane potential has been also observed in vascular smooth muscle cells from hypertensive and diabetic animals (Sobey, 2001). In the present study, the electrophysiological alterations are associated with a decreased level of cyclic GMP and an unchanged level of cyclic AMP in the coronary artery segments. The decrease in cyclic GMP is the result of a reduced cellular production of nitric oxide by the regenerated endothelium as shown in cultured endothelial cells four weeks after angioplasty (Fournet-Bourguignon et al., 2000). In order to study if the changes in membrane potential were related to the alteration of the nitric oxide pathway, the previously denuded blood vessels were exposed to sodium nitroprusside, a donor of nitric oxide, to restore the basal levels in cyclic GMP. Sodium nitroprusside repolarized the vascular smooth muscle cells from coronary arteries with regenerated endothelium, normalizing the resting membrane potential and thus curtailing their spastic activity. By contrast, the nitric oxide donor did not hyperpolarize coronary arteries with native endothelium, in confirmation of earlier observations (Bény & Brunet, 1988). Unlike endothelium-dependent hyperpolarizations of the porcine coronary artery (Quignard et al., 1999; Edwards et al., 2000), the repolarization induced by sodium nitroprusside was not prevented by the combined inhibition of SKCa and IKCa channels with apamin plus charybdotoxin, suggesting that nitric oxide is not an EDHF in this blood vessel. The repolarizing effect of sodium nitroprusside is cyclic GMP-dependent, as it was blocked by ODQ, an inhibitor of soluble guanylate cyclase. These results suggest that NO via the production of cyclic GMP inhibits an inward depolarizing current and/or activate an outward potassium current, both possibly altered in the previously denuded blood vessels. Indeed, NO can interact directly with different potassium channels: ATP-sensitive K+ channels (KATP), large conductance Ca2+ activated and voltage-dependent K+ channels (BKCa) and voltage-gated K+ channels (KV) (Bolotina et al., 1994; Miyoshi et al., 1994; Yuan et al., 1996; Peng et al., 1996; Li et al., 1997; Bychkov et al., 1998). These effects depend on the type of blood vessels and the species studied (Quignard et al., 2000). Furthermore, the function of several types of vascular potassium channels is altered during cardiovascular diseases (Sobey, 2001). Therefore, the effects of specific potassium blockers on the repolarization evoked by sodium nitroprusside was investigated. Under the present experimental conditions, KATP and BKCa channels do not contribute to the resting membrane potential and to the repolarizing effect of sodium nitroprusside, as demonstrated by the absence of effect of glibenclamide and iberiotoxin, respectively. However, KV channels are implicated in the control of the resting membrane potential in both type of coronary arteries, with an increased sensitivity to 4-aminopyridine in the previously denuded blood vessels. 4-Aminopyridine also induces contraction and contractile oscillations of aortic rings taken from atherosclerotic but not those from control mice (Jiang et al., 1999). In the presence of regenerated endothelium most of the vascular preparations when exposed to 4-aminopyridine, show spontaneous changes in membrane potential, small oscillations, burst of spikes or sustained spiking activity. By contrast, this blocker depolarized the arteries with native endothelium without generating abnormal electrical activities. Application of sodium nitroprusside in the presence of 4-aminopyridine did not repolarize the coronary arteries with native endothelium, demonstrating that KV channels are not the target of NO in normal porcine coronary arteries. Furthermore, 4-aminopyridine did not inhibit the effect of sodium nitroprusside in the coronary arteries with regenerated endothelium, indicating that also under these conditions, the repolarizing effect of NO is not mediated by KV channels. The main effect of sodium nitroprusside is to cause a rapid disappearance of spontaneous depolarizations induced by 4-aminopyridine in the arteries with regenerated endothelium, suggesting that the difference in membrane potential between the two types of arteries is the result of an increased depolarizing rather than a decreased repolarizing current. The present results do not permit further speculation as to the nature of the depolarizing current(s) involved (L or T calcium currents, sodium current, calcium-dependent chloride current and/or cationic currents).
