Abstract
Intracellular recordings were made from isolated frog sciatic-sartorius nerve-muscle preparations, and the effects of sphingosine 1-phosphate (S1-P) on miniature endplate potentials (MEPPs) were studied. Extracellular application of S1-P (1 and 30 μM) had no significant effects on the frequency and amplitude of MEPPs. Delivery into nerve terminals by liposomes containing 10−5, 10−4 or 10−3 M S1-P was associated with a concentration-dependent increase in MEPP frequency of 37, 63 and 86%. The per cent of median MEPP amplitude was not significantly changed, but there was an increase in the number of ‘giant' MEPPs. Pre-exposure of the preparations to S1-P 10−5 but not 10−8 M entrapped in liposomes for 15 min blocked the effects of subsequent superfusion of S1-P (10−4 M)-filled liposomes on MEPP frequency. Thus, intracellular S1-P receptors seem to undergo ‘desensitization' to higher concentrations of S1-P. The result provides the first evidence that S1-P acting intracellularly but not extracellularly enhances spontaneous transmitter release at the frog neuromuscular junction.
Keywords: Sphingosine 1-phosphate, cyclic ADP ribose, IP3, miniature endplate potentials, nicotinic acid adenine dinucleotide phosphate
Introduction
The release of neurotransmitters from the nerve terminal is critically dependent on a transient increase in intracellular Ca2+ [Ca2+]i, which may be caused by influx of Ca2+ from extracellular milieu or release from intracellular stores (Silinsky, 1985; Petersen & Cancela, 1999). Major calcium stores in the nerve terminal include smooth endoplasmic reticulum (SER) (Pezzati et al., 2001), mitochondria (Calupca et al., 2001), and secretory vesicles (Pezzati et al., 2001).
Second messengers can activate or mobilize these stores. For example, injection of IP3, cyclic ADP ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) into the presynaptic site of Aplysia neurons enhances transmitter output (Chameau et al., 2001). Similarly, liposomal delivery of IP3, cADPR or NAADP into frog motor nerve endings increases spontaneous transmitter release (Brailoiu & Miyamoto, 2000; Brailoiu et al., 2001). Similar results were reported at a glutamatergic synapse where injection of IP3 or its stable analogue adenophostin A enhances transmitter release at crayfish motor nerve endings (Dixon & Atwood, 1989).
Sphingomyelin metabolism occurs in neurons including motoneurons (Irie & Hirabayashi, 1999). One of the sphingomyelin metabolites is sphingosine, which is phosphorylated by sphingosine kinase to yield the lipid molecule sphingosine 1-phosphate (S1-P) (Meyer zu Heringdorf et al., 1997). S1-P, which is located intracellularly with SER (Ghosh et al., 1994), can cause a release of Ca2+ from thapsigargin-sensitive Ca2+ stores (Ghosh et al., 1990). The topographic distribution renders S1-P ideally suited as an intracellular signalling molecule. In this regard, results from a number of recent studies suggest that S1-P may produce its effect by interacting with extracellular receptors (Hla et al., 1999).
In view of a demonstrated role of IP3, cADPR and NAADP on neurosecretion, we were interested in the possibility that S1-P might also affect transmitter release. Although S1-P has been shown to produce its effect by interacting with extracellular receptors in several tissues, the strategic location in the SER raises the possibility that S1-P may act intracellularly. Therefore, we explored the extracellular as well as intracellular effects of S1-P on spontaneous transmitter release at the frog neuromuscular junction.
Methods
Preparation of liposomes
Reverse-phase evaporation vesicles (REVs liposomes) were prepared from 60 mg ml−1 egg yolk phosphatidylcholine according to the procedure of Szoka & Papahadjopoulos (1978). Stock solution of S1-P (10−2 M dissolved in 30% methanol) was dissolved in 140 mM KCl, which was adjusted previously to pH 6.9 in order to obtain 2.5 ml of 10−3, 10−5 and 10−8 M as a final concentration of S1-P. Separately, 150 mg phosphatidylcholine were dissolved in 7.5 ml diethylether. These two mixtures (phosphatidylcholine/diethylether and S1-P/KCl) were mixed together. After additional vortexing of the emulsion for 5 min, the organic solvents (diethylether and methanol) were evaporated in vacuo using a rotary evaporator at 20°C. Liposome batches were dialyzed (Sigma dialysis sacs) against control Ringer solution (1/600 (v v−1, 150 min) to remove non-incorporated agent, and the Ringer solution was changed every 30 min. Control liposomes were similarly made, except 2.25 ml of 140 mM KCl solution were dissolved in 0.25 ml of 30% methanol. Liposome suspensions were administered by continuous perfusion (1.5 ml min−1) after 1/20 (v v−1) dilution in control Ringer solution.
