Abstract
Deoxycorticosterone acetate (DOCA) salt hypertension is associated with an endothelin-1 (ET-1)-dependent increase in arterial resistance and mean circulatory filling pressure. Contraction of endothelium-intact arteries and veins from sham and DOCA-salt hypertensive rats to agonists of the ETA (ET-1(1–31)) and ETB receptor (sarafotoxin 6c; S6c) was investigated in tissue baths as was expression of mRNA for ET-1 and mRNA and protein for the ETA and ETB receptor.
ET-1(1–31) contracted aorta and vena cava from sham rats with a 30 fold lower potency than ET-1. Contraction was not altered by the ETB receptor antagonist BQ788 (100 nM) but was abolished by the ETA receptor antagonist ABT-627 (30 nM).
In DOCA-salt thoracic aorta, maximum contraction to ET-1 and ET-1(1–31) was reduced (36.6±6.3 and 13.3±4.4% of sham response, respectively); aorta did not contract to S6c.
In vena cava from DOCA-salt rats, contraction to ET-1 and ET-1(1–31) was not reduced compared to sham contraction; vena cava from sham and DOCA-salt rats contracted to S6c with a similar potency.
Real time RT–PCR revealed that prepro ET-1 mRNA was increased 6.6±3.3 fold and 8.7±3.9 fold greater in DOCA-salt aorta and vena cava, respectively, compared to sham. Vena cava expressed a higher content of ETA and ETB receptor mRNA than aorta (P<0.05), but no differences were observed between sham and DOCA-salt tissues. ETA and ETB receptor protein was identified in all tissues. Immunoreactive ETA receptor, observed as a 65, 30 and 28 kDa bands, was expressed 400% greater in DOCA-salt aorta compared to sham, but was not altered in vena cava. Immunoreactive ETB receptor, observed as 120, 45 and 30 kDa bands, tended to be higher in vena cava compared to aorta, but was not different in sham and DOCA-salt vena cava.
These results suggest that ETA receptor function is impaired in aorta but not vena cava of DOCA-salt rats. The ETB receptor was present in the aorta but, unlike in veins, does not mediate contraction directly. A sustained response to ET-1 in the venous circulation may contribute to the elevated blood pressure in the DOCA-salt model.
Keywords: ET-1, ET-1(1–31), venous smooth muscle, mineralocorticoid hypertension
Introduction
The DOCA-salt model of hypertension is associated with elevated arterial levels of the peptide endothelin (ET-1) (Lariviere et al., 1993). ET-1 has been implicated in multiple cardiovascular functions and diseases, but recent evidence suggests a significant involvement in hypertension (Li et al., 1994; Verhagen et al., 1998; Matsumura et al., 1999; Schiffrin, 2001). The endothelins combine with two receptor subtypes to exert their physiological effect, the ETA and ETB receptors. The ETB receptor can be activated selectively by sarafotoxin 6c (S6c) and antagonized by BQ788. Several receptor antagonists, including ABT-627 and BQ610, are selective for the ETA receptor and recently the chymase-derived peptide ET-1(1–31) has been described as a relatively selective agonist of ETA receptors (Hanson et al., 1997; Nagata et al., 2000; Maguire et al., 2001).
We are interested in changes in venous function that may contribute to the elevated blood pressure observed in the DOCA-salt model. We and others have demonstrated that endothelin receptor antagonists reduce total peripheral resistance in the DOCA-salt rat and have recently observed that endothelin receptor antagonists also reduce mean circulatory filling pressure, indicating a possible contribution of increased venous smooth muscle contractility to determination of blood pressure in the DOCA-salt model (Fink et al., 2000; Johnson et al., 2001). Notably, veins are more sensitive to contraction induced by ET-1 than are arteries. This increased sensitivity, observed as a lower threshold concentration of ET-1 and higher potency, has been observed by several groups; the contractile potency of ET-1 in veins has been reported as anywhere from 3–10 times more than in arteries (Cocks et al., 1989; D'orleans-Juste et al., 1989). Importantly, small unpressurized mesenteric veins from the DOCA-salt rat maintain their responsiveness to ET-1 whereas arteries from the same site have a depressed contraction to ET-1 (Nguyen et al., 1992; Fujita et al., 1995; Laurant & Berthelot, 1996; Zhao et al., 2000; Johnson et al., 2002).
Thus, we currently test the hypothesis that veins maintain endothelin responsiveness in the face of overexpression of ET-1. We used large arteries and veins for two reasons. First, this was done to test the principle that the maintained responsiveness is occurring in large veins as it is in small veins. Second, arteries and veins of this size allow us to perform biochemical experiments to measure mRNA and protein for the ETA receptor and ETB receptor.
Methods
Model of hypertension
Male Sprague-Dawley rats (0.225–0.250 kg; Charles River, Portage, MI, U.S.A.) were uninephrectomized and deoxycorticosterone acetate (DOCA, 200 mg kg−1 in silicone rubber) implanted subcutaneously. Postoperatively, the rats were given a solution of 1% NaCl and 0.2% KCl for drinking. Sham normotensive rats were uninephrectomized, received no DOCA and drank normal tap water. All rats were given free access to standard pelleted rat chow (Harlan/Teklad 8640 rodent diet). Animals remained on this regimen for 4 weeks prior to use.
Blood pressure measurement
Systolic blood pressures of rats were determined in the conscious state by the tail cuff method (pneumatic transducer, Narco, TX, U.S.A.).
Isolated smooth muscle contractility measurement
Rats were deeply anaesthetized with pentobarbitone (50 mg kg−1, i.p.) to the point of a loss of eyelid reflex and lack of withdrawal from painful stimuli. Aorta and vena cava were placed in physiologic salt solution consisting of (in mM) NaCl 130; KCl 4.7; KH2PO4 1.18; MgSO4-7H2O 1.17; CaCl2-2H2O 1.6; NaHCO3 14.9; dextrose, 5.5; and CaNa2EDTA, 0.03. Aorta and vena cava were cleaned of fat and connective tissue, left with an intact endothelium, mounted as rings (3–4 mm long) on stainless steel hooks and placed on stainless steel holders in tissue baths (30 ml) for isometric tension recordings using Grass polygraphs and transducers (Astro-Med, West Warwick, RI, U.S.A.) or PowerLab® for the Macintosh (ADInstruments, Dover, NH, U.S.A.). Tissues were placed under optimum resting tension (4000 mg for aorta, 1000 mg for vena cava; determined in preliminary experiments). Tissues from sham and DOCA-salt rats were placed in the same bath, controlling for experimental variations. Muscle baths were filled with warmed (37°C), aerated (95% O2, 5% CO2) physiological salt solution. Tissues were challenged with a maximal concentration of α adrenergic agonist (noradrenaline for vein, phenylephrine for artery). Phenylephrine was not used in the vena cava as it could not stimulate a reproducible contraction in our hands. Phenylephrine was, however, used for aorta so as to be able to relate these findings to those generated from our laboratory in the past. Functional integrity of the endothelial cells was evaluated by testing relaxation caused by acetylcholine (1 uM) in strips contracted with α adrenergic agonist (10–100 nM). Cumulative concentration response curves to agonists were generated. Endothelin receptor agonists contract tissues slowly, so tissues were exposed to each concentration of endothelin receptor agonist (ET-1, S6c, ET-1(1–31)) for a minimum of 5 min prior to adding a higher concentration of agonist. Antagonists, inhibitors or vehicle incubated with vessels for 1 h prior to addition of agonists.