Consequences of altered production of NO during relaxation to bradykinin
Four weeks after balloon denudation, porcine coronary arteries with regenerated endothelium selectively lose endothelium-dependent relaxations to serotonin while those to bradykinin are maintained (Shimokawa et al., 1987; 1989; Borg-Capra et al., 1997). The production of cGMP by cells from regenerated endothelium in response to both agonists is reduced (Fournet-Bourguignon et al., 2000). Thus, one aim of the present study was to analyse the consequences of this altered production of NO on the tissue content in cGMP during relaxations evoked by bradykinin, and to evaluate the contribution of all endothelial factors in this relaxation. To do so, hyperpolarizations, relaxations and the content of cyclic nucleotides were measured simultaneously in the same vascular segments. The alterations in resting membrane potential previously described in unstretched blood vessels with regenerated endothelium were similar to those observed after applying tension. The contraction induced by prosta-glandin F2α was associated with a depolarization of smooth muscle cells from coronary arteries with normal and regenerated endothelium. In a previous set of experiments using coronary arteries from control pigs (data not shown), depolarization with PGF2α was not observed in unstretched segments but was present under applied tension, suggesting that the depolarization was related to the contraction and not to the presence of the contracting agent per se. In the present study, under control conditions, with the contribution of all endothelial factors to the relaxation, the concentration of bradykinin used induced a complete relaxation of both types of blood vessels. A modest increase in content of cAMP was observed after application of bradykinin with no difference between coronary arteries with native or regenerated endothelium. The inhibition of cyclo-oxygenase by indo-methacin did not affect the amplitude of the relaxation. These Rndings strongly suggest that prostacyclin contributes minimally to endothelium-dependent relaxations in response to bradykinin in porcine coronary arteries with either native or regenerated endothelium. The levels of cGMP after exposure to bradykinin were lower in the previously denuded arteries than in the controls both in the absence or presence of indomethacin. When nitric oxide synthase was blocked with Nω-nitro-L-arginine, as demonstrated by the absence of an increase in cGMP levels after exposure to bradykinin, the relaxation was reduced but not suppressed, indicating a modest contribution of nitric oxide to endothelium-dependent relaxations not only in rings with native (Richard et al., 1990; Nagao & Vanhoutte, 1992) but also those with regenerated endothelium. Thus, the present study points to EDHF as the major factor underlying this response. The hyperpolarization was not modiRed by inhibitors of nitric oxide synthase and cyclo-oxygenase indicating an absence of regulation of the EDHF-pathway by either nitric oxide or prostacyclin under the present experimental conditions. At this point, it is necessary to underline that the hyperpolarization measured in stretched blood vessels corresponds to repolarization plus hyperpolarization. Indeed, the augmented hyperpolarization to bradykinin observed under basal conditions in the more depolarized smooth muscle cells from unstretched coronary arteries with regenerated endothelium (Thollon et al., 1999a), did not occur in the present experiments, presumably because of the further depolarization induced by the contracting agent. These results emphasize the influence of the experimental conditions, underlining the caution needed to extrapolate from endothelium-dependent hyperpolarizations under resting conditions to endothelium-dependent relaxations. In porcine coronary arteries, the endothelium-dependent hyperpolarizations resistant to LNA and indomethacin, evoked by bradykinin (30 nM) and substance P (100 nM) were blocked by a combination of apamin and charybdotoxin (but not IbTX) (Quignard et al., 1999; Edwards et al., 2000) as in various vascular tissues (for review Büsse et al., 2002), probably by the inhibition of the SKCa and IKCa of endothelial cells. Nevertheless, the nature of EDHF in these blood vessels has not been clearly established. First, epoxyeicosatrienoic acids (EETs), products of cytochrome P450 epoxygenases, suggested to be EDHF in porcine coronary arteries, did not mimic exactly the EDHF response in these blood vessels (Edwards et al., 2000). Secondly, barium plus ouabain did not suppress the hyperpolarizations induced by bradykinin or substance P in these arteries excluded that EDHF is simply K+ derived from the endothelial cells (Quignard et al., 1999; Edwards et al., 2000). Furthermore, in these coronary segments, incubation with Gap 27, a blocker of gap junctions, abolished the hyperpolarization induced by substance P in SMC impaled from the adventitial side but not those impaled from the intimal side suggesting that myocyte–myocyte coupling are strongly implicated in the EDHF response (Edwards et al., 2000). The present findings do not throw further light on the nature of EDHF in pathological blood vessels. However, the results demonstrated that the hyperpolarization, measured near the site of its production in subintimal vascular SMC, can spread to the other layers of the SMC, electronically or via a diffusion of a factor, despite the thickening and remodelling and thus can generate quite normal relaxation. However, lower kinetics of relaxation of coronary segments with high thickening have been observed, suggesting the need of more time for diffusion among the different layers of the SMC (data not shown).