Preparations and solutions
Frogs (Rana pipiens) were decapitated and rapidly double-pithed, and sciatic-sartorius nerve-muscle preparations were isolated. Every effort was made to use the minimum number of animals required for valid statistical analyses. Procedures were reviewed and approved by the East Tennessee State University Committee for Animal Care. Muscles were mounted in a 3-ml Sylgard-lined Petri dish bath, which was continuously perfused with Ringer solution using a dual-chambered roller pump. The Ringer solution contained (mM): 110 NaCl, 2.5 KCl, 1.8 CaCl2, 2.0 tris(hydroxymethyl) aminomethane (Tris, pH 7.2) and 5.6 glucose.
Electrophysiological techniques
MEPPs were recorded using conventional microelectrode (3 M KCl, 5–15 MΩ) techniques similar to those previously described (Brailoiu & Miyamoto, 2000). Selection of recordings was made from impalements that showed large MEPP size (>0.3 mV), good signal-to-noise ratio (baseline peak-to-peak noise <0.1 mV), and high and stable muscle resting membrane potential (>−80 mV, with <3 mV decline during the control period). Resting potentials ranged between −80 and −90 mV in different fibres. Impaled muscle fibres that showed more than 10% drop in the resting membrane potential during an experiment were not used. Experiments were conducted at the ambient room temperature (21–22°C), and only one trial was carried out on each muscle. Preparations were equilibrated for at least 30 min before use. Signals were fed into a high impedance preamplifier (A-M Systems, Carlsborg, WA, U.S.A.) and viewed on a R5103N oscilloscope (Tektronix, Beaverton, OR, U.S.A.). Signal-to-noise ratio was increased with a band-pass filter (1 kHz) and boosted for interfacing with a data acquisition unit with 1 MHz digitization frequency (RC Electronics, Goleta, CA, U.S.A.). MEPPs were recorded with a modified videocassette recorder (AM Vetter, Rebersburg, PA, U.S.A.) for off-line analysis.
Data analysis
MEPP amplitudes (100 samples for each time point) were measured using stored digitized data and a grid template on a flat screen monitor. To minimize the effects of junction-to-junction variation, data for each experiment were expressed as per cent of values at time zero, and results from six single experiments averaged (plots show mean±s.e.mean). Analysis of statistical differences was made by comparing each point with points obtained in control Ringer, with P<0.05 indicating significant differences (paired t-test). Occasionally, MEPPs of much larger amplitude, referred to herein as giant MEPPs (gMEPPs), were recorded. Giant MEPPs are spontaneous potentials with amplitudes of more than twice that of the regular MEPPs, and with a slower, smoother rising phase (Alkadhi, 1988).
Drugs
Sphingosine 1-phosphate, phosphatidylcholine and all other chemicals were from Sigma (St. Louis, MO, U.S.A.).
Results
Extracellular administration of S1-P
Local application of S1-P, in concentrations that elicited responses in other tissues (1 and 30 μM) (Hla et al., 1999), had no effect on the frequency and amplitude of MEPPs in any of the muscle endplates tested. The MEPP frequency and amplitude 4 min after superfusion of S1-P (1 and 30 μM) were 98±3% (P=0.58797, n=6), 101±4% (P=0.78656, n=6), 99±1% (P=0.8561; n=6) and 100±1% (P=0.69519; n=6) of control, respectively.
Intracellular delivery of S1-P by liposomes
Perfusion with liposomes containing a low concentration of S1-P (10−8 M) or control liposomes (filled only with 140 mM KCl) did not significantly change the MEPPs frequency and amplitude (n=6).
Exposure of muscles to liposomes containing S1-P (10−5, 10−4 and 10−3 M) caused a significant increase in MEPP frequencies of 37, 63 and 86% over the control period (Figures 1 and 2). It should be mentioned that the final concentration of S1-P within the nerve terminal was estimated to be 100 fold less than that in the aqueous phase. In all cases, there was a fairly rapid time to peak; i.e., 3 min for 10−4 and 10−3 M, and 4 min for 10−5 M, followed by a gradual decline (Figure 2). On the other hand, the medium amplitude of MEPPs before and during superfusion of S1-P-filled liposomes was not significantly changed (Figure 3). The MEPP frequency-amplitude histograms of six experiments before and 3 min after superfusion with S1-P (10−3 M)-filled liposomes are shown in Figure 3. Although there was no shift in the amplitude distribution, there was a consistent increase in the number of gMEPPs after S1-P treatment in all six experiments. The gMEPPs had an amplitude two times higher than the median of regular MEPPs, and a time to peak of 3.37±0.31 ms (Figure 3).