Real time RT–PCR
Two step RT–PCR was performed using a GeneAMP 5700 Real Time PCR machine (Applied Biosystems, Foster City, CA, U.S.A.). Total RNA was isolated using standard TRIzol® procedures (GIBCO Life Technologies, Rockville, MD, U.S.A.). Vena cava and thoracic aorta from both sham and DOCA-salt animals were isolated concurrently. Vena cava from three animals were pooled so that sufficient RNA could be isolated. Concentration/purity/integrity of RNA was ascertained spectrophotometrically (A260/A280) and by running a qualitative 1% agarose gel to visualize samples with ethidium bromide (18S/28S check). Two micrograms of total RNA were reverse transcribed using a Taqman® reverse transcriptase kit (Applied Biosystems, Foster City, CA, U.S.A.; buffer, MgCl2 5.5 mM, dNTP 500 uM, of each random hexamer 2.5 uM, RNase inhibitor 0.4 u ul−1 and MultiScribe Reverse Transcriptase 1.25 u ul−1; 10 min hold at 25°C, 30 min hold at 48°C, 5 min hold at 95°C). Samples were always paired with ones in which reverse transcriptase was not included. One-tenth of this cDNA was taken through polymerase chain reaction using a SYBR® Green Master Mix (Applied Biosystems, Foster City, CA, U.S.A.), forward primer and reverse primer for 40 cycles [AmpliTAQ® activation, 10 min hold at 95°C (hot start); cycle=15 s 95°C, 60 s 60°C]. Primer concentration (25 pM–500 nM) was optimized and primers designed using Primer Express® 1.5 (Applied Biosystems, Foster City, CA, U.S.A.) and checked for selectivity using BLAST® within the program MacVector® 1.5 (Accelrys, San Diego, CA, U.S.A.). Samples from sham and DOCA-salt tissues were run in parallel as well as samples containing primers for GAPDH to use as a calibrator reference. Samples without cDNA were also taken along as no template controls. A dissociation protocol (60–95°C melt) was performed at the end of the experiment to verify that one product was present and melted at the appropriate temperature. Final PCR product was examined on a 2% agarose gel stained with ethidium bromide (0.5 ug ml−1) to determine that the appropriately sized amplicon was amplified. Gels were visualized and captured using a Bio-Rad® Fluor-S imaging station (Hercules, CA, U.S.A.). Rodent-derived primers that were used are GAPDH forward, reverse (purchased from Applied Biosystems, Foster City, CA, U.S.A.) with a Tm of 86°C and 228 base pairs (bp). All other primers were synthesized by the Macromolecular Structures and Synthesis Facility at Michigan State University; ET-1 forward=TCT GGG TCA ACA CTC CCG A, ET-1 reverse=AGG ATC GCT TAG ACC TAG AAG GG (Tm=83°C, 69 bp); ETA forward=GGA ATG GGA GCT TGC GG; ETA reverse=TTT GCC ACC TCT CGA CGC (Tm=84°C, 61 bp); ETB forward=CAA AGG AGG GAG GGT GGC, ETB reverse=CAA TTT TTC GTT GGC ACG G (Tm=84°C, 65 bp).
Western analysis
Tissues were isolated directly from the animal, cleaned and placed directly into liquid nitrogen. In liquid nitrogen, tissues were ground to a powder and ice-cold homogenation buffer added [1 ml for one-half an aorta; 0.2 ml for one vena cava; 125 mM Tris (pH 6.8), 4% SDS, 20% glycerol, 0.5 mM phenylmethylsulphonyl fluoride, 1 mM orthovanadate, 10 ug ml−1 aprotinin, 10 ug ml−1 leupeptin]. Homogenates were vortexed, sonicated briefly and transferred to a plastic centrifuge tube and spun at 4°C to pellet debris. Supernatant was separated from the pellet and analysed for protein concentration (BCA protein kit, Sigma Chemical Co., St. Louis, U.S.A.). Eighty micrograms of total protein for measurement of the ET receptors were heated at 37°C for 30 min with standard 4 : 1 sample buffer. This approach was taken to minimize aggregation of the heptahelical receptors. Proteins were separated on 1 mm-thick, 12% SDS polyacrylamide gels using a Mini Bio-Rad III apparatus. Membranes were blocked overnight in 5% milk (4°C, phosphate buffered saline +0.025% NaN3). Primary antibodies (1 : 200 for ETA and 1 : 1000 for ETB receptor from Alomone Laboratories, Jerusalem, Israel) incubated with blots from 48 h. In some experiments, the antibodies were quenched with a competing peptide (microgram/microgram; CP) so as to visualize those bands identified specifically by the antibody. Blots were then rinsed three times in Tris-buffered saline (TBS)+Tween (0.1%) with a final rinse in TBS and incubated with an horseradish peroxidase-linked antirabbit secondary antibody (1 : 2000; Cell Signaling Technology, Beverly, MA, U.S.A.) for 1 h at 4°C (rocking). ECL® reagents (Amersham Life Sciences, Arlington Heights, IL, U.S.A.) were used to visualize bands. Gels were stained with Gel Code Blue® (Pierce, Rockford, IL, U.S.A.) to verify protein loading and blots were reprobed with smooth muscle α-actin primary antibody (1 : 1000; Oncogene Research Products, Boston, MA, U.S.A.) to ensure equal protein loading.
Data analysis
Data are presented as means±standard error of the mean for the number of animals. Contraction is reported as force (milligrams) or as a percentage of response to maximum contraction or phenylephrine/noradrenaline (10 uM). pD2 values (negative logarithm of the agonist concentration necessary to produce a half-maximal response [M]) were determined using non-linear regression analysis in GraphPad Prism® (San Diego, CA, U.S.A.). When comparing two groups, the appropriate Student's t-test was used or, in concentration response curves, ANOVA with repeated measures. ANOVA followed by Student Newman Keuls post hoc test was performed when comparing three or more groups. In all cases, a P value less than or equal to 0.05 was considered statistically significant. Band density was quantified using the public domain program NIH Image (v 1.62). For RT–PCR, an n of 1 for vena cava actually represent three animals whose tissues were pooled. CT values were derived as the threshold cycle at which product was first detected and are reported as cycle numbers.
Materials
ET-1 and sarafotoxin 6c were obtained from Peninsula Laboratories (Belmont, CA, U.S.A.). ET-1(1–31) was obtained from Peptide International (Lexington, KY, U.S.A.). These peptides were solubilized in deionized water or 0.1% acetic acid. BQ788 (solubilized in dimethylsulphoxide) was purchased from Peninsula Laboratories (Belmont, CA, U.S.A.) and ABT-627 (dimethylsulphoxide) was a gift from Abbott Laboratories. Acetylcholine chloride, deoxycorticosterone acetate, noradrenaline hydrochloride and phenylephrine hydrochloride were solubilized in water and purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.).
Results
Blood pressures
For all studies, rats were on DOCA-salt for 4 weeks priorto experimentation. Systolic blood pressures were 120±2 mmHg in sham rats (n=48) and DOCA-salt rats were hypertensive (n=48; 192±9 mmHg; P<0.05). We validated that the endothelium was intact in these preparations as the muscarinic agonist acetylcholine (1 uM) relaxed sham vena cava (60.1±8.7% relaxation) and DOCA-salt vena cava (59.1±9.6; P>0.05) which were contracted with a half-maximal concentration of α adrenoreceptor agonist. As expected, acetylcholine relaxed the DOCA-aorta (29.1±12%) significantly less than in sham aorta (62.2±6.2%; P<0.05) as endothelium-dependent arterial relaxation is reduced in this model (Lockette et al., 1986).