In summary, in porcine coronary arteries with regenerated endothelium, there is za specific impairment of the production of NO both at rest and under stimulation by agonists such as bradykinin. Under basal conditions, this alteration is associated with a depolarization of the vascular smooth muscle cells and abnormal spontaneous electrical activities which both can be counteracted by exogenous NO. This suggests that both alterations are linked and that donors of NO can exert part of their anti-spastic activity in human arteries by suppression of abnormal spiking activities of smooth muscle cells. Furthermore, in coronary arteries with regenerated endothelium, the production of NO during the relaxation induced by bradykinin is reduced while the EDHF component is not increased. Thus, the production of EDHF explains the maintained relaxation to bradykinin despite the reduced production of NO, implying that the sensitivity to the hyperpolarizing signal is augmented.
Abbreviations
- 4-AP
4-aminopyridine
- BK
bradykinin
- BKCa
large conductance Ca2+-activated K+ channels
- cAMP
cyclic adenine monophosphate
- cGMP
cyclic guanosine monophosphate
- ChTX
charybdotoxin
- EDHF
endothelium-derived hyperpolarizing factor
- IbTX
iberiotoxin
- IKCa
intermediate-conductance Ca2+-activated K+ channels
- Indo
indomethacin
- KATP
ATP-sensitive K+ channels
- Kv
voltage-dependent K+ channels
- LNA
Nω-nitro-L-arginine
- NO
nitric oxide
- ODQ
1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one
- PGF2α
prostaglandin F2α
- RMP
resting membrane potential
- SKCa
small conductance Ca2+-activated K+ channels
- SMC
smooth muscle cell
- SNP
sodium nitroprusside
References
- BÉNY J.L., BRUNET P.C. Neither nitric oxide nor nitroglycerin accounts for all the characteristics of endothelially mediated vasodilatation of pig coronary arteries. Blood Vessels. 1988;25:308–311. [PubMed] [Google Scholar]
- BOLOTINA V.M., NAJIBI S., PALACINO J.J., PAGANO P.J., COHEN R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- BORG-CAPRA C., FOURNET-BOURGUIGNON M.P., JANIAK P., VILLENEUVE N., BIDOUARD J.P., VILAINE J.P., VANHOUTTE P.M. Morphological heterogeneity with normal expression but altered function of G proteins in porcine cultured regenerated coronary endothelial cells. Br. J. Pharmacol. 1997;122:999–1008. doi: 10.1038/sj.bjp.0701475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÜSSE R., EDWARDS G., FELETOU M., FLEMING I., VANHOUTTE P.M., WESTON A.H.EDHF: bringing the concepts together Trends Pharmacol. Sci. 2002(in press) [DOI] [PubMed]
- BYCHKOV R, GOLLASCH M., STEINKE T., RIED C., LUFT F.C., HALLER H. Calcium-activated potassium channels and nitrate-induced vasodilation in human coronary arteries. J. Pharmacol. Exp. Ther. 1998;285:293–298. [PubMed] [Google Scholar]
- COHEN R.A., ZITNAY K.M., HAUDENSCHILD C.C., CUNNINGHAM D. Loss of selective endothelial cell vasoactive functions caused by hypercholesterolemia in pig coronary arteries. Circ. Res. 1988;63:903–910. doi: 10.1161/01.res.63.5.903. [DOI] [PubMed] [Google Scholar]
- COWAN C.L., COHEN R.A. Two mechanisms mediate relaxation by bradykinin of pig coronary artery: NO-dependent and -independent responses. Am. J. Physiol. 1991;261:H830–H835. doi: 10.1152/ajpheart.1991.261.3.H830. [DOI] [PubMed] [Google Scholar]