Figure 1.
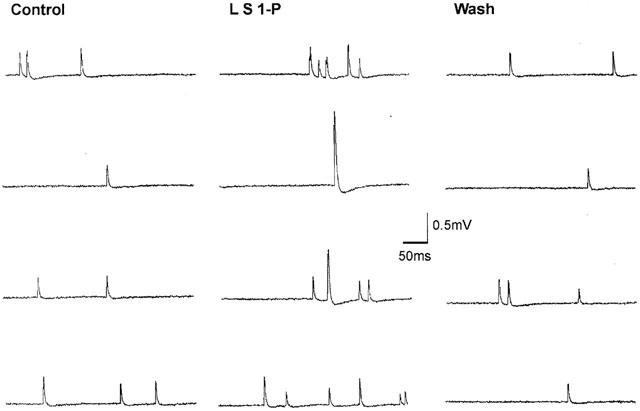
Sample recordings of miniature endplate potentials (MEPPs) in normal Ringer solution before, during, and after superfusion of S1-P (10−3 M) entrapped in liposomes. Note the increase in MEPP frequency and the presence of ‘giant' MEPPs during S1-P superfusion.
Figure 2.
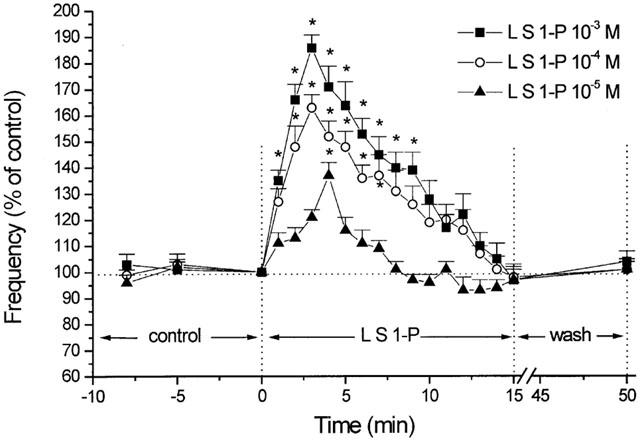
Percent changes in MEPP frequency as a function of time. The effects of 10−5, 10−4 and 10−3 M S1-P delivered by liposomes (L S1-P) on MEPP frequency are superimposed for comparison. The peak effect occurs at 3 min for 10−4 and 10−3 M, and at 4 min for 10−5 M S1-P. MEPP frequency 100%=0.974 s−1 (10−5 M S1-P), 1.12 s−1 (10−4 M S1-P) and 0.983 s−1 (10−3 M S1-P). Note that the MEPP frequency return to control level, e.g., at time=8 min for S1-P 10−5 M. Each point represents the mean from six different experiments. Asterisks denote statistically significant differences (P<0.05) from control.
Figure 3.
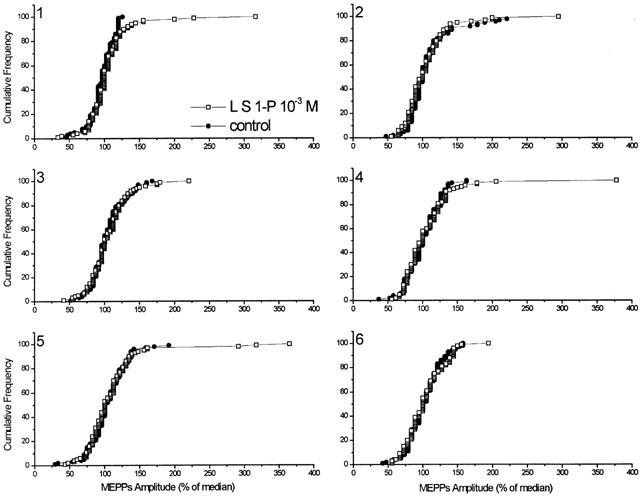
Histogram analysis (cumulative frequency) of the changes in MEPP amplitude distribution before (control) and after S1-P delivered by liposomes (L S1-P). For each of the six single experiments, MEPP amplitudes at time=0 min (pre-exposure) and time=3 min (post-exposure) are expressed as a per cent of the median amplitude (100 samples each). Histograms reveal no change in the shape of the unimodal amplitude-frequency distribution of MEPPs (exp. 1 median=0.348 mV; exp. 2 median=0.317 mV; exp. 3 median=0.392 mV; exp. 4 median=0.329 mV; exp. 5 median=0.377 mV; exp. 6 median=0.366 mV) before and after administration of liposomes containing 10−3 M S1-P. Results indicate no significant changes in the median MEPP amplitude before and after S1-P treatment; however, there is an increase in the number of gMEPPs after S1-P treatment.