Effect of ET-1
Figure 1a depicts vessel contraction to ET-1 as a percentage of initial contraction to phenylephrine (PE) or noradrenaline (NA) (left) and as a percentage of maximum contraction (right) for aorta and vena cava. ET-1 contracted all vessels but a few distinct and important differences were observed. First, the vena cava of sham and DOCA-salt animals had a lower threshold for ET-1 than in aorta (−log threshold [M] aorta=9.25±0.20, vena cava=10.43±0.22; P<0.05 using grouped data). Second, ET-1 was 2.5 times more potent in vena cava (pD2 [M]=8.29±0.03) than in aorta (7.99±0.08; P<0.05). Significantly, the maximum response of the aorta to ET-1 was markedly diminished in DOCA-salt tissue as compared to sham tissues (upper left). This decrease was not due to a change in the affinity of ET receptors present as the potency of ET in aorta from DOCA-salt and sham rats was not different (upper right). By contrast, the efficacy of ET-1 was not diminished in the vena cava of the DOCA-salt rat when compared to the sham (lower left), nor did the potency of ET-1 change (lower right). Thus, these data suggest that ET receptors or contractile elements used by ET receptors are desensitized in the aorta but not in vena cava.
Figure 1.
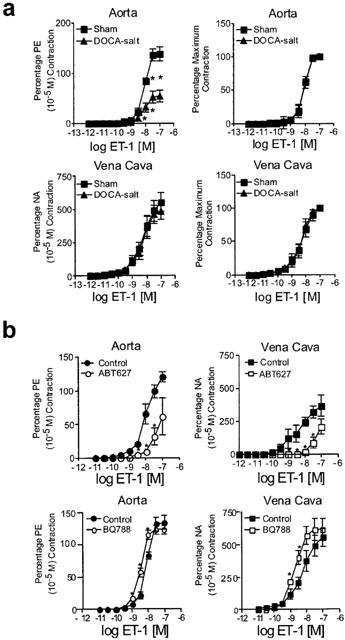
(a) Effect of endothelin-1 (ET-1) on aorta and vena cava. Data are presented as means±s.e.mean and as a percentage of an initial phenylephrine (PE) or noradrenaline (NA) response (left) or as a percentage maximal response (right) for an n of 5–6 animals. (b) Effect of ETA receptor antagonist ABT-627 (30 nM; top) and ETB receptor antagonist BQ788 (100 nM; bottom) on ET-1-induced contraction in aorta (left) and vena cava (right) isolated from sham normotensive rats. Data are presented as means±s.e.mean and as a percentage of an initial phenylephrine (PE) or noradrenaline (NA) response (left) for an n of 5–6 animals. * P<0.05, ANOVA repeated measures.
Effect of ET receptor antagonists on ET-1-induced contraction
To establish the subtype of receptor primarily responsible for mediating ET-1-induced contraction in the normotensive state, we performed experiments examining the ability of the ETA receptor antagonist ABT-627 (30 nM) and ETB receptor antagonist BQ788 (100 nM) to alter ET-1-induced contraction. ABT-627 antagonized contraction to ET-1 in both normal aorta and vena cava (Figure 1b). By contrast, the ETB receptor antagonist BQ788 did not reduce but significantly potentiated contraction to ET-1 in both aorta (Figure 1b, left) and vena cava (Figure 1b, right). This was reflected by a significant change in the pD2 value for ET-1 [aorta control: 8.00±0.12; aorta BQ788: 8.45±0.12, P<0.05; vena cava control: 8.23±0.03; vena cava BQ788 8.72±0.06, P<0.05]. These studies indicate that in both aorta and vena cava, the ETA receptor is the predominant contactile receptor and the ETB receptor modestly depresses contraction to ET-1.
Effect of noradrenaline
We next examined contraction to the α adrenoceptor agonist noradrenaline in contractile experiments as a comparative agonist to ET-1. The adrenoceptor and endothelin receptors use similar signalling elements. Aortae from the DOCA-salt hypertensive rat were more sensitive to NA than aorta from sham rats (pD2 [M] sham=7.42±0.07; DOCA-salt=7.9±0.03; P<0.05) (Figure 2a). The maximum aortic response to NA was not altered in DOCA-salt hypertension. The efficacy of NA was not altered in DOCA-salt hypertension in vena cava, nor was the potency of NA in the vena cava altered (pD2 [M] sham=6.3±0.05; DOCA-salt=6.3±0.12; P>0.05). These data support that the decrease in response to ET-1 in the aorta was not because of general hyporeactivity of aorta from DOCA-salt hypertensive rats.
Figure 2.
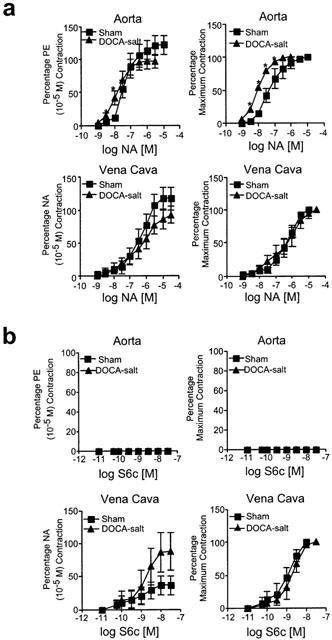
(a) Effect of noradrenaline (NA) on aorta and vena cava. Data are presented as means±s.e.mean and as a percentage of an initial phenylephrine (PE), or noradrenaline (NA) response (left) or as a percentage maximal response (right) for an n of 5–6 animals. (b) Effect of sarafotoxin 6c (S6c) on aorta and vena cava. Data are presented as means±s.e.mean and as a percentage of an initial phenylephrine (PE) or noradrenaline (NA) response (left) or as a percentage maximal response (right) for an n of 5–6 animals. *P<0.05, ANOVA repeated measures.
Effect of sarafotoxin 6c
Figure 2b demonstrates that S6c was without activity in aorta of either sham or DOCA-salt rats, indicating that the ETB receptor does not play a direct role in modifying contraction. However, S6c caused a concentration-dependent contraction in the vena cava of both sham and DOCA-salt rats; in small unpressurized veins, we have demonstrated the ability of BQ788 to block S6c-induced contraction (Fink et al., 2000; Johnson et al., 2001). The maximum contraction to S6c was variable, and was approximately 10–15% that elicited by ET-1. Neither the potency (pD2 [M] sham=9.14±0.14; DOCA-salt=8.76±0.09) or maximum contraction to S6c in the vena cava was different under conditions of DOCA-salt hypertension. Thus, these findings are consistent with veins but not arteries possessing ETB receptors which directly mediate contraction and venous smooth muscle ETB receptor being maintained in DOCA-salt hypertension.
Effect of ETA receptor agonist ET-1(1–31)
In related experiments, we examined arterial and venous contraction to the peptide ET-1(1–31) as a selective agonist of ETA receptors. Preliminary experiments in vena cava and aorta from animals with normal blood pressure were performed to validate the selectivity of this peptide. Both vena cava and thoracic aorta from normotensive rats contracted to ET-1(1–31) (100 nM) to approximately 50–60% of the maximal response to ET-1 (Figure 3a). ET-1(1–31)-induced contraction in both vessels could not be reduced by the ETB receptor antagonist BQ788 but was abolished by the ETA receptor antagonist ABT-627 (Figure 3a,b). These data support ET-1(1–31) as a selective agonist for the ETA receptor.