- DAVIES S.F., YEUNG A.C., MEREDITH I.T., CHARBONNEAU F., GANZ P., SELWYN A.P., ANDERSON T.J. Early endothelial dysfunction predicts the development of transplant coronary artery disease at 1 year post-transplant. Circulation. 1996;93:457–462. doi: 10.1161/01.cir.93.3.457. [DOI] [PubMed] [Google Scholar]
- DIXON J.L., STOOPS J.D., PARKER J.L., LAUGHLIN M.H., WEISMAN G.A., STUREK M. Dyslipidemia and vascular dysfunction in diabetic pigs fed an atherogenic diet. Arterioscler. Thromb. Vasc. Biol. 1999;19:2981–2992. doi: 10.1161/01.atv.19.12.2981. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., THOLLON C., GARDENER M.J., FELETOU M., VILAINE J.P., VANHOUTTE P.M., WESTON A.H. Role of gap junctions and EETs in endothelium-dependent hyperpolarization of porcine coronary artery. Br. J. Pharmacol. 2000;129:1145–1154. doi: 10.1038/sj.bjp.0703188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOURNET-BOURGUIGNON M.P., CASTEDO-DELRIEU M., BIDOUARD J.P., LEONCE S., SABOUREAU D., DELESCLUSE I., VILAINE J.P., VANHOUTTE P.M. Phenotypic and functional changes in regenerated porcine coronary endothelial cells. Increased uptake of modified LDL and reduced production of NO. Circ. Res. 2000;86:854–861. doi: 10.1161/01.res.86.8.854. [DOI] [PubMed] [Google Scholar]
- GOLENHOFEN K., MANDREK K., SCHAPER W., WEINHEIMER G., WEIGAND W. Mechanical activity of isolated canine coronary arteries after coronary occlusion. Basic. Res. Cardiol. 1981;76:480–484. doi: 10.1007/BF01908347. [DOI] [PubMed] [Google Scholar]
- JIANG J., THOREN P., CALIGIURI G., HANSSON G.K., PERNOW J. Enhanced phenylephrine-induced rhythmic activity in the atherosclerotic mouse aorta via an increase in opening of KCa channels: relation to KV channels and nitric oxide. Br. J. Pharmacol. 1999;128:637–646. doi: 10.1038/sj.bjp.0702855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALSNER S. Coronary artery reactivity in human vessels: some questions and some answers. Federation Proc. 1985;44:321–325. [PubMed] [Google Scholar]
- LI P.-L., ZOU A.-P., CAMPBELL W.B. Regulation of Potassium channels in coronary arterial smooth muscle by endothelium-derived vasodilators. Hypertension. 1997;29:262–267. doi: 10.1161/01.hyp.29.1.262. [DOI] [PubMed] [Google Scholar]
- LÜSCHER T.F. Endothelial dysfunction as a therapeutic target. The ENCORE trials. Eur. Heart J. 2000;2 Suppl.:D20–D25. [Google Scholar]
- MIYOSHI H., NAKAYA Y., MORITOKI H. Nonendothelial-derived nitric oxide activates the ATP-sensitive K channel of vascular smooth muscle cells. FEBS. 1994;345:47–49. doi: 10.1016/0014-5793(94)00417-x. [DOI] [PubMed] [Google Scholar]
- NAGAO T., VANHOUTTE P.M. Hyperpolarization as a mechanism for endothelium-dependent relaxations in the porcine coronary artery. J. Physiol. 1992;445:355–367. doi: 10.1113/jphysiol.1992.sp018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENG W., HOIDAL J.R., FARRUKH I.S. Regulation of Ca2+-activated K+ channels in pulmonary vascular smooth muscle cells: role of nitric oxide. J. Appl. Physiol. 1996;81:1264–1272. doi: 10.1152/jappl.1996.81.3.1264. [DOI] [PubMed] [Google Scholar]