S1-P receptor desensitization
Receptors to several intracellular signalling molecules including IP3, cyclic ADP ribose and NAADP appear to undergo homologous desensitization (Lee, 2001). In the case of S1-P, prior exposure of the nerve-muscle preparations to liposomes filled with a low concentration of S1-P (10−8 M) for 15 min did not significantly alter the responses caused by subsequent application of S1-P (10−4 M)-filled liposomes. Thus, second exposure of the muscles to S1-P (10−4 M)-filled liposomes induced an increase in MEPP frequency of 57% (Figure 4A). This increase was also transient, with a peak at 3 min. MEPP amplitudes (% of median) were not changed. On the other hand, prior exposure of the nerve-muscle preparations to liposomes filled with a higher concentration of S1-P (10−5 M) for 15 min blocked the responses caused by subsequent application of S1-P (10−4 M)-filled liposomes (Figure 4B). There was no change in MEPP amplitudes (% of median).
Figure 4.
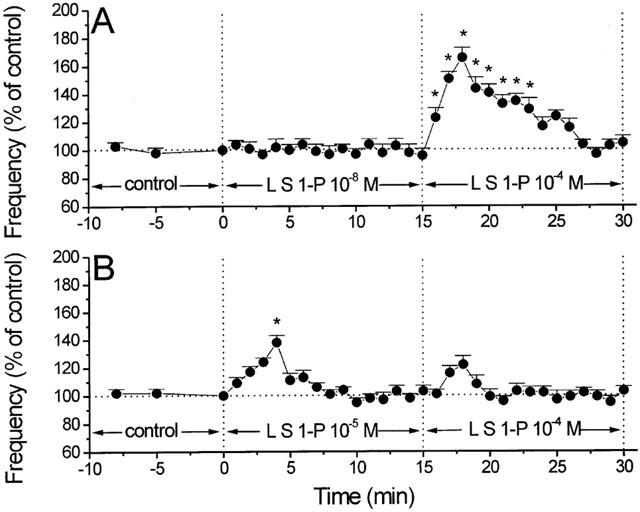
Responses of second liposomal delivery of S1-P following a low and higher concentration of liposomal delivery of S1-P on MEPP frequency. (A) Perfusion with liposomes containing S1-P 10−8 M (L S1-P 10−8 M) had no significant effect on MEPP frequency. A subsequent administration of S1-P 10−4 M-filled liposomes (L S1-P 10−4 M) increased the MEPP frequency to a degree similar to that of muscle preparations treated with S1-P 10−4 M-filled liposomes alone (P>0.05) (n=6). Control MEPP frequency (min 0)=1.17 s−1. (B) Administration of S1-P 10−4 M-filled liposomes (L S1-P 10−4 M) to preparations pre-exposed to S1-P 10−5 entrapped liposomes (L S1-P 10−5 M) induced no significant changes in MEPP frequency (n=6). Control MEPP frequency (min 0)=0.88 s−1. In all cases, asterisks denote statistically significant differences (P<0.05) from control.
Discussion
The major observation made in this study is that extracellular application of S1-P in a concentration as high as 30 μM has no appreciable effect on neurosecretion at the frog motor nerve terminals, which is similar to that reported in PC12 cells (Alemany et al., 2001). Instead, intracellular delivery of S1-P via liposomes enhances neurotransmitter release, as evidenced by an increase in MEPP frequency. This is the first report demonstrating a second messenger role of S1-P in regulating transmitter release from the intact nerve terminals.