Figure 3.
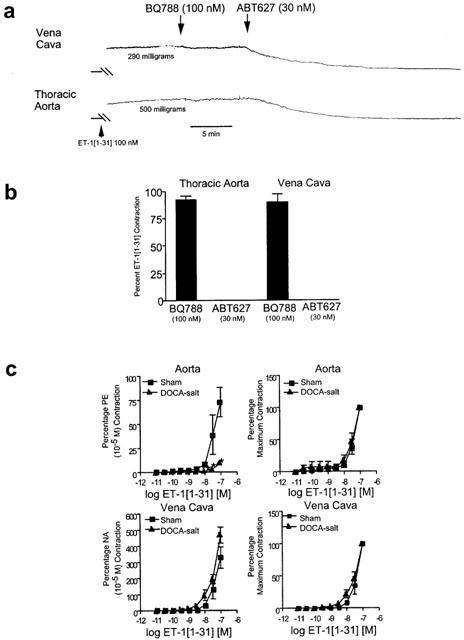
(a) Tracing of vena cava and aortic response to ET-1(1–31) in the presence of the ETB receptor antagonist BQ788 (100 nM) or ETA receptor antagonist ABT-627 (30 nM). (b) Quantitation of effect of receptor antagonists on contraction induced by ET-1(1–31) (100 nM). Bars represent±s.e.mean for n=4–6 animals. (c) Effect of ET-1(1–31) on aorta and vena cava. Data are presented as means±s.e.mean and as a percentage of an initial phenylephrine (PE) or noradrenaline (NA) response (left) or as a percentage maximal response (right) for an n of six animals. *P<0.05, ANOVA repeated measures.
We next compared the response of tissues from normotensive and hypertensive animals. Figure 3c, left shows that aorta from DOCA-salt rats were virtually unresponsive to ET-1(1–31). These tissues are clearly functional as they contracted to phenylephrine (10 uM) to a magnitude similar to that of all other aorta from DOCA-salt rats. By contrast, the concentration-dependent contraction elicited by ET-1(1–31) in vena cava from DOCA-salt rats was similar to that observed in vena cava from normotensive sham rats (Figure 3c). Thus, it is clear that ETA receptor function in aorta but not vena cava is severely depressed in DOCA-salt hypertension. This depressed functional response has been proposed to occur through desensitization of the ETA receptor because of overexpression of ET-1 (Nguyen et al., 1992; Schiffrin, 2001). To determine whether ET-1 overexpression occurs in the vena cava, we performed real time RT–PCR to measure prepro ET-1.
Real time RT–PCR for prepro ET-1, ETA and ETB receptor mRNA
Figure 4a depicts ethidium bromide-stained products of real time RT–PCR for prepro ET-1 mRNA. We observed one product of the expected size (69 bp) and melting temperature (TM=83) and product was observed in all samples. GAPDH was used as a calibrator control. We confirm previous findings in the arteries that preproET-1 mRNA was increased in aorta from DOCA-salt rats as compared to sham, and demonstrate that prepro ET-1 mRNA was elevated in the vena cava from DOCA-salt rats as compared to sham. When normalized to the CT value of GAPDH, DOCA-aorta expressed 6.6±3.3 and DOCA-vena cava 8.7±3.9 times greater prepro ET-1 mRNA than their corresponding sham tissues (P<0.05). Thus, while the cellular site of increased prepro ET-1 mRNA cannot be pinpointed in this experiment, prepro ET-1 mRNA levels were increased on both the arterial and venous side.
Figure 4.
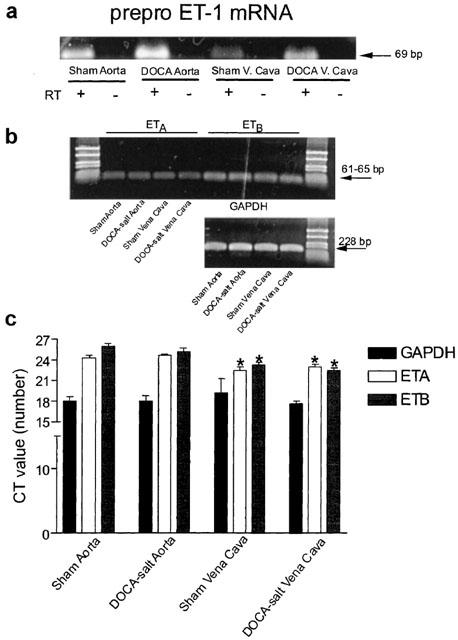
(a) Final product of real time RT–PCR performed for prepro ET-1 (40 cycles, 200 nM primers). Half of the final PCR product was used for this gel (2% agarose). RT=reverse transcriptase. Representative of three separate isolations performed in duplicate. (b) Final product of real time RT–PCR performed for ETA, ETB and GAPDH mRNA in sham and DOCA-salt aorta and vena cava run on 2% agarose and stained with ethidium bromide (40 cycles, 200 nM primers). Half of the final PCR product was used for this gel. Representative of four separate isolations performed in duplicate. (c) Quantitation of CT values for GAPDH, ETA and ETB receptor mRNA in experimental broups. Bars represent±s.e.mean for n=4 performed in duplicate. *Indicates statistically significant differences (P <0.05) vs aortic comparison within experimental group.
With a decreased arterial contraction to ET-1 and ET-1(1–31), one explanation for the decrease in arterial response to ET-1 in the arteries as compared to veins is that ET receptors may be downregulated under conditions of DOCA-salt hypertension in the arteries only. Using the same cDNA as used in experiments displayed in Figure 4a, we performed real time RT–PCR to measure expression of ETA and ETB receptor mRNA in arteries and veins. Figure 4b displays a visualized ethidium bromide-stained gel for the ETA receptor, ETB receptor and GAPDH. Messenger RNA for the ETA and ETB receptor was observed in all tissues and the CT values for GAPDH were statistically similar (P>0.05) between all four groups. When comparing sham vena cava to sham aorta and DOCA-salt vena cava to DOCA-salt aorta, vena cava from either the sham or DOCA-salt rat possesses a significantly higher density of the ETA and ETB receptor mRNA, as indicated by the lower CT values (Figure 4c). However, there was no significant difference in ETA and ETB receptor mRNA density in DOCA vs sham when comparing between tissue type.
Western analyses for ETA and ETB receptor protein
Western analyses were performed to determine if there were differences in protein density and Figures 5 and 6 show these results. Preliminary experiments using a competing peptide for either the ETA or ETB receptor were first performed to determine the bands of interest. Only those proteins that were reproducibly competed against were considered as specific for the antibody. For the ETA receptor, three bands of approximately 65, 30 and 28 kDa were eliminated reproducibly in the presence of competing peptide (Figure 5a, bands that are boxed); a 37–39 kDa protein was also observed, but was not reproducibly competed against. For the ETB receptor, bands at 120, 45 and faint triplets with the highest at 30 kDa were eliminated in the presence of competing peptide (Figure 6a, bands that are boxed). The pattern of each antibody was distinctive and the ETB receptor competing peptide did not block the ETA receptor antibody function and vice versa (data not shown). Western analyses revealed the presence of the ETA and ETB receptor in all tissues (Figures 5b, 6b) but with several notable differences. First, the ETA receptor was, for the same micrograms of total protein, observed as three bands in aorta but only one in vena cava. ETA receptor density, whether quantified using the 65, 30 or 28 kDa band, was approximately 300–400% greater in the aorta from DOCA-salt as compared to aorta from sham (Figure 5c); such a difference was not observed for the single band detected by the ETA receptor in vena cava from sham and DOCA-salt rats (Figure 5c). Second, for the same micrograms of total protein, the ETB receptor tended to be more densely expressed in vena cava when compared to aorta but was expressed in both vessel types (Figure 6b,c). When comparing sham to DOCA-salt, aortic expression of the ETB receptor was similar except for a small increase of the 45 kDa band (Figure 6c).