- QUIGNARD J.F., FÉLÉTOU M., CORRIU C., CHATAIGNEAU T., EDWARDS G., WESTON A.H., VANHOUTTE P.M. 3-Morpholinosydnonimine (SIN-1) and K+ channels in smooth muscle cells of the rabbit and guinea-pig carotid arteries. Eur. J. Pharmacol. 2000;399:9–16. doi: 10.1016/s0014-2999(00)00372-1. [DOI] [PubMed] [Google Scholar]
- QUIGNARD J.F., FÉLÉTOU M., THOLLON C., VILAINE J.P., DUHAULT J., VANHOUTTE P.M. Potassium ions and endothelium-derived hyperpolarizing factor in guinea-pig carotid and porcine coronary arteries. Br. J. Pharmacol. 1999;127:27–34. doi: 10.1038/sj.bjp.0702493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARD V., TANNER F.C., TSCHUDI M., LÜSCHER T.F. Different activation of L-arginine pathway by bradykinin, serotonin, and clonidine in coronary arteries. Am. J. Physiol. 1990;259:H1433–H1439. doi: 10.1152/ajpheart.1990.259.5.H1433. [DOI] [PubMed] [Google Scholar]
- ROSS G., STINSON E., SCHROEDER J., GINSBURG R. Spontaneous phasic activity of isolated human coronary arteries. Cardiovasc. Res. 1980;14:613–618. doi: 10.1093/cvr/14.10.613. [DOI] [PubMed] [Google Scholar]
- SHIMOKAWA H., AARHUS L.L., VANHOUTTE P.M. Porcine coronary arteries with regenerated endothelium have a reduced endothelium-dependent responsiveness to aggregating platelets and serotonin. Circ. Res. 1987;61:256–270. doi: 10.1161/01.res.61.2.256. [DOI] [PubMed] [Google Scholar]
- SHIMOKAWA H., FLAVAHAN N.A., VANHOUTTE P.M. Natural course of the impairment of endothelium-dependent relaxations after balloon endothelium removal in porcine coronary arteries. Circ. Res. 1989;65:740–753. doi: 10.1161/01.res.65.3.740. [DOI] [PubMed] [Google Scholar]
- SOBEY C.G. Potassium channel function in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2001;21:28–38. doi: 10.1161/01.atv.21.1.28. [DOI] [PubMed] [Google Scholar]
- THOLLON C., BIDOUARD J.P., CAMBARRAT C., DELESCLUSE I., VILLENEUVE N., VANHOUTTE P.M., VILAINE J.P. Alteration of endothelium-dependent hyperpolarizations in porcine coronary arteries with regenerated endothelium. Circ. Res. 1999a;84:371–377. doi: 10.1161/01.res.84.4.371. [DOI] [PubMed] [Google Scholar]
- THOLLON C., FOURNET-BOURGUIGNON M.P., CAMBARRAT C., SABOUREAU D., DELESCLUSE I., VANHOUTTE P.M., VILAINE J.P.Contribution of nitric oxide and endothelium-derived hyperpolarizing factor to the relaxation evoked by bradykinin in porcine coronary arteries four weeks after removal of the endothelium Endothelium-derived hyperpolarizing factor 1999b38335–341.ed. Vanhoutte, P.M. [Google Scholar]
- YAMAMOTO Y., TOMOIKE H., EGASHIRA K., NAKAMURA M. Attenuation of endothelium-related relaxation and enhanced responsiveness of vascular smooth muscle to histamine in spastic coronary arterial segments from miniature pigs. Circ. Res. 1987;61:772–778. doi: 10.1161/01.res.61.6.772. [DOI] [PubMed] [Google Scholar]
- YUAN X.-Y., TOD M.L., RUBIN L.J., BLAUSTEIN M.P. NO hyperpolarizes pulmonary artery smooth muscle cells and decreases the intracellular Ca2+ concentration by activating voltage-gated K+ channels. Proc. Natl. Acad. Sci. 1996;93:10489–10494. doi: 10.1073/pnas.93.19.10489. [DOI] [PMC free article] [PubMed] [Google Scholar]