In DDT1MF-2 cell smooth muscle line, S1-P appears to be generated in the endoplasmic reticulum membrane (Ghosh et al., 1994) and induces Ca2+ release from thapsigargin-sensitive Ca2+ pool, via a non-IP3 receptor (Ghosh et al., 1994; Mattie et al., 1994). With respect to the site of action of S1-P within the motor nerve terminal, S1-P may enhance neurosecretion by mobilization of Ca2+ stores from SER and/or synaptic vesicles (Pezzati et al., 2001). In contrast to SER where ceramide is metabolized to S1-P, ceramide fails to produce S1-P in the synaptic vesicle (Shinghal et al., 1993). For this reason, it is unlikely that S1-P enhances neurosecretion by releasing Ca2+ from synaptic vesicles (Shinghal et al., 1993). By inference, S1-P may enhance spontaneous transmitter release by mobilizing Ca2+ from SER stores, similar to the effect of IP3 and cADPR (Brailoiu & Miyamoto, 2000; Brailoiu et al., 2001; Chameau et al., 2001). Ca2+ released from SER may, in turn, facilitate the exocytosis of synaptic vesicles. The observation that the increase in MEPP frequency occurs rapidly, starting in the first minute after liposome perfusion, suggests that S1-P activates the ‘ready releasable vesicular pool' rather than the ‘storage pool'.
Although the exact concentration of S1-P present in the nerve endings is not known and is probably 100 fold less than that in the aqueous phase, it is important to point out that S1-P produced an increase in MEPP frequencies that was concentration-dependent and was reversible, similar to that reported for liposomal delivery of IP3, cADPR or NAADP (Brailoiu & Miyamoto, 2000; Brailoiu et al., 2001).
Intracellular Ca2+ channel receptors, i.e. IP3 and NAADP, are subject to desensitization (Clapper & Lee, 1985; Lee, 2001). However, the pharmacology of desensitization appears to be different. For example, IP3 receptors are desensitized by exposure to a relatively high concentration (μM range) of IP3, whereas, NAADP receptors are desensitized by pre-exposure with a low concentration (nM range) of NAADP. Under our experimental conditions, pre-exposure of the preparations with S1-P 10−5 but not 10−8 M, entrapped in liposomes for 15 min, blocks the effects of subsequent superfusion of S1-P (10−4 M)-filled liposomes on MEPP frequency. In contrast to NAADP receptors, which undergo ‘desensitization' to a low concentration of NAADP, intracellular S1-P receptors seem to undergo ‘desensitization' to a high concentration of S1-P, as in the case of IP3. Desensitization may also explain the phasic effect in enhancing the MEPP frequency observed with liposomes containing 10−5, 10−4 or 10−3 M S1-P (see Figure 2).
At the frog neuromuscular junction, gMEPPs are described as spontaneous potentials with amplitudes of more than twice that of the average of the modal MEPPs and a slower rising phase (Alkadhi, 1988). It is of interest to note that S1-P treatment increased the number of these ‘giant' potentials. The mechanism by means of which S1-P may increase the formation of gMEPPs remains to be studied.
In conclusion, our study indicates that intracellular but not extracellular S1-P can enhance spontaneous transmitter release at the frog neuromuscular junction, by a mechanism likely involving a mobilization of intracellular Ca2+ sources.
Acknowledgments
This work was supported by NIH Grants NS18710 and NS39646 from the Department of Health and Human Services.
Abbreviations
- cADPR
cyclic ADP ribose
- MEPPs
miniature endplate potentials
- NAADP
nicotinic acid adenine dinucleotide phosphate
- S1-P
Sphingosine 1-phosphate
- SER
smooth endoplasmic reticulum
References
- ALEMANY R., KLEUSER B., RUWISCH L., DANNEBERG K., LASS H., HASHEMI R., SPIEGEL S., JAKOBS K.H., MEYER ZU HERINGDORF D. Depolarisation induces rapid and transient formation of intracellular sphingosine-1-phosphate. FEBS Lett. 2001;509:239–244. doi: 10.1016/s0014-5793(01)03168-4. [DOI] [PubMed] [Google Scholar]
- ALKADHI K.A. Emetine increases giant miniature endplate potential population at the frog neuromuscular junction. Brain Res. 1988;447:293–298. doi: 10.1016/0006-8993(88)91132-8. [DOI] [PubMed] [Google Scholar]