Figure 5.
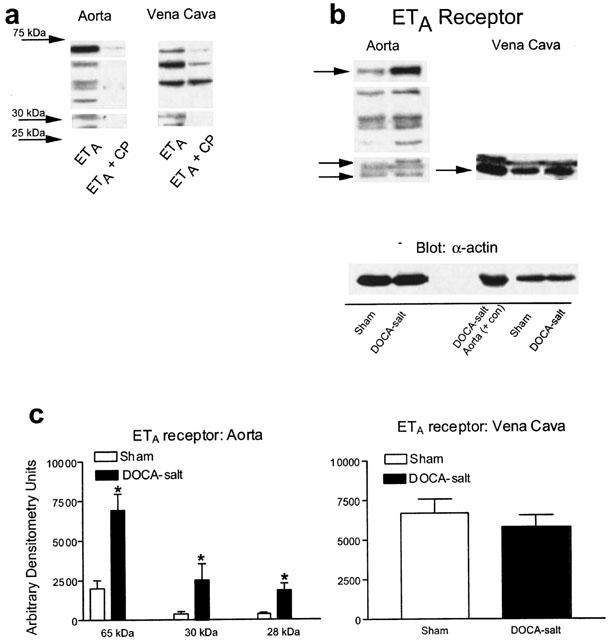
(a) Visualized Western blot showing immunoreactive bands that drop out in the presence of a competing peptide for the ETA receptor. (b) Representative blots for the ETA receptor in aorta and vena cava of sham and DOCA-salt rats. (c) Densitometry results for ETA receptor immunoreactive bands in aorta and vena cava. Bars represent means±s.e.mean for seven to eight animals. *Indicates statistically significant differences (P<0.05) in sham vs DOCA-salt comparison.
Figure 6.
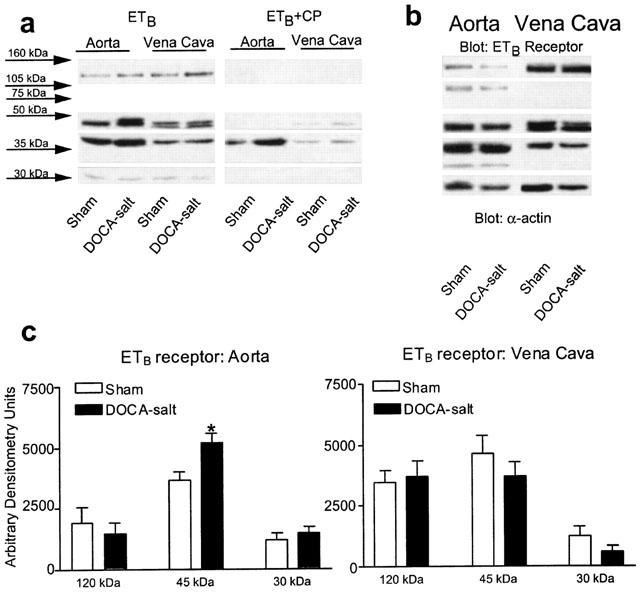
(a) Visualized Western blot showing immunoreactive bands that drop out in the presence of a competing peptide for the ETB receptor. (b) Representative blots for the ETB receptor in aorta and vena cava of sham and DOCA-salt rats. (c) Densitometry results for ETB receptor immunoreactive bands in aorta and vena cava. Bars represent means±s.e.mean for seven to eight animals. *Indicates statistically significant differences (P<0.05) in sham vs DOCA-salt comparison.
Discussion
These studies were undertaken to determine whether arteries and veins display similar changes in responsiveness to ET-1 in DOCA-salt hypertension, with the long term goal of understanding the potential role of the venous circulation in contributing to the elevation of blood pressure. The DOCA-salt model was the model of choice as it has been well established as ET-1-dependent (Lariviere et al., 1993; Matsumura et al., 1999; Schiffrin, 2001). We found aortic contraction to ET-1 significantly depressed in DOCA-salt hypertension. This is in agreement with previous work demonstrating a decreased arterial contraction to ET-1 (Nguyen et al., 1992; Fujita et al., 1995; Laurant & Berthelot, 1996; Zhao et al., 2000; Johnson et al., 2002). This was in marked contrast to the unchanged ET-1-induced contraction in vena cava. A majority of the experiments performed were done to determine whether the pharmacology of the receptor response between artery and vein, sham and DOCA, was similar and to begin to investigate potential mechanisms for a maintained venous response to ET-1.
Arterial responsiveness
The predominant receptor present in arterial tissue appears to be the ETA receptor. This is supported by functional studies in multiple different arteries from several species (rat thoracic aorta (White et al., 1993), rat mesenteric artery, rabbit carotid artery (Calo et al., 1996), human coronary artery, human pulmonary artery, rabbit femoral artery (Eguchi et al., 1997) and human pial artery (Pierre & Davenport, 1998). ET-1 causes a contraction with greater potency than ET-3 and the ETB receptor agonist S6c is virtually inactive in these arteries. By contrast, in coronary and pulmonary arteries, the ETB receptor appears to play a direct role in modulating contractility (Teerlink et al., 1994; Calo et al., 1996; White et al., 1993) and some arteries show a mixed response (Bax et al., 1994; Lodge et al., 1995; Mickley et al., 1997). In our experiments, it is clear that under normal conditions the ETA receptor is important to ET-1-induced contraction in both aorta and vena cava. This is supported by the significant blockade of ET-1-induced contraction by the ETA receptor antagonist ABT-627 and the concentration-dependent contraction elicited by the ETA receptor agonist ET-1(1–31).