- BRAILOIU E., MIYAMOTO M.D. Inositol phosphates and cyclic adenosine diphosphate-ribose increase quantal transmitter release at frog nerve terminals: possible involvement of smooth endoplasmic reticulum. Neurosci. 2000;95:927–931. doi: 10.1016/s0306-4522(99)00509-6. [DOI] [PubMed] [Google Scholar]
- BRAILOIU E., MIYAMOTO M.D., DUN N.J. Nicotinic acid adenine dinucleotide phosphate enhances quantal neurosecretion at frog neuromuscular junction: possible action on synaptic vesicles in the releasable pool. Mol. Pharmacol. 2001;60:718–724. [PubMed] [Google Scholar]
- CALUPCA M.A., PRIOR C., MERRIAM L.A., HENDRICKS G.M., PARSONS R.L. Presynaptic function is altered in snake K+-depolarized motor nerve terminals containing compromised mitochondria. J. Physiol. (Lond.) 2001;532:217–227. doi: 10.1111/j.1469-7793.2001.0217g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMEAU P., VAN DE VREDE Y., FOSSIER P., BAUX G. Ryanodine-, IP3- and NAADP-dependent calcium stores control acetylcholine release. Pflugers Arch. 2001;443:289–296. doi: 10.1007/s004240100691. [DOI] [PubMed] [Google Scholar]
- CLAPPER D.L., LEE H.C. Inositol trisphosphate induces calcium release from nonmitochondrial stores in sea urchin egg homogenates. J. Biol. Chem. 1985;260:13947–13954. [PubMed] [Google Scholar]
- DIXON D., ATWOOD H.L. Conjoint action of phosphatidylinositol and adenylate cyclase systems in serotonin-induced facilitation at the crayfish neuromuscular junction. J. Neurophysiol. 1989;62:1251–1259. doi: 10.1152/jn.1989.62.6.1251. [DOI] [PubMed] [Google Scholar]
- GHOSH T.K., BIAN J., GILL D.L. Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science. 1990;248:1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- GHOSH T.K., BIAN J., GILL D.L. Sphingosine-1-phosphate generated in the endoplasmic reticulum membrane activates release of stored calcium. J. Biol. Chem. 1994;269:22628–22635. [PubMed] [Google Scholar]
- HLA T., LEE M.J., ANCELLIN N., LIU C.H., THANGADA S., THOMPSON B.D., KLUK M. Sphingosine-1-phosphate: extracellular mediator or intracellular second messenger. Biochem. Pharmacol. 1999;58:201–207. doi: 10.1016/s0006-2952(99)00086-6. [DOI] [PubMed] [Google Scholar]
- IRIE F., HIRABAYASHI Y. Ceramide prevents motoneuronal cell death through inhibition of oxidative signal. Neurosci. Res. 1999;35:135–144. doi: 10.1016/s0168-0102(99)00077-2. [DOI] [PubMed] [Google Scholar]
- LEE H.C. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Ann. Rev. Pharmacol. Toxicol. 2001;41:317–345. doi: 10.1146/annurev.pharmtox.41.1.317. [DOI] [PubMed] [Google Scholar]
- MATTIE M., BROOKER G., SPIEGEL S. Sphingosine-1-phosphate, a putative second messenger, mobilizes calcium from internal stores via an inositol trisphosphate-independent pathway. J. Biol. Chem. 1994;269:3181–3188. [PubMed] [Google Scholar]
- MEYER ZU HERINGDORF D., VAN KOPPEN C.J., JAKOBS K.H. Molecular diversity of sphingolipid signalling. FEBS Lett. 1997;410:34–38. doi: 10.1016/s0014-5793(97)00320-7. [DOI] [PubMed] [Google Scholar]
- PETERSEN O.H., CANCELA J.M. New Ca2+-releasing messengers: are they important in the nervous system. Trends in Neurosci. 1999;22:488–495. doi: 10.1016/s0166-2236(99)01456-3. [DOI] [PubMed] [Google Scholar]
- PEZZATI R., MELDOLESI J., GROHOVAZ F. Ultra rapid calcium events in electrically stimulated frog nerve terminals. Biochem. Biophys. Res. Comm. 2001;285:724–727. doi: 10.1006/bbrc.2001.5241. [DOI] [PubMed] [Google Scholar]
- SHINGHAL R., SCHELLER R.H., BAJJALIEH S.M. Ceramide 1-phosphate phosphatase activity in brain. J. Neurochem. 1993;61:2279–2285. doi: 10.1111/j.1471-4159.1993.tb07470.x. [DOI] [PubMed] [Google Scholar]
- SILINSKY E.M. The biophysical pharmacology of calcium-dependent acetylcholine secretion. Pharmacol. Rev. 1985;37:81–132. [PubMed] [Google Scholar]
- SZOKA F., JR, PAPAHADJOPOULOS D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. U.S.A. 1978;75:4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]