A recent elegant study by Maguire suggests that ET-1(1–31) may have affinity for the ETB receptor in the human heart (Maguire et al., 2001). Our functional studies and those of others were not able to find a significant interaction of ET-1(1–31) with the ETB receptor, and thus we used this peptide as a relatively selective agonist of the ETA receptor. As such, it is important that contraction to ET-1(1–31) was reduced in aorta from DOCA-salt hypertensive rats. Because the aorta has a significant ETA receptor component, the reduced contraction to ET-1(1–31) suggests a downregulation in the ETA receptor. However, real time RT–PCR demonstrated the presence of a similar amount of ETA receptor mRNA in sham and DOCA-salt aorta, and Western analyses revealed that the ETA receptor was more densely expressed in aorta from DOCA-salt as compared to sham arteries, regardless of whether putative monomers (28 and 30 kDa) or potential oligomeric bands were measured. These findings indicate that the ETA receptor, while present, is uncoupled from contraction in the aorta in DOCA-salt hypertension. Reduced arterial contraction to ET-1 and increased or normal receptor binding has been observed in the coronary artery of the DOCA-salt rat (Giulumian et al., 2002). Moreover, in ETB receptor deficient rats, plasma ET-1 is increased as it is in the DOCA-salt rat but ETA receptor-mediated contraction in small mesenteric arteries was reduced compared to normal rats, but the ETA receptor membran protein density was increased (Perry et al., 2001). Thus, our findings are consistent with those of others. It is important, however, to understand the limitations of the Western experiments herein, and these limitations are true for experiments measuring both ETA and ETB receptor density. First, we have used a whole tissues preparation as opposed to a membrane preparation. Thus, we may have detected proteins located intracellularly in the process of degradation and thus observed proteins smaller (28, 30 kDa) than the size reported for use with this antibody (39 kDa and approximately 58–60 kDa). Second, we did not boil the homogenates prior to loading on the gel but instead warmed the samples at 37°C for 30 min so as to minimize oligomeric formation. It is possible that full receptors were degraded during this time. Thus, the measures of protein we have done represent a picture of the ET proteins in all aspects, not just that at the membrane level. It may be useful to perform radioligand binding studies to measure the membrane and thus functional ETA receptor population, but there are inherent difficulties with such an experiment. It is difficult to choose a receptor antagonist, which is ideal for such studies, with which to label the receptors because evidence suggests that the ETA and ETB receptor may interact physically and alter the pharmacological sensitivity of the ETA receptor to blockade by ETA receptor antagonists (Lodge et al., 1995). While agonists could certainly be used as radioligands, they are fraught with their own difficulties. Thus, these studies represent an initial step in measuring protein density.
Another important finding is that mRNA and protein for the ETB receptor is present in the aorta. The ETB receptor may be located in the endothelial cell or smooth muscle cell because endothelial-cell intact homogenates were made for isolation of mRNA and protein; future immunohistochemical studies will enable this distinction. Evidence supporting a direct function of the ETB receptor in arteries is limited. The ETB receptor, in an endothelium-dependent manner, may mediate ET-1 and/or ET-3-induced relaxation in several arteries (Karaki et al., 1993; Magazine & Srivastava, 1996). The aorta of either the sham or DOCA-salt rat does not possess a contractile ETB receptor as S6c was inactive. However, blockade of the ETB receptor with BQ788 resulted in a small but significant leftward shift in ET-1-induced contraction in arteries, suggesting that the ETB receptor can modulate contraction, either through blocking ETB receptor-mediated vasorelaxation or by clearing ET-1 from the extracellular fluid (Sato et al., 1995; Pollock, 2001). It is difficult to determine which of these functions of the ETB receptor is responsible. These two possibilities could not be distinguished even with removal of the endothelium since this cell type mediates both ET-1-induced relaxation and clearance of ET-1. Given the fact that endothelial cell mass is small compared to smooth muscle mass in the aorta, it is likely the ETB receptor is expressed in aortic smooth muscle. While the ETB receptor does not appear to mediate contraction directly, the receptor may serve other functions in the aorta.
Venous responsiveness
Both the ETA and ETB receptor mediate contraction in venous smooth muscle. This mixed receptor population has been reported for the rabbit saphenous vein (Douglas et al., 1995; Sudjarwo & Karaki, 1995), rabbit portal vein (Wang et al., 1997), rabbit jugular vein and human saphenous vein (Wang et al., 1997). There are exceptions such as the dog pial vein in which the predominant receptor appears to be the ETA receptor (Fernandez et al., 1995). Presumably, ETB receptors are present in both smooth muscle and endothelial cells. In the vena cava, both ETA and ETB receptors directly modulate contraction as evidenced by concentration-dependent contraction to the ETA receptor agonist ET-1(1–31) and the ETB receptor agonist S6c. Interestingly, while the ETA receptor antagonist ABT-627 antagonized ET-1-induced contraction in vena cava, the ETB receptor antagonist BQ788 did not; in fact, BQ788 enhanced contraction to ET-1 in vena cava. One interpretation of these data is that the clearance function of the ETB receptor may overwhelm the contractile function of the ETB receptor such that the net effect observed is a potentiation of contraction to ET-1 rather than a reduction. There is also the possibility that ETA and ETB receptors may influence the function of one another (Lodge et al., 1995; Fukuroda et al., 1996; Adner et al., 2001). The ETA receptor, as evidenced by contractile and immunoblot analysis, is expressed and functional in mediating contraction in the vena cava of the DOCA-salt rat. By contrast, the ETA receptor in the aorta of the DOCA-salt rat is highly expressed but dysfunctional in mediating contraction. One explanation for this difference may be that the aorta is exposed to a higher level of ET-1 than the vein, causing desensitization in the aorta but not in the vein. The relative amount of prepro ET-1 mRNA was elevated in the DOCA-salt vena cava compared to the sham, indicating that both vena cava and aorta have the potential of higher than normal concentrations of ET-1. Recent work by Wang et al. (2002) demonstrated that while aortic ET-1 content was increased in DOCA-salt hypertension, vena cava ET-1 was not. However, basal levels of ET-1 in vena cava were higher than in the aorta such that levels of ET-1 in vena cava and DOCA-salt aorta are similar. One can speculate that ET-1 may actually be secreted at a higher rate in veins compared to arteries, and this would not be reflected when measuring ET-1 content.
Speculation
The question remains as to why veins and arteries respond to ET-1 so differently in DOCA-salt hypertension. We speculate that the ETB receptor, present and functional in the smooth muscle of the vena cava but not in the aorta, may protect the tissue from desensitization to ET-1, as the ETB receptor serves as a receptor of clearance and is degraded rapidly within lysosomes (Bremnes et al., 2000). It is clear that the ETB receptor is present in both tissue types, but the coupling or processing of the ETB receptor must be significantly different in a vein and artery for the differences we have observed to occur. Moreoever, the recycling/handling of the ETA receptor in arteries and veins is necessarily different in the condition of DOCA-salt hypertension given the observed results.
A maintained response to ET-1 is important not only to contraction elicited by ET-1, but in the ability of ET-1 to facilitate or potentiate contraction to important modulators of venomotor tone such as noradrenaline (Shimamoto et al., 1992; Kita et al., 1998). We speculate that the ETB receptor may be important in hypertension as S6c, given chronically, induces a hypertension that cannot be reduced by the ETA receptor antagonist ABT-627 (Fink et al., 2001). These studies suggest that activation of the ETB receptor can result in hypertension, but we have yet to prove whether the ETB receptor is necessary for DOCA-salt hypertension. Another interesting finding was the observation of potential multimers or high molecular weight complexes of the ET receptors and this will be the focus of future studies.
In summary, the ETA receptor is the primary receptor mediating ET-1-induced contraction in aorta and vena cava from normotensive rats. Additionally, the vena cava has a ETB receptor component relevant directly to contraction as the ETB receptor agonist S6c contracts veins but not arteries. Under the condition of DOCA-salt hypertension, contraction elicited by either ET-1 or the ETA receptor agonist ET-1(1–31) was profoundly depressed in the aorta but not in the vena cava; ETA receptor density was increased in DOCA-salt aorta but not in DOCA-salt vena cava. Both vessel types appear to have increased prepro ET-1 mRNA levels in DOCA-salt hypertension and express the ETB receptor. The presence of a functional ET receptor in veins, in the face of dysfunctional receptors in arteries, may be permissive for maintaining responsiveness to ET-1 in DOCA-salt hypertension.
Acknowledgments
NIH HL58489.
Abbreviations
- DOCA
deoxycorticosterone acetate
- ET-1
endothelin-1
- NA
noradrenaline
- PE
phenylephrine
- S6c
sarafotoxin 6c
References
- ADNER M., SHANKLEY N., EDVINSSON L. Evidence that ET-1, but not ET-3 and S6b, ETA receptor mediated contractions in isolated rat mesenteric arteries are modulated by co-activation of ETB receptors. Br. J. Pharmacol. 2001;133:927–935. doi: 10.1038/sj.bjp.0704135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAX W.A., AGHAI Z., VAN TRICHT C.L., WASSENAAR C., SAXENA P.R. Different endothelin receptors involved in endothelin-1- and sarafotoxin S6B-induced contractions of the human isolated coronary artery. Br. J. Pharmacol. 1994;113:1471–1479. doi: 10.1111/j.1476-5381.1994.tb17162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREMNES T., PAASCHE J.D., MEHLUM A., SANDBERG C., BREMNES B., ATTRAMADAL H. Regulation and intracellular trafficking pathways of the endothelin receptors. J. Biol. Chem. 2000;275:17596–17604. doi: 10.1074/jbc.M000142200. [DOI] [PubMed] [Google Scholar]
- CALO G., GRATTON J.P., TELEMAQUE D'ORLEANS-JUSTE P., REGOLI D. Pharmacology of endothelins: vascular preparations for studying ETA and ETB receptors. Mol. Cell. Biochem. 1996;154:31–37. doi: 10.1007/BF00248458. [DOI] [PubMed] [Google Scholar]
- COCKS T.M.J., FAULKNER N.L., SUDHIR K., ANGUS J. Reactivity of endothelin-1 on human and canine large veins compared with larger arteries in vitro. Eur. J. Pharmacol. 1989;171:17–24. doi: 10.1016/0014-2999(89)90425-1. [DOI] [PubMed] [Google Scholar]
- D'ORLEANS-JUSTE P., FINET M., DE NUCCI G., VANE J.R. Pharmacology of endothelin-1 in isolated vessels: effect of nicardipine, methylene blue, hemoglobin and gossypol. J. Cardiovasc. Pharmacol. 1989;13 Suppl 5:19–22. [PubMed] [Google Scholar]
- DOUGLAS S.A., BECK G.R., ELLIOTT J.D., OHLSTEIN E. Pharmacological evidence for the presence of three distinct functional endothelin receptor subtypes in the rabbit lateral saphenous vein. Br. J. Pharmacol. 1995;113:1529–1540. doi: 10.1111/j.1476-5381.1995.tb14936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGUCHI D., NISHIMURA J., KOBAYASHI S., KOMORI K., SUGIMACHI K., KANAIDE H. Down-regulation of endothelin-B receptors in autogenous saphenous veins grafted into the arerial circulation. Cardiovasc. Res. 1997;35:360–367. doi: 10.1016/s0008-6363(97)00103-x. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ N., MONGE L., GARCIA-VILLALON A.L., GARCIA J.L., GOMEZ B., DIEGUEZ G. Endothelin-1-induced in vitro cerebral venoconstriction is mediated by endothelin ETA receptors. Eur. J. Pharmacol. 1995;294:483–490. doi: 10.1016/0014-2999(95)00577-3. [DOI] [PubMed] [Google Scholar]
- FINK G.D., BALLEW J.R., GALLIGAN J.J. Role of ETA receptor in hypertension secondary to ETB receptor stimulation or antagonisms in conscious rats. Hypertension. 2001;38:518. [Google Scholar]
- FINK G.D., JOHNSON R.J., GALLIGAN J.J. Mechanisms of increased venous smooth muscle tone in desoxycorticosterone acetate-salt hypertension. Hypertension. 2000;35:464–469. doi: 10.1161/01.hyp.35.1.464. [DOI] [PubMed] [Google Scholar]
- FUJITA K., MATSUMURA Y., KITA S., MIYAZAKI Y., HISAKI K., TAKAOKA M., MORIMOTO S. Role of endothelin-1 and the ETA receptor in the maintenance of deoxycorticosterone acetate salt induced hypertension. Br. J. Pharmacol. 1995;114:925–930. doi: 10.1111/j.1476-5381.1995.tb13292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKURODA T., OZAKI S., IHARA M., ISHIKAWA K., YANO M., MIYAUCHI T., ISHIKAWA S., ONIZUKA M., GOTO K., NISHIKIBE M. Necessity of dual blockade of endothelin ETA and ETB receptor subtypes for antagonism of endothelin-1-induced contraction in human bronchi. Br. J. Pharmacol. 1996;117:959–999. doi: 10.1111/j.1476-5381.1996.tb16688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIULUMIAN A.D., MOLERO M.M., REDDY V.B., POLLOCK J.S., POLLOCK D.M., FUCHS L.C. Role of ET-1 receptor binding and [Ca(2+)](i) in contraction of coronary arteries from DOCA-salt hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1944–H1949. doi: 10.1152/ajpheart.00627.2001. [DOI] [PubMed] [Google Scholar]
- HANSON G.C., ANDERSSON K.E., GYLLSTEDT E., HOGESTATT E.D., LINDBERG B.F. Hydrolysis of big endothelin-1 by a serine protease in the membrane fraction of human lung. Regulatory Peptides. 1997;68:63–69. doi: 10.1016/s0167-0115(96)02105-2. [DOI] [PubMed] [Google Scholar]
- JOHNSON R.J., GALLIGAN J.J., FINK G.D. Factors affecting endothelin-induced venous tone in conscious rats. J. Cardiovasc. Pharmacol. 2001;37:187–195. doi: 10.1097/00005344-200102000-00006. [DOI] [PubMed] [Google Scholar]
- JOHNSON R.J., FINK G.D., WATTS S.W., GALLIGAN J.J. Endothelin receptor function in mesenteric veins from deoxycorticosterone acetate-salt hypertensive rats. J. Hypertension. 2002;20:665–676. doi: 10.1097/00004872-200204000-00024. [DOI] [PubMed] [Google Scholar]
- KARAKI H., SUDJARWO S.A., HORI M., SAKATA K., URADE Y., TAKAI M., OKADA T. ETB receptor antagonist, IRL 1038, selectively inhibits the endothelin-induced endothelium-dependent vascular relaxation. Eur. J. Pharmacol. 1993;231:371–374. doi: 10.1016/0014-2999(93)90112-u. [DOI] [PubMed] [Google Scholar]
- KITA S., TAGUCHI Y., MATSUMURA Y. Endothelin-1 enhances pressor responses to noradrenaline: involvement of the endothelin-B receptor. J. Cardiovasc. Pharmacol. 1998;31:S119–S121. doi: 10.1097/00005344-199800001-00036. [DOI] [PubMed] [Google Scholar]
- LARIVIERE R., THIBAULT G., SCHIFFRIN E.L. Increased endothelin-1 content in blood vessels of deoxycorticosterone acetate-salt hypertensive but not in spontaneously hypertensive rats. Hypertension. 1993;21:294–300. doi: 10.1161/01.hyp.21.3.294. [DOI] [PubMed] [Google Scholar]
- LAURANT P., BERTHELOT A. Endothelin-1-induced contraction in isolated aortae from normotensive and DOCA-salt hypertensive rats: effect of magnesium. Br. J. Pharmacol. 1996;119:1367–1374. doi: 10.1111/j.1476-5381.1996.tb16048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI J.S., LARIVIERE R., SCHIFFRIN E.L. Effect of a nonselective endothelin antagonist on vascular remodling in deoxycorticosterone acetate-salt hypertensive rats. Evidence for a role of endothelin in vascular hypertrophy. Hypertension. 1994;24:183–188. doi: 10.1161/01.hyp.24.2.183. [DOI] [PubMed] [Google Scholar]
- LOCKETTE W., OTSUKA Y., CARRETERO O. The loss of endothelium-dependent vascular relaxation in hypertension. Hypertension. 1986;8 Suppl II:61–66. doi: 10.1161/01.hyp.8.6_pt_2.ii61. [DOI] [PubMed] [Google Scholar]
- LODGE N.J., ZHANG R., HALAKA N.N., MORELAND S. Functional role of endothelin ETA and ETB receptors in venous and arterial smooth muscle. Eur. J. Pharmacol. 1995;287:279–285. doi: 10.1016/0014-2999(95)00494-7. [DOI] [PubMed] [Google Scholar]
- MAGAZINE H.I., SRIVASTAVA K.D. Thrombin-induced vascular reactivity is modulated by ETB receptor-coupled nitric oxide release in rat aorta. Am. J. Physiol. 1996;271:C923–C928. doi: 10.1152/ajpcell.1996.271.3.C923. [DOI] [PubMed] [Google Scholar]
- MAGUIRE J.J., KUC R.E., DAVENPORT A.P. Vasoconstrictor activity of novel endothelin peptide, ET-1(1–31), in human mammary and coronary arteries in vitro. Br. J. Pharmacol. 2001;134:1360–1366. doi: 10.1038/sj.bjp.0704384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUMURA Y., HASHIMOTO N., TAIRA S., KURO T., KITANO R., OHKITA M., OPGENORTH T.J., TAKAOKA M. Different contributions of endothelin-A and endothelin-B receptors in the pathogenesis of deoxycorticosterone acetate-salt-induced hypertension in rats. Hypertension. 1999;33:759–765. doi: 10.1161/01.hyp.33.2.759. [DOI] [PubMed] [Google Scholar]
- MICKLEY E.J., GRAY G.A., WEBB D.J. Activation of endothelin ETA receptors masks the constrictor role of endothelin ETB receptors in rat isolated small mesenteric arteries. Br. J. Pharmacol. 1997;120:1376–1382. doi: 10.1038/sj.bjp.0701036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGATA N., NIWA Y., NAKAYA Y. A novel 31-amino acid length endothelin, ET-1 (1–31), can act as a biologically active peptide for vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2000;275:595–600. doi: 10.1006/bbrc.2000.3292. [DOI] [PubMed] [Google Scholar]
- NGUYEN P., PARENT A., DENG L., FLUCKIGER J.P., THIBAULT G., SCHIFFRIN E. Endothelin vascular receptors and responses in deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 1992;19:98–104. doi: 10.1161/01.hyp.19.2_suppl.ii98. [DOI] [PubMed] [Google Scholar]
- PERRY M.G., MOLERO M.M., GIULUMIAN A.D., KATAKAM P.V., POLLOCK J.S., POLLOCK D.M., FUCHS L.C. ET(B) receptor-deficient rats exhibit reduced contraction to ET-1 despite an increase in ET(A) receptors. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H2680–H2686. doi: 10.1152/ajpheart.2001.281.6.H2680. [DOI] [PubMed] [Google Scholar]
- PIERRE L.N., DAVENPORT A.P. Relative contribution of endothelin A and endothelin B receptors to vasoconstriction in small arteries from human heart and brain. J. Cardiovasc. Pharmacol. 1998;31 Supp l:S74–S76. doi: 10.1097/00005344-199800001-00024. [DOI] [PubMed] [Google Scholar]
- POLLOCK D.M. Contrasting pharmacological ETB receptor blockade with genetic ETB deficiency in renal responses to big ET-1. Physiol. Genomics. 2001;6:39–43. doi: 10.1152/physiolgenomics.2001.6.1.39. [DOI] [PubMed] [Google Scholar]
- SATO K., OKA M., HASUNUMA K., OHNISHI M., SATO K., KIRA S. Effects of separate and combined ETA and ETB blockade on ET-1-induced constriction in perfused rat lungs. Am. J. Physiol. 1995;269:L668–L672. doi: 10.1152/ajplung.1995.269.5.L668. [DOI] [PubMed] [Google Scholar]
- SCHIFFRIN E.L. Role of endothelin-1 in hypertension and vascular disease. Am. J. Hypertens. 2001;14:83S–89S. doi: 10.1016/s0895-7061(01)02074-x. [DOI] [PubMed] [Google Scholar]
- SHIMAMOTO H., BOURREAU J.P., KWAN C.Y., DANIEL E.E. Amplification of alpha adrenergic vasoconstriction in canine isolated mesenteric artery and vein. J. Pharmacol. Exp. Ther. 1992;260:1119–1127. [PubMed] [Google Scholar]
- SUDJARWO S.A., KARAKI H. Role of protein kinase C in the endothelin-induced contraction in the rabbit saphenous vein. Eur. J. Pharmacol. 1995;294:261–269. doi: 10.1016/0014-2999(95)00542-0. [DOI] [PubMed] [Google Scholar]
- TEERLINK J.R., BREU V., SPRECHER U., CLOZEL M., CLOZEL J.-P. Potent vasoconstriction mediated by endothelin ETB receptors in canine coronary arteries. Circ. Res. 1994;74:105–114. doi: 10.1161/01.res.74.1.105. [DOI] [PubMed] [Google Scholar]
- VERHAGEN A.M., RABELINK T.J., BRAAM B., OPGENORTH T.J., GRONE H.J., KOOMANS H.A., JOLES J.A. Endothelin A receptor blockade alleviates hypertension and renal lesions associated with chronic nitric oxide synthase inhibition. J. Am. Soc. Nephrol. 1998;9:755–762. doi: 10.1681/ASN.V95755. [DOI] [PubMed] [Google Scholar]
- WANG H., CHEN A.F., WATTS S.W., GALLIGAN J.J., FINK G.D. Endothelin-1 content in arteries and veins of DOCA-salt hypertensive rats. FASEB J. 2002;16:A382.31. [Google Scholar]
- WANG H.G., SHIBAMOTO T., MIYAHARA T. Endothelin-1 selectively contracts portal vein through both ETA and ETB receptors in isolated rabbit liver. Am. J. Physiol. 1997;273:G1036–G1043. doi: 10.1152/ajpgi.1997.273.5.G1036. [DOI] [PubMed] [Google Scholar]
- WHITE D.G., CANNON T.R., GARRATT H., MUNDIN J.W., SUMNER M.J., WATTS I.S. Endothelin ETA and ETB receptors mediate vascular smooth muscle contraction. J. Cardiovasc. Pharmacol. 1993;22:S144–S148. doi: 10.1097/00005344-199322008-00039. [DOI] [PubMed] [Google Scholar]
- ZHAO H., JOSHUA I.G., PORTER J.P. Microvascular responses to endothelin in deoxycorticosterone acetate–salt hypertensive rats. Am. J. Hypertens. 2000;13:819–826. doi: 10.1016/s0895-7061(00)00260-0. [DOI] [PubMed] [Google Scholar]


